Diabetes and hypertension are the leading causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD), but little is known about the genes involved. The present study by Dhande et al.1 validated two regions of the genome that together completely account for CKD in Stroke-prone Spontaneously Hypertensive Rats (SpSHR). This study is highly significant since one in seven Americans have CKD, and the Medicaid costs for treatment of patients with ESRD and CKD are 34 and 64 billion dollars/yr., respectively.2 Most patients with mild or moderate hypertension do not develop proteinuria or significant renal injury indicating that there are differences in the genetic susceptibility.3,4 Renoprotection is also seen in Spontaneously Hypertensive Rats (SHR) and angiotensin-dependent rodent models of hypertension that is associated with an elevation in renal vascular resistance that prevents transmission of systemic pressure to the glomerular capillaries.3,4 However, renal autoregulation is often impaired in patients with diabetes and African Americans with low renin forms of hypertension that exhibit increased susceptibility to proteinuria and glomerulosclerosis in response to even modest elevations in pressure.3,4 Thus, there is tremendous interest in defining the genes and pathways that influence the susceptibility to hypertension-induced renal injury. Whole genome-wide association studies (GWAS) have uncovered susceptibility loci on nearly every chromosome.5 However, progress in identifying the causal genes remains limited. To date, only variants in apolipoprotein 1 and cubulin have been found to elevate the risk of CKD in African Americans, and albuminuria in diabetic patients.5
Genetic mapping studies using strains of rats that are susceptible (Fawn-Hooded Hypertensive, Dahl salt-sensitive, Buffalo, Munich Wistar Furth, and SpSHR) and resistant (Spontaneously hypertensive, Brown-Norway, Wistar Kyoto, Lewis, August Copenhagen Irish rats) to renal disease have identified many genomic regions associated with glomerular injury, proteinuria, and renal interstitial fibrosis.5 However, the only molecular variant that has yet to be confirmed by transgenic rescue is a loss of function mutation in the Rab38 gene that inhibits reabsorption of filtered protein in Fawn-Hooded Hypertensive (FHH) rats.6 Part of the difficulty in identifying the causal genes in previous studies is that the susceptible and resistant strains were chosen to maximize genetic diversity, so the number of potential sequence variants in candidate regions is overwhelming. An alternative approach taken in the present study by Dhande et al.1 was to use closely-related SpSHR (SHR-A3 in Dhande et al.1) and SHR (SHR-B2 in Dhande et al.1) as the susceptible and resistant strains. These strains were derived during the development of SHR by inbreeding a subline susceptible to stroke after the alleles for hypertension were fixed.1,5 As such, they are genetically identical across 87% of the genome and differ in only 121 distinct haplotype blocks which greatly simplifies gene mapping.7 In a previous study, this group headed by Dr. Doris, reported that a 10 Mb haplotype block on chromosome 17 was linked to differences in blood pressure in an F2 cross of SpSHR and SHR rats.7 In another study, they identified a highly polymorphic 9 Mb region on chromosome 6, harboring 230 genes encoding for heavy chain immunoglobulins (IgH) of which 49% exhibited non-synonymous variation in SpSHR versus SHR.8 This was associated with differences in the expression of IgG isotypes and increased renal injury in SpSHR that was ameliorated by immunosuppression.8 The authors hypothesized that variants in the IgH genes on chromosome 6 might contribute to renal injury in SpSHR by altering B cell and immune function.8
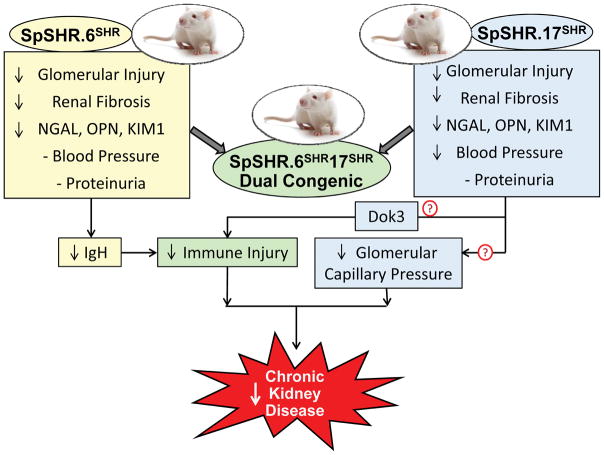
In the present study, Dhande et al.1 introgressed the regions on chromosomes 6 and 17 from SHR into the SpSHR genetic background and phenotyped the congenic strains for blood pressure and renal injury. As summarized in Figure 1, they found that glomerular injury and renal interstitial fibrosis, but not proteinuria, were lower in the SpSHR.6SHR congenic strain than in SpSHR rats. Blood pressure was not altered in the SpSHR.6SHR congenic strain. Glomerular injury and renal interstitial fibrosis, but not proteinuria, were also reduced in the SpSHR.17SHR congenic strain, and blood pressure fell to the same level as that seen in SHR. Transfer of both regions in a dual congenic strain (SpSHR.6SHR17SHR), lowered blood pressure, glomerular injury, renal interstitial fibrosis and the excretion of urinary biomarkers of renal tubular injury to the same levels seen in SHR. These results confirm that a gene variant on chromosome 17 is responsible for the greater degree of hypertension and renal injury in SpSHR, and that one or more of the genes in the IgH region on chromosome 6 contribute to renal injury in SpSHR. The authors further established that the altered expression of IgG subtypes in SpSHR was normalized in the chromosome 6 congenic strain. This finding supports the view that the genes in this region enhance renal injury in SpSHR by altering B and T cell function and the immune response. The authors also found that there are nonsynonymous sequence differences in 9 of the 74 genes on chromosome 17 in SpSHR versus SHR. They suggested that a variant found in the DoK3 in SpSHR was of particular interest as it participates in the immune response. However, the remaining candidate genes in this region do not appear to be involved in immune signaling, suggesting that another renoprotective mechanism might be involved. In this regard, previous studies have indicated that enhanced renal myogenic response and renal blood flow autoregulation protect the glomerular circulation from barotrauma up to 180 mmHg in SHR.3,4 Blood pressure exceeds this limit in SpSHR, and there is evidence that renal and cerebral autoregulation maybe impaired in this strain.9 Thus, the renoprotection in the chromosome 6 congenic strain may simply be secondary to the fall in blood pressure. This is consistent with a previous finding that reducing blood pressure below a critical level of 190 mmHg can prevent and even reverse renal injury in SpSHR.10
Figure 1.
Summary of the effects of transfer of regions of chromosomes 6 (SpSHR.6SHR) and 17 (SpSHR.17SHR) from Spontaneously Hypertensive Rats (SHR, SHR-B2 in Dhande et al.1) to Stroke-prone SHR (SpSHR, SHR-A3 in Dhande et al.1) on blood pressure and renal injury. Transfer of these regions lowered glomerular injury, renal interstitial fibrosis and urinary excretion of neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN) and kidney injury molecule 1 (KIM1), but had no effect on proteinuria. Blood pressure was reduced in the SpSHR.17SHR and SpSHR. 6SHR17SHR dual congenic rats but not SpSHR.6SHR. The fall in renal injury in the SpSHR.17SHR congenic strain might be due to the reduction of blood pressure back into the autoregulatory range and a reduction in glomerular capillary pressure (Pgc). The renoprotection afforded by chromosome 6 is likely due to variants in IgH genes that influence B cell and immune function.
The most unexpected finding in the present study is that while transfer of genes on chromosome 6 and 17 from SHR normalized blood pressure, glomerulosclerosis, and renal interstitial fibrosis in SpSHR, they had no effect on the urinary albumin/creatinine concentration ratio. This suggests that proteinuria in SpSHR is determined by genes on other chromosomes, and is consistent with previous results that identified loci for proteinuria in SpSHR on chromosomes 1, 4, 10 and 16.5 The failure to detect a fall in proteinuria in the congenic strains is also hard to understand as it is likely that the reduction in glomerulosclerosis lowered the filtration of albumin. This suggests albuminuria in SpSHR may not be caused by glomerular injury alone, but may be related to a progressive loss of the ability to reabsorb filtered protein as seen in FHH rats6 and some diabetic models.4
Overall, the present study is an important step forward in the search for genes that contribute to hypertension-induced nephrosclerosis. It confirms that a region on chromosome 17 is responsible for the higher blood pressure in SpSHR than SHR, and that genes on chromosomes 6 and 17 mediate the enhanced susceptibility to renal injury, but not proteinuria in SpSHR. The next step will be to identify and validate the sequence variants involved using subcongenic or transgenic rescue strains. Discovery of these causal genes for renal injury could lead to novel therapies to slow or even reverse the progression of CKD in patients with hypertension.
Acknowledgments
Sources of Funding
This work was partially supported by grants DK104184, AG050049 and GM104357 from the National Institutes of Health and 16GRNT31200036 from the American Heart Association.
Footnotes
Disclosures
None
The opinions expressed in this editorial are not necessarily in line with those of the editors of Hypertension.
References
- 1.Dhande IS, Cranford SM, Zhu Y, Kneedler SC, Hicks J, Wenderfer SE, Braun MC, Doris PA. Susceptibility to hypertensive renal disease in the spontaneously hypertensive rat is influenced by two loci affecting blood pressure and the immunoglobulin repertoire. HYPERTENSION. 2018 doi: 10.1161/HYPERTENSIONAHA.117.10593. XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. [Google Scholar]
- 3.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–8. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol. 2014;12:845–58. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz A, Kreutz R. Mapping genetic determinants of kidney damage in rat models. Hypertens Res. 2012;35:675–94. doi: 10.1038/hr.2012.77. [DOI] [PubMed] [Google Scholar]
- 6.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol. 2005;16:852–6. doi: 10.1681/ASN.2005010029. [DOI] [PubMed] [Google Scholar]
- 7.Bell R, Herring SM, Gokul N, Monita M, Grove ML, Boerwinkle E, Doris PA. High-resolution identity by descent mapping uncovers the genetic basis for blood pressure differences between spontaneously hypertensive rat lines. Circ Cardiovasc Genet. 2011;4:223–31. doi: 10.1161/CIRCGENETICS.110.958934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun MC, Herring SM, Gokul N, Monita M, Bell R, Zhu Y, Gonzalez-Garay ML, Wenderfer SE, Doris PA. Hypertensive renal injury is associated with gene variation affecting immune signaling. Circ Cardiovasc Genet. 2014;7:903–10. doi: 10.1161/CIRCGENETICS.114.000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Davis G, Johns EJ. Effect of nitrendipine on autoregulation of perfusion in the cortex and papilla of kidneys from Wistar and stroke-prone spontaneously hypertensive rats. Br J Pharmacol. 1994;111:111–116. doi: 10.1111/j.1476-5381.1994.tb14031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin KA, Polichnowski A, Litbarg N, Picken M, Venkatachalam MA, Bidani AK. Critical blood pressure threshold dependence of hypertensive injury and repair in a malignant nephrosclerosis model. Hypertension. 2014;64:801–7. doi: 10.1161/HYPERTENSIONAHA.114.03609. [DOI] [PMC free article] [PubMed] [Google Scholar]