Abstract
Electrochemotherapy is a new therapeutic option for patients with locally spread melanoma. It is based on the phenomenon of reversible electroporation, i.e. a transient increase in permeability of cell membranes under the influence of an appropriately modulated electric field. This allows multiplication of toxicity of a cytostatic agent entering the tumour cell. It is highly effective, especially in the palliative treatment of cancers located in the integument of the human body (skin and subcutaneous tissue). Available literature provides a mandate both for the application of this method in the aforementioned cases as well as for further work on its development.
This paper focuses on reviewing the literature concerning the use of electrochemotherapy in the treatment of melanoma.
Keywords: electrochemotherapy, mel-anoma, bleomycin
Introduction
Tumours, irrespective of their histology, can cause metastases to the skin or subcutaneous tissue. Between 8% and 45% of patients diagnosed with melanoma experience skin metastases of which 22.7% are satellite and in-transit tumours; 50.2% are metastases in regional lymph nodes and 28.1% are distant metastases [1]. In the case of superficial lesions local therapies include: surgical intervention, radiotherapy, cryotherapy, laser therapy, RFA ablation, local chemotherapy, local immunotherapy with BCG, isolated limb perfusion and as a last resort in the case of limbs – amputation [2–7]. In addition to local treatment, systemic therapy is increasingly used. Progress in new targeted therapies has been tremendous in recent years [2, 7]. Despite this, some of the superficial lesions are initially unresectable, e.g. due to their size or location – e.g. face. Complications such as bleeding, infections, psychological aspects often require application of local therapies, even in stage 4 of the disease [8].
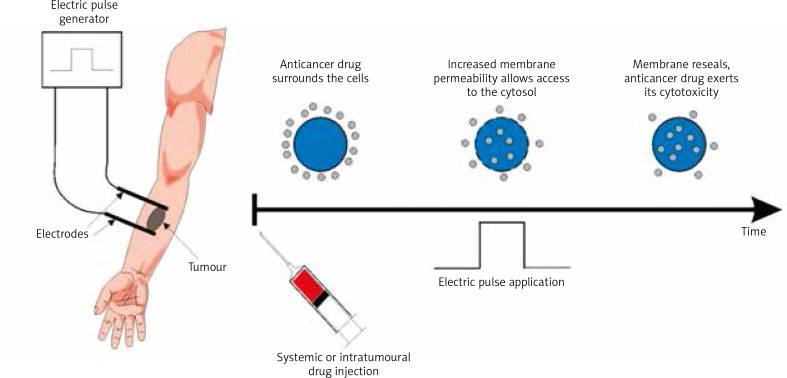
One of the newer therapies applied in metastatic melanoma is electrochemotherapy (ECT) which combines physical properties of electroporation using electric current with chemical properties of chemotherapeutics. In this method, by applying an electric current to the tissue, we induce a temporary increase in permeability of the cell membrane, thus enabling a free flow of large molecules into the cell, including cytostatics that at baseline are not transported to the cytosol. As a result, their potential toxicity increases considerably [9]. A flow chart presenting the procedure of electrochemotherapy is shown in Fig. 1.
Fig. 1.
Flow chart presenting steps in electrochemotherapy (by courtesy of IGEA Ltd.)
Combinations of different cytostatic agents and electroporation have been studied in many pre-clinical studies and clinical trials. These studies concerned, inter alia: bleomycin, cisplatin, carboplatin, mitomycin-c, and cyclophosphamide [10–13]. The highest rate of cytotoxic enhancement after application of electroporation was reported for bleomycin, which increased in toxicity by up to 1000 times and for cisplatin – up to 80 times.
The first results concerning application of ECT with the use of intravenous bleomycin for melanoma were described by Rudolf et al. in 1995, and a year later Glass et al. described their first experience with ECT and bleomycin administered intratumourally [11, 14]. Both studies showed similar results with ORR of 92%. ECT based on cisplatin is equally effective; however fewer clinical data are available describing its use, mainly due to the necessity to administer this cytostatic agent locally – into the tumour, which in the case of disseminated skin lesions in one patient is often infeasible [12].
The ECT procedure is presented in detail in two papers: ESOPE (European Standard Operating Procedures of Electrochemotherapy) and SOP (Standard Operating Procedures) [9, 15]. In the case of qualification for treatment of patients with small, single lesions (< 7 lesions and < 2 cm diameter of each lesion), both bleomycin and cisplatin can be used locally – intratumourally. The procedure starts with administration of a chemotherapeutic agent, and after 1 minute electroporation is performed. If the skin lesions in the patient are above 2 cm in diameter or there are more than 7 lesions, bleomycin administered intravenously is the optimal cytostatic drug. Also in this case the procedure starts with administration of the cytostatic agent, and the supply of electric pulses begins after 8 minutes. The optimum concentration of the chemotherapeutic agent is maintained in the bloodstream from 8 to about 28 minutes after administration. Depending on the planned length of the procedure, it may be performed under local or general anaesthesia.
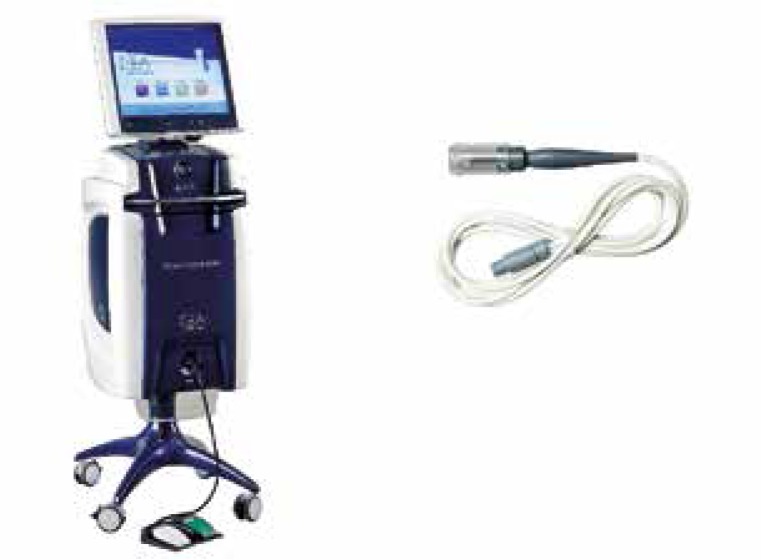
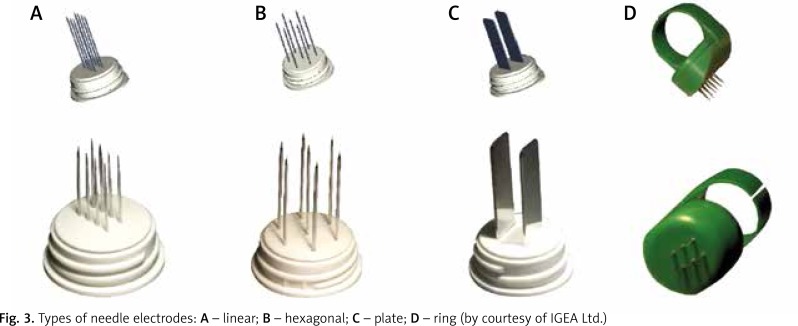
The ECT standards have been developed based on the use of a device called a Cliniporator (IGEA, Modena, Italy). This system provides several types of needle applicators that deliver current pulses to the tissue. The longest electrodes have a length of about 3 cm, which is the maximum depth for an effective treatment of lesions present in the skin and subcutaneous tissue (Figs. 2, 3) [16].
Fig. 2.
Basic device Cliniporator™ with an applicator (by courtesy of IGEA Ltd.)
Fig. 3.
Types of needle electrodes: A – linear; B – hexagonal; C – plate; D – ring (by courtesy of IGEA Ltd.)
The main contraindications to ECT include renal failure, allergy to bleomycin or cisplatin, pulmonary fibrosis (in the case of bleomycin), epilepsy, and a pacemaker. Nevertheless, according to the literature, ECT demonstrates a low toxicity profile and limited, mostly minor side effects. The main complications are local and include local pain, swelling, redness, ulcers (due to necrosis of originally exophytic tumours), and depigmentation [9, 16].
Material and methods
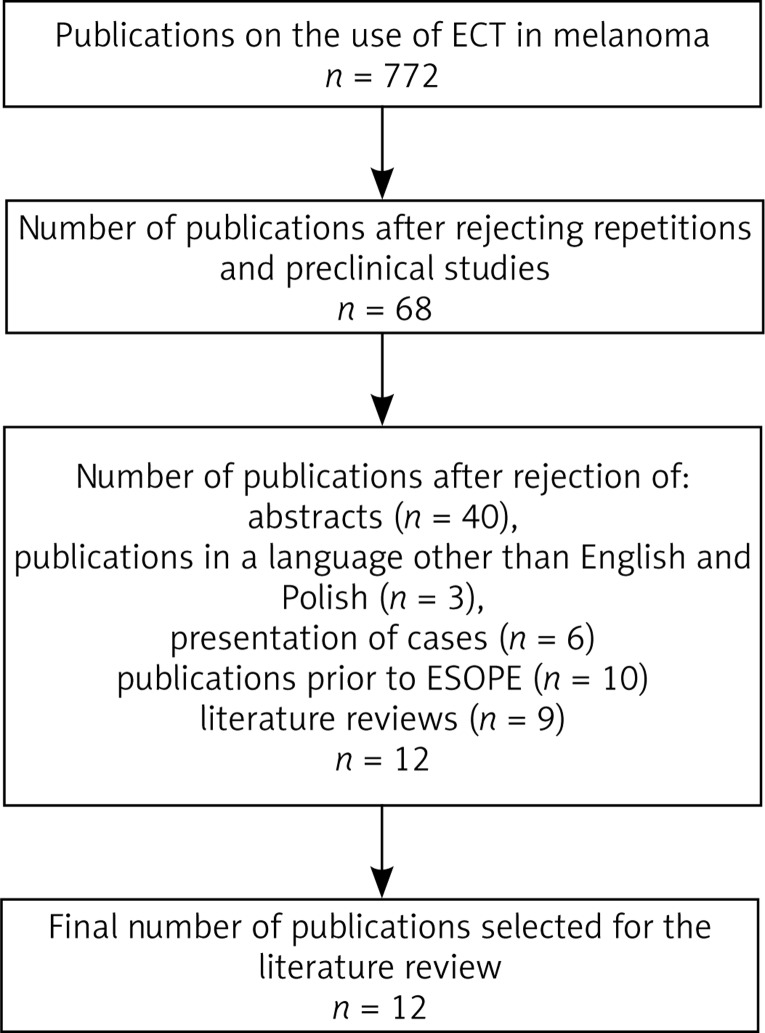
In the present paper, the method of electrochemotherapy was discussed and a review of literature was conducted concerning the treatment of melanoma with the use of ECT. This review included five databases: PubMed, HighWire Press, Science Direct, Wiley Online Library, and Google Scholar. Initially, 772 publications were selected by searching the phrases “electrochemotherapy” and “melanoma”. We searched for publications on studies carried out after the publication of ESOPE standards, i.e. from 08.2006 until 07.2017. After a preliminary analysis we rejected the following: papers repeated in different databases, papers on preclinical studies, works only in the form of abstracts, publications in languages other than English or Polish, works in the form of presentations of clinical cases, reviews of literature and publications prepared on the basis of data from before the ESOPE study. We also excluded papers that described results of ECT on heterogeneous groups of patients, where a group of patients with melanoma was not clearly isolated. Ultimately, 11 original papers were qualified to the literature review. The scheme for selection of publications is presented in Fig. 4.
Fig. 4.
The process of selection of publications included in the literature Review
Results
The paper containing the latest standards in ECT in the treatment of metastatic melanoma is the aforementioned ESOPE [9]. The study included 61 patients from 4 European centres. ECT was performed using bleomycin i.t. or i.v. or cisplatin i.t. In this publication patients with melanoma constituted the largest homogeneous group. In 20 patients included in the study ECT was performed on 99 skin lesions, achieving an OR (overall response) of 80.6% and CR (complete response) of 66.3%.
One of the first works published after ESOPE was the paper by Gaudy et al. in which patients were randomised to two arms of the study – in the first arm the patients were treated with ECT using bleomycin administered intratumourally while in the other arm they received only bleomycin intratumourally [17]. The study ultimately gathered 40 patients, 24 of whom were randomised to the ECT group and 16 to the group with bleomycin alone. OR and CR for group 1 were 46% and 36%, respectively, and 25% and 8% for group 2, respectively. Treatments were performed under local anaesthesia and the most common side effects were painful muscle spasms. Although a statistically significant difference was reported in CR, the group of patients with no response or progression of the disease dampened enthusiasm for this method because the group comprised 54% of all patients included in the study.
The best results with a representative group of 30 patients and 654 treated skin lesions were published by Ricotti et al. in 2014 [18]. They evaluated CR for 20% and PR (partial response) for 80% which together amounted to 100% of OR. A similar OR result was reported by Skarlatos et al.; however there were 5 patients with melanoma in this study [19].
A group of 60 patients was gathered by Caraco et al. who after 3 months of follow-up achieved OR of 86.6%, and in 27.7% they achieved a CR to the treatment with a follow-up of at least 27.5 months [20].
A large group of patients with metastatic melanoma was treated with ECT by Campana et al. who achieved very good results. OR of 92% (CR 48%, PR 44%) [21]. After 2 years of follow-up LPFS (local progression-free survival) was 87%, and after 26 months a local recurrence was observed in 6 patients from the originally CR group. The most common complications associated with the treatment included postoperative pain, vomiting, nausea, fever, and skin lesions (ulcers, discolouration).
Another paper presenting very good results was the publication of Quaglino et al. [22]. This paper described results of application of ECT on a group of 14 melanoma patients, and OR amounted to 93% after 8 weeks of follow-up. After 24 months of follow-up, 74.5% of CR patients had no local recurrence.
In 2014, Solari presented the results of his studies on ECT in melanoma [23]. In a group of 20 patients he used ECT with intravenous bleomycin and achieved CR and PR of 10% and 45%, and SD (stable disease) and PD (progressive disease) in 15% and 30%, respectively. It should be noted, however, that he used a longer follow-up period after which he assessed his patients, i.e. 6 months.
In 2017, Kunte and an international team published a study InspECT based on 151 patients demonstrating high efficacy of ECT [24]. The overall response was 73% with a minimum follow-up 60 days. One year overall survive was 67%. The most common side effects were related to hyperpigmentation and ulceration (42%, G3 in 2 patients), and others included: post-operative pain (39%), flu-like symptoms (4%), nausea (3%), and swelling (3%). This paper drew some important conclusions regarding ECT indications. In multivariate analysis, factors positively associated with overall response were tumour size < 3 cm, absence of visceral metastases, treatment of non-irradiated areas, presence of lymphoedema, and coverage of deep margins and lateral margins. This publication was a prospective multicentre study on a large group of patients and therefore these results strongly confirm the conclusions of the previous publications and prove the effectiveness of the method.
All data collected from the publications included in the literature review are presented in Table 1.
Table 1.
Selected publications on the use of electrochemotherapy in the treatment of melanoma
| Author (year) | Number of patients | Number of lesions | Drug applied/delivery route | Number of ECT courses | Response to treatment | Follow-up | Local tumor control rate | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete (CR) (%) | Partial (PR) (%) | Stabilization(SD) (%) | Progression(PD) (%) | |||||||
| Scarlatos et al. 2011 [19] |
5 | ND | bleomycin i.v. or i.t. | 7 (avg. 1.4) | 60 | 40 | 0 | 0 | 60 days | ND |
| Gaudy et al. 2006 [17] | 12 | 30 | bleomycin i.t. | ND | 36 | 10 | 29 | 25 | min. 12 weeks | ND |
| Marty et al. 2006 [9] | 20 | 98 | bleomycin i.v. or i.t.; cisplatin i.t. | ND | 66.3 | 14.3 | ND | ND | min. 60 days, average of 133 days (from 60 to 380 days) | ND |
| Mir-Bonafe et al. 2014 [42] | 31 | ND | bleomycin i.v. | 57 (avg. 1.54) from 1 to 3 courses | 23 | 49 | 0 | 28 | 360 days | ND |
| Kreuter et al. 2014 [43] |
20 | ND | bleomycin i.v. | 46 (avg. 2.3) | 20 | 30 | 20 | 30 | ND | ND |
| Ricotti et al. 2014 [18] | 30 | 654 | bleomycin i.v. | Avg. 1.32 | 20 | 80 | 0 | 0 | min. 4 weeks, average of 20 months | LTCR 72% after 24 months |
| Solari et al. 2014 [23] | 20 | ND | bleomycin i.v. | ND | 10 | 45 | 15 | 30 | 6 months | ND |
| Caraco et al. 2013 [20] | 60 | ND | bleomycin i.v. | 100 (avg. 1.66) from 1 to 5 courses | 48.4 | 38.3 | 0 | 13.3 | min.3 months, average of 27.5 months | CR 27.7% after 27.5 months |
| Campana et al. 2009 [44] | 34 | 373 | bleomycin i.v or i.t. | ND | 50 | ND | ND | ND | min. 8 weeks, average of 9 months | ND |
| Quaglino et al. 2008 [22] | 14 | 233 | bleomycin i.v. | 24 (avg. 1.71) | 50 | 43 | 7 | 0 | min. 8 weeks, average of 21 months | LTCR 74.5% after 24 months |
| Campana et al. 2012 [21] |
85 | 268 | bleomycin i.v, i.t. or combination | 226 (avg. 2.66) | 48 | 44 | 6 | 1,9 | min 4 weeks, average of 26 months | LPFS after 24 months 87% |
| Kunte et al. 2017 [24] | 151 | 394 | bleomycin i.v. or i.t. | 188 (avg. 1.3) | 58 | 19 | 20 | 2 (1% not evaluable) | min. 60 days, average 116 days | OR 73% after 60 days |
| Total/average | 502 | 2050 | 40.1 | 34 | ||||||
| ~OR = 74% | ||||||||||
i.v. – intra venous; i.t. – intra tumorally; ND – no data
Conclusions
In the case of limited in-transit melanoma metastases to the skin and subcutaneous tissue surgery remains the main therapeutic option. The most available and, therefore, most frequent intervention used in the case of cutaneous metastatic cancer in the case of ineligibility for surgery is a systemic chemotherapy and immunotherapy. Other methods used include radiotherapy and techniques of local therapies e.g. PDT (photodynamic therapy) and local chemotherapy (intralesional therapy – ILT). A meta-analysis published in 2014 presenting the results of 47 prospective studies compared five available therapies used for skin cancers (ECT, RT [radiotherapy], PDT, topical treatment – injection of a cytostatic drug into the tumour [ILT], and systemic treatment) – in the case of ECT the OR result was 75.4% (CR 47.5%), with a low toxicity profile (≥ 3 toxicity based on the CTC toxicity scale in less than 6% of patients). OR for RT was 62.7%; OR for PDT was 67.8%; OR for ILT was 21.4%; and OR for systemic therapy amounted to 12.9% [25]. In the presented meta-analysis melanoma recurrence accounted for 83,3% of cases.
The efficacy of ECT has been proven in the treatment of many cancers, including melanoma, Kaposi’s sarcoma, leiomyosarcoma, breast cancer spread, head and neck cancers, and primary skin cancers [19, 26–28]. Most often it is a palliative treatment when other methods do not bring a positive effect [29]. However, due to its proven efficacy, ECT can be considered a method of choice in the case of contraindications to surgery and radiotherapy, e.g. for a palliative provision of bleeding ulcers, thus improving the quality of life of patients. The advantage of ECT over other therapies is that it can be applied when chemotherapy, radiotherapy, and surgery have been used earlier and it can be repeated in the same area several times.
Different mechanisms are postulated to explain the achieved anti-tumour effect of ECT. The best documented include: an which increase in the intracellular concentration and, consequently, in activity of cytostatic drugs administered locally or systemically [30, 31]; induction of ischaemia in the mechanism of local contraction of arterioles (including the so called “vascular lock” – a delayed wash-out of the drug from the treated area) [32, 33] and the toxicity for endothelial cells [34, 35]; promotion of inflammatory infiltration in the treated area [36, 37]; toxicity associated with electrolysis and migration of electrolytes in the intracellular matrix and with disruptions of the transmembrane potential [38, 39].
An increasing number of studies showing the influence of ECT on immunological response were the basis of combining ECT with immunotherapy. Two recent studies seeking to evaluate the potency of the combination of ipilimumab and ECT have produced very encouraging results [40, 41]. In the case report published by Brizio et al. [40], local ECT treatment of cutaneous lesions of melanoma was followed by ipilimumab administration, resulting in the complete regression of all the cutaneous and visceral metastases for at least 1 year. Interestingly, vitiligo-like lesions developed exclusively around the sites of previous ECT, suggesting that a prior ECT-driven immune activation was enhanced by ipilimumab. The other study reported that the volume of distant non-ECT-treated tumours decreased or was stabilised in nine patients out of 15, possibly through ipilimumab-induced regulatory T cell depletion [41].
In the near future we can expect further studies on new combinations of ECT with immunotherapy. Not only drugs but also nucleic acids can be transferred into target cells via the delivery of electroporation, e.g. electro-gene therapy, i.e. the electroporation-mediated transfer of therapeutic genes, which is currently under clinical evaluation and can potentially bring us to the next level of cancer fighting.
These methods, however, are relatively new and requires further studies, especially in large randomised groups of patients.
Footnotes
The authors declare no conflict of interest.
References
- 1.Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, Garbe C. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70. doi: 10.1046/j.1365-2133.2002.04867.x. [DOI] [PubMed] [Google Scholar]
- 2.Eapen S, Dutcher JP. A review of evidence-based treatment of stage IIB to stage IV melanoma. Cancer Invest. 2005;23:323–37. doi: 10.1081/cnv-58865. [DOI] [PubMed] [Google Scholar]
- 3.Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol. 2004;30:1107–12. doi: 10.1016/j.ejso.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Lindner P, Doubrovsky A, Kam P, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–36. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 5.McKay AJ, Byrne DS. Other treatments. In: Thompson JF, Morton DL, Kroon BBR, editors. Textbook of Melanoma. London: Martin Dunitz; 2004. pp. 438–44. [Google Scholar]
- 6.Stevens G, Thompson JF, Firth I, O’Brien CJ, McCarthy WH, Quinn MJ. Locally advanced melanoma: results of postoperative hypofractionated radiation therapy. Cancer. 2000;88:88–94. doi: 10.1002/(sici)1097-0142(20000101)88:1<88::aid-cncr13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Hersey P, Coates AS, McCarthy WH, et al. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol. 2002;20:4181–90. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 8.Balch CM, Buzaid AC, Atkins MB, et al. A new American Joint Committee on Cancer staging system for cutaneous melanoma. Cancer. 2000;88:1484–91. doi: 10.1002/(sici)1097-0142(20000315)88:6<1484::aid-cncr29>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4:3–13. [Google Scholar]
- 10.Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9:319–25. doi: 10.1097/00001813-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Glass LF, Pepine ML, Fenske NA, Jaroszeski M, Reintgen DS, Heller R. Bleomycin-mediated electrochemotherapy of metastatic melanoma. Arch Dermatol. 1996;132:1353–7. [PubMed] [Google Scholar]
- 12.Sersa G, Stabuc B, Cemazar M, Miklavcic D, Rudolf Z. Electrochemotherapy with cisplatIn: clinical experience in malignant melanoma patients. Clin Cancer Res. 2000;6:863–7. [PubMed] [Google Scholar]
- 13.Mir LM, Tounekti O, Orlowski S. BleomycIn: revival of an old drug. Gen Pharmacol. 1996;27:745–8. doi: 10.1016/0306-3623(95)02101-9. [DOI] [PubMed] [Google Scholar]
- 14.Rudolf Z, Stabuc B, Cemazar M, et al. Electrochemotherapy with bleomycin. The firms clinical experience in malignant melanoma patients. Radiol Oncol. 1995;29:229–35. [Google Scholar]
- 15.Lluis M, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator by means of invasive or non-invasive electrodes. EJC Supplements. 2006;4:14–25. [Google Scholar]
- 16.Kis E, Oláh J, Ócsai H, Baltas E, Gyulai R, Kemény L, Horvath AR. Electrochemotherapy of cutaneous metastases of melanoma – a case series study and systematic review of the evidence. Dermatol Surg. 2011;37:1–9. doi: 10.1111/j.1524-4725.2011.01951..x. [DOI] [PubMed] [Google Scholar]
- 17.Gaudy C, Richard MA, Folchetti G, Bonerandi JJ, Grob JJ. Randomized controlled study of electrochemotherapy in the local treatment of skin metastases of melanoma. J Cutan Med Surg. 2006;10:115–21. doi: 10.2310/7750.2006.00037. [DOI] [PubMed] [Google Scholar]
- 18.Ricotti E, Giuliodori K, Cataldi I, Campanati A, Ganzetti G, Ricotti G, Offidani A. Electrochemotherapy: an effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatologic Therapy. 2014;27:148–52. doi: 10.1111/dth.12098. [DOI] [PubMed] [Google Scholar]
- 19.Scarlatos I, Kyrgias G, Mosa E, et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In Vivo. 2011;25:265–74. [PubMed] [Google Scholar]
- 20.Caraco C, Mozzillo N, Marone U, et al. Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer. 2013;13:564. doi: 10.1186/1471-2407-13-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campana LG, Valpione S, Mocellin S, Sundararajan R, Granziera E, Sartore L, Chiarion-Sileni V, Rossi CR. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99:821–30. doi: 10.1002/bjs.8749. [DOI] [PubMed] [Google Scholar]
- 22.Quaglino P, Mortera C, Osella-Abate S, Barberis M, Illengo M, Rissone M, Savoia P, Bernengo MG. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–22. doi: 10.1245/s10434-008-9976-0. [DOI] [PubMed] [Google Scholar]
- 23.Solari N, Spagnolo F, Ponte E, Quaglia A, Lillini R, Battista M, Queirolo P, Cafiero F. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patient treated with palliative intent. J Surg Oncol. 2014;19:270–4. doi: 10.1002/jso.23481. [DOI] [PubMed] [Google Scholar]
- 24.Kunte C, Letule V, Gehl J, et al. Electrochemmotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br J Dermatol. 2017;176:1475–85. doi: 10.1111/bjd.15340. [DOI] [PubMed] [Google Scholar]
- 25.Spratt DE, Gordon Spratt EA, Wu S, DeRosa A, Lee NY, Lacouture ME, Barker CA. Efficiency of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–55. doi: 10.1200/JCO.2014.55.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sersa G, Miklavcic D, Cemazar M, et al. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34:232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Moller M, Salwa S, Soden D, et al. Electrochemotherapy as an adjunct or alternative to other treatments for unresectable or in-transit melanoma. Expert Rev Anticancer Ther. 2009;9:1611–30. doi: 10.1586/era.09.129. [DOI] [PubMed] [Google Scholar]
- 28.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 29.Snoj M, Cemazar M, Slekovac Kolar B, et al. Effective treatment of multiple unresectable skin melanoma metastases by electrochemotherapy. Croat Med J. 2007;48:391–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Mir LM, Orlowski S, Belehradek J, et al. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 31.Jaroszeski MJ, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201–8. doi: 10.1097/00001813-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Sersa G, Cemazar M, Parkins CS, Chaplin DJ. Tumour blood flow changes induced by application of electric pulses. Eur J Cancer. 1999;35:672–7. doi: 10.1016/s0959-8049(98)00426-2. [DOI] [PubMed] [Google Scholar]
- 33.Jarm T, Cemazar M, Miklavcic D, et al. Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev Anticancer Ther. 2010;10:729–46. doi: 10.1586/era.10.43. [DOI] [PubMed] [Google Scholar]
- 34.Cemazar M, Parkins CS, Holder AL, Chaplin DJ, Tozer GM, Sersa G. Electroporation of human microvascular endothelial cells: evidence for an anti-vascular mechanism of electrochemotherapy. Br J Cancer. 2001;84:565–70. doi: 10.1054/bjoc.2000.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markelc B, Sersa G, Cemazar M. Differential Mechanisms Associated with Vascular Disrupting Action of Electrochemotherapy: Intravital Microscopy on the Level of Single Normal and Tumor Blood Vessels. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0059557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlowski S, An D, Belehradek J, et al. Antimetastatic effects of electrochemotherapy and of histoincompatible interleukin-2-secreting cells in the murine Lewis lung tumor. Anticancer Drugs. 1998;9:551–6. doi: 10.1097/00001813-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Serša G, Miklavcic D, Cemazara M, Belehradek J, Jarm T, Mir LM. Electrochemotherapy with CDDP on LPB sarcoma: Comparison of the anti-tumor effectiveness in immunocompetent and immunodeficient mice. Bioelectrochemistry and Bioenergetics. 1997;43:279–83. [Google Scholar]
- 38.Nilsson E, von Euler H, Berendson J, et al. Electrochemical treatment of tumours. Bioelectrochemistry. 2000;51:1–11. doi: 10.1016/s0302-4598(99)00073-2. [DOI] [PubMed] [Google Scholar]
- 39.Turler A, Schaefer H, Schaefer N, Wagner M, Maintz D, Qiao JC, Hoelscher AH. Experimental low-level direct current therapy in liver metastases: influence of polarity and current dose. Bioelectromagnetics. 2000;21:395–401. [PubMed] [Google Scholar]
- 40.Brizio M, Fava P, Astrua C, Cavaliere G, Savoia P. Complete regression of melanoma skin metastases after electrochemotherapy plus ipilimumab treatment: an unusual clinical presentation. Eur J Dermatol. 2015;25:271–2. doi: 10.1684/ejd.2015.2522. [DOI] [PubMed] [Google Scholar]
- 41.Mozzillo N, Simeone E, Benedetto L, Curvietto M, Giannarelli D, Gentilcore G, et al. Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology. 2015;4:e1008842. doi: 10.1080/2162402X.2015.1008842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mir-Bonafe JM, Vilalta A, Alarcon I, et al. Electrochemotherapy in the Treatment of Melanoma Skin Metastases: a report on 31 cases. Actas Dermosifiliogr. 2015;106:285–291. doi: 10.1016/j.ad.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Kreuter A, van Eijk T, Lehmann P, et al. Electrochemotherapy in advanced skin tumors and cutaneous metastases – a retrospective multicenter analysis. J Dtsch Dermatol Ges. 2015;13:308–15. doi: 10.1111/ddg.12583. [DOI] [PubMed] [Google Scholar]
- 44.Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16:191–9. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]