Abstract
The immune response to biomaterial implants critically regulates functional outcomes such as vascularization, transplant integration/survival, and fibrosis. To create “immunologically smart” materials, the host-material response may be engineered to optimize the recruitment of pro-regenerative leukocyte subsets which mature into corresponding wound-healing macrophages. We have recently identified a unique feature of pro-regenerative Ly6Clow monocytes that is a higher expression of both the bioactive lipid receptor sphingosine-1-phosphate receptor 3 (S1PR3) and the stromal derived factor-1α (SDF-1α) receptor CXCR4. Therefore, we designed a bifunctional hydrogel to harnesses a mechanistic synergy between these signaling axes to enhance the recruitment of endogenous pro-regenerative monocytes. To overcome the challenge of codelivering two physiochemically distinct molecules—a large hydrophilic protein and hydrophobic small molecule—we engineered a dual affinity hydrogel that exploits the growth factor affinity of a heparin derivative (Hep−N) and lipid chaperone activity of albumin. The sphingosine analog FTY720 and SDF-1α are successfully loaded and coreleased from the Hep−N-functionalized PEG-DA hydrogels while maintaining bioactivity. Placement of these hydrogels into a murine partial thickness skin wound demonstrates that corelease of FTY720 and SDF-1α yields superior recruitment of myeloid cells to the implant interface compared to either factor alone. Although in vivo delivery of FTY720 or SDF-1α individually promotes the enhanced recruitment of Ly-6Clow anti-inflammatory monocytes, codelivery enhances the early accumulation and persistence of the differentiated wound healing CD206+ macrophages in the tissue surrounding the gel. Co-delivery similarly promoted the synergistic expansion of vasculature adjacent to the implant, a key step in tissue healing. Taken together, these findings suggest that the combination of chemotactic molecules may provide additional maturation signals to the infiltrating leukocytes to facilitate macrophage transition and vascular network expansion, thus, ultimately, potentiating tissue repair. The coupling of multiple pro-regenerative biological cues provides a foundation for more fine-tuned immunoregenerative modulation to facilitate tissue repair.
Keywords: stromal derived factor-1α (SDF-1α), bioactive lipids, heparin hydrogels, immunoregenerative engineering
Graphical Abstract

INTRODUCTION
Endogenous inflammatory response in sterile injury and tissue trauma is characterized by the infiltration of circulating mononuclear phagocytes to clear debris, remodel extracellular matrix and vasculature, and signal to circulating and parenchymal cells that help restore the native cellular and matrix composition.1 Loss of function studies show that early myeloid cell input is critical for effective repair of tissue injury in the complex tissue regeneration models of the salamander limb and zebrafish tail fin.2,3 Monocytes and their macrophage progeny participate in processes of angiogenesis4 and arterio-genesis5 while orchestrating healing across myriad injury contexts including liver,6 kidney,7 cardiac and skeletal muscle,8–11 and peripheral nervous tissue.12,13 Circulating blood monocytes are comprised of at least two functionally distinct subsets14 and the inflammatory index, or balance of regenerative to inflammatory myeloid cells, in the tissue is correlated with the healing outcome.12,15–17 Classical “inflammatory monocytes” (IMs) are characterized by high Ly6C and low CX3CR1 surface expression in mice and CD14+CD16− in humans. Nonclassical “anti-inflammatory monocytes” (AMs), express low levels of Ly6C and high CX3CR1 in mice and CD14lowCD16+ in humans.1,14 Monocyte-derived macrophages similarly exhibit functional and phenotypic heterogeneity with a spectrum of M1 “inflammatory” to M2 “regenerative” macrophages and the dominant presence of M2 versus M1 macrophages is related to positive healing outcomes.1 Biomaterial-mediated strategies to change the inflammatory index of the tissue improves metrics of regeneration;12,15,16,18 therefore, development of materials that can effectively control the recruitment, polarization, and activity of myeloid cells presents an opportunity for supporting a powerful endogenous repair system.15,16
Controlled drug delivery can facilitate spatial and temporal control over presentation of biological or pharmacological factors within a wounded tissue. Using biomaterials to produce in vivo gradients of immunomodularory cues can facilitate recruitment of endogenous pro-regenerative cells to the injury microenvironment.1,16,19 To control the frequency and selectivity of endogenous inflammatory cell recruitment, we have investigated receptor signaling axes that govern selective recruitment of monocyte subsets. Two such control points for manipulating the balance of functional phenotypes of monocytes/macrophages within inflamed tissues are the bioactive lipid receptor sphingosine 1-phosphate receptor 3 (S1PR3) and CXC-type chemokine receptor 4 (CXCR4).15,16,19 AMs display a higher expression of S1PR315 and CXCR416 at the cell surface compared to IMs. Following inflammatory injury, upregulation of stromal derived factor-1α (SDF-1α), the natural ligand of CXCR4, promotes tissue repair in part by attracting and retaining myeloid cells near vessels to coordinate leukocyte-assisted angiogenesis and arteriogenesis.20,21 Antagonism of CXCR4 during the injury response leads to a significant reduction in recruited myeloid cells and a failure of neovascularization.20 Localized biomaterial-mediated delivery of exogenous SDF-1α enhances the recruitment of AMs to inflamed tissue vasculature and concomitant expansion of microvascular networks within the injury niche.16 Similarly, delivery of FTY720, a small molecule agonist of S1PR3, from degradable polymers stimulates selective recruitment of AMs from blood and their pro-regenerative on-site education, as indicated by their strategic perivascular positioning, attenuated secretion of inflammatory cytokines, and differentiation to CD206+ M2 macrophages.15 Molecular cross-talk between S1PR3 and CXCR4 evidenced by transactivation of CXCR4 upon S1PR3 stimulation in endothelial progenitor cells suggests potential synergy between these axes for chemotaxis.22 Stimulation of S1PR3 also enhances CXCR4-mediated chemotaxis of AMs toward SDF-1α, whereas IMs fail to migrate robustly toward SDF-1α.15 S1PR3 agonism enhances CXCR4 activation in endothelial progenitor cells, thereby enhancing their homing efficacy in the treatment of hind limb ischemia.22 These results suggest that dual stimulation of S1PR3 and CXCR4 signaling axes in vivo through localized gradient release within an inflammatory injury niche would promote synergistic recruitment of pro-regenerative monocytes/macrophages and enhance microvascular growth and remodeling.1,16,21
To investigate the functional synergy of the CXCR4 and S1PR3 signaling axes, we engineered a dual-affinity heparin-based biomaterial carrier to corelease SDF-1α and S1P receptor targeted small molecule FTY720. Heparin is a glycosaminoglycan that binds cationic proteins such as SDF-1α and protects them from denaturing conditions.23–25 We have previously demonstrated that matrices functionalized with a heparin derivative protect proteins against denaturation to maximize bioactivity and lower the payload requirement for in vivo efficacy.24,26 To reduce the anticoagulant activity of heparin for safe use in vivo, we incorporated a cross-linkable heparin derivative that was selectively desulfated at the -N position (Hep−N)24 within a poly(ethylene glycol) diacrylate (PEG-DA) network. To overcome the physiochemical disparities between SDF-1α, a 10 kDa hydrophilic protein, and FTY720, a 307 Da hydrophobic small molecule lipid, we further engineered the scaffolds for dual affinity by encapsulation of albumin within the matrix during cross-linking (aHep−N) as an affinity-based carrier for the small molecule FTY720 because of its endogenous chaperone binding capacity for bioactive lipids.27
In this study, we investigated inflammatory and arteriogenic responses to aHep−N-PEG-DA hydrogel implants releasing either SDF-1α or FTY720 or both factors combined. We show that SDF-1α and FTY720 are coreleased from aHep−N-PEG-DA gels over several days in vitro. The dorsal skinfold window chamber model, a mouse model of excisional skin injury, was used to longitudinally assess the recruitment of innate immune cells and associated microvascular network expansion. Synergy between SDF-1α and FTY720 was observed with regard to leukocyte attachment to the implant surface and structural enlargement of arterioles in the peri-implant tissue. Accumulation of macrophages in the early phase of repair and wound-resolving macrophages in the later phase was enhanced by codelivery of SDF-1α and FTY720 compared to either factor alone. These results suggest an exciting potential of the aHep−N-PEG-DA hydrogel technology to release multiple distinct biomolecular cues and locally tune the innate immune response in favor of regeneration.
EXPERIMENTAL MATERIALS AND METHODS
Heparin Modification
N-Desulfated heparin methacrylamide (Hep−N-MAm) was fabricated as described previously.16 Briefly, heparin sodium salt (Sigma) was dissolved in dH2O at a concentration of 10 mg/mL and desalted using Dowex 50WX4 resin (mesh size 100–200, Sigma). Pyridine was added dropwise to result in a pH of ~6, after which time excess pyridine was removed using a rotatory evaporator.24 The solution was flash frozen and lyophilized to yield heparin pyridinium salt. Next, heparin pyridinium salt was dissolved in 90% DMSO/10% ddH2O (v/v) at a concentration of 1 mg/mL, mixed for 2 h at 50 °C using a rotary evaporator, cooled and precipitated with 95% ethanol (VWR) saturated with sodium acetate (VWR). The precipitate was centrifuged, collected, and dissolved in dH2O and this solution was dialyzed for 3 days followed by lyophilization to yield N-desulfated heparin. Finally, the product was functionalized with methacrylamide groups with an 8.0 molar excess of of N-(3-aminopropyl) methacrylamide hydrochloride (APMAm, Polysciences), N-hydroxysuccinimide (NHS, Acros Organics) and N-3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride (EDC, Sigma) at an acidic pH. After functionalization, Hep−N-MAm was dialyzed, lyophilized, and stored at −20 °C until use. Proton nuclear magnetic resonance (1H NMR) was performed to determine the degree of methacrylamide functionalization whereby Hep−N-MAm was dissolved in deuterated water (10 mg/mL) and 1H NMR spectra were recorded on a Bruker Avanace III 400 spectrometer at 400 MHz.
Hydrogel Fabrication
As described previously,16 PEG-DA and Hep-N-MAm were UV sterilized, combined in a 9:1 PEG-DA:Hep-N-MAm ratio, and then added to a sterile phosphate buffered saline (PBS) solution comprised of GMP-quality bovine serum albumin (BSA, Sigma), ammonium persulfate (APS, 0.018M, Sigma), and N,N,N′,N′-tetramethylethylenediamine (TEMED, 0.018M, Sigma). Final concentrations of components in the hydrogel precursor solution were as follows: PEG-DA, 10.0% w/v; Hep-N-MAm, 1.1% w/v; BSA, 5.6% w/v; APS, 0.41% w/v; and TEMED, 0.21% w/v.″ The hydrogel precursor solution was pipetted between two sterile glass slides with an inner clearance of 0.5 mm, allowed to gel for 10 min at room temperature, and then punched with 2 mm diameter biopsy punches (Miltex) to form gels of 0.5 mm thickness and 2 mm diameter.
SDF-1α/FTY720 Loading and Release in Vitro
Following fabrication, gels were placed in ultralow binding 24-well plates and rinsed with PBS for 3 h. For FTY720 only gels, a 10 µL droplet containing 2.38 × 10−8 moles of either FTY720 (Cayman Chemical) or fluorescent 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-FTY720 (Cayman Chemical) was added to the top of each gel and the gels were incubated overnight at 4 °C. After loading, gels were rinsed for 3 h at room temperature with 500 µL of PBS. For SDF-1α only gels, PBS was removed and a 10 µL droplet of SDF-1α (0.1 µg; PeproTech) was added to each gel followed by incubation overnight at 4 °C. For dual-loaded gels, FTY720 was loaded overnight first, followed by SDF-1α. (For parity in sample treatment, in gels with either FTY720 or SDF-1α only, the samples would undergo both overnight incubations, but with a 10 µL droplet of PBS in place of the drug that was not being loaded.)
After loading, the wells were filled with 500 µL of PBS and incubated at 37 °C for the release study. At each time point, PBS was exchanged and stored at −80 °C for subsequent analysis. The concentration of SDF-1α in supernatant was quantified using a Mouse CXCL12/SDF-1α Quantikine ELISA Kit (R&D Systems) and the concentration of 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-FTY720 was quantified using a fluorescence plate reader with λex = 485 nm and λem = 515 nm. Two batches of Hep−N -MAm were required to complete all studies, and therefore FTY720 and SDF-1α release from hydrogels made with both Hep −N-MAm batches were averaged to obtain final release curves (n = 6 hydrogels per group across 2 batches of Hep−N-MAm).
In Vitro Migration
Bioactivity of released SDF-1α/FTY720 was assayed in vitro as described previously. Briefly, albumin-embedded gels loaded with FTY720 and SDF-1α (aHep−N-FTY+SDF), SDF-1α alone (aHep−N-SDF), or neither factor (aHep−N) were incubated with 650 µL of Iscove’s modified Dulbecco’s medium (Fisher) containing 0.5% fatty acid-free bovine serum albumin (Fisher) for 24h at 37 °C. A transwell assay was assembled by transferring hydrogel-conditioned media to the chamber below a 5 µm pore size membrane, and seeding 4 × 105 cells from C57BL/6 mouse bone marrow aspirate on top. After 4h of migration at 37 °C and 5% CO2, migrated cells were stained with DRAQ5 dye according to manufacturer’s protocol (Cell Signaling Technologies). Relative migration was quantified by fluorescence intensity using an Odyssey CLx Infrared Imaging System (LI-COR Biosciences). Statistical analysis was performed using oneway ANOVA, Geisser-Greenhouse correction for nonsphericity, and Tukey posthoc multiple comparisons test (n = 5).
Dorsal Skinfold Window Chamber and Hydrogel Implantation
Male C57BL/6 mice aged 8–12 weeks of age were anesthetized by i.p. injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) in sterile 0.9% saline or by inhaled isoflurane and surgically fitted with sterile dorsal skinfold window chambers (APJ Trading Co.) as previously described 15,19,28,29 Prior to surgery, dorsal skin was shaved, depilated, and sterilized via triplet washes of 70% ethanol and chlorhexidine. A double-layered skin fold was elevated off the back of the mouse and the back side of the titanium window chamber frame was surgically fixed to the underside of the skinfold. Surgical microscissors were used to remove the epidermis and dermis from the top-side of the skinfold in a circular area (diameter = 12 mm) to reveal the vasculature underlying the reticular dermis. Exposed tissue was kept hydrated with sterile saline. The front side of the titanium frame was then mounted on the top of the skin and attached to its underlying counterpart. The dorsal skin was sutured to the titanium frame, and mice were implanted with one Hep−N internal control hydrogel and one aHep−N experimental hydrogel (unloaded or loaded albumin-containing gel with SDF-1α, FTY720, or both factors) placed on opposite sides of the window. The exposed tissue was then overlaid with a protective sterile glass coverslip. Mice were allowed to recover in heated cages and administered sustained release buprenorphine via i.p. injection as a postoperative analgesic. All mice received a standard laboratory diet and water ad libitum throughout the course of the experiment.
Flow Cytometry and Identification of Immunophenotypes
To measure the recruitment of cells to tissue surrounding the hydrogels, the dorsal tissue circumscribing the gel position was collected and digested with 1 mg/mL collagenase IA (Sigma) in Krebs-Ringers solution at 37 °C, and further disaggregated with a cell strainer and pestle to create a single cell suspension. To examine cells at the hydrogel interface, cells were removed from the hydrogel surface by incubation with trypsin for 10 min at 37 °C and combined with a fixed volume of flow cytometry counting beads for quantification (CountBright Absolute Counting Beads, Life Technologies). Immuno-staining and flow cytometry analyses were performed according to standard procedures and analyzed on a FACS-AriaIIIu flow cytometer (BD Biosciences). The following antibodies were used for cell phenotyping: APC/Cy7- or BV510-conjugated anti-CD11b (BioLegend, M1/70), APC- or BV421-conjugated anti-Ly-6C (BioLegend, HK1.4), PE- or PerCP/Cy5.5-conjugated anti-CXCR4 (eBiosciences, 2B11), PE-conjugated anti-MerTK (R&D Systems, 108928), BV711-conjugated anti-CD64 (BioLegend, X54–5/7.1), and BV605-con-jugated anti-CD206 (BioLegend, C068C2). Monocyte populations were defined as CD11b+SSClowLy-6Chigh/low and were confirmed to be Ly-6G−N (BioLegend, 1A8) to exclude neutrophils. “AMs” are CD11b+SSClowLy-6Clow and “IMs are CD11b+SSClowLy-6Chigh. Macrophages were identified with high fidelity as MerTK+CD64+ cells,30 which excludes dendritic cells.30,31 Further confirming macrophage identity of the MerTK+CD64+ cells, nearly all cells identified as macrophages expressed F4/80 (data not shown).
Statistical analysis in Figure 2 and 4 was conducted using one-way ANOVA, Geisser-Greenhouse correction for nonsphericity, and Tukey posthoc multiple comparisons test. The number of replicates are as follows: Figure 2, n = 5–6; Figure 4A, n = 7–9; Figure 4B, C, n = 2–4.
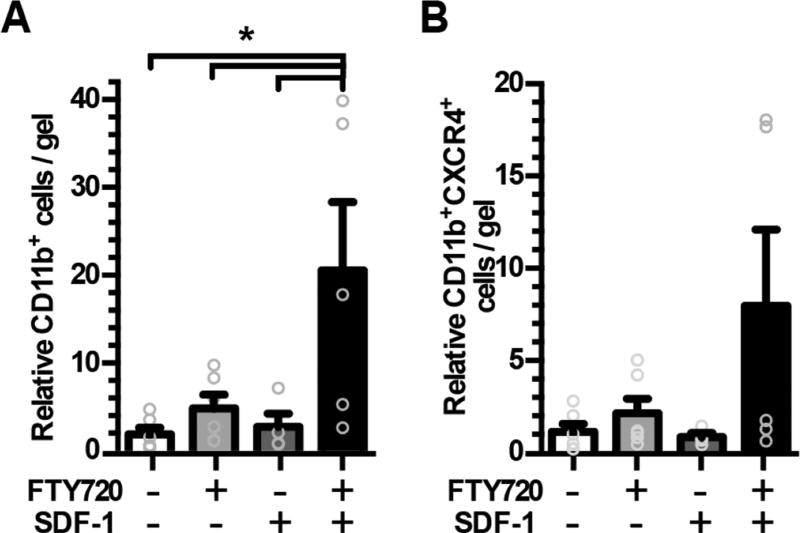
Figure 2.
Dual release of SDF-1α and FTY720 from aHep−N-PEG-DA hydrogels promotes synergistic recruitment of leukocytes to the gel surface. Gels were implanted in the dorsal skin window chamber for 3 days and explanted to evaluate cell association with the gel by flow cytometric analysis of (A) CD11b+ and (B) CD11b+CXCR4+ populations. (n = 5–6, ANOVA, *p < 0.05).
Figure 4.
Recruitment of mononuclear phagocyte subsets to tissue surrounding aHep−N-PEG-DA gels. Tissue surrounding the hydrogel was analyzed 3 days after surgery and implantation for the immunophenotype of the recruited myeloid populations. (A) Either FTY720 or SDF-1α lead to an increase in AM (SSClowCD11b+Ly6Clow) recruitment to the gel as a percent of monocytes (SSClowCD11b+) (n = 7–9, ANOVA, *p < 0.05). (B) Dual release of FTY720 and SDF-1α significantly increased the presence of MerTK+CD64+ macrophages within the tissue as a percent of total cells (n = 2–4, ANOVA of control, SDF-1, and FTY720/SDF-1 groups (FTY720 excluded due to low sample size)). (C) Dual release similarly produced a trend of increased CD206+MerTK+CD64+ cells as a percent of total cells (n = 2–4, ANOVA of control, SDF-1, and FTY720/SDF-1 groups, p = 0.07).
Confocal Intravital Microscopy and Quantification of CX3CR1dim Macrophages
Male heterozygous B6.129P-Cx3cr1tm1Litt/J (CX3CR1-EGFP) mice were utilized to visualize monocytes and macrophages in live mice based on their selective surface expression of CX3CR1.14–16 Confocal intravital microscopy was performed on day 1 or day 6 after surgery and gel implantation. To label perfused vasculature, mice were anesthetized with isoflurane and injected with high-molecular-weight TRITC-conjugated dextran (2 MDa; Life Technologies). For subsequent microscopy, the mouse was secured to the microscope stage in a custom adapter, the glass window was removed, and dorsal tissue was irrigated with sterile saline. Z-stack images were acquired immediately proximal to the gel using a 20× water immersion objective (NA = 1.0) on a Zeiss LSM710 NLO microscope. For 3D analysis in Imaris (Bitplane), time-lapse images (period = 30 s, duration = 15 min) were acquired to visualize immune cell migration in the close surrounding tissue. Cells expressing CX3CR1-GFP were identified in Imaris using the surface tool. CX3CR1+ surfaces were identified by smoothing with a 2 µm grain size and an automatic threshold on absolute intensity. Touching objects were split using a seed points diameter of 10 µm. Vessels were identified in Imaris by drawing a surface on the TRITC-dextran fluorescent channel with a 3 µm grain size, manually selected threshold value (determined based on each image), and manually selected volume filter to remove small debris. The automatic cell tracking feature was selected, and the following metrics were exported for statistical analysis: track displacement, track length, and track straightness. For statistical analysis of cell migration metrics in Figure 3, Mann-Whitney rank test was performed on the distribution comprised of all cells analyzed across 2 mice per group and 2 ROI per mouse (FTY720+SDF-1, n = 3095; internal control, n = 2119).
Figure 3.
Myeloid cells have greater directional motility around dual releasing gel. Myeloid cell movements were captured by intra vital microscopy of the dorsal window chamber in the CX3CR1GFP/+ transgenic mouse model 1 day after surgery and gel implantation. Myeloid cells (green) and vessels (red) were visible adjacent to the unloaded Hep−N-PEG-DA internal control gel (A) and the dual releasing aHep−N-PEG-DA SDF-1α + FTY720 gel (B) in the same window chamber. Fifteen minute videos were acquired and cell tracking analysis was done in IMARIS software. Cell displacement (C) was enhanced in the region surrounding the experimental gel but not track length (D), suggesting that the cells are traveling in a straighter and more directed path (E). (n = 2119–3095 cells, 2 mice/group, 2 regions of interest each, Mann-Whitney rank test, *p < 0.05).
Whole-Mount IHC, Confocal Microscopy, And Analysis of CD68+ and CD206+ Cells
on day 7 after surgery and gel implantation, mouse vasculature was perfused with warm 0.9% saline and then 4% PFA until tissues were fixed. Dorsal tissue was explanted and drop-fixed in 4% PFA for 10 min, permeabilized with 0.2% saponin in PBS for 16–24 h at 4 °C, blocked with 10% mouse serum in PBS for 16–24 h, and stained with combinations of the following monoclonal antibodies diluted into a solution of 0.5% BSA, 5% mouse serum, and 0.1% saponin in PBS: Cy3-conjugated anti-α-smooth muscle actin (αSMA, 1:300, Sigma), AF594-conjugated anti-CD31 (1:100, BioLegend), AF647-conjugated anti-CD68 (1:200, AbD Serotec), and AF488-conjugated anti-CD206 (1:200, AbD Serotec). Images of macrophage accumulation in dorsal tissue were acquired as 3-dimensional z-stacks in confocal mode using a Zeiss LSM710 NLO microscope at 20× magnification. To quantify the number of CD68+CD206+ macrophages per unit area, Imaris (Bitplane) software was used. Surfaces were created in the CD68 and CD206 channels independently, and the number of CD68+ surfaces overlapped by CD206+ surfaces were counted. In Figure 6, cell density is reported for n = 2–11 regions of interest per group across 2–3 animals per group.
Figure 6.
Dual release of SDF-1 and FTY720 increases abundance of CD206+ macrophages. (A) Whole-mount IHC and confocal microscopy shows accumulation of CD68+ macrophages and CD206+CD68+ macrophages in tissue proximal to the gels at day 7 (CD206, green; CD68, blue scale bar 100 µm). (B, C) Quantification of CD206+CD68+ macrophages (n = 2–3 mice, 2–11 ROI each, ANOVA, *p < 0.05).
Bright-Field Intravital Microscopy and Analysis of Arteriolar Diameter
Immediately following surgery and on day 3, mice were maintained under isoflurane anesthesia, the glass window was removed, and dorsal tissue was flooded with 1 mM adenosine in Ringer’s solution to maximally dilate all vessels and maintain tissue hydration. The mouse was then mounted to a custom microscope stage mount and a tile scan of the entire window was acquired noninvasively at 5× magnification on a Zeiss Imager.D2 microscope with AxioCam MRc 5 color digital camera (Carl Zeiss). To measure changes in the diameter of arterioles, we identified arteriole-venule pairs within a 3 mm radius of the center of each gel. Arterioles and venules were identified on the basis of size and morphology at day 0. Internal diameters based on blood column width in brightfield images were measured using Zen Blue (Zeiss) and recorded longitudinally for each vessel segment on day 0 and 3. Quantification of arteriolar diameter was restricted to the microvasculature by analyzing arterioles with diameters <40 µm on day 0.16,32 Analysis was limited to arterioles visible at both time points (1–7 arterioles/gel).
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). All statistical analysis was executed in GraphPad Prism software. Statistical tests are reported in the methods and legends associated with each figure. Unless otherwise noted, p < 0.05 was considered statistically significant.
RESULTS
aHep-n-PEG-DA Gels Corelease FTY720 and Bioactive SDF-1α in Vitro
The sphingosine analog FTY720 enhances the migration of AMs toward an SDF-1α gradient in vitro.15 To harness this biological synergy, we sought to develop a biomaterial that could release both the small molecule FTY720 and the protein SDF-1α to locally deliver these agents in vivo. Hydrogels containing a heparin derivative and embedded with albumin were engineered to sequester and release these physiochemically distinct biomolecules. The albumin provides an affinity carrier for small bioactive lipids or small molecule analogs such as FTY720,27 while the heparin derivative provides a platform for loading and stabilizing heparin-binding growth factors.16,24,25 The fabrication and composition of aHep−N hydrogels encapsulating the chemokine protein SDF-1α and hydrophobic small molecule FTY720 are shown in Figure 1A. The dual loaded gels release 24.9 ± 7.2% of total SDF-1α payload by day 1 and 26.0 ± 0.2% by day 7 in vitro (Figure 1B), whereas 43.0 ± 4.0% of FTY720 is released by day 1 and 87.2 ± 2.1% by day 7 (Figure 1B). Both FTY720 and SDF-1α released from the gels maintain bioactivity as illustrated by their ability to induce chemotaxis of primary murine bone marrow cells toward 24h hydrogel-conditioned media in an in vitro transwell migration assay. Media conditioned by gels releasing SDF-1α induced a 1.5-fold increase in chemotaxis compared to control gels containing only embedded albumin, while gels releasing both FTY720 and SDF-1α induced a 2.0-fold increase compared to control (Figure 1C).
Figure 1.
Hep−N-PEG-DA hydrogels corelease bioactive SDF-1α and FTY720 in vitro. (A) Fabrication and postgelation loading of SDF-1α and/or FTY720 into albumin-embedded Hep−N-PEG-DA gels (aHep−N). (B) SDF-1α and FTY720 are released over 7 days in vitro (n = 6 gels, 2 independent studies). (C) In vitro migration of primary murine bone marrow cells toward media conditioned by 24h of hydrogel release. *Indicates significance compared to all other groups (n = 5, ANOVA, p < 0.05).
Dual Release of FTY720 and SDF-1α Promotes Synergistic Recruitment of Leukocytes to the Surface of aHep−N-PEG-DA Gels
To measure the ability of aHep−N-PEG-DA gels to recruit endogenous cells in vivo, we utilized the dorsal skinfold window chamber model, a mouse model of excisional skin injury that enables intravital microscopic (IVM) assessment of host responses to biomaterial implants, including microvascular remodeling and associated recruitment of immune cells. aHep−N gels were implanted in wild-type C57Bl/6 mice for 3 days and explanted for analysis of cell recruitment to the implant-tissue interface. Brightfield microscopy of explanted gels reveals increased cell attachment to the surface of gels coreleasing SDF-1α and FTY720 compared to either factor alone or unloaded control (data not shown). Cells were digested from the gel surface and characterized by flow cytometry. Recruitment of myeloid leukocytes (CD11b+) is greatest in response to codelivery of FTY720 and SDF-1α in a pattern that indicates synergy between these two molecules (Figure 2A). Myeloid leukocytes (CD11b+) expressing the SDF-1α receptor (CXCR4) exhibit a similar, but nonsignificant, pattern of synergy in recruitment to the gel indicating that the responding cells may be equipped to respond to SDF-1α released from the gel (Figure 2B). One day after implantation of either an unloaded Hep−N internal control gel or an aHep−N gel coreleasing FTY720 and SDF-1α into CX3CR1GFP/+ mice that allow the tracking of myeloid cells by GFP fluorescence, myeloid cells can be seen extravasating and migrating in the tissue adjacent to each gel (Figure 3A–B). Cells in the peri-implant region of the dual releasing gel had a greater displacement (Figure 3C) and directional migratory patterns compared to cells in the peri-implant region of the unloaded Hep−N gel (Figure 3D–E) suggesting that the dual affinity gel alters the migratory behavior of infiltrating myeloid cells.
Release of Either FTY720 or SDF-1α Alone Recruits AMs (Ly6Clow Monocytes) to Injured Tissue
To assess the immunophenotypes of recruited cells in the tissue surrounding each gel at day 3, a 4 mm biopsy of dorsal tissue under the hydrogel was harvested and digested for flow cytometric analysis. We have previously shown that AMs (Ly6Clow monocytes) can be functionally distinguished from IMs (Ly6Chigh monocytes) by higher surface expression of CXCR4 and S1PR3.15,16 In the present study, recruitment of AMs to the peri-implant tissue increased approximately 1.5-fold in response to delivery of either FTY720 or SDF-1α alone compared to animals implanted with the albumin containing control gel; however, codelivery of FTY720 and SDF-1α did not significantly increase AM recruitment (Figure 4A).
Codelivery of FTY720 and SDF-1α Release Increases Abundance of Wound Healing Macrophages Surrounding the Hydrogel
Although both FTY720 and SDF-1α both individually increased recruitment of AMs to the tissue adjacent to the hydrogel, the dual delivery gel did not exhibit the expected synergy of localized AM recruitment in these two chemotactic signaling axes. Previous studies suggest that AMs recruited by FTY720 can differentiate into CD206+ wound healing macrophages within the injury niche.15 We hypothesized that codelivery of FTY720 and SDF-1α may support an accelerated monocyte-to-macrophage differentiation prior to the day 3 time point. In agreement with this hypothesis, corelease of FTY720 and SDF-1α increased the abundance of macrophages (MerTK+CD64+)30,31 in tissue surrounding the implanted biomaterial at day 3 (Figure 4B). The abundance of CD206+MerTK+CD64+ macrophages out of total cells at day 3 followed an increasing trend that paralleled the increase in total macrophages (Figure 4C). To further investigate this finding, we assessed in situ macrophage accumulation at later stages of repair by complementary microscopy techniques. The CX3CR1GFP/+ transgenic mouse model was selected to enable intra vital microscopic visualization of monocyte and macro-phage populations based on GFP expression and cell morphology (Figure 5A).33,34 Implantation of SDF-1α or dual-loaded gels in the CX3CR1gfp/+ mouse followed by confocal intra vital microscopy at day 6 revealed phenotypic differences in CX3CR1-GFP+ cell accumulation and morphology. The SDF-1α group had a higher proportion of rounded monocytes identified as CX3CR1-GFP+ (Figure 5B, D), whereas the dual release FTY720 + SDF-1α group was enriched in CX3CR1-GFPdim and more elongated cells, features that are consistent with macrophage morphology34 (Figure 5C, E). Whole-mount immunohistochemistry in wild-type C57Bl/6 mice at day 7 revealed increased accumulation of CD68+CD206+ wound-healing macrophages in the tissue surrounding gels coreleasing FTY720 + SDF-1α compared to either factor alone or control (Figure 6A–C). A higher proportion of CD68+ cells were also CD206+ in the dual treatment group (Figure 6C). Taken together, these results suggest that corelease of FTY720 and SDF-1α increases the early accumulation of CD68+CD206+ wound-healing macrophages compared to release of either factor alone.
Figure 5.
Dual release of SDF-1α and FTY720 alters CX3CR1+ cell morphology. (A) CX3CR1−GFP/+ mice were fitted with a dorsal window chamber and after 6 days were injected (i.v.) with rhodamine-dextran to visualize perfused vessels. Confocal intravital imaging was then conducted in the region directly surrounding the hydrogel implant. (B, D) Tissue surrounding the SDF hydrogel had rounded CX3CR1-GFP+ cells surrounding the vasculature, whereas (C, E) tissue surrounding the SDF-1α + FTY720 hydrogel had CX3CR1dim myeloid cells with elongated morphology consistent with macrophage phenotype. Scale bar 100 µm. (representative images from n = 3 ROI per gel).
Co-release of FTY720 and SDF-1α Synergistically Increases the Caliber of Arterioles in the Tissue Surrounding aHep−N-PEG-DA Gels
Mononuclear phagocytes serve a prominent regulatory role in arteriogenesis, which is critical to restoring perfusion to ischemic tissues.5,32,35 To determine whether recruitment of pro-regenerative monocyte and macrophage populations by dual-loaded aHep−N gels is associated with enhanced arteriogenesis, we assessed the change in diameter of arterioles in the tissue surrounding aHep−N gels (Figure 7A–C). At day 3 during the acute phase of healing, FTY720-releasing gels cause a mean decrease in arteriolar diameter of −21.7% while SDF-1α-releasing and coreleasing gels stimulate an increase of 20.1% and 25.4%, respectively (Figure 7B). At day 7 during the later phase of healing, gels coreleasing FTY720 + SDF-1α stimulated greater arteriolar diameter enlargement (Figure 7C). In addition, the dual releasing gel produced a more densely packed network of microvasculature compared to an internal control Hep−N gel implanted in the same window chamber (Figure 7D–F). Taken together, these data demonstrate that corelease of FTY720 and SDF-1α from bifunctional aHep−N-PEG-DA gels provides a regenerative environment that supports the recruitment of CD206+ macrophages and a coordinate increase in vascular remodeling.
Figure 7.
Dual release of SDF-1α and FTY720 synergistically increases the caliber of arterioles in tissue surrounding Hep-N-PEG-DA gels. (A) Bright-field intravital microscopy was used to measure enlargement of arterioles in the microcirculation surrounding implanted gels at (B) day 3 and (C) day 7 (ANOVA, *p < 0.05, n = 8–10 at day 3, n = 3–5 at day 7). (D–F) Whole mount immunofluorescent imaging of vessels (CD31+SMA+) surrounding unloaded control gels or aHep-N-FTY720 + SDF-1 gels within the same window chamber (n = 3).
DISCUSSION
The immune response to biomaterial implants critically regulates functional healing outcomes such as vascularization, tissue regeneration, transplant integration, and extent of fibrosis. By mimicking the presentation of natural inflammatory and regenerative signals, we have an opportunity to engineer the host-material interaction in the design of “immunologically smart” materials. Because of the complexity of the immune response, materials that can release multiple complementary factors are likely necessary to fine-tune an engineered inflammatory response. We have previously shown that either SDF-1α or FTY720 released in a localized gradient from a biomaterial can support the enhanced recruitment of Ly6ClowAMs and improved vascular remodeling. The inflammatory process is inherently complex and dynamic; tissues and infiltrating inflammatory cells integrate and respond to a multiplicity of signals which may be enhanced by localized release of multiple complementary therapeutic agents.36 In order to move toward higher level of control of the local inflammatory environment, we have developed a bifunctional hydrogel that targets a mechanistic synergy between chemokine protein SDF-1α and the S1P signaling axis to enhance the recruitment and behavior of endogenous pro-regenerative leukocytes. Dual affinity aHep-N-PEG-DA hydrogels were engineered to achieve simultaneous in vivo release of SDF-1α and FTY720 with the goal of modulating local innate immunity to promote vascular remodeling that will support wound healing. We found that both SDF-1α and FTY720 recruit Ly6C lowAMs to the peri-implant region; however, the combination of these two factors increases the number of CD206+ macrophages within the tissue suggesting a complex cellular response that can be achieved by combination of two signaling factors compared to either factor alone.
To overcome the challenge of codelivering two physiochemically distinct molecules—a hydrophilic protein and hydro-phobic small molecule—we engineered a dual affinity hydrogel that exploits the growth factor affinity of heparin and lipid chaperone activity of albumin. In the present study, albumin-embedded Hep−N-PEG-DA hydrogels released bioactive SDF-1α and FTY720 in vitro with approximately ~25% of the total SDF-1α payload and ~85% of the FTY720 payload released by day 7 (Figure 1). SDF-1α, loaded by charge-based interaction with a heparin derivative covalently linked in the hydrogel, was delivered primarily in a burst release within the first 24–36 h (Figure 1). Heparin-based growth factor loading provides an advantage of protecting the stability of the growth factor cargo under various conditions that may otherwise lead to denaturation or degradation. 23,24 To enable safe in vivo use of heparin and maintain its protective effect on growth factor cargo, we utilized a selectively -N desulfated heparin derivative (Hep-N) with reduced anticoagulant activity. 3,2 In previous studies, we have demonstrated that this amount of SDF-1α released from aHep-N hydrogels in vivo stimulates a localized recruitment of Ly6C low AMs to the tissue surrounding the gel within 3 days.
To make the hydrogel bifunctional, we encapsulated albumin within the Hep−N hydrogels as a lipid carrier for codelivery with protein cargo and a novel means to display bioactive lipids in a biomaterial context.27 Chaperone proteins facilitate stability and transport of lipids within blood and tissue compartments. Albumin is one of the main chaperone proteins that reversibly binds S1P in the blood and has also been found to bind FTY72027. Nearly 35% of S1P is found in albumin-rich fractions of blood and approximately 65% is found in complex with ApoM high-density lipoprotein.38 FTY720, which is loaded by affinity for albumin entrapped in the bulk gel, released with a burst and continued sustained release over 7 days for total release of approximately 85% of the loaded drug in vitro (Figure 1). The differences in cumulative release between SDF-1α and FTY720 are likely due to the distinct means of loading within the aHep−N matrix As we have observed previously with FTY720 released from PLGA polymer films, release of FTY720 alone from aHep−N gels increased recruitment of AMs to the peri-implant niche suggesting in vivo bioactivity (Figure 3). This highly localized recruitment is a desirable feature in managing local versus off-target effects. Although these gels were designed as 2 mm disk gels to create a localized response within the 12 mm dorsal skinfold window chamber injury, the size, shape, and loading could be altered to achieve a broader tissue coverage area, thus scaling the response. Future iterations of the material could include degradable elements to extend the opportunity for cell infiltration into the material.
Selective recruitment of Ly6ClowAMs and CD206+ MerTK+CD64+ “wound-resolving” macrophages are associated with enhanced tissue repair.12,15–17,39 The extent to which macrophage heterogeneity arises from inherent plasticity versus microenvironmental polarization (“on-site education”),40 selective or sequential recruitment of monocyte subsets, or preferential survival of specific subtypes remains controversial because of the complex nature of the wound healing cascade in different injury contexts. Cells on the M2 end of the macrophage continuum are responsible for both stimulating the deposition of extracellular matrix and degrading/remodeling the matrix during wound healing; if left unchecked, these macrophages can contribute to pathologic fibrotic tissue.41 A careful balance of the appropriate local cues must be struck to steer the inflammatory process toward healing and not chronic inflammatory fibrosis. Hydrogels were implanted in excisional skin wounds to evaluate synergy between S1PR3 and CXCR4 signaling axes with regard to pro-regenerative cell recruitment and microvascular remodeling. Dual release of SDF-1α and FTY720 synergistically increased the recruitment of CD11b+ myeloid leukocytes and CXCR4+ cells to the implant-tissue interface relative to control or either factor alone supporting our hypothesis of functional synergy between these two pathways (Figure 2). Dual-loaded gels altered the early migratory behavior of monocytes that extravasated near the loaded implant, but not an unloaded control implant within the same window-chamber, indicating that release of these molecules affects the local behavior of monocytes in the tissue (Figure 3). Interestingly, within the peri-implant tissue, although both SDF-1α and FTY720 individually increased the proportion of Ly6ClowAMs in the tissue, with dual release, we observed a synergistic increase in macrophages (MerTK+CD64+) but not monocytes (Figure 4). Dendritic cells were excluded from our analysis based on the literature findings that skin dendritic cells do not express high levels of CD64.31 Increased macrophage accumulation was further supported by the presence of myeloid cells with an elongated morphology consistent with macrophage phenotype in the CX3CR1GFP/+ mouse (Figure 5) and the presence of more CD206+ cells in whole mount immunohistochemistry of the peri-implant tissue (Figure 6). CD206 is most highly expressed on M2a macrophages, but may also be expressed at lower levels on M2c cells.42 Detection of synergy in the macrophage pool but not their recruited monocyte precursors may be explained by enhanced differentiation. These findings suggest that the combination of these two factors in vivo produces a more complex synergy than we have previously observed in vitro or that has been described in the literature. Although priming monocytes with FTY720 increases their migration toward SDF-1α in vitro in an S1PR3-dependent manner,15 treating inflamed tissue with both SDF-1α and FTY720 causes an increase in cell recruitment and fate transition within the injury niche. We have previously shown that FTY720 released from PLGA thin films enhances AMs entrance into tissue from circulation as well as their differentiation into macrophages.15 Although the mechanism of this enhanced differentiation is incompletely under-stood,15 S1PR3 activation by FTY720 regulates reduced secretion of inflammatory cytokines from monocytes and increases in anti-inflammatory/pro-regenerative protein secretion from endothelial cells.15 Thus, FTY720 may serve as a tissue conditioning cue that provides on-site education to encourage pro-regenerative differentiation of recruited monocytes into macrophages and their polarization toward wound-resolving phenotypes. The precise mechanistic details of this phenomenon will require further investigation.
Recruited leukocytes facilitate blood vessel remodeling during angiogenesis and arteriogenesis via strategic perivascular positioning, secretion of growth factors, and matrix-remodeling enzymes.5,20,43–45,32,46 Expansion of the vascular network through formation of new vessels and increased caliber of existing vessels is crucial for supplying sufficient nutrients to promote tissue repair. Wound-resolving macrophages on the M2 end of the spectrum in particular are associated with enhanced vascular remodeling.5,47,48 In the present study, dual release of SDF-1α and FTY720 synergistically enhanced arteriolar structural enlargement in conjunction with greater accumulation of wound-resolving macrophages. Although we cannot definitively say there is a causal relationship between the macrophages and vascular remodeling in this study, based on prior literature, we believe there is a strong link between the macrophage recruitment and increased vascular diameter and network density. Thus, the dual affinity aHep−N-PEG-DA platform has potential to increase tissue perfusion during repair which has been linked to improved therapeutic outcomes after ischemia, trauma, and tissue transplantation. It remains unclear whether this phenomenon is the result of increased total myeloid recruitment, increased differentiation of M2-like macrophages, or alterations to the behavior of the recruited cells.15 Taken together, these results indicate that cooperativity between the SDF-1α/CXCR4 and FTY720/S1PR3 signaling axes can be exploited to localize pro-regenerative macrophages to sites of injury and enhance microvascular network remodeling.
CONCLUSIONS
Because of the complexity of the immune response, materials that can release multiple complementary factors are likely necessary to fine-tune an engineered inflammatory response. Immunologically smart biomaterials are those in which the host-material response is engineered to control the recruitment of pro-regenerative leukocyte subsets which mature into corresponding wound healing macrophages within the injury niche. Here we have shown that in vivo delivery of SDF-1α or FTY720 separately promotes the recruitment of Ly-6Clow anti-inflammatory monocytes and that codelivery enhances the accumulation of pro-regenerative CD206+ macrophages to orchestrate microvascular network growth and remodeling. These findings demonstrate that control of the recruitment and function of distinct therapeutic immune cell subtypes can be achieved by rational design of dual affinity biomaterials that exploit unique synergy between distinct signaling modules. Further, the aHep−N-PEG-DA platform provides a flexible tool for exploiting emerging knowledge of local bioactive lipid signaling in the inflammatory response to biomaterials and investigating cooperativity with protein signals. By recruiting and educating endogenous immune cells that coordinate healing, such “immuno-regenerative” approaches may produce longer-lasting therapeutic effects and fewer undesirable off-target effects compared to direct delivery of potent growth factors.
Acknowledgments
We gratefully acknowledge the funding sources for this work including: National Institutes of Health grants R01AR056445 and R01DE019935 to E.A.B.; Petit Faculty Fellowship to J.S.T.; and American Heart Association Fellowship 16PRE31110023 to J.R.K. We thank and acknowledge the technical assistance and expertise of the core facilities personnel and animal care personnel of the Institute for Bioengineering and Bioscience at Georgia Institute of Technology.
Footnotes
The authors declare no competing financial interest.
References
- 1.Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. (London, UK.) 2016;241(10):1084–97. doi: 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc. Natl. Acad. Sci. U.S.A. 2013;110(23):9415–20. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrie TA, Strand NS, Tsung-Yang C, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. 2014;141(13):2581–2591. doi: 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–40. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de, Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479(7371):122–6. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, Ransohoff RM, Charo IF, Jenne CN, Kubes P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 2015;212(4):447–56. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney, differentiate into functionally distinct populations. J. Immunol. 2009;183(10):6733–43. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 8.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory, reparative phases in the infarcted myocardium. Circ. Res. 2014;114(10):1611–22. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold L, Perrin H, de Chanville CB, Saclier M, Hermand P, Poupel L, Guyon E, Licata F, Carpentier W, Vilar J, Mounier R, Chazaud B, Benhabiles N, Boissonnas A, Combadiere B, Combadiere C. CX3CR1 deficiency promotes muscle repair, regeneration by enhancing macrophage ApoE production. Nat. Commun. 2015;6:8972. doi: 10.1038/ncomms9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31(2):384–96. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 11.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38(3):555–69. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 15.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. USA. 2013;110(34):13785–90. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger JR, Ogle ME, McFaline-Figueroa J, Segar CE, Temenoff JS, Botchwey EA. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1alpha-releasing hydrogels enhances microvascular network remodeling. Biomaterials. 2016;77:280–90. doi: 10.1016/j.biomaterials.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative Expression of Proinflammatory Antiinflammatory Genes Reveals Differences between Healing Nonhealing Human Chronic Diabetic Foot Ulcers. J. Invest. Dermatol. 2015;135(6):1700–3. doi: 10.1038/jid.2015.30. [DOI] [PubMed] [Google Scholar]
- 18.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a proregenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352(6283):366–70. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogle ME, Sefcik LS, Awojoodu AO, Chiappa NF, Lynch K, Peirce-Cottler S, Botchwey EA. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater. 2014;10(11):4704–14. doi: 10.1016/j.actbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P, Levkau B, Zeiher AM, Dimmeler S, Haendeler J. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler., Thromb. Vasc. Biol. 2007;27(2):275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 23.Miller T, Goude MC, McDevitt TC, Temenoff JS. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014;10(4):1705–19. doi: 10.1016/j.actbio.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seto SP, Miller T, Temenoff JS. Effect of selective heparin desulfation on preservation of bone morphogenetic protein-2 bioactivity after thermal stress. Bioconjugate Chem. 2015;26(2):286–93. doi: 10.1021/bc500565x. [DOI] [PubMed] [Google Scholar]
- 25.Tellier LE, Miller T, McDevitt TC, Temenoff JS. Hydrolysis sulfation pattern effects on release of bioactive bone morphogenetic protein-2 from heparin-based microparticles. J. Mater. Chem. B. 2015;3(40):8001–8009. doi: 10.1039/C5TB00933B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35(25):7228–38. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai J-P, Parepally J, Men A. Clinical Pharmacology and Biopharmaceutics Review (Fingolimod) Center for Drug Evaluation and Research, U.S. Food and Drug Administration; Silver Spring, MD: 2010. [Google Scholar]
- 28.Sefcik LS, Petrie Aronin CE, Awojoodu AO, Shin SJ, Mac Gabhann F, MacDonald TL, Wamhoff BR, Lynch KR, Peirce SM, Botchwey EA. Selective activation of sphingosine 1-phosphate receptors 1 and 3 promotes local microvascular network growth. Tissue Eng., Part A. 2011;17(5–6):617–629. doi: 10.1089/ten.tea.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29(19):2869–77. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ Immunological Genome C. Gene-expression profiles transcriptional regulatory pathways that underlie the identity diversity of mouse tissue macrophages. Nat. Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39(5):925–38. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Bruce AC, Kelly-Goss MR, Heuslein JL, Meisner JK, Price RJ, Peirce SM. Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model. Arterioscler., Thromb. Vasc. Biol. 2014;34(9):2012–22. doi: 10.1161/ATVBAHA.114.303399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Molecular and cellular biology. 2000;20(11):4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5 CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117(1):185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ. Res. 2004;94(5):671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 36.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadir R, Baleux F, Grosdidier A, Imberty A, Lortat-Jacob H. Characterization of the stromal cell-derived factor-1alpha-heparin complex. J. Biol. Chem. 2001;276(11):8288–96. doi: 10.1074/jbc.M008110200. [DOI] [PubMed] [Google Scholar]
- 38.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlback B, Kono M, Proia RL, Smith JD, Hla T. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signaling. 2015;8(389):ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Segar CE, Hughley BB, Bowers DT, Botchwey EA. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials. 2013;34(38):9853–62. doi: 10.1016/j.biomaterials.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic, arteriogenic accessory cells. J. Exp. Med. 2013;210(12):2611–25. doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wermuth PJ, Jimenez SA. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clin Transl Med. 2015;4:2. doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes, macrophage accumulation is severely depressed in op/op mice. J. Leukocyte Biol. 2006;80(1):59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 44.Hamm A, Veschini L, Takeda Y, Costa S, Delamarre E, Squadrito ML, Henze AT, Wenes M, Serneels J, Pucci F, Roncal C, Anisimov A, Alitalo K, De Palma M, Mazzone M. PHD2 regulates arteriogenic macrophages through TIE2 signalling. EMBO Mol. Med. 2013;5(6):843–57. doi: 10.1002/emmm.201302695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front. Physiol. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–62. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. Journal of pathology. 2013;229(2):176–85. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 48.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–18. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]