Abstract
PURPOSE
Despite the enormity of the problem and the lack of new therapies, research in the pre-clinical arena specifically using pediatric traumatic brain injury (TBI) models is limited. In this review, some of the key models addressing both the age spectrum of pediatric TBI and its unique injury mechanisms will be highlighted. Four topics will be addressed, namely, 1) unique facets of the developing brain important to TBI model development, 2) a description of some of the most commonly used pre-clinical models of severe pediatric TBI including work in both rodents and large animals, 3) a description of the pediatric models of mild TBI and repetitive mild TBI that are relatively new, and finally 4) a discussion of challenges, gaps and potential future directions to further advance work in pediatric TBI models.
METHODS
This narrative review on the topic of pediatric TBI models was based on review of PUBMED/Medline along with a synthesis of information on key factors in pre-clinical and clinical developmental brain injury that influence TBI modeling.
RESULTS
In the contemporary literature, six types of models have been used in rats including weight drop, fluid percussion injury (FPI), impact acceleration, controlled cortical impact (CCI), mechanical shaking, and closed head modifications of CCI. In mice, studies are largely restricted to CCI. In large animals, FPI and rotational injury have been used in piglets and shake injury has also been used in lambs. Most of the studies have been in severe injury models, although more recently studies have begun to explore mild and repetitive mild injuries to study concussion.
CONCLUSIONS
Given the emerging importance of TBI in infants and children, the morbidity and mortality that is produced, along with its purported link to the development of chronic neurodegenerative diseases, studies in these models merit greater systematic investigations along with consortium type approaches and long-term follow-up to translate new therapies to the bedside.
Keywords: Head trauma, Developmental, immature, abusive head trauma, shaken baby syndrome, excitotoxicity, apoptosis, oxidative stress, axonal injury, inflammation
Introduction
Traumatic brain injury (TBI) in infants and children is an important problem worldwide. In the United States alone, according to the Centers for Disease Control, >300,000 children suffered TBI in 2012 [1]. With the recognition of epidemic number of cases of mild TBI (mTBI) and repetitive mTBI, and given the newly recognized link between TBI and the development of a variety of neurodegenerative diseases, such as chronic traumatic encephalopathy and dementia, the total life-long burden of TBI in children world-wide across the injury spectrum is undoubtedly enormous. As is the case for TBI in adults, although a number of therapies are used clinically as part of standard of care, particularly in the setting of severe TBI, no new therapies have translated from bench to bedside. Thus novel therapies are badly needed.
Despite the enormity of the problem, and the lack of new therapies, overall the amount of pre-clinical research that has been carried out specifically addressing pediatric TBI is limited. In this narrative review, some of the key contributions to the field in the area of pre-clinical research in pediatric TBI are highlighted. Several topics will be addressed, namely, 1) unique facets of the developing brain important to TBI model development, 2) models of severe pediatric TBI including work in both rodents and large animals, 3) pediatric models of mTBI and repetitive mTBI, and finally 4) a discussion of challenges, gaps and potential future directions to further advance work in pediatric TBI models.
Unique facets of the developing brain—relevance to pediatric TBI model development
There is a clear mechanistic rationale for having pediatric models of TBI. Age dependent changes in many factors that may be important to the evolution of secondary damage after TBI have been shown including myelination, neurotransmitter and neurotrophin development, programmed cell death, synaptogenesis and synaptic reorganization, gliogenesis, antioxidant status, cerebral blood flow (CBF), cerebral oxidative and glycolytic metabolism, blood-brain barrier (BBB) function, and cerebrospinal fluid (CSF) dynamics, among others. For a comprehensive review of developmental neurobiology and neurophysiology relevant to both pre-clinical and clinical aspects of TBI, the reader is referred to other sources [2]. In addition, age-related differences in the biomechanical response to TBI are almost certainly important [3], however, these have seen less investigation than age-related differences between adult and pediatric cellular or molecular mechanisms of secondary injury. A much less well characterized, albeit important issue, is the long-term consequences of injury during development on outcome. This includes assessment of both behavioral and histological outcomes. Finally, studies exploring the link between TBI and the development of chronic neurodegenerative diseases have not been performed—and are only beginning to be addressed in adult or aged animal models.
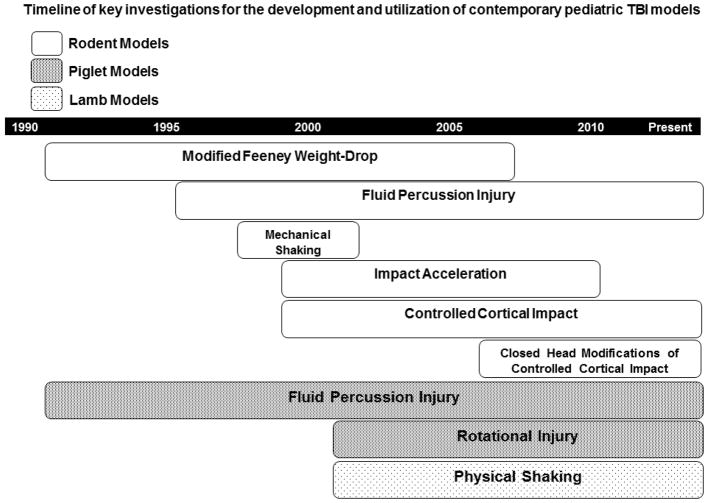
In general, to model pediatric TBI, rodents 11–21 days of age (post-natal day [PND] 11–21) have been used to model the injury response in humans ranging from a term infant (using PND 7–11) to a toddler (using PND 17–21) [4–17]. This contrasts work in the area of neonatal brain injury where PND 7 rats are generally studied, predominantly using the Rice-Vanucci model of hypoxic-ischemic injury. Although this approach to TBI in developing rodents has allowed the comparison of studies done across various laboratories, not all aspects of brain development mature in parallel between rodents and humans, and thus only some aspects of brain development contributing to secondary injury and outcome may be appropriately modeled at any one time. An early review on this topic, in the era of contemporary pediatric TBI models was published by Prins and Hovda in 2003 that serves as an excellent foundation for understanding how the field has built on the more seminal studies [18]. Modeling pediatric TBI in large animals has been somewhat less commonly done than in rodents, and focused predominantly in a small number of laboratories. In those studies, piglets of between 1 and 5 days of age (PND 1–5) have been used to model infant TBI victims. Notably, the most common form of TBI in infants < 1 year of age is abusive head trauma (AHT), also known as the shaken baby syndrome [19]. In addition, a substantial body of work (discussed later in this review) has also focused on cerebrovascular derangements in juvenile piglets (3–4 weeks of age) in order to model findings to the neurocritical care of children with severe TBI [20–23]. A large animal model of developmental brain injury was also designed to study AHT using 7–10 day old lambs [24–27]. There were a few early reports on the use of PND 6–8 rats exposed to shaking plus hypoxemia to model AHT [28–30]. More recently several reports in PND 11 rats have used single or multiple closed head impacts also to model AHT in infants [15, 31–33]. A timeline of model development and utilization in pediatric TBI is provided in Figure 1.
Figure 1.
Timeline of key investigations for the development and utilization of contemporary pediatric traumatic brain injury (TBI) models. Rats have been used across all of the rodent pediatric TBI models, while mice have been largely limited to controlled cortical impact. All of these models have been used to study severe TBI, except that the closed head modifications of controlled cortical impact have been used to study mild TBI and repetitive mild TBI given the emerging importance of mild TBI and concussion in the current era. Please see text for details.
Given their importance to pediatric model development, of the many potential age-related differences in cellular, molecular, and functional underpinnings of secondary damage and its assessment in TBI, we have selected four areas for brief discussion, namely, 1) oxidative stress, 2) programmed cell death and excitotoxicity, 3) inflammation, and 4) behavioral outcome assessments important to developing TBI models.
Oxidative stress is of special importance after TBI in the developing brain related to the well-described age-related differences in antioxidant defenses. This concept represents a prime example that illustrates the need to study therapies specifically in developmental models of TBI. Bayır et al [34] reviewed the topic of antioxidant enzyme activities and antioxidant levels in immature versus adult brain showing that some antioxidant pathways, particularly pertinent to clearance of hydrogen peroxide, exhibit markedly lower activity in the developing rat brain, specifically in PND 14–17 versus adult rats. This can have important modeling and therapeutic consequences. For example, transgenic adult mice overexpressing superoxide dismutase are highly protected in pre-clinical models of acute brain injury, while in immature mice, a paradoxical increase in damage is seen [35]. This has been suggested to result from the fact that the developing brain has inadequate glutathione peroxidase activity which leads to the accumulation of toxic hydrogen peroxide in neurons of superoxide dismutase overexpressing mice—given that hydrogen peroxide is produced when superoxide anion is dismutated [34, 35]. Consistent with this work, PND 21 glutathione peroxidase transgenic mice demonstrate improved spatial memory and neuronal survival in dentate versus wild type littermates after TBI [36]. Substantial benefit has also been shown with the use of mitochondrial targeting antioxidant therapies in PND 17 rats after TBI produced by controlled cortical impact (CCI) supporting the potential relevance of these findings to drug development for pediatric TBI targeting oxidative stress [37].
Another molecular mechanism of the secondary injury cascade that exhibits important age-related differences is programmed cell death via apoptosis. This was clearly illustrated in the classic report of Yakovlev et al [8] over 15 years ago. In that work, age-dependent expression of caspase 3, the key regulator of both the intrinsic and extrinsic pro-apoptotic cell death pathways was shown. Progressive reductions in caspase 3 mRNA and protein levels in cerebral cortex were observed in developing rats at PND 7, PND 14, and PND 60. Vulnerability to apoptotic neurodegeneration has been suggested to represent an important therapeutic target for TBI in the developing brain, including both the intrinsic and extrinsic pathways, based on the successful use of pan caspase inhibitors in reducing neuronal death after TBI in developing rats in the work of Bittigau et al [11]. However, classic developmental apoptosis such as that resulting from ethanol exposure in utero, is not dependent on caspase 3 [38]. A role for Bax is suggested [39]. This work and a number of related studies on developmental apoptosis also suggest that consideration to anesthetic choice might be important in pediatric TBI models. The roots of this link can be traced back to early work showing age-related differences in excitotoxicity during development. Critical age-related differences in the response to glutamate were initially shown in the classic report of McDonald and Johnston [40] when PND 7 rats were highly vulnerable to injection of the N-methyl-D-aspartate (NMDA) receptor agonist glutamate. Anesthetics that block NMDA receptor activation appear to trigger developmental apoptosis, most notably ketamine, nitrous oxide, and isoflurane, among others. This could be important in studies where the animal is anesthetized for a prolonged period, such as for neuro-monitoring. This has been reported in rodent models but has also been shown in primates where in PND 5–6 rhesus monkeys, exposure to isoflurane (1%) for a period of 8 hours results in obvious neuronal damage in the frontal cortex [41]. A variety of anesthetics have been used in pre-clinical studies of pediatric TBI, although isoflurane is certainly the most common. Prolonged duration of exposure has been suggested to be essential in the studies of developmental apoptosis (5–24 hours depending on the study), so that brief exposures generally used to produce TBI may not be complicated by this process. However, there could be interactions between anesthesia and injury and caution is advised. Appropriate anesthesia sham controls are thus particularly important in pediatric TBI models.
A third pathway demonstrating important age-related differences in the secondary injury response that may influence model development is inflammation. A comprehensive review of the inflammatory response to TBI was recently reported [42] and suggested clinical and pre-clinical support for a particularly robust inflammatory response in infants and children vs. adults with severe TBI, for mechanisms such as macrophage and lymphocyte activation, and the microglial response. Specifically, the acute inflammatory response to brain injury appears to be particularly robust early in development and represent a promising therapeutic target. For example, a body of work from the laboratory of Hagberg et al [43] has demonstrated a marked microglial response to ischemic brain injury in PND 7 rat pups, mediated in part by Toll receptors. Similarly, Kannan et al [44] demonstrated a marked microglial response in vulnerable brain regions in newborn rabbits in a model of cerebral palsy. An impressive therapeutic benefit was seen with N-acetyl-cysteine delivered via nanoparticles. In both of these examples, the inflammatory response was notably prominent in white matter—which could have special importance in the setting of developmental TBI—given the importance of axonal injury in TBI. However, cell death pathways in oligodendrocyte precursor cells relevant to periventricular leukomalacia may be operating in cerebral ischemia models in PND 7 rat pups but these cells are largely absent from TBI models generally carried out in PND 17–21 rats [45]. Thus the inflammatory triggers may critically differ across the age spectrum during development; however, a comprehensive and systematic comparison of the inflammatory response to TBI across the age spectrum in a pre-clinical model remains to be performed.
Finally, a fourth age-related difference merits consideration when establishing and/or interpreting data from pediatric TBI models. The most common tool used to assess cognitive outcome in TBI models is the Morris water maze (MWM). Regarding model development and therapy testing, it is important to note that developing rats do not reach adult levels of proficiency in the MWM until PND 21–23 [31]. The MWM has thus, with this caveat, been successfully used to assess behavioral outcomes in developmental TBI model for over 15 years including CCI, fluid percussion (FPI), and impact acceleration diffuse brain injury [9,14,16].
Pediatric models of severe TBI in rodents
Weight drop
Early pre-clinical investigations in the area of TBI in pediatric models used a modification of the Feeney weight-drop model [46, 47]. Briefly, in this model, a brass rod is allowed to fall from a fixed height to impact the cortical surface through a glass guide tube. This model produces a focal contusion, and as applied in most of the early investigations in pediatric TBI, targeted the parietal cortex in PND 21–28 male Wistar rats [4–7]. Early work with this model focused on the cerebrovascular response to TBI and demonstrated a more hyperemic CBF response in developing rats, in contrast to marked CBF depression after injury in aged (14–15-month-old) rats [6]. In that model, an early assessment of the impact of therapeutic hypothermia was performed which surprisingly has mirrored subsequent clinical studies [4]. Specifically, early posttraumatic brain edema at 4 h after injury was attenuated vs. normothermia, while no benefit was seen on a more longer-term outcome, namely, histology assessed at 5 days after injury. Modifications of this model have been used to assess developmental apoptosis and inflammation in PND 7 rats, among other mechanisms [11, 48]. Although contributing importantly to some of the earliest work in pediatric TBI modeling, this model was supplanted by the CCI model which affords a high level of precision and reproducibility.
Impact acceleration
In an attempt to develop a pediatric model that mimics the diffuse swelling so often seen in pediatric patients with severe TBI [49, 50], Adelson et al [9] used a modified version of the impact acceleration model of TBI that had been previously developed in adult rats [51]. In the pediatric version of the impact acceleration model, TBI was produced in PND 17 male Sprague-Dawley rats using a 150-gram brass weight allowed to free-fall through a plexi-glass guide tube (19 mm inner diameter, 2.5 m length) from a pre-determined height to impact the closed skull. To prevent skull fracture, a metal disk (3 mm wide, 10 mm diameter) was cemented to the skull in the midline and incorporated into an acrylic helmet. In this model, the rat is placed on a foam bed of known spring constant. The foam bed is slid under the tip of the guide tube such that the impact is centered over the metal disk. This model, at injury levels that were associated with survival, produced a diffuse injury, but did not produce neuronal death or robust diffuse swelling. However, the model produce substantial motor deficits and enduring deficits in performance on the MWM—remarkably through 90 days post-TBI. Thus, although the focus of the model was to target diffuse swelling, it produced an outcome that might serve to allow exploration of therapies targeting behavioral outcomes. The lack of neuronal death in this model, although felt to represent a limitation back in 2000, when most research focused on severe TBI, might have greater relevance now to pediatric TBI given the importance of mTBI and concussion which have emerged—and where neuronal death is uncommon [52].
Lateral FPI
Shortly after the above investigations, Giza et al [14, 53] carried out a series of studies in a pediatric model of TBI using a modification of FPI in rats. Using PND 17 or 19 male Sprague-Dawley rat pups, a classic approach to FPI was used which entailed placement of a 3-mm diameter craniotomy 2-mm posterior to bregma and 6-mm lateral to midline. A plastic cap was then placed which was filled with saline and attached to the fluid percussion device. The FPI device, initially described for work in rats by Dixon et al [54] is comprised of a Plexiglas cylinder filled with physiological saline and connected at one end to the cap on the craniotomy site. At the other end, the cylinder is closed with a Plexiglas cork mounted on O-rings. Injury is then produced by striking the cork with a pendulum dropped from a pre-defined height. Work with that model has characterized cell death pathways, NMDA-receptor subunit changes, gene induction, and experience-dependent behavioral plasticity after injury, among other outcomes [14, 53, 55, 56]. Strengths of this model for use in pediatric TBI include its diffuse injury pattern, robust MWM deficits even with limited histological damage, and axonal injury. It is particularly useful at low to moderate injury levels. However, the model is limited by the production of impact apnea due to brainstem compression as the injury level is increased—which can produce mortality or require resuscitation, complicating data interpretation. It also requires a craniotomy. To our knowledge, it has not been used in immature mice, limiting transgenic or knockout studies in pediatric applications.
CCI
The most widely used pediatric TBI model is CCI. Initially described for use in adult rats [57], CCI is induced using a pneumatic impactor rigidly mounted on a cross bar either angled to be perpendicular to the dural surface or vertical. The tip of the impactor can have a varying surface either flat or round. The impactor tip is driven at a predetermined velocity, depth of penetration and duration of tissue deformation [58]. In pediatric applications most commonly PND 17 rats have been used with various insult parameters, often at a velocity of 4 m/sec and with a depth ranging generally from 1.5–2.5 mm. In general, hippocampal neuronal death seems to be somewhat more difficult to produce in PND 17 rats than in adult rats as suggested in early work in developmental CCI by Jenkins et al [10]. MWM deficits are seen in male PND 17 rats subjected to 2.0 mm or 2.5 m deformations [16] and various pediatric versions of the CCI model have been used by numerous laboratories to study a wide variety of outcomes and/or therapies including proteomic assessments [10, 59], oxidative stress [60], mitochondrial disturbances [37, 61], calpain activation [62], and various therapies such as ketones [13], progesterone [63] and novel antioxidant strategies [37] among many others. In mice, the pediatric version of the CCI model is also established and generally, PND 21 male mice are used [36, 64]. Injury parameters of 4–4.6 m/sec at a depth of 0.5 mm produced a contusion with hippocampal neuronal death when a 3-mm impactor tip was used. Strengths of CCI include its reproducibility, simplicity—allowing rapid through-put, and ability to generate reliably a number of distinct therapeutic targets (contusion, hippocampal neuronal death, edema, and behavioral deficits). It is limited by the need for a craniotomy, and by its predominantly focal nature of injury.
Rodent models of AHT
In 1998 Smith et al [28] reported on a model of shaking plus hypoxemia in PND 6 rats in an attempt to model AHT. Injury was induced in the model using a shaker producing cortical hemorrhages and loss of cortical tissue, along with production of markers of oxidative stress. Cortical tissue loss exceeded 28% by 14 days post injury. In an early pharmacological study in that model, the anti-excitotoxic glutamate release inhibitor riluzole reduced cortical neurodegeneration, while the anti-oxidant tirilazad was ineffective [29]. In a subsequent report by a separate investigative group PND 8 rats were also injured using a rotating shaker (15 second exposure) and once again hemorrhagic lesions were observed, in this case, in a widespread distribution including cortex, periventricular white matter, corpus callosum, brain stem and cerebellar white matter [30]. Cystic lesions were seen out to 31 days. Once again, an anti-excitotoxic treatment, this time the glutamate receptor antagonist MK-801, was shown to confer some benefit. The model, however, has not generated a significant body of follow up investigations by other laboratories—although the problem of AHT remains important and deserving of additional investigation.
Pediatric models of TBI in large animals
FPI in piglets
In an initial report in 1994, Armstead et al [20] published seminal findings on the hemodynamic responses to FPI in newborn (PND 1–5) versus juvenile (3–4 week old) piglets. In addition to the FPI, which was produced in a parallel fashion to the rodent models, a cranial window and intracranial pressure monitoring have been routinely incorporated into the model in many of the studies to assess the cerebral microcirculation and intracranial dynamics. Profound pial artery vasoconstriction and cerebral hemoglobin desaturation was observed in the newborn piglet after FPI, despite similar injury levels in the original report. Following that work, the Armstead laboratory has used this model to study a large number of secondary injury mechanisms, with a general focus on studies germane to the cerebral circulation and its regulation. A complete review of the many studies performed is beyond the scope of this review, however, key issues such as the mechanisms of autoregulation and its impairment after injury [21], sex differences in both the injury and vascular responses as well as consequences of therapy [22], and reports on pre-clinical approaches to optimizing perfusion and mitigate neuronal death after injury [23] highlight some of the areas of investigation. Insight into the roles of prostanoids, oxidative signaling, kinases, and endothelin in vascular dysregulation has been provided. Several of these recent reports should foster the development of clinical studies to evaluate translation of the findings. For example, recent work has suggested marked improvements in cerebral autoregulation and histology after injury with the use of the pressor agent phenylephrine [23]—with the greatest benefit in the female piglets. Choice of pressor after severe TBI in children to optimize cerebral perfusion pressure remains somewhat arbitrary in clinical care, and thus this study could translate to benefit if confirmed in clinical investigations and/or use.
Rotational head injury in the piglet
A rotational model of TBI has been in use in the laboratory of Susan Margulies since 2002. In that model, 3–5 day-old piglets [19] are subjected to a rapid non-impact axial rotation of the head. The model produced immediate coma, impact apnea in 5 of 7 piglets, and a number of features of diffuse axonal injury assessed at 6 h after the insult. In a follow up study, both a mild (142 rad/sec) and moderate (188 rad/sec) injury level have been reported [65]. The moderate injury level produced behavioral deficits on exploring their environment and in visual-based problem solving vs. sham and the moderate but not mild injury levels led to axonal injury as quantified by amyloid precursor protein immunohistochemistry. A number of additional studies have been carried out in this model including studies of non-invasive monitoring of CBF and cerebral blood oxygenation [66] and treatments such as folic acid [67]. Strengths of the studies in large animals include the use of a gyrencephalic animal mimicking the human condition with regard to the amount of white matter, the ability to carry out clinically relevant physiological monitoring, and similarities between humans and pigs with regard to pharmacology and physiology. Limitations include lack of a mechanical impact in the rotational injury model, the small number of molecular tools available for use in pigs, less well established behavioral outcome tasks than in rodents, and cost.
Mild TBI and Repetitive TBI
Pediatric models of mild TBI and repetitive TBI in rodents
Closed head CCI
The most commonly used models to produce mTBI or repetitive mTBI in pediatric applications have been variants of the CCI model using a closed head approach [15, 17, 32, 33, 68]. In these studies PND 11 rats have been used to model AHT, PND 18 rats to model toddlers, and PND 35 have been used to model sports concussion in adolescent athletes. In these studies, either a conventional CCI impactor tip has been used or a customized rubber ball tip (9.5 mm in diameter). In some cases, a closed head CCI approach was used to produce mTBI with a single exposure. In general, the approaches used targeted mTBI or concussion and were designed to avoid skull fracture. These models produce axonal injury, neuroinflammation (particularly microglial activation) and calpain activation, but little or no neuronal death. As anticipated, repeated injury magnifies the pathology. Traditional MWM assessments generally have not been consistently useful in these models, but other behavioral outcome tasks such as novel object recognition or fear conditioning are often more useful. In the closed head CCI models described above, several therapies such as minocycline, and FK506 have been tested, however, they have shown less efficacy vs. those reported in adult TBI models in rodents [32, 33].
Pediatric models of repetitive TBI in large animals
Rotational head injury in the piglet
Reports on repetitive TBI in large animal models are scarce. Friess et al [69] used the previously described rapid non-impact rotational TBI model (at a moderate injury level) in 3–5 day old piglets and evaluated multiple impacts either one day or one week apart. Despite a moderate injury level, multiple impacts led to mortality in 43% of the piglets. In surviving animals, behavioral deficits in visual problem solving and axonal injury assessed with APP staining were observed.
Shaking injury in infant lambs
In an attempt to model AHT in a large animal, Finnie and co-workers [24–27] developed a model using anesthetized 7–10 day old lambs. Remarkably the insult was produced by physical shaking—studied in part to answer questions related to the effects of shaking in the human condition. In these studies, the lambs were grasped under the axilla and vigorously shaken for 20 seconds. Multiple shaking exposures were used (10 episodes over a period of 30 minutes). The injury produced ranged from diffuse BBB damage and axonal injury to brainstem injury and damage at the cranio-cervical junction and/or mortality.
Challenges, gaps, potential future directions, and conclusions
Given the importance of the problem, it is clear that additional translational investigations are needed in pediatric TBI. Based on the work that has been discussed, huge gaps remain. Although several important differences in the secondary injury cascades were outlined and discussed, it remains unclear exactly how any aspect of the secondary injury response differentially impacts the brain across the pediatric age spectrum. How important key targets such as neuro-inflammation, excitotoxicity, neuronal death, axonal injury, and other components of the secondary injury cascade compare in infants, children, and adults remains unclear. It is also evident that age-related differences in injury mechanism (i.e., accidental injuries vs AHT) are also important. Pre-clinical studies in pediatric TBI also have only begun to wrestle with factors that still stymie investigations in adult models, such as the effect of anatomical injury phenotype including contusion, diffuse axonal injury, diffuse swelling, and subdural, epidural and subarachnoid hemorrhage, among others. Similarly, genetic differences have only begun to be explored in the pediatric TBI arena—and even the most basic genetic factors such as sex differences have seen limited investigation. The previously discussed reports from the laboratory or William Armstead (22, 23) suggest translational potential. Another gap in pre-clinical pediatric TBI investigations is in penetrating brain injury—which has been systematically studied only in adult TBI models. In pre-clinical research, the issue of reproducibility and rigor has also been raised as an important concern and may be contributing to the failure of clinical translation of new therapies. Pre-clinical research, in adult models, has begun to address this concern through work in Operation Brain Trauma Therapy [70, 71] and some work on this important facet of preclinical TBI research is also beginning to be addressed in pediatric TBI [72]. These types of collaborative investigators should be expanded. On the clinical front, studies of the epidemiology of severe TBI and the response to current therapies through investigations in the ADAPT trial [73–75] or via the use of big data [76] have great potential to begin to outline the most favorable approach to the management of severe pediatric TBI in the intensive care unit. Given the fact that its importance has only recently been recognized, pediatric models of mTBI and repetitive mTBI have only begun to be explored, and study of potential link between TBI in infants and children and long-term neurodegenerative diseases is another priority that must be addressed.
Acknowledgments
We thank NIHCD T32 HD 040686 for support (JW)
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Patrick M. Kochanek, Ake N. Grenvik Professor of Critical Care Medicine, Vice Chair, Department of Critical Care Medicine, Professor of Anesthesiology, Pediatrics, Bioengineering, and Clinical and Translational Science, Director, Safar Center for Resuscitation Research, Children’s Hospital of Pittsburgh of UPMC, John G. Rangos Research Center – 6th Floor, 4401 Penn Avenue, Pittsburgh, PA 15224, Tel: 412-692-7700.
Jessica S. Wallisch, Pediatric Critical Care Medicine Fellow, Children’s Hospital of Pittsburgh of UPMC, NIH T32 Post-Doctoral Scholar, Safar Center for Resuscitation Research, 4401 Penn Ave, Faculty Pavilion, Suite 02000, Pittsburgh, PA 15224.
Hülya Bayır, Professor, Department of Critical Care Medicine, Children’s Hospital of Pittsburgh of UPMC, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, 4401 Penn Avenue, Suite Floor 5, Pittsburgh, PA 15224.
Robert S.B. Clark, Chief, Pediatric Critical Care Medicine, Children’s Hospital of Pittsburgh of UPMC, Associate Professor, Critical Care Medicine and Pediatrics, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, 4401 Penn Avenue, Suite Floor 5, Pittsburgh, PA 15224.
References
- 1. [Accessed 6 May 2017]; https://www.cdc.gov/traumaticbraininjury/get_the_facts.html.
- 2.Jenkins LW, Kochanek PM. Developmental neurobiology, neurophysiology, and the PICU. In: Nichols DG, Shaffner DH, editors. Rogers’ Textbook of Pediatric Intensive Care. 5. Wolters Kluwer; Philadelphia: 2016. pp. 861–876. [Google Scholar]
- 3.Margulies SS, Thibault KL. Infant skull and suture properties: measurements and implications for mechanisms of pediatric brain injury. J Biomech Eng. 2000;122:364–371. doi: 10.1115/1.1287160. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield RT, Schiding JK, Hamilton RL, Kochanek PM. Effects of hypothermia on traumatic brain injury in immature rats. J Cereb Blood Flow Metab. 1996;16:244–252. doi: 10.1097/00004647-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Grundl PD, Biagas KV, Kochanek PM, Schiding JK, Barmada M, Nemoto EM. Early cerebrovascular response to head injury in immature and mature rats. J Neurotrauma. 1994;11:135–148. doi: 10.1089/neu.1994.11.135. [DOI] [PubMed] [Google Scholar]
- 6.Biagas KV, Grundl PD, Kochanek PM, Schiding JK, Nemoto EM. Posttraumatic cerebral hyperemia in rats: autoradiographic determination of age-related differences in the response to percussive injury. J Neurotrauma. 1996;13:189–200. doi: 10.1089/neu.1996.13.189. [DOI] [PubMed] [Google Scholar]
- 7.Clark RS, Kochanek PM, Schwarz MA, Schiding JK, Turner DS, Chen M, Carlos TM, Watkins SC. Inducible nitric oxide synthase expression in cerebrovascular smooth muscle and neutrophils after traumatic brain injury in immature rats. Pediatr Res. 1996;39:784–790. doi: 10.1203/00006450-199605000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao W-L, Yoshihara K, Faden AI. Differential expression of apoptotic protease-activating factor-1 and case-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21:7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adelson PD, Dixon CE, Kochanek PM. Long-term dysfunction following diffuse traumatic brain injury in the immature rat. J Neurotrauma. 2000;17:277–286. doi: 10.1089/neu.2000.17.273. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins LW, Peters GW, Dixon CE, Zhang X, Clark RSB, Skinner JC, Marion DW, Adelson PD, Kochanek PM. Conventional and functional proteomics using large format two-dimensional gel electrophoresis 24 hours after controlled cortical impact in postnatal day 17 rats. J Neurotrauma. 2002;19:715–740. doi: 10.1089/08977150260139101. [DOI] [PubMed] [Google Scholar]
- 11.Bittigau P, Sifringer M, Felderhoff-Mueser U, Hansen HH, Ikonomidou C. Neuropathological and biochemical features of traumatic injury in the developing brain. Neurotox Res. 2003;5:475–490. doi: 10.1007/BF03033158. [DOI] [PubMed] [Google Scholar]
- 12.Bittigau P, Sifringer M, Felderhoff-Mueser U, Ikonomidou C. Apoptotic neurodegeneration in the context of traumatic injury to the developing brain. Exp Toxicol Pathol. 2004;56:83–99. doi: 10.1016/j.etp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Prins ML, Fujima LW, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- 14.Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Huh JW, Widing AG, Raghupathi R. Repetitive mild non-contusive brain trauma in immature rats exacerbates traumatic axonal injury and axonal calpain activation: A preliminary report. J Neurotrauma. 2007;24:15–27. doi: 10.1089/neu.2006.0072. [DOI] [PubMed] [Google Scholar]
- 16.Adelson PD, Fellows-Mayle W, Kochanek PM, Dixon CE. Morris Water Maze function and histologic characterization of two age-at-injury experimental models of controlled cortical impact in the immature rat. Childs Nerv Syst. 2013;29:43–53. doi: 10.1007/s00381-012-1932-4. [DOI] [PubMed] [Google Scholar]
- 17.Fidan EG, Lewis J, Kline AE, Garman RH, Alexander H, Cheng JP, Bondi CO, Clark RSB, Dezfulian C, Kochanek PM, Kagan VE, Bayır H. Repetitive mild traumatic brain injury in the developing brain: effects on long-term functional outcome and neuropathology. J Neurotrauma. 2016;33:641–651. doi: 10.1089/neu.2015.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 19.Raghypathi R, Marguilies SS. Traumatic axonal injury after closed head injury in the neonatal pig. J Neurotrauma. 2002;19:843–853. doi: 10.1089/08977150260190438. [DOI] [PubMed] [Google Scholar]
- 20.Armstead WM, Kurth CD. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J Neurotrauma. 1994;11:487–497. doi: 10.1089/neu.1994.11.487. [DOI] [PubMed] [Google Scholar]
- 21.Armstead WM, Riley J, Vavilala MS. Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr Crit Care Med. 2013;14:e103–e111. doi: 10.1097/PCC.0b013e3182712b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstead WM, Riley J, Vavilala MS. Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr Crit Care Med. 2016;17:e130–e137. doi: 10.1097/PCC.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Curvello V, Hekierski H, Riley J, Vavilala M, Armstead WM. Sex and age differences in phenylephrine mechanisms and outcomes after piglet brain injury. Pediatr Res. 2017 Apr 26; doi: 10.1038/pr.2017.83. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Finnie JW, Blumbergs PC, Manavis J, turner RJ, Helps S, Vink R, Byard RW, Chidlow G, Sandoz B, Dutschke J, Anderson RW. Neuropathological changes in a lamb model of non-accidental head injury (the shaken baby syndrome) J Clin Neurosci. 2012;19:1159–1164. doi: 10.1016/j.jocn.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Sandoz B, Dutshke J, Liu Q, Manavis J, Finnie JW, Vink R, Anderson RWG. In vivo biomechanical response of ovine heads to shaken baby syndrome events. Comput Methods Biomech Biomed Engin. 2012;15(Suppl 1):293–294. doi: 10.1080/10255842.2012.713640. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RW, Sandoz B, Dutschke JK, Finnie JW, Turner RJ, Blumbergs PC, Manavis J, Vink R. Biomechanical studies in an ovine model of non-accidental head injury. J Biomech. 2014;47:2578–2583. doi: 10.1016/j.jbiomech.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Dutschke JK, Finnie JW, Manavis J, Anderson RW. Semiquantitation of axonal injury in traumatically damaged brains using color deconvolution. Appl Immunohistochem Mol Morphol. 2017;25:277–281. doi: 10.1097/PAI.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 28.Smith SL, Andrus PK, Gleason DD, Hall ED. Infant rat model of the shaken baby syndrome: preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneration. J Neurotrauma. 1998;15:693–705. doi: 10.1089/neu.1998.15.693. [DOI] [PubMed] [Google Scholar]
- 29.Smith SL, Hall ED. Tirilazad widens the therapeutic window for riluzole-induced attenuation of progressive cortical degeneration in an infant rat model of the shaken baby syndrome. J Neurotrauma. 1998;15:707–719. doi: 10.1089/neu.1998.15.707. [DOI] [PubMed] [Google Scholar]
- 30.Bonnier C, Mesplès B, Carpentier S, Henin D, Gressens P. Delayed white matter injury in a murine model of shaken baby syndrome. Brain Pathol. 2002;12:320–328. doi: 10.1111/j.1750-3639.2002.tb00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanlon LA, Huh JW, Raghupathi R. Minocycline transiently reduces microglia/macrophage activation but exacerbates cognitive deficits following repetitive traumatic brain injury in the neonatal rat. J Neuropathol Exp Neurol. 2016;75:214–226. doi: 10.1093/jnen/nlv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanlon LA, Raghupathi R, Huh JW. Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp Neurol. 2017;290:1–14. doi: 10.1016/j.expneurol.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dileonardi AM, Huh JW, Raghupathi R. Differential effects of FK506 on structural and functional axonal deficits after diffuse brain injury in the immature rat. J Neuropathol Exp Neurol. 2012;71:959–972. doi: 10.1097/NEN.0b013e31826f5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayır H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28:420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- 35.Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res. 1996;39:204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Tsuru-Aoyagi K, Potts MB, Trivedi A, Pfankuch T, Raber J, Wendland M, Claus CP, Koh SE, Ferriero D, Noble-Haeusslein LJ. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji J, Kline AE, Amoscato A, Arias AS, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kaga VE, Bayır H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci. 2012;15:1407–1415. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young C, Roth KA, Klocke BJ, West T, Holtzman DM, Labruyere J, Qin Y-Q, Dikranian K, Olney JW. Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis. 2005;20:608–614. doi: 10.1016/j.nbd.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Hayes SR, Deshpande JK. Newly postulated neurodevelopmental risks of pediatric anesthesia. Curr Neurol Neurosci Rep. 2011;11:205–210. doi: 10.1007/s11910-010-0177-4. [DOI] [PubMed] [Google Scholar]
- 40.McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- 41.Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011;33:592–597. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Simon DW, McGeachy M, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13:171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 44.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R, Kannan RM. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 47.Dail WG, Feeney DM, Murray HM, Linn RT, Boyeson MG. Responses to cortical injury: II. Widespread depression of the activity of an enzyme in cortex remote from a focal injury. Brain Res. 1981;211:79–89. doi: 10.1016/0006-8993(81)90068-8. [DOI] [PubMed] [Google Scholar]
- 48.Sifringer M, Stefovska V, Endesfelder S, Stahel PF, Genz K, Dzietko M, Ikonomidou C, Felderhoff-Mueser U. Activation of caspase-1 dependent interleukins in developmental brain trauma. Neurobiol Dis. 2007;25:614–622. doi: 10.1016/j.nbd.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Cordobes F, Lobato RD, Rivas JJ, Portillo JM, Sarabia M, Munoz MJ. Post-traumatic diffuse brain swelling: isolated or associated with cerebral axonal injury. Clinical course and intracranial pressure in 18 children. Childs Nerv Syst. 1987;3:235–238. doi: 10.1007/BF00274055. [DOI] [PubMed] [Google Scholar]
- 50.Kochanek PM. Pediatric Traumatic Brain Injury: Quo Vadis. Dev Neurosci. 2006;28(4–5):244–255. doi: 10.1159/000094151. [DOI] [PubMed] [Google Scholar]
- 51.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 52.Blennow K, Brody D, Kochanek PM, Levin H, McKee A, Ribbers GM, Yaffe K, Zetterberg H. Traumatic brain injuries. Nature Rev Dis Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 53.Giza CC, Prins ML, Hovda DA, Herschman HR, Feldman JD. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity. J Neurotrauma. 2002;19:387–402. doi: 10.1089/08977150252932352. [DOI] [PubMed] [Google Scholar]
- 54.Dixon CE, Lyeth BG, Povlishock JT, Findling RL, Hamm RJ, Marmarou A, Young HF, Hayes RL. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 55.Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prins ML, Povlishock JT, Phillips LL. The effects of combined fluid percussion traumatic brain injury and unilateral entorhinal deafferentation on the juvenile rat brain. Brain Res Dev Brain Res. 2003;140:93–104. doi: 10.1016/s0165-3806(02)00588-6. [DOI] [PubMed] [Google Scholar]
- 57.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 58.Kochanek PM, Dixon CE. Animal Models of Traumatic Brain Injury. In: Winn HR, editor. Youmans Neurological Surgery. 6. Elsevier Inc; Philadelphia: 2011. pp. 3300–3304. [Google Scholar]
- 59.Kochanek AR, Kline AE, Gao WM, Chadha M, Lai Y, Clark RS, Dixon CE, Jenkins LW. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev Neurosci. 2006;28:410–419. doi: 10.1159/000094167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bayır H, Tyurin VA, Tyurin YY, Viener R, Ritov V, Amoscato A, Zhao Q, Zhang X, Feldman-Janesko KL, Alexander H, Clark RSB, Kochanek PM, Kagan VE. Selective early cardiolipin oxidation after brain trauma: A lipidomics analysis. Ann Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 61.Robertson CL, Bucci CJ, Fiskum G. Mitochondrial response to calcium in the developing brain. Brain Res Dev Brain Re. 2004;151:141–148. doi: 10.1016/j.devbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Huh JW, Franklin MA, Widing AG, Raghupathi R. Regionally distinct patterns of calpain activation and traumatic axonal injury following contusive brain injury in immature rats. Dev Neurosci. 2006;28:466–476. doi: 10.1159/000094172. [DOI] [PubMed] [Google Scholar]
- 63.Robertson CL, Saraswati M. Progesterone protects mitochondrial function in a rat model of pediatric traumatic brain injury. J Bioenerg Biomembr. 2015;47:43–51. doi: 10.1007/s10863-014-9585-5. [DOI] [PubMed] [Google Scholar]
- 64.Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: Characterization of regional vulnerability. Exp Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- 65.Friess SH, Ichord RN, Owens K, Ralston J, Rizol R, Overall KL, Smith C, Helfaer MA, Margulies SS. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp Neurol. 2007;204:234–243. doi: 10.1016/j.expneurol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou C, Eucker SA, Durduran T, Yu G, Ralston J, Friess SH, Ichord RN, Margulies SS, Yodh AG. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J Biomed Opt. 2009;14:034015. doi: 10.1117/1.3146814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naim MY, Friess S, Smith C, Ralston J, Ryall K, Helfaer MA, Margulies SS. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev Neurosci. 2010;32:466–479. doi: 10.1159/000322448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prins ML, Hales A, Reger M, Giza CC, Hovda DA. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci. 2010;32:510–518. doi: 10.1159/000316800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Friess SH, Ichord RN, Ralston J, Ryall K, Helfaer MA, Smith C, Margulies SS. Repeated traumatic brain injury affects composite cognitive function in piglets. J Neurotrauma. 2009;26:1111–1121. doi: 10.1089/neu.2008.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kochanek PM, Bramlett HM, Dixon CE, Shear DA, Dietrich WD, Schmid KE, Mondello S, Wang KKW, Hayes RL, Povlishock JT, Tortella FC. Operation Brain Trauma Therapy: Approach to modeling therapy evaluation, drug selection, and biomarker assessments, for a multi-center pre-clinical drug screening consortium for acute therapies in severe traumatic brain injury. J Neurotrauma. 2016;33:513–522. doi: 10.1089/neu.2015.4113. [DOI] [PubMed] [Google Scholar]
- 71.Kochanek PM, Bramlett HM, Shear DA, Dixon CE, Mondello S, Dietrich WD, Hayes RL, Wang KKW, Poloyac SM, Empey PE, Povlishock JT, Mountney A, Browning M, Deng-Bryant Y, Yan HQ, Jackson TC, Catania M, Glushakova O, Richieri SP, Tortella FC. Operation Brain Trauma Therapy: Synthesis of findings, current investigations, and future directions. J Neurotrauma. 2016;33:606–614. doi: 10.1089/neu.2015.4133. [DOI] [PubMed] [Google Scholar]
- 72.Margulies SS, Kilbaugh T, Sullivan S, Smith C, Propert K, Byro M, Saliga K, Costine BA, Duhaime AC. Establishing a clinically relevant large animal model platform for TBI therapy development: Using cyclosporine A as a case study. Brain Pathol. 2015;25:289–303. doi: 10.1111/bpa.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell MJ, Adelson PD, Hutchison JS, Kochanek PM, Tasker RC, Vavilala MS, Beers SR, Fabio A, Kelsey SF, Wisniewski SR the Multiple Medical Therapies for Pediatric Traumatic Brain Injury Workgroup. Differences in medical therapy goals for children with severe traumatic brain injury – An international study. Pediatr Crit Care Med. 2013;14:811–818. doi: 10.1097/PCC.0b013e3182975e2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller Ferguson N, Sarnaik A, Miles D, Shafi N, Peters MJ, Truemper E, Vavilala MS, Bell MJ, Wisniewski SR, Luther JF, Hartman AL, Kochanek PM for the Investigators of the ADAPT Trial. Abusive head trauma and mortality—An analysis from an international comparative study of children with severe traumatic brain injury. Crit Care Med. 2017 Apr 20; doi: 10.1097/CCM.0000000000002378. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kochanek PM, Bell MJ. Tackling the challenges of clinical trials for severe traumatic brain injury in children: Screening, phenotyping, and adapting. Crit Care Med. 2015;43:1544–1546. doi: 10.1097/CCM.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 76.Shein SL, Ferguson NM, Kochanek PM, Bayır H, Clark RSB, Fink EL, Tyler-Kabara EC, Wisniewski SR, Tian Y, Bell MJ. Effectiveness of pharmacological therapies for intracranial hypertension in children with severe traumatic brain injury—results from an automated data collection system time-synched to drug administration. Pediatr Crit Care Med. 2016;17:236–245. doi: 10.1097/PCC.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]