Abstract
Metabolomics is maturing as an experimental approach in nutrition science, and it is a useful analysis for revealing systems biology outcomes associated with changes in diet. A major goal of this review is to present the rapidly evolving body of scientific literature that seeks to reveal connections between an individual’s metabolic profile and experimentally manipulated or naturally varied dietary intakes. Metabolite profiles in tissue, serum, urine, or stool reflect changes in metabolic pathways that respond to dietary intervention which makes them accessible samples for revealing metabolic effects of diet. Three broadly defined areas of investigation related to dietary-metabolomic strategies include: (1) describing the metabolite variation within and between dietary exposures or interventions; (2) characterizing the metabolic response to dietary interventions with respect to time; and (3) assessing individual variation in baseline nutritional health and/or disease status. An overview of metabolites that were responsive to dietary interventions as reported from original research in human or animal studies is provided and illustrates the breadth of metabolites affected by dietary intervention. Advantages and drawbacks for assessing metabolic changes are discussed in relation to types of metabolite analysis platforms. A combination of targeted and non-targeted global profiling studies as a component of future dietary intervention trials will increase our understanding of nutrition in a systems context.
Keywords: Nutrition, metabolomics, dietary intervention, metabolism, analytical platforms
INTRODUCTION
Nutritional metabolomics has emerged as a high-throughput and sensitive approach to identify and characterize biochemical pathways that underlie complex relationships between dietary exposures and chronic diseases with altered metabolic phenotypes [1–3]. The ability to identify novel correlations between dietary patterns and health, or between consumption of specific foods and disease-related outcomes has presented challenges due to individual variability in complex metabolic pathways and digestion, as well as novel metabolite identification [4, 5]. Food-omics refers to the metabolite profiling of foods prior to consumption [6, 7], while nutritional metabolomics has emerged with two major goals: (1) to determine the effects of dietary compounds on host metabolism after consumption for a defined period of time, and (2) to identify dietary intake or phytochemical dose-dependent associated metabolite biomarkers.
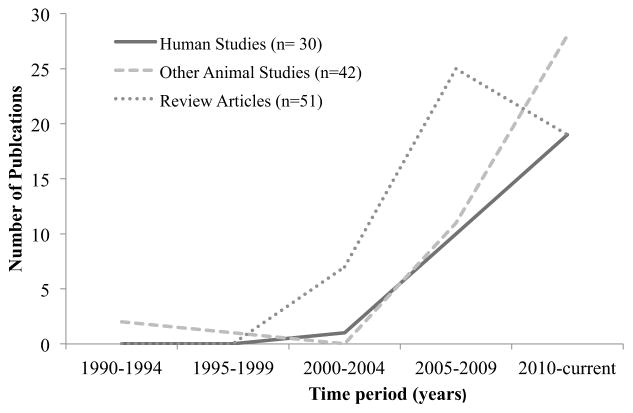
The number of nutritional metabolomics studies has substantially increased in the last decade, as evidenced by the number of original research articles cited in Pubmed Central with the terms ‘metabolome’ and ‘diet’ (Fig. 1). The solid line illustrates the number of reports from human studies, the dashed line represents papers reporting metabolite data across animal models (e. g. rodents, pigs, dogs etc.), and the dotted line shows review articles published in this field. Fig. (1) demonstrates an increasing trend in nutritional metabolomics investigations and future studies will benefit from a critical synthesis of this data prior to conducting new experiments. For example, dietary exposure studies are designed to improve our understanding of disease-fighting and health-promoting properties of medical foods, phytochemicals, food ingredients, food-associated toxicants, and dietary patterns. The results of such studies can guide future dietary intervention studies that seek to examine mechanistic relationships between metabolic changes and healthy organs or diseased tissues. Unfortunately, the nutritional research community lacks a synthesis of original nutritional metabolome data across trial designs, species, biological samples and analytical platforms. Previous review articles have largely emphasized the opportunities, limitations and importance of diet-metabolome research. In this review, we summarize nutritional metabolome data from the literature. As further discussed below, the literature reveals a breadth of metabolites influenced in dietary intervention studies. Thus, a renewed focus for nutritional metabolome investigations should include both targeted (biased to a select group of metabolites) and non-targeted (analysis of all detectable metabolites) studies. We discuss the application of metabolomics to nutritional investigations with a focus on experimental design and biological interpretation unique to food components, including the influence of the gut microbiome.
Fig. 1. Number of publications on “metabolomics and diet”* cited in PubMed Central (n= 123).
The following search criteria was used in PubMed Central: ‘metabolome AND diet’ and ‘fecal metabolites AND diet’. Review articles also included summaries from conferences and opinion articles.
OVERVIEW OF NUTRITIONAL METABOLOMICS LITERATURE
A review of the literature was performed for nutritional metabolomics studies targeted toward metabolites detected in urine, blood, and stool (Tables 1–3, respectively). Diet responsive metabolites included nucleotides, sterols, lipids, carbohydrates, and amino acids. This collection of metabolites was derived from animal and human studies, which spanned a variety of dietary exposures and represented both acute and long-term changes. Bolded metabolites in all three tables indicate those metabolites that were classified as ‘diet modifiable’ in three or more distinct, independent studies.
Table 1.
Dietary Modulation of the Urine Metabolome
| Sample | Metabolites Identified by Platform
|
|||
|---|---|---|---|---|
| GC-MS | LC-MS | NMR | Other* | |
|
| ||||
| Urine | Hippurate | Trimethylamine-N-oxide | Proline | Niacin |
| 4-hydroxphenylacetic acid | Phenylalanine | Betaine | Proline | |
| Tartrate | Histidine | 4- hydroxyhippurate | Betaine | |
| Ethanol | Citrate | Fumarate | Hesperidin | |
| Mannitol | Acetaminophen | Lactate | Narirutin | |
| 3-methyl-oxovalerate | Acetate | Glucose | ||
| Nitrogen | Choline | Glycine | ||
| Creatinine | Phenylacetylglutamine | Methylamine | ||
| Succinate | Taurine | Phenylacetylglycine | ||
| Putrescine | Methionine | Formate | ||
| Threonine | Urocanate | Branched Chain Amino Acids | ||
| 3-hydroxyisovalerate | Sucrose | P-cresolsulfate | ||
| Arginine | Cis-aconitate | Trigonelline | ||
| Acetone | Methylhistidine | Theobromine | ||
| N (1) -methyl-2-pyridone-5-carboxylamide (PYR) | Dimethylsulfone | Caffeine | ||
| Tyrosine | Prolinebetaine | |||
| Ascorbate derivatives | Hydroxynicotnic acid | Hesperidin | ||
| 4-cresylsulfate | Urolithin B glucuronide | Narirutin | ||
| S-methyl-l-cysteine sulfoxide | Urolithin A | β-aminoisobutyrate | ||
| Urolithin B | Oxodecanoic acid | |||
| 2-oxoglutarate | Acylcarnitines | |||
| Fumarate | Creatinine | |||
| Hippurate | Trimethylamine-N-oxide | |||
| Lactate | Hippurate | |||
| Creatinine | Phemylacetylglutamine | |||
| Succinate | Acetone | |||
|
| ||||
| References | [59, 60] | [9, 59, 61, 62] | [11, 14, 17, 22, 24, 63–66] | [67–69] |
Other Platforms used in Identifying Metabolites: ICP-OES: Inductively Coupled Plasma Spectroscopy, DAD-MS/MS: Diode Array Detector Mass Spectrometry, FIEI-MS: Flow Injection Electrospray-ionisation.
Table 3.
Dietary Modulation of the Fecal Metabolome
| Sample | Metabolites Identified by Platform
|
|||
|---|---|---|---|---|
| GC-MS | LC-MS | NMR | Other | |
|
| ||||
| Stool | Galactonic Acid | 9-octadecenoic Acid | Propanoic Acid | Cholic Acid |
| E. rectale | Lithocholic acid | Fumaric Acid | Deoxycholic Acid | |
| Coprostanol | Deoxycholic acid | Glycine | Lithocholic Acid | |
| Cholestanone | β-muricholic acid | Homocysteine | Hyodeoxycholic Acid | |
| Deoxycholic acid | Chenodeoxycholic acid | Enterobacteriaceae | ||
| Betahyocholic acid | Benzaldehyde | Cholic acid | ||
| Lithocholic acid | Urolithin A Cytotoxic | Hyodeoxycholic acid | ||
| Cholic acid | haem metabolite (haem factor) | Valerate | ||
| Benzoic acid | Isovalerate | |||
| Acetophenones | Isobutyrate | |||
| Cholesterol | Lactate | |||
| Cholestanol | Benzene | |||
| Coprostanone | Acetic Acid | |||
| Cholestenone | Butonic Acid | |||
| Glutamic Acid | ||||
| Alanine | ||||
|
| ||||
| References | [51, 77] | [29, 78] | [33, 79] | [33, 67, 79–81] |
Other Platforms used in Identifying Metabolites: ICP-OES: Inductively Coupled Plasma Spectroscopy, DAD-MS/MS: Diode Array Detector Mass Spectrometry, FIEI-MS: Flow Injection Electrospray-ionisation.
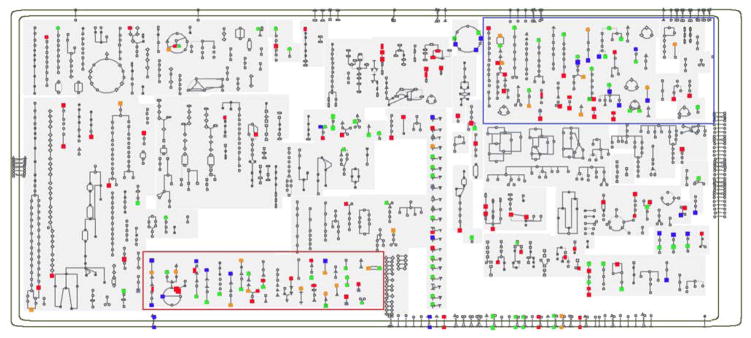
All of the metabolites reported in the tables were overlaid onto the Meta Cyc human metabolism pathway map (Fig. 2). This metabolic mapping overview visualizes the extent of metabolism that is influenced by the diet, suggesting that narrowing analytical focus to a short list of target metabolites could prevent detection of important, sensitive, and subtle metabolic reactions that lie outside the scope of the targeted list.
Fig. 2.
The metabolites listed in the tables were overlaid onto the core metabolic map offered at Meta Cyc for Homo sapiens. Red points represent diet-responsive metabolites in serum, blue points in urine, and orange points in fecal samples. Green points represent metabolites that were found to be diet-responsive in two or more biofluids. Particularly rich coverage is provided in amino acid metabolism (red box) and catabolism (blue box).
EXPERIMENTAL DESIGN IN NUTRITIONAL METABOLOMICS
While some may have portrayed omics experiments as descriptive, ‘fishing expeditions’, a proper experimental design allows for both hypothesis-driven research, as well as generating new hypotheses. Experimental design is particularly essential for nutritional metabolomics research given the thousands of monitored metabolites and the biological variation inherent to clinical studies [8]. As an example, Xu et al. recently reported the results of a study using 1H NMR spectroscopy to assess the effects of diet (lactovegetarian or omnivorous) and gender (male or female) on the urinary metabolome [9]. It was found that the most influential low molecular weight metabolites responsible for the differences between the diet groups were N-acetyl glycoprotein (NAG), succinate, citrate, trimethylamine-N-oxide (TMAO), taurine, glycine, hippurate, phenylalanine, methylhistidine and formate. The study was sufficiently powered and enabled the distinction between diet and gender effects on the metabolome. Often, however, metabolite studies are underpowered. In such a case, while metabolite variation would have been noted, the data would not have been able to support the influence of diet and gender found in the study [9]. Below, we discuss important considerations in experimental design for nutritional metabolomics studies including study size, study controls, time-points, sample matrix, individual metabolite variation, and the influence of the gut microbiome.
Study Size
The minimum number of biological replicates for statistically significant evaluation of a metabolomic dataset is determined based on pilot data and/or previously reported variation. Typically, the number of replicates required for a given statistical power should be estimated based on the variance and magnitude of the hypothesized response variable. In the metabolomics setting, the parameters (fold-change and variance) are dependent on both the metabolite and instrument platform. Thus, the number of replications is generally driven by study limitations such as subject availability and experimental costs, though increased numbers of biological replication will always provide higher confidence and statistical power. Biological replication should not be confused with analytical replication, in which a given extract of a sample is measured several times on the same instrument. Analytical replication is performed to address variation induced by the metabolite detection platform, and involves the collection of duplicate or triplicate data for each biological sample. Biological replication requires sampling from several subjects (recommended absolute minimum n=5 replicates), followed by independent metabolite extraction and data acquisition of those samples. These two sources of variation are independent, and analytical (instrumental or measurement) variation is typically considerably smaller than biological variation [10].
Study Controls
The scientific discovery of meaningful and reproducible metabolome changes in response to dietary interventions requires consistent, detectable metabolite levels, and also a clear understanding of variation in a baseline metabolite profile. Statistical analyses can articulate a change in a single metabolite over a personal baseline, which may be unique for each individual. The identification of statistically significant changes in a dietary treatment group relative to a control group can be more complex [11, 12].
Dietary intervention studies can be inherently difficult to control when compared to drug treatment trials because there is not a true placebo. Nutrients and phytochemicals have been shown to have less functional relevance when isolated for use as single agents, and rather exist as a complex network of essential and nonessential components [13]. In lieu of a true placebo, control groups are commonly comprised of non-intervention participants that follow existing dietary recommendations and guidelines. A recent example comes from a parallel intervention trial with 5 dietary intervention groups where study participants were randomly assigned to a 6-month low-fat diet that differed by various combinations of low/high glycemic index and low/high protein [14]. The control group did not follow any specific glycemic index recommendations. The goal of this trial was to evaluate the impact of dietary protein and glycemic index on weight (re) gain in a large number of families that suffer from obesity or overweight [15], yet the control participant metabolite profiles were excluded from the metabolomic investigation due to variation in glycemic load of the diet [14]. Thus, the design of this study exemplifies the problem of introducing bias when estimating the intervention’s true “metabolite effect” because the natural “non-intervention” metabolomic variation was not characterized. Although the study revealed that urinary hippurate was associated with dietary fiber intake at a group level in this population, we also know that this urine metabolite may be changed to some extent in a control population as a microbial byproduct and result of flavonoid metabolism (Table 1) [16].
Time-points
For nutritional studies, metabolite variation is assessed at multiple timepoints to consider the time-dependent nature of responses to a nutritional intervention. While the dynamics of the response are the most obvious motivation for monitoring several timepoints, additional sampling provides an opportunity to visualize trends and increase confidence in the results, particularly when the effect observed may be subtle. Furthermore, the results are more robust when proper study participant controls are included at each time point [17, 18]. Terminology regarding study duration is an essential distinction for reporting diet-affected metabolites, and is referred to herein as a short-term (acute, transient response) or a long-term response (chronic metabolic phenotype). Examples of short-term experimental designs have consisted of metabolite assessments after a few hours to 28 days [19, 20]. Long-term trial designs include samples collected for analysis after one month to years post-intervention [21, 22]. These long-term trials represent metabolite assessments that incorporate the complex interactions between diet-derived phytochemicals and nutrients, hormone flux, gut microbiota and temporal and spatial metabolite variations [23]. Limited evidence exists for assessing multiple timepoints in the same trial, restricting the ability to perform comparisons that allow classification of metabolites as either short-term or long-term responders [24, 25].
Sample Matrix
Blood plasma, serum, urine, stool, saliva, muscle, and liver metabolomes may all reflect different aspects of dietary intakes and responses [17, 26–29]. Thus, the rationale for selecting a particular biofluid to extract for analysis will be essential for biological interpretation of the observed metabolome. For example, if the goal of the study is to examine bioavailability, serum is traditionally assessed given the transmission of metabolites from food to gut to liver to blood. However, urine and/or stool may be more appropriate to evaluate degradation or detoxification pathways. Furthermore, as discussed in more detail below, the fecal metabolome might offer otherwise elusive insight into the response of the microbiome to nutritional interventions [19, 29–34].
The benefit of including multiple biofluids is illustrated in a recent study that showed that cluster analysis of blood and urine identified 3 distinct dietary patterns on the basis of the energy contribution assessment of different food groups in 160 individuals [35]. The combination of three-day diet records, plasma fatty acid profiles and 1H NMR spectra of urine metabolites were used to evaluate associations with the intake of specific food groups. Specifically, there were fatty acid profiles across percentiles of red-meat intake that showed significant differences in plasma oleic acid concentrations, and increased urinary O-acetylcarnitine content in the red-meat diet group. Oleic acid has been typically associated with olive oil intake, yet showed a stronger relationship as the primary monounsaturated fatty acid in beef [36]. The vegetarian cluster group showed increased urinary glycine and phenylacetyl glutamine [35]. Thus the analysis of both plasma and urine allowed for more comprehensive and robust diet-metabolite relationships.
Individual Variation
Metabolomics studies in mammals have advanced our understanding of inter- and intra-individual metabolite variation [37–39]. Nutritional metabolomics study design is further complicated by the diverse and dynamic biochemical makeup of cells and tissues, and because the metabolite profile is only a snapshot of metabolism at a given time [11]. Metabolite fluctuations occur due to many factors, including but not limited to, diet history and environmental exposures, presence and severity of infections or chronic diseases, and genetics [39]. The integrated nature of these factors contributes to individual metabolite variation that is inherently difficult to control for.
Crossover studies (e. g. run-in diets prior to an intervention [40] or wash-out periods [41]) are common trial design strategies to reduce baseline variation in study participants prior to beginning a dietary intervention; though the optimal amount of time for the normalization period is not well understood. A run-in diet may initiate a short-term, transient metabolic response and confound the biological mechanisms under investigation. Furthermore, the variation by which individuals are uniquely affected by a run-in diet is not well understood. These study parameters merit methodical, focused research to better characterize fluctuations in metabolic status during different life stages [40].
Influence of Gut Microbiome
Gut and serum metabolism is also affected by variation in gut microbiome composition [42]. The gut microbiome and its relation to the diet are important to evaluate in dietary intervention trials with metabolomic endpoints. A recent comparison between germ-free mice colonized by human baby flora and conventional mice demonstrated the complexity of diet modifiable microbiome/metabolome covariation. In this study, the effect of the intestinal microbiome on plasma metabolites revealed that more than 10% of the plasma metabolome is directly dependent upon the microbiome [43]. Some examples for microbial dependent compounds in plasma include phenylalanine metabolism (e. g. cinammic acid), glycine conjugated compounds that can lead to the formation of hippuric acid (Table 2), and other plasma metabolites derived from gut anaerobes (e. g. phenyl-propionylgylcine). The gut microbiome also directly affects the host’s ability to metabolize lipids, carbohydrates and proteins, and can carry out a number of phase II detoxification mechanisms [43]. Additionally, there is evidence that gut microbes metabolize nonnutritive phytochemicals [44, 45]. For example, Wang et al. recently showed that levels of three microbiome-dependent diet-derived metabolites, choline, trimethylamine N-oxide, and betaine, could predict risk for cardiovascular disease in mice [23].
Table 2.
Dietary Modulation of the Blood Metabolome
| Sample | Metabolites Identified by Platform
|
|||
|---|---|---|---|---|
| GC-MS | LC-MS | NMR | Other* | |
|
| ||||
| Blood | Betaine | Butyrylcarnitine | B-hydroxybutyrate | Caffeic Acid |
| N-dimethylglycine | L-tryptophan | Lactate | Sulphonatemethylepicatechin | |
| Dimethyl sulfone | Choline | Acetate | ||
| Stearic Acid | Cysteine | Betaine/trimethylamine-N-oxide | ||
| Oleic Acid | Glycine | Glycine betaine | ||
| LysoPC 14:0 | Methionine | β-glucose | ||
| LysoPC 18:0 | Serine | α-glucose | ||
| LysoPC 18:1 | Urate | inosine/adenosine and nucleotides | ||
| LysoPC 18:2 | Phenylalanine | Campesterol | ||
| LysoPC 20:2 | Alanine | DHA | ||
| L-Carnitine | Histidine | Cholestenol | ||
| L-Valine | Branched-chain amino acids | Sphingosine moiety | ||
| D-Pipecolic acid | Isobutyrate | Nervonic acid | ||
| L-Tyrosine | Palmitic acid | Erythrosphingosine | ||
| L-Leucine | EPA | Threosphingosine | ||
| Propionylcarnitine | Cholic acid | 3-O-methylsphingosine | ||
| SIRT1 | Saccharic acid | 5-O-methylsphingosine | ||
| 11-dehydro thromboxane B (2) | Sucrose | N-methylalanine | ||
| 3-hydroxybutanoic acid | γ-linoleic acid | palmitoleic acid | ||
| Sulfoglycolithocholic acid | Phenylacetylglutamine | 3-hydroxy-3-methylglutaric acid | ||
| Threitol | Tryptophan | Idonic acid | ||
| Hydrocinnamic acid | Taurine | Lactobionic acid | ||
| α-ketoglutaric acid | Citric Acid | 3-phenyllactic acid | ||
| Docosapentaenoic acid | L-proline | Glycerol-3-galactoside | ||
| O-acetylcarnitine | α-tocopherol | Raffinose | ||
| Proline | Betasitosterol | Actetoacetate | ||
| Tyrosine | Urolithin A glucuronide | Glucose | ||
| Carnitine | Niacine | Creatinine | ||
| SIRT1 | Biuret | Triacylglycerol | ||
| Creatine | Glycerol | |||
| Oleic Acid | Ascorbic acid | |||
| Hippuric Acid | urolithin C (trihydroxydibenzopyranone) | |||
| Adenine | Acetone | |||
| LysoPC | ||||
| Uric Acid | ||||
| Creatinine | ||||
| Leucine | ||||
| Putrescine | ||||
|
| ||||
| References | [2, 38, 59, 70–72] | [38, 40, 59, 61, 73–76] | [11, 20, 21] | [1, 67, 71] |
Other Platforms used in Identifying Metabolites: ICP-OES: Inductively Coupled Plasma Spectroscopy, DAD-MS/MS: Diode Array Detector Mass Spectrometry, FIEI-MS: Flow Injection Electrospray-ionisation.
Long term diet patterns and geographical location also correlate with unique gut microbiomes [46–48], supporting an association between gut microbial function and the nutritionally modulated metabolome [49, 50]. Stool is a relevant biological sample for microbial metabolic assessment (Table 3), whereby coprastanol, the microbial derived metabolite of cholesterol, was decreased in excreted stool during an 8 week study observing cholesterol metabolism in humans when calcium phosphate was supplemented [51]. Using NMR, significant amounts of amino acids and fatty acids were also detected in fecal water from people consuming a vegetarian diet [29]. However, an important limitation of fecal analysis is the inability to detect metabolites that have increased intestinal bioavailability, and thus are actually absorbed by the host colonic epithelium. New analytical methods are under development for the quantitative analysis of tissue microbial metabolites and this represents an emerging, integral part of global metabolomics platforms [52], and was recently reviewed in [53].
TECHNICAL CONSIDERATIONS IN NUTRITIONAL METABOLOMICS
In general, there are two approaches to a metabolomics experiment: targeted or non-targeted. A targeted approach involves the directed analysis of a pre-determined panel of metabolites relevant to the hypothesis of the study. The advantage of this type of approach is that it can be optimized for the detection of specific molecules, which enables increased sensitivity and absolute quantitation. It is limiting in scope, however, in that it requires a priori knowledge of the metabolites of interest. Alternatively, a non-targeted approach is performed in a broad and unbiased manner to enable the detection of many metabolites. The results of a non-targeted approach tend to be hypothesis-generating and drive the next set of experiments to validate the findings. The advantage of a non-targeted approach is the potential for novel discoveries. However, a substantial disadvantage of this approach is the challenge of metabolite annotation [54]. It is possible to combine a targeted and non-targeted experimental design to enable unbiased profiling while simultaneously monitoring a set of known metabolites within the data.
Ultimately, the choice of experimental design will depend on the goal of the study, such that metabolite profiling data can be hypothesis generating with a non-targeted approach and may enable the identification of novel metabolic pathways in response to a dietary change. However, if the goal is to assess a specific metabolic pathway or set of molecules with known mechanisms of action, than a targeted metabolite profiling approach could more appropriate as it can be optimized for selectivity and sensitivity for these targets.
Sample Preparation
The preparation methods may vary based on sample type, target metabolites, and analytical platform. While the stated goal of metabolomics is to profile the entire metabolome, this is technically impossible using a single analytical platform or sample extraction procedure. As a result, having a list of metabolites of interest is important when designing an appropriate sample extraction method. The sample extraction method should provide reproducible recovery of target compounds for profiling purposes, and additionally provide complete recovery for absolute quantitation. Sample integrity (frozen until extraction) and sample homogeneity (to ensure a representative subsample and efficient extraction) are critical factors to consider. Specifically, a method that incorporates solvents capable of solubilizing the target compounds is generally accomplished through adjustment of the solvent polarity, ranging from highly polar (water, often pH adjusted or buffered), through moderate polarity (water-methanol mixtures) to non-polar solvents such as chloroform-methanol mixtures designed for lipid extraction. When the goal is a broad, global profile, selecting a solvent of moderate polarity will provide a representative sample compatible with multiple instrument platforms. The limitation is that the method is not optimized for any metabolite, limiting the quantitative accuracy (when quantitation is desirable). It is advised to reference the literature and replicate methods used in previous studies when the optimal extraction methodology is unclear.
Analytical Platform
Nutritional metabolomics has been performed using either mass spectrometry (MS) or nuclear magnetic resonance spectroscopy (NMR). Mass spectrometry generally excels in sensitivity and breadth of coverage, while NMR offers more readily interpreted structural information. The largest portion of metabolomics experiments utilizes mass spectrometry coupled to a chromatography system. The chromatographic platforms most frequently used are liquid chromatography (LC), gas chromatography (GC), or capillary electrophoresis (CE), with LC being the most frequently used and CE the least. Each separation method has both advantages and limitations. The ability to couple chromatography to MS is the most significant reason MS is used more frequently than NMR for metabolomics experiments. GC-MS is typically used to monitor small polar metabolites, such as monosaccharides, amino acids, organic acids, nucleosides, and also routinely detects more non-polar compounds such as fatty acids and sterols. Its major limitation is the requirement for volatility, which is achieved through chemical derivatization of small molecule extract. Extensive mass spectral libraries aid in the identification of compounds detected. The various LC-MS platforms eliminate the need for derivatization and are not limited to small molecules, as volatility is not a limiting criterion for detection. Mass spectral libraries for LC-MSMS are also developing rapidly, though the growth curve lags behind that for GC-MS spectra. NMR offers the advantage of simple sample prep and data collection, as well as being a useful tool for structure identification. Its major limitation is the lack of chromatographic separation: sample complexity is more limiting with NMR than chromatographically coupled MS systems. The lists of metabolites identified from nutritional metabolomics studies are provided according to the biological sample detected and alongside the actual platform utilized (Tables 1–3).
Metabolic
Flux
The flux of metabolites through metabolic pathways can be measured with the use of stable isotopes. Isotope-based metabolic flux analysis has traditionally provided fundamental knowledge on cooperating actions in a complex network of genes, transcripts, proteins, and metabolites. A detailed description of studies that use isotope labeling to inform metabolic flux from dietary exposures is beyond the scope of this review, yet metabolomics analysis of samples collected from these trials may provide invaluable and innovative advances to the field of nutritional, non-targeted metabolomics and may provide unique mechanistic insights [56].
EXAMPLE NUTRITIONAL METABOLOMICS DATASET
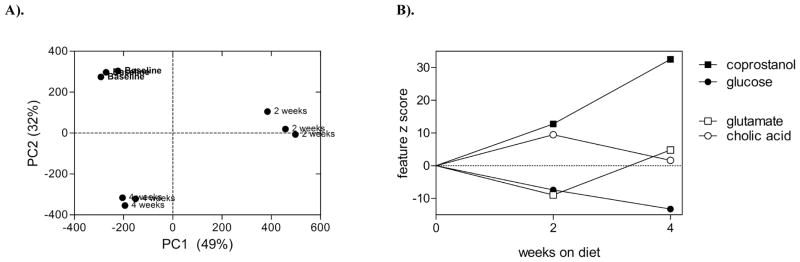
To illustrate the challenges associated with time-point effects on metabolites, a pilot dataset from an ongoing dietary intervention trial (conducted under approved protocols from the Colorado State University Research Integrity and Compliance Review Office and University of Colorado Health Institutional Review Boards) is shown in (Fig. 3). In this case, a single individual’s data revealed dramatic changes in the global metabolite profile after 2 and 4 weeks of substantially increased navy bean intake. Fecal metabolites were extracted using an aqueous-methanol solvent (n = 3 replicates per timepoint), and a non-targeted GC-MS profiling technique was applied as previously described [57]. Fecal metabolite profiles were assessed by principal component analysis (PCA), and the model explained 81% of the variation (Fig. 3A). The PC scores indicated fecal metabolite profiles changed due to the dietary intervention. PC1 explained variation observed at the 2-week time-point, as baseline and 4-week samples had similar x-axis coordinates (approximately −200 units). The 2-week time-point can be interpreted to represent a transient effect, whereby some of the metabolites that increased or decreased at 2-weeks did not differ between baseline and 4-week samples. This transient effect explained most of the variation in the model (49% out of 81% visualized on this plot). Conversely, PC2 explained variation associated with a steady change in metabolite content over time, as the baseline, 2-week, and 4-week clusters were equidistant across the y-axis. Four metabolites associated with PC1 and PC2, are shown as examples of transient and response phase effects associated with each component (Fig. 3B). Z-scores (a test statistic indicating the number of standard deviations a sample group differed from baseline) for glutamate and cholate indicate the metabolite content changed after 2-weeks, and then reverted closer to base line at 4-weeks. On the other hand, glucose and coprostanol steadily changed compared to baseline over the course of the 4-week diet. Note that all metabolites varied at 2-weeks, but the different trends over time may infer distinct types of metabolite-responses. In the case of glucose and coprostanol (the latter a strictly gut microbe associated metabolite), the changes would eventually reach a new steady state level, whereas the glutamate/cholate-like trend is not in a consistent direction. The temporal response in this dietary intervention can be divided into three stages:
Fig. 3.
A pilot example of fecal metabolite profiling results from a healthy adult that participated in a dietary navy bean intervention trial for 28 days. (A) Principal component analysis scores plot for three timepoints: baseline, 2-weeks, and 4-weeks. (B) Selected metabolites that change in a transient or steady pattern at each timepoint examined.
Baseline phase: The baseline metabolite profile is intended to be an accurate representation of the biochemical makeup of a given biological sample prior to intervention.
Transient phase: The transient metabolite profile consists of small molecules that may differ via natural variation among foods in the regular diet. The transient effect may be distinct across diet studies and among individuals. This is commonly referred to as an acclimation or adjustment period to a new food or dietary supplement.
Response phase: The metabolite profile response phase represents small molecules that change with a trend that is distinct from baseline and control samples. Response metabolites vary due to the consistent intake of new dietary components. This phase can also be considered a new steady-state level following a dietary intervention.
Fig. (4). shows that the baseline, transient, and response phases are reflected in the PCA. Individual metabolite identifications allow for determination of affected metabolic pathways-networks following a dietary intervention. Investigations into specific biochemical pathways may be difficult to interpret when there is large variation in baseline metabolite profiles of one experimental group. Thus, for pathway-focused analyses derived from omic-level data, a common first step is to combine multivariate and univariate statistical tests to determine metabolites that vary. One can perform ANOVA on principal components to identify components that vary according to the response phase, and subsequently select metabolites that contribute to the response variation by conducting outlier-like tests for each principal component’s loadings. Biased multivariate methods such as partial least squares-discriminant analysis can achieve similar results, but may fail to describe trends in the transient phase if the analysis is initially biased towards the response phase. In (Fig. 3B), one may focus interpretation around the biochemical relationship between coprostanol and glucose, which both exhibited significant loadings for the PC2 described in (Fig. 3A). Thus, this example dataset illustrates that multivariate statistics can be used to identify sets of metabolites that vary according to baseline, transient, or response phases and that trends for each set of metabolites can be independently assessed in the context of biochemical pathways.
Fig. 4.
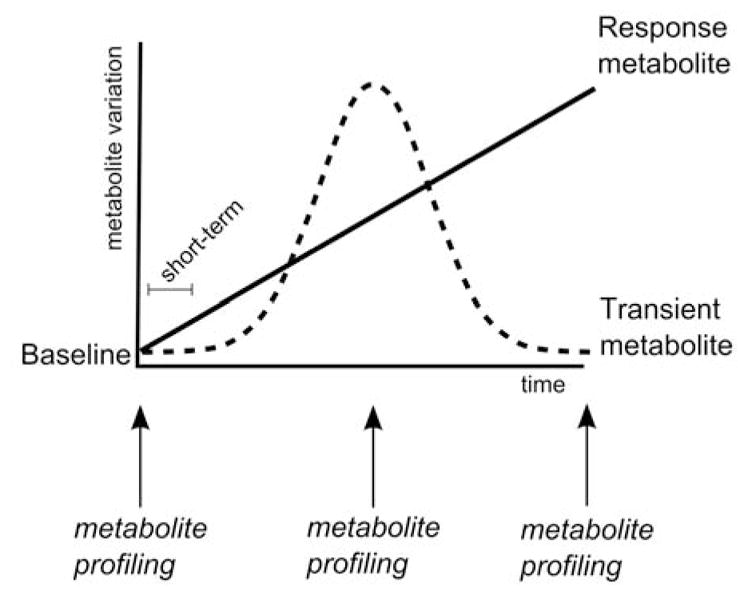
Model for interpretation of three distinct effect phases that may occur during a dietary intervention study, and that can be observed via metabolomic profiling.
SUMMARY
Nutritional metabolomics investigations have utilized both targeted and non-targeted experimental approaches in a variety of non-invasively collected biological samples. Additional studies are needed to gain a better understanding of dietary-associated changes in small molecules detected in organs and tissues. This review focused on evaluation and detection of short and long-term diet responsive metabolites reported in the literature from blood, urine, and stool, as well as specific experimental considerations in the design of nutritional metabolomics studies.
Advances in our knowledge of the host metabolome response across dietary exposure or intervention trials alongside careful consideration of experimental design have provided compelling data for future dietary-focused studies. Given the emerging role and evidence that gut microbiota influences host metabolism, cross-omic or trans-omics data integration will be instrumental for over all results interpretation [50, 58].
The full potential of metabolomics to the nutritional science, dietetics, and public health nutrition community has become better realized over the past few years, and will continue to advance by the availability of digital repositories of raw and/or annotated data sets useful for mechanistic and metabolic pathway analysis and inform the field of nutrition on diet-modifiable metabolic networks.
Acknowledgments
Declared none.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Mihalik SJ, Michaliszyn SFJ, de las Heras F, Bacha S, Lee DH, Chace VR, DeJesus J, Vockley, Arslanian SA. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care. 2012;35:605–611. doi: 10.2337/DC11-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purushotham A, Xu Q, Li X. Systemic SIRT1 insufficiency results in disruption of energy homeostasis and steroid hormone metabolism upon high-fat-diet feeding. FASEB J. 2012;26:656–667. doi: 10.1096/fj.11-195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS, Milner JA. Bioactive food components and cancer-specific metabonomic profiles. J Biomed Biotechnol. 2011:721213. doi: 10.1155/2011/721213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodge JK. Symposium 2: Modern approaches to nutritional research challenges: Targeted and non-targeted approaches for metabolite profiling in nutritional research. Proc Nutr Soc. 2010;69:95–102. doi: 10.1017/S0029665109991704. [DOI] [PubMed] [Google Scholar]
- 5.Gibney MJ, Walsh M, Brennan L, Roche HM, German B, Ommen van B. Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005;82:497–503. doi: 10.1093/ajcn.82.3.497. [DOI] [PubMed] [Google Scholar]
- 6.Puiggros F, Sola R, Blade C, Salvado MJ, Arola L. Nutritional biomarkers and foodomic methodologies for qualitative and quantitative analysis of bioactive ingredients in dietary intervention studies. J Chromatogr A. 2011;1218:7399–7414. doi: 10.1016/j.chroma.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Heuberger AL, Lewis MR, Chen MH, Brick MA, Leach JE, Ryan EP. Metabolomic and Functional Genomic Analyses Reveal Varietal Differences in Bioactive Compounds of Cooked Rice. Plos One. 2010;5 doi: 10.1371/journal.pone.0012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown M, Dunn WB, Dobson P, Patel Y, Winder CL, Francis-McIntyre S, Begley P, Carroll K, Broadhurst D, Tseng A, Swainston N, Spasic I, Goodacre R, Kell DB. Mass spectrometry tools and metabolite-specific databases for molecular identification in metabolomics. The Analyst. 2009;134:1322–1332. doi: 10.1039/b901179j. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Yang S, Cai S, Dong J, Li X, Chen Z. Identification of biochemical changes in lactovegetarian urine using 1H NMR spectroscopy and pattern recognition. Anal Bioanal Chem. 2010;396:1451–1463. doi: 10.1007/s00216-009-3338-z. [DOI] [PubMed] [Google Scholar]
- 10.Masson P, Spagou K, Nicholson JK, Want EJ. Technical and biological variation in UPLC-MS-based untargeted metabolic profiling of liver extracts: application in an experimental toxicity study on galactosamine. Anal chem. 2011;83:1116–1123. doi: 10.1021/ac103011b. [DOI] [PubMed] [Google Scholar]
- 11.Krug S, Kastenmuller G, Stuckler F, Rist MJ, Skurk T, Sailer M, Raffler J, Romisch-Margl W, Adamski J, Prehn C, Frank T, Engel KH, Hofmann T, Luy B, Zimmermann R, Moritz F, Schmitt-Kopplin P, Krumsiek J, Kremer W, Huber F, Oeh U, Theis FJ, Szymczak W, Hauner H, Suhre K, Daniel H. The dynamic range of the human metabolome revealed by challenges. FASEB J. 2012;26:2607–2619. doi: 10.1096/fj.11-198093. [DOI] [PubMed] [Google Scholar]
- 12.Kemsley EK, Le Gall G, Dainty JR, Watson AD, Harvey LJ, Tapp HS, Colquhoun IJ. Multivariate techniques and their application in nutrition: a metabolomics case study. Br J Nutr. 2007;98:1–14. doi: 10.1017/S0007114507685365. [DOI] [PubMed] [Google Scholar]
- 13.Lila MA. From beans to berries and beyond - Teamwork between plant chemicals for protection of optimal human health. Ann Ny Acad Sci. 2007;1114:372–380. doi: 10.1196/annals.1396.047. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen LG, Winning H, Savorani F, Ritz C, Engelsen SB, Astrup A, Larsen TM, Dragsted LO. Assessment of dietary exposure related to dietary GI and fibre intake in a nutritional metabolomic study of human urine. Genes & nutrition. 2012;7:281–293. doi: 10.1007/s12263-011-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen TM, Dalskov S, van Baak M, Jebb S, Kafatos A, Pfeiffer A, Martinez JA, Handjieva-Darlenska T, Kunesova M, Holst C, Saris WH, Astrup A. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries - a comprehensive design for long-term intervention. Obe Rev. 2010;11:76–91. doi: 10.1111/j.1467-789X.2009.00603.x. [DOI] [PubMed] [Google Scholar]
- 16.Vazquez-Fresno R, Llorach R, Alcaro F, Rodriguez MA, Vinaixa M, Chiva-Blanch G, Estruch R, Correig X, Andres-Lacueva C. (1)H-NMR-based metabolomic analysis of the effect of moderate wine consumption on subjects with cardiovascular risk factors. Electrophoresis. 2012;33:2345–2354. doi: 10.1002/elps.201100646. [DOI] [PubMed] [Google Scholar]
- 17.Walsh MC, Brennan L, Malthouse JP, Roche HM, Gibney MJ. Effect of acute dietary standardization on the urinary, plasma, and salivary metabolomic profiles of healthy humans. Am J Clin Nutr. 2006;84:531–539. doi: 10.1093/ajcn/84.3.531. [DOI] [PubMed] [Google Scholar]
- 18.Winnike JH, Busby MG, Watkins PB, O’Connell TM. Effects of a prolonged standardized diet on normalizing the human metabolome. Am J Clin Nutr. 2009;90:1496–1501. doi: 10.3945/ajcn.2009.28234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill CI, McDougall GJ, Glidewell S, Stewart D, Shen Q, Tuohy K, Dobbin A, Boyd A, Brown E, Haldar S, Rowland IR. Profiling of phenols in human fecal water after raspberry supplementation. J Agric Food Chem. 2010;58:10389–10395. doi: 10.1021/jf1017143. [DOI] [PubMed] [Google Scholar]
- 20.Robertson DG, Ruepp SU, Stryker SA, Hnatyshyn SY, Shipkova PA, Aranibar N, McNaney CA, Fiehn O, Reily MD. Metabolomic and transcriptomic changes induced by overnight (16 h) fasting in male and female Sprague-Dawley rats. Chem Res Toxicol. 2011;24:481–487. doi: 10.1021/tx200074f. [DOI] [PubMed] [Google Scholar]
- 21.Moazzami AA, Zhang JX, Kamal-Eldin A, Aman P, Hallmans G, Johansson JE, Andersson SO. Nuclear magnetic resonance-based metabolomics enable detection of the effects of a whole grain rye and rye bran diet on the metabolic profile of plasma in prostate cancer patients. J Nutr. 2011;141:2126–2132. doi: 10.3945/jn.111.148239. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen LG, Winning H, Savorani F, Toft H, Larsen TM, Dragsted LO, Astrup A, Engelsen SB. Assessment of the effect of high or low protein diet on the human urine metabolome as measured by NMR. Nutrients. 2012;4:112–131. doi: 10.3390/nu4020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt E, Fu BX, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzmann SS, Merrifield CA, Rezzi S, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Stability and robustness of human metabolic phenotypes in response to sequential food challenges. J Proteome Res. 2012;11:643–655. doi: 10.1021/pr2005764. [DOI] [PubMed] [Google Scholar]
- 25.Duggan GE, Hittel DS, Hughey CC, Weljie A, Vogel HJ, Shearer J. Differentiating short- and long-term effects of diet in the obese mouse using (1) H-nuclear magnetic resonance metabolomics. Diabetes Obes Metab. 2011;13:859–862. doi: 10.1111/j.1463-1326.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 26.Kleemann R, Verschuren L, van Erk MJ, Nikolsky Y, Cnubben NH, Verheij ER, Smilde AK, Hendriks HF, Zadelaar S, Smith GJ, Kaznacheev V, Nikolskaya T, Melnikov A, Hurt-Camejo E, van der Greef J, van Ommen B, Kooistra T. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8:R200. doi: 10.1186/gb-2007-8-9-r200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Wang L, Yan X, Liu Q, Yu C, Wei H, Li Y, Zhang X, He F, Jiang Y. A proton nuclear magnetic resonance metabonomics approach for biomarker discovery in nonalcoholic fatty liver disease. J Proteome Res. 2011;10:2797–2806. doi: 10.1021/pr200047c. [DOI] [PubMed] [Google Scholar]
- 28.Zeisel SH, Freake HC, Bauman DE, Bier DM, Burrin DG, German JB, Klein S, Marquis GS, Milner JA, Pelto GH, Rasmussen KM. The nutritional phenotype in the age of metabolomics. J Nutr. 2005;135:1613–1616. doi: 10.1093/jn/135.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettersson J, Karlsson PC, Choi YH, Verpoorte R, Rafter JJ, Bohlin L. NMR metabolomic analysis of fecal water from subjects on a vegetarian diet. Biol Pharm Bull. 2008;31:1192–1198. doi: 10.1248/bpb.31.1192. [DOI] [PubMed] [Google Scholar]
- 30.Cao H, Huang H, Xu W, Chen D, Yu J, Li J, Li L. Fecal metabolome profiling of liver cirrhosis and hepatocellular carcinoma patients by ultra performance liquid chromatography-mass spectrometry. Analytica chimica acta. 2011;691:68–75. doi: 10.1016/j.aca.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Dixon E, Clubb C, Pittman S, Ammann L, Rasheed Z, Kazmi N, Keshavarzian A, Gillevet P, Rangwala H, Couch RD. Solid-phase microextraction and the human fecal VOC metabolome. PloS one. 2011;6:e18471. doi: 10.1371/journal.pone.0018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao X, Pujos-Guillot E, Sebedio JL. Development of a quantitative metabolomic approach to study clinical human fecal water metabolome based on trimethylsilylation derivatization and GC/MS analysis. Analytical chemistry. 2010;82:6447–6456. doi: 10.1021/ac1006552. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Haraguchi T, Iwanaga S, Tomotake H, Okazaki Y, Mineo S, Moriyama A, Inoue J, Kato N. Consumption of some polyphenols reduces fecal deoxycholic acid and lithocholic acid, the secondary bile acids of risk factors of colon cancer. J Agric Food Chem. 2009;57:8587–8590. doi: 10.1021/jf900393k. [DOI] [PubMed] [Google Scholar]
- 34.Saric J, Wang Y, Li J, Coen M, Utzinger J, Marchesi JR, Keiser J, Veselkov K, Lindon JC, Nicholson JK, Holmes E. Species variation in the fecal metabolome gives insight into differential gastrointestinal function. Journal of proteome research. 2008;7:352–360. doi: 10.1021/pr070340k. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr. 2011;93:314–321. doi: 10.3945/ajcn.110.000950. [DOI] [PubMed] [Google Scholar]
- 36.Saadatian-Elahi M, Slimani N, Chajes V, Jenab M, Goudable J, Biessy C, Ferrari P, Byrnes G, Autier P, Peeters PHM, Ocke M, de Mesquita BB, Johansson I, Hallmans G, Manjer J, Wirfalt E, Gonzalez CA, Navarro C, Martinez C, Amiano P, Suaerz LR, Ardanaz E, Tjonneland A, Halkjaer J, Overvad Jakobsen K, Berrino MU, Pala FV, Palli D, Tumino R, Vineis P, de Magistris MS, Spencer EA, Crowe FL, Bingham S, Khaw KT, Linseisen J, Rohrmann S, Boeing H, Noethlings U, Olsen KS, Skeie G, Lund E, Trichopoulou A, Oustoglou E, Clavel-Chapelon F, Riboli E. Plasma phospholipid fatty acid profiles and their association with food intakes: results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 89:331–346. doi: 10.3945/ajcn.2008.26834. [DOI] [PubMed] [Google Scholar]
- 37.Lenz EM, Bright J, Wilson ID, Morgan SR, Nash AF. A 1H NMR-based metabonomic study of urine and plasma samples obtained from healthy human subjects. J Pharm Biomed Anal. 2003;33:1103–1115. doi: 10.1016/s0731-7085(03)00410-2. [DOI] [PubMed] [Google Scholar]
- 38.Colyer A, Gilham MS, Kamlage B, Rein D, Allaway D. Identification of intra- and inter-individual metabolite variation in plasma metabolite profiles of cats and dogs. Br J Nutr. 2011;106(Suppl 1):S146–149. doi: 10.1017/S000711451100081X. [DOI] [PubMed] [Google Scholar]
- 39.Gruden K, Hren M, Herman A, Blejec A, Albrecht T, Selbig J, Bauer C, Schuchardt J, Or-Guil M, Zupancic K, Svajger U, Stabuc B, Ihan A, Kopitar AN, Ravnikar M, Knezevic M, Rozman P, Jeras M. A “crossomics” study analysing variability of different components in peripheral blood of healthy caucasoid individuals. PloS one. 2012;7:e28761. doi: 10.1371/journal.pone.0028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandre-Gouabau MC, Courant F, Le Gall G, Moyon T, Darmaun D, Parnet P, Coupe B, Antignac JP. Offspring metabolomic response to maternal protein restriction in a rat model of intrauterine growth restriction (IUGR) Journal of proteome research. 2011;10:3292–3302. doi: 10.1021/pr2003193. [DOI] [PubMed] [Google Scholar]
- 41.Xie G, Zhao A, Zhao L, Chen T, Chen H, Qi X, Zheng X, Ni Y, Cheng Y, Lan K, Yao C, Qiu M, Jia W. Metabolic Fate of Tea Polyphenols in Humans. J Proteome Res. 2012 doi: 10.1021/pr300318m. [DOI] [PubMed] [Google Scholar]
- 42.Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, Rezzi S, Ramadan Z, van Bladeren P, Fay LB, Kochhar S, Lindon JC, Holmes E, Nicholson JK. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wikoff WR, Anfora AT, Liu J, Schultz P, Lesley GSA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee HC, Jenner AM, Low CS, Lee YK. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol. 2006;157:876–884. doi: 10.1016/j.resmic.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Hervert-Hernandez D, Pintado Rotger CR, Goni I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int J Food Microbiol. 2009;136:119–122. doi: 10.1016/j.ijfoodmicro.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 46.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grzeskowiak L, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, Isolauri E, Salminen S. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr. 2012;54:812–816. doi: 10.1097/MPG.0b013e318249039c. [DOI] [PubMed] [Google Scholar]
- 48.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone C, Lauber AC, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dougal K, Harris PA, Edwards A, Pachebat J, Blackmore ATM, Worgan HJ, Newbold CJ. A comparison of the microbiome and the metabolome of different regions of the equine hind-gut. FEMS Microbiol Ecol. 2012 doi: 10.1111/j.1574-6941.2012.01441.x. [DOI] [PubMed] [Google Scholar]
- 50.Sellitto M, Bai G, Serena G, Fricke WF, Sturgeon C, Gajer P, White JR, Koenig SS, Sakamoto J, Boothe D, Gicquelais R, Kryszak D, Puppa E, Catassi C, Ravel J, Fasano A. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PloS one. 2012;7:e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ditscheid B, Keller S, Jahreis G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr. 2005;135:1678–1682. doi: 10.1093/jn/135.7.1678. [DOI] [PubMed] [Google Scholar]
- 52.Koek MM, Muilwijk B, van der Werf MJ, Hankemeier T. Microbial metabolomics with gas chromatography/mass spectrometry. Anal Chem. 2006;78:1272–1281. doi: 10.1021/ac051683+. [DOI] [PubMed] [Google Scholar]
- 53.Baidoo EE, Benke PI, Keasling JD. Mass spectrometry-based microbial metabolomics. Methods Mol Biol. 2012;881:215–278. doi: 10.1007/978-1-61779-827-6_9. [DOI] [PubMed] [Google Scholar]
- 54.Theodoridis G, Gika HG, Wilson ID. Mass spectrometry-based holistic analytical approaches for metabolite profiling in systems biology studies. Mass Spectrom Rev. 2011 doi: 10.1002/mas.20306. [DOI] [PubMed] [Google Scholar]
- 55.Wisselink HW, Cipollina C, Oud B, Crimi B, Heijnen JJ, Pronk JT, van Maris AJ. Metabolome, transcriptome and metabolic flux analysis of arabinose fermentation by engineered Saccharomyces cerevisiae. Metabolic engineering. 2010;12:537–551. doi: 10.1016/j.ymben.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Hiller K, Metallo C, Stephanopoulos G. Elucidation of cellular metabolism via metabolomics and stable-isotope assisted metabolomics. Curr Pharm Biotechnol. 2011;12:1075–1086. doi: 10.2174/138920111795909096. [DOI] [PubMed] [Google Scholar]
- 57.Ryan EP, Heuberger AL, Weir TL, Barnett B, Broeckling CD, Prenni JE. Rice bran fermented with saccharomyces boulardii generates novel metabolite profiles with bioactivity. J Agric Food Chem. 2011;59:1862–1870. doi: 10.1021/jf1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oberbach A, Bluher M, Wirth H, Till H, Kovacs P, Kullnick Y, Schlichting N, Tomm JM, Rolle-Kampczyk U, Murugaiyan J, Binder H, Dietrich A, von Bergen M. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J Proteome Res. 2011;10:4769–4788. doi: 10.1021/pr2005555. [DOI] [PubMed] [Google Scholar]
- 59.Hall JA, Brockman JA, Jewell DE. Dietary fish oil alters the lysophospholipid metabolomic profile and decreases urinary 11-dehydro thromboxane B(2) concentration in healthy Beagles. Vet Immunol Immunopathol. 2011;144:355–365. doi: 10.1016/j.vetimm.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 60.Ivers DJ, Veum TL. Effect of graded levels of niacin supplementation of a semipurified diet on energy and nitrogen balance, growth performance, diarrhea occurrence, and niacin metabolite excretion by growing swine. J Anim Sci. 2012;90:282–288. doi: 10.2527/jas.2011-4035. [DOI] [PubMed] [Google Scholar]
- 61.Kim JY, Park JY, Kim OY, Ham BM, Kim HJ, Kwon DY, Jang Y, Lee JH. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS) J Proteome Res. 2010;9:4368–4375. doi: 10.1021/pr100101p. [DOI] [PubMed] [Google Scholar]
- 62.Llorach R, Urpi-Sarda M, Jauregui O, Monagas M, Andres-Lacueva C. An LC-MS-based metabolomics approach for exploring urinary metabolome modifications after cocoa consumption. J Proteome Res. 2009;8:5060–5068. doi: 10.1021/pr900470a. [DOI] [PubMed] [Google Scholar]
- 63.Bertram HC, Yde CC, Zhang X, Kristensen NB. Effect of dietary nitrogen content on the urine metabolite profile of dairy cows assessed by nuclear magnetic resonance (NMR)-based metabolomics. J Agric Food Chem. 2011;59:12499–12505. doi: 10.1021/jf204201f. [DOI] [PubMed] [Google Scholar]
- 64.Edmands WM, Beckonert OP, Stella C, Campbell A, Lake BG, Lindon JC, Holmes E, Gooderham NJ. Identification of human urinary biomarkers of cruciferous vegetable consumption by metabonomic profiling. J Proteome Res. 2011;10:4513–4521. doi: 10.1021/pr200326k. [DOI] [PubMed] [Google Scholar]
- 65.Vazquez-Fresno R, Llorach R, Alcaro F, Rodriguez MA, Vinaixa M, Chiva-Blanch G, Estruch R, Correig X, Andres-Lacueva C. (1) H-NMR-based metabolomic analysis of the effect of moderate wine consumption on subjects with cardiovascular risk factors. Electrophoresis. 2012;33:2345–2354. doi: 10.1002/elps.201100646. [DOI] [PubMed] [Google Scholar]
- 66.Winning H, Roldan-Marin E, Dragsted LO, Viereck N, Poulsen M, Sanchez-Moreno C, Cano MP, Engelsen SB. An exploratory NMR nutri-metabonomic investigation reveals di-methyl sulfone as a dietary biomarker for onion intake. Analyst. 2009;134:2344–2351. doi: 10.1039/b918259d. [DOI] [PubMed] [Google Scholar]
- 67.Espin JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–10485. doi: 10.1021/jf0723864. [DOI] [PubMed] [Google Scholar]
- 68.Lloyd AJ, Beckmann M, Fave G, Mathers JC, Draper J. Proline betaine and its biotransformation products in fasting urine samples are potential biomarkers of habitual citrus fruit consumption. Br J Nutr. 2011;106:812–824. doi: 10.1017/S0007114511001164. [DOI] [PubMed] [Google Scholar]
- 69.Lloyd AJ, Fave G, Beckmann M, Lin W, Tailliart K, Xie L, Mathers JC, Draper J. Use of mass spectrometry fingerprinting to identify urinary metabolites after consumption of specific foods. Am J Clin Nutr. 2011;94:981–991. doi: 10.3945/ajcn.111.017921. [DOI] [PubMed] [Google Scholar]
- 70.Mellert W, Kapp M, Strauss V, Wiemer J, Kamp H, Walk T, Looser R, Prokoudine A, Fabian E, Krennrich G, Herold M, van Ravenzwaay B. Nutritional impact on the plasma metabolome of rats. Toxicol Lett. 2011;207:173–181. doi: 10.1016/j.toxlet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 71.Sha W, da Costa KA, Fischer LM, Milburn MV, Lawton KA, Berger A, Jia W, Zeisel SH. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J. 2010;24:2962–2975. doi: 10.1096/fj.09-154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao L, Wu J, Wang Y, Yang J, Wei J, Gao W, Guo C. Cholesterol metabolism is modulated by quercetin in rats. J Agric Food Chem. 2011;59:1104–1108. doi: 10.1021/jf1035367. [DOI] [PubMed] [Google Scholar]
- 73.Barri T, Holmer-Jensen J, Hermansen K, Dragsted LO. Metabolic fingerprinting of high-fat plasma samples processed by centrifugation- and filtration-based protein precipitation delineates significant differences in metabolite information coverage. Analytica chimica acta. 2012;718:47–57. doi: 10.1016/j.aca.2011.12.065. [DOI] [PubMed] [Google Scholar]
- 74.Bertram HC, Larsen LB, Chen X, Jeppesen PB. Impact of high-fat and high-carbohydrate diets on liver metabolism studied in a rat model with a systems biology approach. J Agric Food Chem. 2012;60:676–684. doi: 10.1021/jf203994k. [DOI] [PubMed] [Google Scholar]
- 75.Castro-Perez JM, Roddy TP, Shah V, McLaren DG, Wang SP, Jensen K, Vreeken RJ, Hankemeier T, Johns DG, Previs SF, Hubbard BK. Identifying static and kinetic lipid phenotypes by high resolution UPLC-MS: unraveling diet-induced changes in lipid homeostasis by coupling metabolomics and fluxomics. J Proteome Res. 2011;10:4281–4290. doi: 10.1021/pr200480g. [DOI] [PubMed] [Google Scholar]
- 76.Park Y, Kim S, Wang BB, Blanco RA, Le NA, Wu S, Accardi CJ, Alexander RW, Ziegler TR, Jones DP. Individual variation in macronutrient regulation measured by proton magnetic resonance spectroscopy of human plasma. Am J Physiol Regul Integr Comp Physiol. 2009;297:R202–209. doi: 10.1152/ajpregu.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poroyko V, Morowitz M, Bell T, Ulanov A, Wang M, Donovan S, Bao N, Gu S, Hong L, Alverdy JC, Bergelson J, Liu DC. Diet creates metabolic niches in the “immature gut” that shape microbial communities. Nutr Hosp. 2011;26:1283–1295. doi: 10.1590/S0212-16112011000600015. [DOI] [PubMed] [Google Scholar]
- 78.Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ, Duthie GG, Flint HJ. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr. 2011;93:1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- 79.de Vogel J, Jonker-Termont DS, van Lieshout EM, Katan MB, van der Meer R. Green vegetables, red meat and colon cancer: chlorophyll prevents the cytotoxic and hyperpro-liferative effects of haem in rat colon. Carcinogenesis. 2005;26:387–393. doi: 10.1093/carcin/bgh331. [DOI] [PubMed] [Google Scholar]
- 80.Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, Lacroix C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. The Journal of nutrition. 2012;142:271–277. doi: 10.3945/jn.111.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Owen RW, Dodo M, Thompson MH, Hill MJ. Fecal steroids and colorectal cancer. Nutr Cancer. 1987;9:73–80. doi: 10.1080/01635588709513914. [DOI] [PubMed] [Google Scholar]