Abstract
Background
After a series of positive studies for mechanical thrombectomy in large vessel occlusion acute ischemic stroke, the question remains, can symptomatic patients with distal vessel occlusion benefit from mechanical thrombectomy?
Purpose
To assess the safety and efficacy of the 3MAX reperfusion system as frontline therapy for M2 and M3 occlusions.
Methods
This study retrospectively collected data on 58 patients treated for M2 and M3 occlusions between January and September 2016. Of these 58 patients, 31 had an isolated M2 or M3 occlusion. Eligible patients were treated with 3MAX by adirect first pass aspiration (ADAPT) technique within 6 hours following stroke onset. Effectiveness was defined by functional independence (90-day modified Rankin Scale core 0–2) and revascularization to modified Thrombolysis in Cerebral Infarction (mTICI) 2b–3 scores adjudicated by a core laboratory, while complication rates were used to determine safety of the device and the procedure.
Results
Patients with an isolated M2 or M3 occlusion had a mean age of 68.6±13.3 years (range 18–90 years), a median National Institutes of Health Stroke Score of 15 (IQR 9–19), and ASPECTS score of 9 (IQR 8–10). After intervention, 100% (31/31) of patients were revascularized to mTICI 2b–3; 77.4% (24/31) of patients showed revascularization to mTICI 3. Aspiration alone led to revascularization in 83.9% (26/31) of patients. At 90 days, 96.8% (30/31) of patients had achieved functional independence. The incidence of symptomatic intracranial hemorrhage was 0% (0/31).
Conclusions
Results suggest that the 3MAX reperfusion system is safe and effective in achieving successful revascularization and functional independence for patients with acute ischemic stroke secondary to M2 and M3 occlusions using ADAPT, either as frontline monotherapy, or in combination with adjunctive devices.
Keywords: acute ischemic stroke, aspiration thrombectomy, endovascular therapy, distal occlusion
Introduction
After a succession of positive studies for mechanical thrombectomy in large vessel occlusion acute ischemic stroke,1–5 the challenge now is to determine if symptomatic patients with more distal vessel occlusion can safely be treated with mechanical thrombectomy and whether their clinical symptoms would improve. Early trials (Interventional Management of Stroke (IMS) I, IMS II, Prolyse in Acute Cerebral Thromboembolism (PROACT) II)6–8 failed to show a link between reperfusion and good clinical outcome. Recently published data on the endovascular therapy of M2 occlusions in IMS III revealed that anatomic heterogeneity, operational definitions, and the affected M2 segment might have an effect on outcome in the reperfusion of M2 occlusions.9 So far, only case reports have described the usage of a direct first pass aspiration technique (ADAPT) for stroke thrombectomy in small vessel occlusions.10 In our study, consecutive patients with symptomatic stroke and M2 or M3 occlusions were treated with advanced technology based on the ADAPT technique using large-bore and smaller size reperfusion catheters and continuous aspiration using a high-vacuum pump.
Recent articles have assessed the ADAPT technique and found it to be an effective and safe thrombectomy methodology in large cerebral vessels, using state-of-the-art aspiration catheters.11–14 Endovascular therapy is safe and effective in the treatment of an isolated M2 occlusion.15 The ADAPT technique using ACE68, ACE64, ACE60, and 5MAX reperfusion catheters has been shown to reduce overall procedural time and lead to improvements in clinical outcome in large vessel occlusions.12 16 The occlusion of smaller arteries, such as the M2 or M3 segments of the middle cerebral artery (MCA), pericallosal artery, or the posterior cerebral artery, sometimes causes ischemic strokes with pronounced clinical impact on the patient. The benefits of thrombectomy of small arteries must be carefully considered in relation to the risks of the procedure. The diameter of the artery occluded should be taken into account. In particular, the clinical benefit that can be obtained by recanalization is also based on the eloquence of the dependent territory of the artery occluded.
The ACE60, ACE64, and ACE68 catheters have a distal outer diameter of 5.4, 5.75, and 6F, respectively, and may be too large for the caliber of these smaller arteries. The purpose of this study is to assess the safety and efficacy of the Penumbra system including the 3MAX reperfusion system as frontline ADAPT therapy for M2 and M3 occlusions. We also describe our initial experience with the ADAPT technique using smaller catheters (3.8F) in these more distal arteries as the primary method for recanalization.
Methods
This retrospective study collected data on 58 consecutive patients with M2 and M3 occlusions between January 2016 and September 2016. Eligible patients presented with a modified Thrombolysis in Cerebral Infarction (mTICI) score 0–1 in the target region and were treated with 3MAX by the ADAPT technique within 6 hours following stroke symptom onset. Effectiveness was defined by rates of functional independence, defined as modified Rankin scale (mRS) score 0–2 at 90 days and successful revascularization to a mTICI score 2b–3 adjudicated by an independent and blinded core laboratory, while complication rates were used to determine safety of the device and the procedure.
Device description
The Penumbra system consists of a reperfusion catheter (ACE or MAX catheter), a high-vacuum aspiration pump PMX220 with its associated consumables, and large-diameter aspiration tubing with vacuum switch as well as an aspiration canister. In this study we used the reperfusion catheters 5MAX (n=29), ACE64 (n=28), and ACE 68 (n=1) with distal outer diameters of 5.0, 5.75, and 6F, respectively. The 3MAX reperfusion system, consisting of a 4.7F proximal to 3.8F distal outer diameter aspiration catheter in combination with continuous aspiration using a pump, was designed as part of the Penumbra system to rapidly restore flow in small vessel occlusions. The 3MAX has a flexible tip with a distal internal diameter of 0.035 in and proximal internal diameter of 0.043 in, and a working length of 153 cm is provided. This design allows optimal aspiration power at the catheter tip to ingest or secure the clot in small vessel occlusions. In this modified ADAPT technique, the 5MAX or ACE is deployed first, followed by the 3MAX to create a seamless procedure. At the same time, the ACE provides support for further advancement of the 3MAX.
Patient selection
Inclusion criteria were anterior circulation acute cerebral occlusion, age ≥18 years, with National Institutes of Health Stroke Scale (NIHSS) score ≥6 and baseline mRS score of 3–5. When not contraindicated, in stroke <4.5 hours from symptom onset, all patients received intravenous thrombolysis before intervention following the guidelines of the German Society of Neurology. Patients with symptomatic stroke with isolated distal M2 or M3 occlusions or with a combination of large vessel occlusion (internal carotid artery (ICA)/ICA terminus/M1) and distal M2 or M3 occlusion were enrolled. Exclusion criteria included the presence of intracranial hemorrhage or established cerebral infarction according to the Alberta Stroke Programme Early CT Score (ASPECTS ≤7). A neurologist evaluated the NIHSS and mRS scores at admission, at discharge, and at 90 days (mRS only).
Imaging evaluation
At admission and after a clinical evaluation by a neurologist, all patients underwent a baseline CT scan, supra-aortic and cerebral CT angiography, and cerebral perfusion CT. A CT control was performed 24 hours after intervention to rule out intracranial hemorrhage (ICH). An independent core laboratory reviewed all imaging data and assessed the appropriateness of the mTICI scores before and after intervention, and assessed the ASPECT score from the CT preprocedure scan. The independent core laboratory also reviewed the imaging data for complications of emboli to new territory, vessel dissection, vasospasm, vessel perforation, and ICH. Imaging evaluation was conducted blinded to all clinical information except stroke side. Symptomatic ICH was defined by any evidence of a bleed from head CT at 24 hours after the procedure by an independent core laboratory and an increase of ≥4 points on the NIHSS score.
Endovascular procedure
All procedures were performed under general anesthesia. After femoral puncture, the presence of arterial occlusion previously displayed in the CT angiogram was initially confirmed by placing a long sheath (Penumbra Neuron Max 088, Alameda, California, USA) at the origin of the ICA. Through this long sheath the ICA was distally catheterized with a 5.0F/5.75F/6F catheter (Penumbra 5MAX/ACE64/ACE68) over a 3.8F reperfusion catheter (3MAX) and a 0.016 microwire (Medtronic, Silverspeed, Dublin, Ireland). Under roadmap assistance, the 3MAX, guided by this microwire, was advanced and positioned to gently touch the proximal part of the clot. Then the microwire was removed, and the 3MAX catheter was connected to the Penumbra aspiration pump for at least 1–2 min. Next, the reperfusion catheters were slowly removed under aspiration, maintaining the long sheath in position and aspirating the fluid with a 60 mL syringe through the side port. For patients with proximal occlusions, the large-bore catheter (Penumbra 5MAX/ACE64/ACE68) was positioned to touch the proximal part of the clot. After removal of the 3MAX and wire, the retrieval process was performed as described above. In all cases, the Penumbra aspiration pump was hooked to a 3MAX or larger-bore reperfusion catheter, and manual suction using a 60 mL syringe on a NeuronMax long sheath was performed. If necessary after proximal clot removal, the 3MAX was re-advanced towards the remaining distal clot to retrieve it in the same manner as in isolated M2 or M3 occlusions.
Recanalization was considered successful on achieving a mTICI score of 2b–3. If aspiration failed, the reperfusion catheter was quickly placed in position and another aspiration thrombectomy or stent retriever attempt was performed, at the discretion of the operator. The time to recanalization was defined as the time from femoral puncture until achieving, at a minimum, a mTICI 2a score.
Data analysis/statistics
Descriptive statistics included the number of observations, mean and SD, and median and IQR for continuous variables, and counts and percentages for discrete variables. Statistical analysis was performed using SAS v 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Between January 2016 and September 2016, 58 consecutive patients with acute ischemic stroke and distal M2 or M3 occlusions were treated in our hospital with direct aspiration as first intention using the Penumbra reperfusion catheter, as described above (figures 1 and 2). In this study period, approximately 190 patients received mechanical thrombectomy in our institution. Target vessel occlusions were isolated M2 or M3 occlusions in 53.4% (31/58) of cases, and M2 or M3 occlusions with upstream large vessel occlusions (ICA: n=8, ICA terminus: n=8, M1 MCA: n=11) in 46.6% (27/58) of cases. A total of 6.5% (2/31) had an isolated M3 occlusion, 71.0% (22/31) had isolated M2 occlusions and 22.6% (7/31) had M2 and M3 occlusions. The mean age of all patients was 68.8±12.4 years (range 18–94 years); 56.9% (33/58) of the cohort was female. Patients presented with a median NIHSS score of 15 (IQR 10–18) and ASPECTS score of 9 (IQR 7–10). Lytic therapy was administered in 91.4% (53/58) of all patients before mechanical thrombectomy. The baseline characteristics of all patients, of the subgroup with isolated M2 or M3 occlusions, and of the subgroup with proximal large vessel occlusion (LVO) and M2 or M3 occlusions are shown in table 1. In these 58 patients, a total of 69% (40/58) of distal occlusions were treated within one pass; 17% (10/58) required two passes; and 14% (8/58) a third pass.
Figure 1.
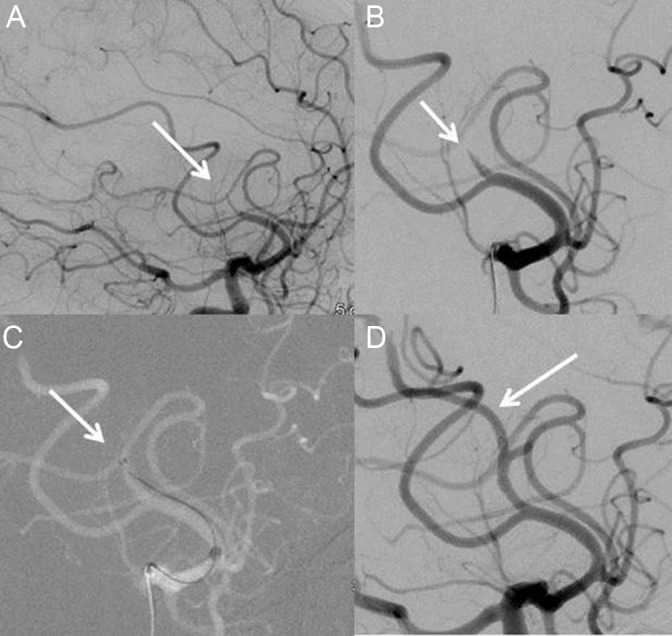
Patient with M2 occlusion of right cerebral artery. (A) Lateral view before treatment showing M2 occlusion of cerebral artery. (B) Close up view of the M2 occlusion. (C) Position of the 3MAX reperfusion catheter tip in front of the thrombus before aspiration. (D) Lateral view of angiogram after first pass treatment with 3MAX.
Figure 2.
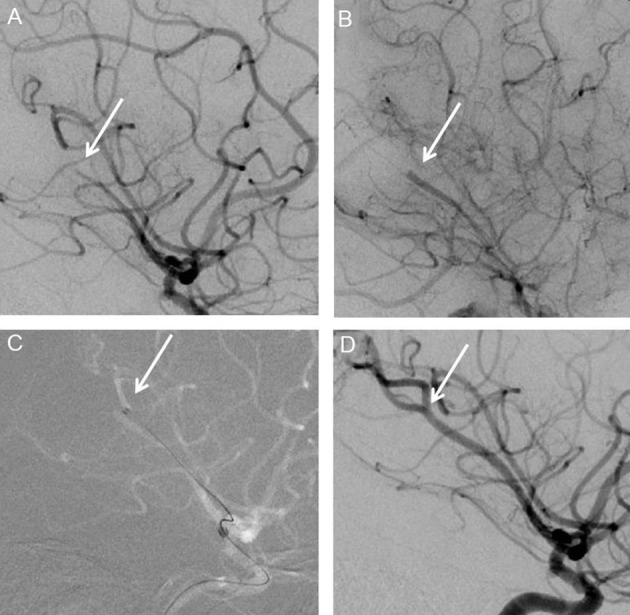
Patient with M2 occlusion of left cerebral artery. (A) Lateral view before treatment showing M2 occlusion of cerebral artery. (B) Close up view of the M2 occlusion. (C) Position of the 3MAX reperfusion catheter tip in front of the thrombus before aspiration. (D) Lateral view of angiogram after successful treatment.
Table 1.
Baseline characteristics
| Characteristics | All patients (n=58) |
Isolated M2 or M3 (n=31) | LVO+M2 or M3, (n=27) | Difference (95% CI)* | p Value |
| Age (mean±SD, min, max), years | 68.8±12.4 (18, 94) | 68.6±13.3 (18, 90) | 69.0±11.4 (48, 94) | 0 (−6 to 6) | 0.9130 |
| Women (n/N (%)) | 33/58 (56.9%) | 18/31 (58.1%) | 15/27 (55.6%) | 2.5% (−23.4% to 28.0%) | 1.0000 |
| Isolated M2 or M3 occlusions (n/N (%)) | 31/58 (53.4%) | 31/31 (100%) | N/A | N/A | N/A |
| LVO in combination with M2 or M3 occlusions (n/N (%)) | 27/58 (46.6%) | N/A | 27/27 (100%) | N/A | N/A |
| Baseline NIHSS (median, IQR) | 15 (10–18) | 15 (9–19) | 15 (12–18) | 0 (−3, 3) | 0.9751 |
| Baseline ASPECTS score (median, IQR) | 9 (7–10) | 9 (8–10) | 8.5 (6–9.5) | 1 (0, 2) | 0.0601 |
| Baseline mRS score (mean±SD) | 4.4±0.7 (range 3–5) | 4.4±0.7 (range 3–5) | 4.4±0.7 (range 3–5) | 0 (0, 0) | 0.7611 |
| Baseline mTICI score of 0 (n/N (%)) |
58/58 (100%) | 31/31 (100%) | 27/27 (100%) | 0%, N/A | 1.0000 |
| Time from symptom onset to door (median, IQR), min | 60 (50–75) | 60 (50–75) | 60 (50–75) | 0 (−10, 10) | 0.9562 |
| IV thrombolysis (n/N (%)) | 53/58 (91.4%) | 29/31 (93.5%) | 24/27 (88.9%) | 4.7% (−20.8% to 29.9%) | 0.6557 |
| Time from symptom onset to IV thrombolysis (median, IQR), min | 82.5 (70–100) | 80 (70–100) | 85 (75–90) | 0 (−10, 10) | 0.7719 |
*Wilcoxon rank sum or Fisher’s exact test for difference between isolated M2 or M3 and LVO + M2 or M3 groups. Hodges-Lehmann estimate and CI was computed for results of Wilcoxon rank sum test. Exact estimate and Clopper-Pearson CI was computed for results of Fisher’s exact test.
ASPECTS, Alberta Stroke Program Early CT Score; LVO, Large vessel occlusion (internal carotid artery (ICA), ICA terminus or M1 middle cerebral artery); mRS, modified Rankin Scale; mTICI, modified Thrombolysis In Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale.
After all interventions, 100% (58/58) of patients were successfully revascularized in the M2 or M3 territory to mTICI 2b–3% and 82.8% (48/58) were revascularized to mTICI 3 by core laboratory assessment. By site Principal Investigator (PI) assessment, 91.4% (53/58) of patients were successfully revascularized to mTICI 2b–3 (table 2). In the isolated M2 or M3 group, 100% (31/31) of patients were successfully revascularized to mTICI 2b–3 and 77.4% (24/31) were revascularized to mTICI 3 by core laboratory assessment. By site PI assessment, 90.3% (28/31) of this subgroup were successfully revascularized to mTICI 2b–3. Median time from groin puncture to revascularization was 45.3 min (IQR 30–55) for the isolated M2 or M3 group. Aspiration alone led to revascularization in 83.9% (26/31) of patients with isolated M2 or M3; a stent retriever was used as adjunctive treatment with a 3MAX in 16.1% (5/31) of cases to remove persistent occlusion in the target vessel. Larger-bore reperfusion catheters (ACE68, ACE64, 5MAX) were used in 100% (31/31) of patients for aspiration in M2 segments. At discharge in the isolated M2 or M3 group, the median final NIHSS score was significantly reduced to 4 (IQR 3–5), p<0.001, with Wilcoxon rank sum test (figure 3A). At discharge, 93.5% (29/31) of patients had returned to mRS 0–2. The mRS score improved significantly from a mean of 4.4±−0.7 at admission to 1.0±−0.9 at discharge, p<0.001, Wilcoxon rank sum test. At 90 days, functional independence was achieved in 96.8% (30/31) of patients with isolated M2 or M3 (figure 3B). In a comparison of patients with isolated versus upstream LVO, there were no statistically significant differences in patient characteristics, angiographic results, or clinical outcomes. Complications of vessel perforations or dissections were not observed. Symptomatic ICH was seen in 0% (0/31) of patients with isolated M2 or M3; 6.5% (2/31) of patients showed asymptomatic small bleeds (table 2).
Table 2.
Procedure Time
| Procedure and outcome | All patients, (n=58) | Isolated M2 or M3 (n=31) | LVO+M2 or M3 (n=27) | Difference (95% CI)* | p Value |
| Time from symptom onset to groin puncture (median, IQR), min | 105 (95–120) | 105 (95–125) | 110 (95–120) | 0 (−10 to 10) | 0.9563 |
| Time from symptom onset to recanalization (median, IQR), min | 130 (115–145) | 127.5 (115–145) | 130 (115–150) | 0 (−15 to 10) | 0.6646 |
| Procedure duration (median, IQR), min | 54.3 (37–65) | 45.3 (30–55) | 63.5 (45–75) | −22 (−40 to 5) | 0.014 |
| Post-treatment mTICI score 2b–3 (n/N (%)), per core laboratory | 58/58 (100%) | 31/31 (100%) | 27/27 (100%) | 0%, N/A | 1.0000 |
| Post-treatment mTICI score 2b–3 (n/N (%)), per site PI | 53/58 (91.4%) | 28/31 (90.3%) | 25/27 (92.6%) | −2.3% (−27.4% to 23.4%) | 1.0000 |
| Post-treatment mTICI score 3 (n/N (%)), per core laboratory | 48/58 (82.8%) | 24/31 (77.4%) | 24/27 (88.9%) | −11.5% (−36.4% to 14.1%) | 0.3110 |
| Post-treatment mTICI score 3 (n/N (%)), per site PI | 41/58 (70.7%) | 22/31 (71.0%) | 19/27 (70.4%) | 1.0% (−25.0% to 26.1%) | 1.0000 |
| Post-treatment NIHSS score (median, IQR) | 4 (3–6) | 4 (3–5) | 5 (3–7) | −1 (−2 to 0) | 0.1909 |
| Good functional outcome at discharge, mRS 0–2 (n/N (%)) | 54/58 (93.1%) | 29/31 (93.5%) | 25/27 (92.6%) | 1.0% (−24.3% to 26.5%) | 1.0000 |
| Good functional outcome at 90 days, mRS 0–2 (n/N (%)) | 55/58 (94.8%) | 30/31 (96.8%) | 25/27 (92.6%) | 4.2% (−21.3% to 29.6%) | 0.5931 |
| Symptomatic ICH (n/N (%)) | 2/58 (3.4%) | 0/31 (0%) | 2/27 (7.4%) | −7.4% (−32.6% to 18.3%) | 0.2123 |
| Asymptomatic ICH (n/N (%)) | 10/58 (17.2%) | 2/31 (6.5%) | 8/27 (29.6%) | −23.2% (−46.6% to 3.0%) | 0.0340 |
| Vessel dissection (n/N (%)) | 0/58 (0%) | 0/31 (0%) | 0/27 (0%) | 0%, N/A | 1.000 |
| Vessel perforation (n/N (%)) | 0/58 (0%) | 0/31 (0%) | 0/27 (0%) | 0%, N/A | 1.000 |
| Major groin complication (n/N (%)) | 0/58 (0%) | 0/31 (0%) | 0/27 (0%) | 0%, N/A | 1.000 |
*Wilcoxon rank sum or Fisher’s exact test for difference between isolated M2 or M3 and LVO + M2 or M3 groups. Hodges-Lehmann estimate and CI was computed for results of Wilcoxon rank sum test. Exact estimate and Clopper-Pearson CI was computed for results of Fisher’s exact test.
ICH, intracranial hemorrhage; LVO, large vessel occlusion; mRS, modified Rankin Scale; mTICI, modified Thrombolysis in Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale; PI, Principal Investigator.
Figure 3.
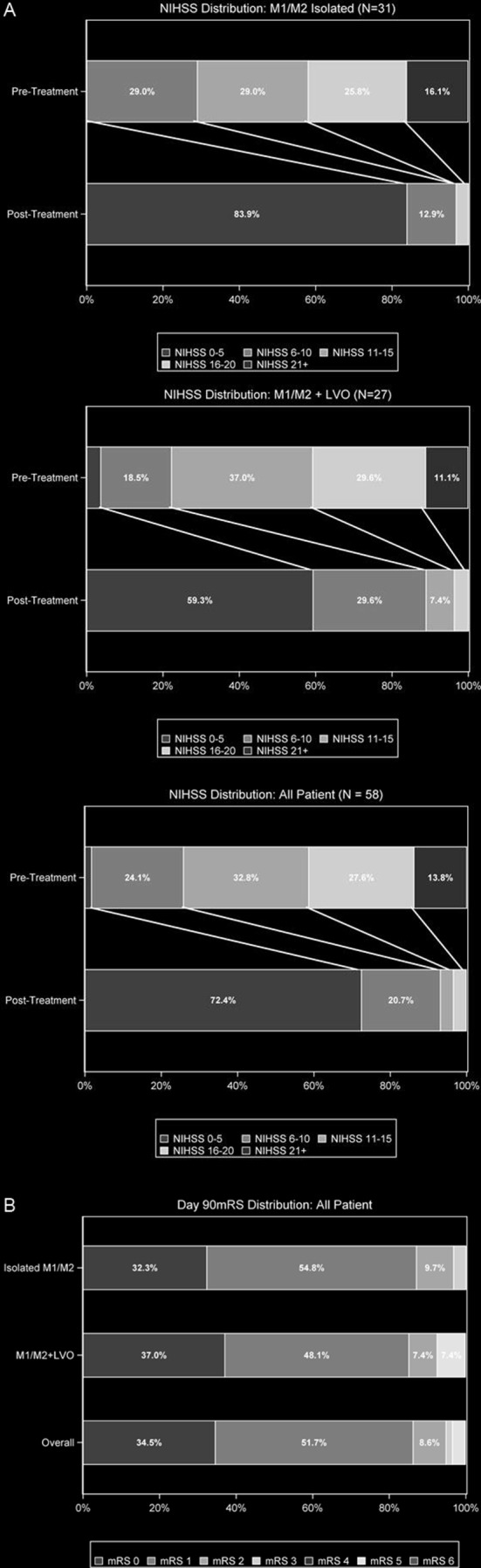
Neurological and functional outcomes. (A) Distribution of National Institutes of Health Stroke Scale (NIHSS) score at baseline and at discharge. (B) Distribution of modified Rankin Scale (mRS) score at 90 days. LVO, large vessel occlusion.
Discussion
In acute stroke, distal cerebrovascular occlusions can be linked to severe clinical symptoms, and treatment by mechanical thrombectomy may have an important clinical impact. A case series review of the Trevo 3×20 device for the treatment of distal intracranial occlusions was published recently, emphasizing this importance.17 In their study of eight patients, with a mean age of 51 years and mean admission NIHSS score of 19, the overall procedure duration was 110 min. The Trevo was used in five M3 MCA, three anterior cerebral artery (two pericallosal artery and one callosomarginal artery), and two posterior cerebral artery (1 P2 and 1 P3) occlusions. TICI 2b–3 flow was achieved in 75% of cases and arterial occlusive lesion grading 3 in 100% of cases. Vasospasm was reported in 62.5%, and two patients had parenchymal hematomas (1 PH1, 1 PH2) in this previous study.
In our study population, patients had a high baseline NIHSS score and proven distal vessel occlusion. No difference between the isolated and LVO groups was observed, possibly because the isolated occlusion was located in key vessels. Subjects had either not responded to intravenous fibrinolytic therapy or this treatment was contraindicated. This procedure was not chosen merely to recanalize an artery but also to achieve a rapid clinical benefit for the treated patient. The development of new reperfusion catheter systems, with better navigability, increased flexibility, less traumatic tips, and improved ingestion force by enhanced high-vacuum pump systems, allows the catheterization of more distal occlusions and the aspiration of these as first intention endovascular treatment (figure 4).
Figure 4.

3MAX reperfusion catheter tip and secured clot.
The 3MAX catheter, commonly used to support catheterization with the ACE/ACE 64 reperfusion catheters, was shown to be an effective catheter for the recanalization of more distal arteries. In our initial series, we achieved recanalization (mTICI 2b–3) in the vast majority of patients without using a stent retriever on the first attempt. In contrast, stent retrievers were used as a bailout therapy in only a few patients. It also was not necessary to combine treatment with a stent retriever. In most cases, it was not required to pass the clot with a microwire, thus minimizing the chances of distal embolization. The rate of good clinical outcomes of the patients as measured by mRS score at 90 days was remarkably high and higher than reported in recent randomized trials on the treatment of LVOs.1–5 This might be because the studies on LVOs did not recommend full recanalization of all distal branches and modern techniques for distal occlusion therapy were not available in these trials, while in this trial, a core lab-adjudicated mTICI 2b–3 rate of 100% was observed, with 83% being mTICI 3. In addition, the experience of the interventionalists might have contributed to the high rate of success. They treated a total of 190 patients with stroke with thrombectomy during the study period in which data were collected on the 58 patients with distal occlusion with a relatively short median procedure time of 20 min.
The experience of the center has improved by streamlining logistics, triage, and in-hospital processes, and interventional techniques. The team used methodology based on published data using the Solumbra technique,18 moving to the ADAPT technique13 and now have applied current techniques to facilitate removal of M2 or M3 thrombi. It also might be speculated that the pathophysiology of M2 or M3 occlusions differs from that of LVOs, and, thus, success rates of thrombectomy in distal arteries might be higher, since distal occlusion is a more localized condition.
Limitations of this study included the highly selective small patient group with severely affected isolated M2 or M3 occlusions referred by a neurologist and the retrospective study design. Key strengths included consecutive enrollment and imaging assessment by an independent core laboratory.
In this study, findings indicate that first-line aspiration is a promising and atraumatic procedure for revascularization of small vessel occlusions. Patients with isolated M2 or M3 occlusions showed similar severity of disease, recanalization rates, and clinical improvement as patients with proximal occlusions in combination with M2 or M3 occlusions. The procedure is recommended to reduce the chances of thrombus disaggregation in distal emboli. Furthermore, selective catheterization with suitable reperfusion catheters of the proximal segment of the artery to be treated may minimize the risk of stroke in new territories and increase the safety of the procedure. The benefits of thrombectomy of small arteries must be carefully considered in relation to the risks. This study had a similar risk profile for symptomatic bleeding and asymptomatic small bleeding and for vessel injuries as those in recently reported controlled clinical trials.1–5
Conclusion
The results of this retrospective analysis in 58 consecutive patients suggest that the 3MAX reperfusion system safely and effectively achieves successful revascularization and functional independence for patients with acute ischemic stroke secondary to M2 and M3 occlusions using ADAPT, either as frontline monotherapy, or in combination with adjunctive devices. However, larger prospective studies are necessary to provide more evidence of the benefits and safety of the procedure.
Acknowledgments
The authors thank Prof. Dr. Arnd Dörfler, Radiology and Neuroradiology University Hospital Erlangen, for serving as an independent core lab for the analysis of the imaging data.
Footnotes
Contributors: All authors made a substantial, direct, and intellectual contribution to the work.
Competing interests: JA: Travel support for presentation at congresses, PENUMBRA; J-OK, SH, RH, CL: None.
Ethics approval: Ruhr-University-Bochum.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data available.
Correction notice: This article has been corrected since it published Online First. The acknowledgments section, table 2 and the results section have been updated.
References
- 1. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 3. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 4. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 5. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 6. IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–11. 10.1161/01.STR.0000121641.77121.98 [DOI] [PubMed] [Google Scholar]
- 7. IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–35. 10.1161/STROKEAHA.107.483131 [DOI] [PubMed] [Google Scholar]
- 8. Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11. [DOI] [PubMed] [Google Scholar]
- 9. Tomsick TA, Carrozzella J, Foster L, et al. Endovascular therapy of M2 occlusion in IMS III: role of M2 segment definition and location on clinical and revascularization outcomes. AJNR Am J Neuroradiol 2017;38:84–9. 10.3174/ajnr.A4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navia P, Larrea JA, Pardo E, et al. Initial experience using the 3MAX cerebral reperfusion catheter in the endovascular treatment of acute ischemic stroke of distal arteries. J Neurointerv Surg 2016;8:787–90. 10.1136/neurintsurg-2015-011798 [DOI] [PubMed] [Google Scholar]
- 11. Turk AS, Spiotta A, Frei D, et al. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 2014;6:231–7. 10.1136/neurintsurg-2013-010713 [DOI] [PubMed] [Google Scholar]
- 12. Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014;6:260–4. 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 13. Kowoll A, Weber A, Mpotsaris A, et al. Direct aspiration first pass technique for the treatment of acute ischemic stroke: initial experience at a European stroke center. J Neurointerv Surg 2016;8:230–4. 10.1136/neurintsurg-2014-011520 [DOI] [PubMed] [Google Scholar]
- 14. Romano DG, Cioni S, Vinci SL, et al. Thromboaspiration technique as first approach for endovascular treatment of acute ischemic stroke: initial experience at nine Italian stroke centers. J Neurointerv Surg 2017;9:6–10. 10.1136/neurintsurg-2016-012298 [DOI] [PubMed] [Google Scholar]
- 15. Delgado Almandoz JE, Kayan Y, Young ML, et al. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either Solumbra or ADAPT techniques. J Neurointerv Surg 2016;8:1123–8. 10.1136/neurintsurg-2015-012122 [DOI] [PubMed] [Google Scholar]
- 16. Sarraj A, Sangha N, Hussain MS, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artert M2 segment. JAMA Neurol 2016;73:1291–6. [DOI] [PubMed] [Google Scholar]
- 17. Haussen DC, Lima A, Nogueira RG. The Trevo XP 3×20 mm retriever (‘Baby Trevo’) for the treatment of distal intracranial occlusions. J Neurointerv Surg 2016;8:295–9. 10.1136/neurintsurg-2014-011613 [DOI] [PubMed] [Google Scholar]
- 18. Mpotsaris A, Bussmeyer M, Weber W. Mechanical thrombectomy with the Penumbra 3D separator and lesional aspiration: technical feasibility and clinical outcome. Clin Neuroradiol 2014;24:245–50. 10.1007/s00062-013-0242-x [DOI] [PubMed] [Google Scholar]


