Abstract
Objectives
To report 4-year imaging outcomes in the RAPID-axSpA (NCT01087762) study of patients with ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA), treated with certolizumab pegol (CZP).
Methods
This phase III, randomised trial was placebo-controlled and double-blind to week 24, dose-blind to week 48 and open-label to week 204. Patients fulfilling the Assessment of Spondyloarthritis International Society (ASAS) axSpA criteria with active disease were stratified (AS/nr-axSpA) according to the modified New York (mNY) criteria at randomisation. Spinal radiographs were assessed using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). MRI inflammation used the Spondyloarthritis Research Consortium of Canada (SPARCC) score for sacroiliac joints (SIJ) and the Berlin spinal score (remission defined as SPARCC <2 and Berlin ≤2, respectively).
Results
MRI improvements from baseline (BL) to week 12 were maintained to week 204 (SPARCC BL: AS=8.5, nr-axSpA=7.5; SPARCC week 204: AS=1.3, nr-axSpA=2.4; Berlin BL: AS=7.4, nr-axSpA=4.4; Berlin week 204: AS=2.6, nr-axSpA=1.9). 66.7% of patients with AS and 69.6% of patients with nr-axSpA with BL SPARCC scores ≥2, and 65.4% of patients with AS and 57.3% of patients with nr-axSpA with BL Berlin score >2, achieved remission at week 204. Mean mSASSS change in AS from BL to week 204 was 0.98 (95% CI 0.34, 1.63); 0.67 (95% CI 0.21,1.13) from BL to week 96; and 0.31 (95% CI 0.02,0.60) from week 96 to week 204. Corresponding nr-axSpA changes were 0.06 (95% CI −0.17,0.28), –0.01 (95% CI −0.19,0.17) and 0.07 (95% CI −0.07,0.20). 4.5% of patients with nr-axSpA fulfilled the mNY criteria at week 204, while 4.3% of patients with AS no longer did so.
Conclusions
In patients with CZP-treated axSpA, rapid decreases in spinal and SIJ MRI inflammation were maintained to week 204. Overall, 4-year spinal progression was low, with less progression during years 2–4 than 0–2. Radiographic SIJ grading changes demonstrated limited progression.
Trial registration number
Keywords: ankylosing spondylitis, magnetic resonance imaging, spondyloarthritis, anti-tnf, inflammation
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease primarily characterised by inflammation of the axial skeleton (the spine and the sacroiliac (SI) joints). Patients with evidence of structural damage to the SI joints (radiographic sacroiliitis), which is identifiable using X-ray imaging and fulfils the modified New York (mNY) classification criteria, are considered to have ankylosing spondylitis (AS; also termed radiographic axSpA). However, many patients with axSpA do not fulfil the mNY criteria; this is termed non-radiographic axSpA (nr-axSpA) and has the potential to develop into AS.1 2 Importantly, the disease burden and clinical features are similar in both subpopulations, representing a spectrum of the same disease.3 4
In contrast to clinical outcomes, long-term imaging data in tumour necrosis factor (TNF) inhibitor-treated patients are limited. There are currently no long-term modified Stoke Ankylosing Spondylitis Spine Score (mSASSS)5 data available for patients with nr-axSpA.
RAPID-axSpA is the only large trial to include both patients with AS and nr-axSpA and previously demonstrated that certolizumab pegol (CZP), a PEGylated fragment-crystallisable (Fc)-free anti-TNF agent, improved the signs and symptoms of axSpA from as early as 12 weeks of treatment, which were maintained over 4 years.4 6–8
Here, we report the imaging outcomes over 4 years of CZP treatment. This represents the longest term MRI imaging study in patients with anti-TNF-treated axSpA to date, and the only data addressing X-ray and MRI imaging of both SI joints and spine in AS and nr-axSpA subpopulations.
Methods
Study design
RAPID-axSpA (NCT01087762) was a 204-week, phase III, randomised, parallel-group, multicentre trial, conducted at 83 centres in Europe, North America and Latin America. The study was placebo-controlled and double-blind until week 24, dose-blind to week 48 and open-label to week 204.
Full details have been published previously.4 Patients were randomised 1:1:1 to placebo or CZP 400 mg at weeks 0, 2 and 4 (loading dose), followed by either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks (online supplementary figure 1).
annrheumdis-2017-212377supp001.docx (517.8KB, docx)
Patients
Full inclusion and exclusion criteria have been reported previously.4 Eligible participants were aged ≥18 years at screening and fulfilled the ASAS axSpA classification criteria, with a clinical diagnosis of adult-onset axSpA of ≥3 months’ duration and active disease defined by Bath Ankylosing Spondylitis Activity Index ≥4, spinal pain ≥4 on a 0–10 Numerical Rating Scale, and either elevated C-reactive protein (>7.9 mg/L) or a positive SI joint MRI assessment.
To define AS and nr-axSpA subpopulations, the most recent SI joint X-rays (performed ≤12 months prior to screening) were locally read to determine the presence/absence of radiographic sacroiliitis.
Study procedures and evaluations
The primary outcome (ASAS20 response at week 12) has been reported previously,4 as have clinical data to week 2046 7 and imaging data to week 96.4 6 8–10 Here we report the long-term imaging results (radiographs and MRI of both SI joints and spine) from the complete 4-year study period.
SI joint X-rays were conducted at baseline and week 204/early withdrawal (if after week 104). Lateral radiographs of the lumbar/cervical spine were performed at baseline, week 96 and week 204. MRI assessments of both the spine and SI joints were conducted at baseline and weeks 12, 48, 96 and 204. MRI and radiograph assessments were each performed independently by two central readers blinded to timepoint, treatment group and clinical data. In the event of disagreement between central readers when grading SI joint radiographs, an additional third reader assessed the radiographs from the patient in question. A third reader was not used for MRI or spinal radiographs.
The short tau inversion recovery sequence of MRI scans was assessed for disease activity using the Spondyloarthritis Research Consortium of Canada (SPARCC) scoring method for SI joints (0–72 scale)11 and the Berlin modification of the Ankylosing Spondylitis spine MRI-activity scoring system for the spine (0–69 scale).12 Spinal radiographs were assessed using the mSASSS scoring method.
Data are reported from the week 204 reading campaign, which included all available images from baseline to week 204 with the exception of SI joint radiographs; only SI joint images from patients with both baseline and week 204/early withdrawal radiographs were included.
Statistical analysis
Data are presented for patients who received ≥1 dose of CZP (200 mg every 2 weeks (Q2W) and 400 mg every 4 weeks (Q4W) groups combined) at any timepoint to week 204, including rerandomised placebo-treated patients. Statistical analyses were conducted assuming data were missing at random.13 The number of images available for each imaging modality is presented in online supplementary table 1.
The MRI set included all randomised patients with valid MRI assessments (either spine or SI joint) at baseline and ≥1 other timepoint during the trial (n=158). Week 12 MRI data were not used from patients randomised to placebo. Average MRI scores of the two readers were considered for statistical analyses, and group least squares (LS) mean Berlin and SPARCC scores were estimated post hoc by mixed-model repeated measures (MMRM) analysis on observed data using ‘visit’ as a fixed factor with an unstructured within-patient covariance matrix. The proportions achieving MRI remission (SPARCC <2 or Berlin ≤2) were estimated by multiple imputation: estimated proportions of patients in MRI remission were pooled from 50 multiply imputed data sets, where missing actual scores were imputed via predicted mean matching, with the predicted value at a visit based on linear regression of values from other visits.14 15 Results were summarised for patients with MRI baseline inflammation (Berlin score >2 or SPARCC score ≥2).
Radiographic data were examined for all CZP-treated patients with ≥1 mSASSS assessment (X-ray set), including those rerandomised from placebo. Based on average scores of the two readers, LS mean mSASSS and changes between visits were estimated using MMRM analyses on observed data, as described above. The online supplementary material includes observed mean changes for subjects with a complete sequence of images at baseline, week 96 and week 204. Radiographic progression rates (an increase of ≥2 points from baseline) at weeks 96 and 204 were estimated using multiple imputation, as described above. Within-patient correlation coefficients were calculated between change from baseline to week 96, and change from week 96 to week 204. Plausibility of the missing-at-random assumption was evaluated by comparing disease activity outcomes of patients with no mSASSS data at week 96/week 204 and those with data at all relevant timepoints. In particular, Ankylosing Spondylitis Disease Activity Score (ASDAS) levels were compared using observed data and last observation carried forward-imputed data. For plots of individual patient mSASSS results, observed data are presented for all patients with ≥2 valid mSASSS assessments. Patients with ≥1 non-bridging or bridging syndesmophyte (defined as a score of 2 or 3, respectively) and syndesmophyte formation (defined as a shift in score from 0 or 1 to 2 or 3) were considered when reported by both readers for a given vertebral edge.
Presence of definitive sacroiliitis (grade ≥2 bilateral or grade 3–4 unilateral) was based on the judgement of two central readers. In the event of disagreement, a third reader’s results were used to provide a majority. Agreement between central readers was calculated using simple kappa (κ) statistics. Statistical analyses were performed with SAS V.9.3 and V.9.4.
Results
Patient disposition and baseline characteristics
Three hundred and twenty-five patients were randomised at week 0, 107 to placebo and 218 to CZP (111 to CZP 200 mg Q2W and 107 to CZP 400 mg Q4W). Of 315 patients (174 AS and 141 nr-axSpA) who received ≥1 dose CZP at any point in the trial, 199 (63.2%) completed the study to week 204. One hundred and fifty-eight patients had valid MRI assessments at baseline and ≥1 other timepoint (MRI set). The baseline characteristics of the MRI set and overall population were similar (table 1, and data not shown).
Table 1.
Baseline characteristics of all CZP patients
| Overall axSpA N=315 |
AS n=174 |
nr-axSpA n=141 |
|
| Mean age, years (SD) | 39.7 (12.0) | 41.5 (11.7) | 37.5 (11.9) |
| Male, % | 62.2 | 73.0 | 48.9 |
| Symptom duration, years, median (min, max) | 7.8 (0.3, 50.9) | 9.1 (0.3, 50.9) | 5.8 (0.3, 41.5) |
| CRP, mg/L, median (min, max) | 13.4 (0.1, 174.8) | 14.2 (0.1, 174.8) | 12.0 (0.1, 156.2) |
| Patients with elevated CRP (>15 mg/L), % | 40.6 | 43.7 | 36.9 |
| BASDAI, mean (SD) | 6.4 (1.5) | 6.4 (1.6) | 6.5 (1.5) |
| BASFI, mean (SD) | 5.4 (2.3) n=314 |
5.7 (2.2) | 4.9 (2.3) n=140 |
| BASMI linear, mean (SD) | 3.8 (1.7) | 4.4 (1.7) | 3.1 (1.5) |
| ASDAS, mean (SD) | 3.9 (0.9) n=313 |
3.9 (0.9) | 3.8 (0.8) n=139 |
| Spinal radiographs | |||
| mSASSS | |||
| Mean (SD)* | 9.5 (16.1) | 13.2 (18.2) | 4.4 (11.0) |
| Median | 1.5 n=190 |
3.0 n=110 |
0.0 n=80 |
| Patients with ≥1 bridging or non-bridging syndesmophyte at baseline, n (%) | 63 (33.2) | 47 (42.7) | 16 (20.0) |
| MRI set | n=158 | n=92 | n=66 |
| SPARCC (SI joints) | |||
| Mean (95% CI)* | 8.1 (6.1, 10.2) | 8.5 (5.6, 11.4) | 7.5 (4.4, 10.6) |
| Median | 2.0 n=151 |
1.0 n=91 |
3.0 n=60 |
| Patients with MRI inflammation (SPARCC ≥2), estimate, % | 51.2 | 47.0 | 57.3 |
| Berlin score (spine) | |||
| Mean (95% CI)* | 6.2 (4.8, 7.5), n=157 | 7.4 (5.6, 9.2) | 4.4 (2.4, 6.5), n=65 |
| Median | 2.0 n=153 |
4.3 | 0.5 n=61 |
| Patients with MRI inflammation (Berlin >2) estimate, % | 50.4 n=157 |
57.6 | 40.1 n=65 |
*Least squares mean scores were estimated using mixed-model for repeated measures analyses. Inflammation was defined as Berlin >2 or SPARCC ≥2. Data are presented for all patients who received ≥1 dose CZP at any point in the trial. To define AS and nr-axSpA subpopulations, the most recent SI joint X-rays (performed ≤12 months prior to screening) were locally read to determine the presence/absence of radiographic sacroiliitis.
AS, ankylosing spondylitis; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CRP, C-reactive protein; CZP, certolizumab pegol; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; nr, non-radiographic; SI, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
One hundred and ninety-six patients had ≥1 mSASSS assessment and were included in the MMRM and multiple imputation analyses of radiograph parameters (X-ray set; online supplementary table 2); this included 45 patients who received 1 mSASSS reading at baseline with no further mSASSS assessments during the study. No major differences in disease activity were observed between those with and without complete mSASSS data (online supplementary table 3). One hundred and thirty-seven patients with SI joint radiographs at baseline and week 204/early withdrawal were assessed for radiographic progression based on the mNY criteria.
MRI data
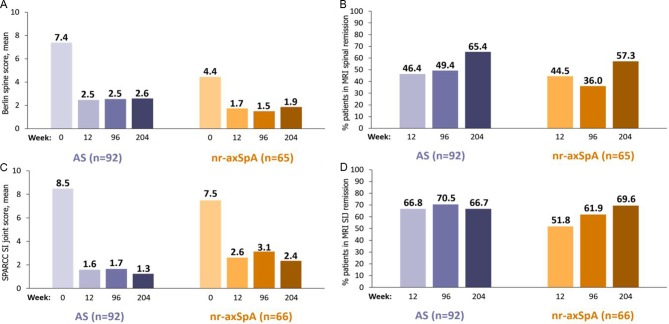
Spinal and SI joint MRI assessments showed reduction of inflammation with rapid improvements from baseline to week 12 maintained to week 204 (figure 1). At baseline, the LS mean spinal inflammation assessed by Berlin score (standard error; SE) for AS and nr-axSpA subpopulations was 7.4 (0.92) and 4.4 (1.03), respectively, which reduced to 2.5 (0.50) and 1.7 (0.40) at week 12, and 2.6 (0.56) and 1.9 (0.44) at week 204. Similarly, the LS mean SPARCC scores for patients with AS and nr-axSpA at baseline were 8.5 (1.45) and 7.5 (1.53), respectively, which were reduced to 1.6 (0.66) and 2.6 (0.67) at week 12, and were maintained at 1.3 (0.46) and 2.4 (0.85) at week 204.
Figure 1.
MRI imaging results to week 204. Sustained improvement in (A) LS mean Berlin score (MMRM), (B) percentage of patients in MRI spinal remission (Berlin score ≤2) to week 204 (missing at random (MAR)) in the subgroup of patients with MRI spinal inflammation at baseline (Berlin score >2), (C) LS mean SPARCC SIJ score (MMRM), and (D) percentage of patients in MRI SIJ remission (SPARCC score <2) to week 204 (MAR) in the subgroup of patients with inflammation at baseline (SPARCC ≥2). AS, ankylosing spondylitis; LS, least squares; MMRM, mixed-model repeated measures; nr-axSpA, non-radiographic axial spondyloarthritis; SIJ, sacroiliac joints; SPARCC, Spondyloarthritis Research Consortium of Canada.
Of patients with respective inflammation at baseline, 66.7% (AS) and 69.6% (nr-axSpA) achieved SI joint MRI remission at week 204, and 65.4% of patients with AS and 57.3% of patients with nr-axSpA achieved spinal MRI remission.
Radiographic progression
Limited changes in SI joint grading were observed to week 204: 2/44 (4.5%) patients with nr-axSpA fulfilled the mNY criteria, while 4/93 (4.3%) patients with AS no longer did so at week 204. Agreement between the two central readers regarding the absence/presence of radiographic sacroiliitis was moderate, with disagreement occurring in 39/158 cases assessed at baseline (κ=0.49). In total, 113/158 (71.5%) images were read by a third reader due to grading disagreements between the main two readers.
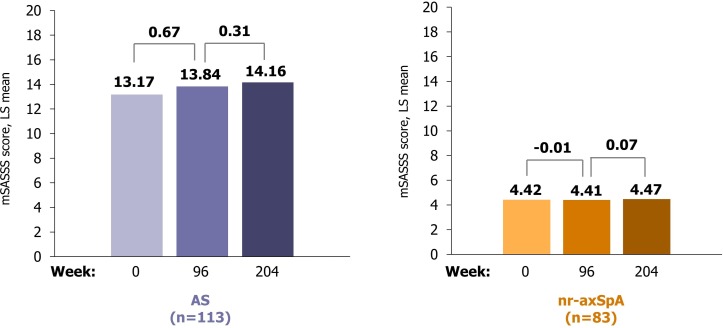
Mean baseline mSASSS scores of 13.2 and 4.4 were observed in patients with AS and nr-axSpA, respectively. Limited spinal radiographic progression occurred in CZP-treated patients, with most progression seen in the AS cohort. In patients with AS, the mean mSASSS change between baseline and week 204 was 0.98 (95% CI 0.34 to 1.63), with the majority of progression observed during the first 2-year period (0.67 (95% CI 0.21 to 1.13)) compared with years 2–4 (0.31 (95% CI 0.02 to 0.60); figure 2). Patients with nr-axSpA exhibited a mean mSASSS change of 0.06 (95% CI −0.17 to 0.28) over 204 weeks. Observed changes in patients with complete mSASSS readings available at baseline, week 96 and week 204 are summarised in online supplementary figure 2; observed mean mSASSS changes between baseline and week 204 were 1.12 and 0.04 for patients with AS and nr-axSpA, respectively. Patients with AS who progressed during years 1 and 2 were more likely to progress in the second 2-year period: within-patient correlation coefficients between change from baseline to week 96, and change from week 96 to week 204, were 0.53 (AS) and 0.05 (nr-axSpA).
Figure 2.
Radiographic imaging results of the spine to week 204. AS, ankylosing spondylitis; LS, least squares; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; nr-axSpA, non-radiographic axial spondyloarthritis.
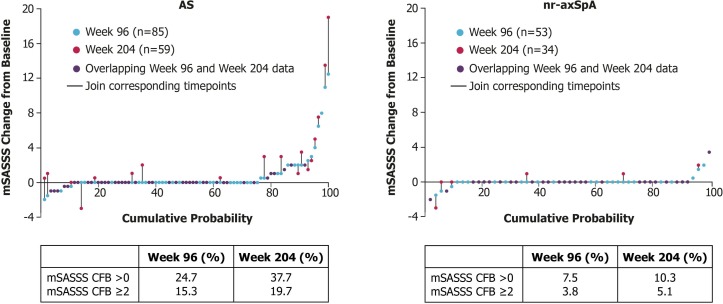
Based on the multiple imputation analysis, 84.2% of patients with AS did not progress (progression was defined as an mSASSS increase of ≥2 points) from baseline to week 96. By week 204, 80.6% of patients with AS had not progressed. Progression was observed in only two patients with nr-axSpA, and therefore multiple imputation analysis was not performed. Of 85 patients with AS, 5 (5.9%) developed ≥1 syndesmophyte by week 96 and only 1 patient with nr-axSpA demonstrated one new syndesmophyte during the study. Sixty-one AS patients were assessed at week 204; at that time, no additional patients were observed to develop syndesmophytes. No patients with an absence of syndesmophytes at baseline developed syndesmophytes at 4 years. Individual patient mSASSS results are presented in figure 3.
Figure 3.
Proportion of patients with spinal progression at years 2 and 4. AS, ankylosing spondylitis; CFB, change from baseline; mSASSS, modified Stoke Ankylosing Spondylitis Spine Score; nr-axSpA, non-radiographic axial spondyloarthritis.
Discussion
RAPID-axSpA is the first long-term, large study to investigate imaging results in AS and nr-axSpA subpopulations when treated with a TNF-inhibitor. Here, CZP treatment rapidly improved axSpA inflammation of the spine and SI joints as observed using MRI in patients with AS and nr-axSpA. These improvements were maintained to week 204, with similar responses observed in both populations.
Changes from baseline in Berlin spine and SPARCC SI joint scores were comparable with other AS and nr-axSpA trials.16 17 However, none of the longer term MRI studies (spanning up to 3 years of therapy) have reported results of both patients with AS and nr-axSpA in parallel.16 18–21 It is important to note that comparisons between clinical trials should be treated with caution, as differences in population, study design and the years in which the trial was conducted can lead to variation between study outcomes. Comparisons between trials commencing at largely different timepoints may introduce chronology bias caused by differences in standard medical practice at the time of each investigation.
Many patients with MRI inflammation at baseline achieved spinal and SI joint MRI remission by week 204, with improvements in both scores seen as early as week 12 in both AS and nr-axSpA cohorts. Spinal MRI inflammation has been shown to be associated with radiographic progression, as vertebral edge inflammation contributes to the development of new syndesmophytes, although it remains to be proven that by reducing MRI inflammation in early axSpA, future structural damage may be prevented.
Limited changes from mNY negative to mNY positive were observed. Net progression to week 204 was minimal (−1.5%); similar proportions of patients ‘progressed’ from nr-axSpA to AS (4.5%) as ‘regressed’ from AS to nr-axSpA (4.3%). Given the low numbers of patients whose disease was reclassified, and the similar movement in both directions between the two populations, this is likely to represent intrareader variability, with little true progression. Previous follow-ups of untreated axSpA cohorts have reported progression rates (from nr-axSpA to AS) between 10% and 12% over 2 years.22–24 In the DEvenir des Spondyloarthrites Indifférenciées Récentes (DESIR) cohort, the net progression from nr-axSpA to radiographic axSpA (AS) was 5.1% over 5 years.25 However, a direct comparison between DESIR and RAPID-axSpA is not recommended since DESIR was a prevalence cohort of early axSpA. RAPID-axSpA also included patients with longer disease duration and higher disease activity. During the ESTHER trial, radiographic progression from nr-axSpA to AS was observed mainly between baseline and year 2 with no patients progressing to AS between years 2 and 4. In EMBARK, in which 161 patients had X-rays available at baseline and week 104, one patient (0.6%) satisfied the mNY criteria at baseline. Of 160 patients with mNY negative scores at baseline, none became mNY positive at week 104.19 26
Recognition of sacroiliitis on pelvic radiographs is generally considered to be difficult, due to both the complexity of the SI joints and the poor visualisation associated with plain radiographs. Previous radiographic studies have observed large intraobserver/interobserver variability in reading SI joint radiographs,27–29 with significant variability reported between central and local readers.22 In RAPID-axSpA, determination of mNY status, used as a stratification factor, was based on local SI X-ray reads, which are more reflective of daily clinical practice. This approach has been used in a number of previous AS trials investigating anti-TNFs, as well as the ABILITY-1 nr-axSpA study.
The rates of spinal radiographic progression in patients with axSpA are variable2; however, in the majority of patients with axSpA, several years may elapse before new bone formation in radiographs can be assessed. Therefore, a minimum 2-year follow-up is required to investigate radiographic progression. Structural spinal damage and inflammation in axSpA have an impact on patient quality of life, especially through reduction of mobility and function.30–32 Recently Poddubnyy et al 33 investigated the effect of radiographic spinal progression and disease activity on function and spinal mobility in anti-TNF-treated patients with established AS. Both functional status and spinal mobility remained stable during 10 years of anti-TNF therapy despite radiographic progression, suggesting that reduction and control of inflammation may counteract the effects of radiographic spinal progression at a group level.
Interestingly, no patients with an absence of syndesmophytes at baseline developed syndesmophytes during 4 years of CZP treatment. Several studies showed that syndesmophyte prevalence predisposes to more rapid radiographic progression, and therefore could be used as a predictor for future radiographic damage despite the variability of progression rates in patients with axSpA.34–36
Here, we observed limited spinal radiographic progression, with a decrease in progression rate with long-term CZP therapy. After 4 years, 80.6% of patients with AS did not progress (<2 mSASSS points change from baseline) and the mean change was 0.98. As expected, patients with AS in RAPID-axSpA were generally more progressive than patients with nr-axSpA. The limited progression over 4 years observed in this study in patients with AS (80.3% non-progression defined as mSASSS change from baseline <2) is consistent with recent reports from the MEASURE 1 trial, in which 79% of patients with AS treated with secukinumab did not progress (<2 mSASSS points change from baseline) over 4 years.37 However, these findings cannot be used in isolation to confirm an impact on disease progression since in both cases a control was absent. In the absence of a control arm, further data are required to elucidate the natural history of AS to better understand the impact of biologic treatment on disease progression. Long-term spinal X-ray data have also been reported for patients with AS in the GO-RAISE study38 (4 years) and in an 8-year follow-up to a randomised controlled trial investigating the effects of infliximab, although this was conducted using a limited number of patients (n=69).39 In GO-RAISE, patients with AS treated with 50 mg or 100 mg golimumab every 4 weeks demonstrated a mean mSASSS change from baseline (SD) of 1.3 (4.1) and 2.0 (5.6), respectively, at week 20838 (although it should be noted that the GO-RAISE study should not be directly compared with RAPID-axSpA due to differing trial designs and study populations).
Two-year radiographic results from RAPID-axSpA exhibited a mean mSASSS change of 0.67 observed in patients with AS to week 96. The decrease in progression rate observed in RAPID-axSpA between years 2 and 4, and the diminished progression observed in long-term anti-TNF studies,40 41 support earlier observations that prolonged use of TNF inhibitors may be associated with reduction of progression. Further evidence suggests a link between disease activity and radiographic progression. Results from the OASIS study, in which patients with AS were followed up for 12 years, found that disease activity was longitudinally associated with radiographic progression. In an AS prospective cohort study, the observed reduction in radiographic progression during anti-TNF treatment appeared to be mediated by a decrease in disease activity.42 Consequently, long-term anti-TNF treatment could have the potential to inhibit structural progression by suppressing disease activity.
The analyses reported here are not without limitation. Patient withdrawal introduces bias, since patients whose symptoms do not improve sufficiently or those who suffer side effects are less likely to continue the trial. In long-term studies, the cumulative impact of missing data is more pronounced. Notably, there was a high proportion of missing MRI and radiographic data in this study. Since disease activity is likely to be associated with radiographic progression, ASDAS outcomes were compared between those with and without complete mSASSS assessments. Given that no major differences were observed, it is unlikely that study dropouts or otherwise missed assessments would have caused major bias to the study results.
Use of the mSASSS scoring system for quantification of radiographic progression could also be considered a limitation. The mSASSS system captures changes at the anterior vertebral corners of both the cervical and lumbar spine,5 but does not evaluate other elements of the axial skeleton, for example, the thoracic spine or facet joints.43 Subsequently, changes in these regions may have gone undetected. Nevertheless, at present, the mSASSS is the preferred scoring method for use in AS and is endorsed by the ASAS and Outcome Measures in Rheumatology (OMERACT).44
As two readers were used to evaluate the presence and formation of syndesmophytes, it was important to establish the level of agreement required. Here, agreement between readers was required at vertebral edge level; this approach is likely to underestimate the prevalence and incidence of syndesmophytes, but has been used in previous trials such as the ASSERT study.32
In conclusion, early improvements in MRI inflammation observed in a CZP-treated axSpA population, including both patients with AS and nr-axSpA, were maintained to week 204. MRI assessments demonstrated a rapid reduction of inflammation and sustained rates of remission in both SI joint and spinal examinations. Radiograph assessments revealed a low rate of spinal progression during the first 2 years of RAPID-axSpA with a decrease in the rate of spinal progression observed between years 2 and 4, and limited SI joint progression during 4 years of study.
Acknowledgments
The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study. The authors also acknowledge Alvaro Arjona, PhD, UCB Pharma, Brussels, Belgium, and Helen Chambers, PhD, CMPP, Costello Medical, Cambridge, UK, for publication coordination, and Eleanor Thurtle, MChem, Costello Medical, Cambridge, UK, for medical writing and editorial assistance.
Footnotes
Handling editor: Josef S Smolen
Contributors: Substantial contributions to study conception/design, or acquisition/analysis/interpretation of data: DvdH, XB, K-GAH, RBML, PMM, WPM, ORD, NdP, BH, LB, TN and JB. Drafting of the publication or revising it critically for important intellectual content: DvdH, XB, K-GAH, RBML, PMM, WPM, ORD, NdP, BH, LB, TN and JB. Final approval of the publication: DvdH, XB, K-GAH, RBML, PMM, WPM, ORD, NdP, BH, LB, TN and JB.
Funding: UCB Pharma sponsored the study and the development of this manuscript, and reviewed the text to ensure that from UCB perspective, the data presented in the publication are scientifically, technically and medically supportable, that they do not contain any information that has the potential to damage the intellectual property of UCB, and that the publication complies with applicable laws, regulations, guidelines and good industry practice. The authors approved the final version to be published after critically revising the manuscript for important intellectual content.
Competing interests: DvdH has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB Pharma, and is the director of Imaging Rheumatology BV. XB has received consulting and/or speaker’s fees and/or research grants from AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Janssen, MSD, Novartis, Pfizer and UCB Pharma. K-GAH has received speaker’s fees for AbbVie, MSD, Pfizer and UCB Pharma. RBML has received consulting fees and/or research grants and/or speaker’s bureau from Abbott, Ablynx, Amgen, AstraZeneca, Bristol-Myers Squibb, Centocor, GlaxoSmithKline, Merck, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma and Wyeth. PMM has received consulting/speaker’s fees from AbbVie, Centocor, Janssen, MSD, Novartis, Pfizer and UCB Pharma. WPM has received consulting and/or speaker’s fees and/or grants from AbbVie, Amgen, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Sanofi and UCB Pharma, and is the Chief Medical Officer of Canadian Research Education (CaRE) Arthritis. ORD, NdP, BH, LB and TN are employees of UCB Pharma. JB has received consulting fees/research grants from Abbott, Bristol-Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche and UCB Pharma.
Patient consent: Obtained.
Ethics approval: The study protocol, amendments and subject informed consent were reviewed by a national, regional or independent ethics committee (IEC) or institutional review board (IRB).
Provenance and peer review: Not commissioned; externally peer reviewed.
Presented at: Data from this study have previously been presented at the ACR/ARHP Annual Meeting 2016 and the European League Against Rheumatism Annual Meeting 2017.
References
- 1. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 2. Baraliakos X, Braun J. Non-radiographic axial spondyloarthritis and ankylosing spondylitis: what are the similarities and differences? RMD Open 2015;1:e000053 10.1136/rmdopen-2015-000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Haibel H, Baraliakos X, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. 10.1002/art.24483 [DOI] [PubMed] [Google Scholar]
- 4. Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Creemers MC, Franssen MJ, van’t Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sieper J, Landewé R, Rudwaleit M, et al. Effect of certolizumab pegol over ninety-six weeks in patients with axial spondyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol 2015;67:668–77. 10.1002/art.38973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Heijde D, Dougados M, Landewé R, et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 2017;56:1498–509. 10.1093/rheumatology/kex174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braun J, Baraliakos X, Hermann KG, et al. Effect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritis. RMD Open 2017;3:e000430 10.1136/rmdopen-2017-000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Heijde D, Maksymowych W, Landewé R, et al. Structural progression of the spine measure by X-ray in patients with axial spondyloarthritis treated with certolizumab pegol over 96 weeks, including ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Rheumatol 2014;66:S248. [Google Scholar]
- 10. Deodhar AA, Dougados M, Landewé R, et al. Safety and Efficacy of Certolizumab Pegol over 204 Weeks in Patients with Axial Spondyloarthritis, Including Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis (abstract). Arthritis Rheumatol 2016;68(suppl 10). Abstract number: 687. [Google Scholar]
- 11. Maksymowych WP, Inman RD, Salonen D, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 2005;53:502–9. 10.1002/art.21337 [DOI] [PubMed] [Google Scholar]
- 12. Braun J, Baraliakos X, Golder W, et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36. 10.1002/art.10883 [DOI] [PubMed] [Google Scholar]
- 13. National Research Council. The prevention and treatment of missing data in clinical trials. Washington, DC: The National Academies Press, 2010. [PubMed] [Google Scholar]
- 14. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–42. 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 15. Vink G, Frank LE, Pannekoek J, et al. Predictive mean matching imputation of semicontinuous variables. Stat Neerl 2014;68:61–90. 10.1111/stan.12023 [DOI] [Google Scholar]
- 16. Braun J, Baraliakos X, Hermann KG, et al. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo- controlled GO-RAISE study. Ann Rheum Dis 2012;71:878–84. 10.1136/annrheumdis-2011-200308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maksymowych WP, Dougados M, van der Heijde D, et al. Clinical and MRI responses to etanercept in early non-radiographic axial spondyloarthritis: 48-week results from the EMBARK study. Ann Rheum Dis 2016;75:1328–35. 10.1136/annrheumdis-2015-207596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cantarini L, Fabbroni M, Talarico R, et al. Effectiveness of adalimumab in non-radiographic axial spondyloarthritis: evaluation of clinical and magnetic resonance imaging outcomes in a monocentric cohort. Medicine 2015;94:e1170 10.1097/MD.0000000000001170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dougados M, Maksymowych WP, van der Heijde D, et al. SAT0405 No Radiological Sacroiliac Joint Progression after 2 Years of Etanercept Treatment in Non-Radiographic Axial Spondyloarthritis: Data from The Embark Study. Ann Rheum Dis 2016;75:816 10.1136/annrheumdis-2016-eular.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun J, Landewé R, Hermann KG, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52. 10.1002/art.21790 [DOI] [PubMed] [Google Scholar]
- 21. Song IH, Hermann KG, Haibel H, et al. Consistently good clinical response in patients with early axial spondyloarthritis after 3 years of continuous treatment with etanercept: longterm data of the ESTHER trial. J Rheumatol 2014;41:2034–40. 10.3899/jrheum.140056 [DOI] [PubMed] [Google Scholar]
- 22. Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369–74. 10.1136/ard.2010.145995 [DOI] [PubMed] [Google Scholar]
- 23. Poddubnyy D, Sieper J. Radiographic progression in ankylosing spondylitis/axial spondyloarthritis: how fast and how clinically meaningful? Curr Opin Rheumatol 2012;24:363–9. 10.1097/BOR.0b013e328352b7bd [DOI] [PubMed] [Google Scholar]
- 24. Sampaio-Barros PD, Bertolo MB, Kraemer MH, et al. Undifferentiated spondyloarthropathies: a 2-year follow-up study. Clin Rheumatol 2001;20:201–6. 10.1007/s100670170066 [DOI] [PubMed] [Google Scholar]
- 25. Dougados M, Sepriano A, Molto A, et al. Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. 10.1136/annrheumdis-2017-211596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dougados M, Maksymowych W, Landewe R, et al. THU0382 Change in sacroiliac joint structural radiographic damage after two years of etanercept therapy in comparison to a contemporary control cohort in non-radiographic axial spondyloarthritis [abstract]. Ann Rheum Dis 2017;76:S350–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis 2003;62:519–25. 10.1136/ard.62.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazici H, Turunç M, Ozdoğan H, et al. Observer variation in grading sacroiliac radiographs might be a cause of ’sacroiliitis' reported in certain disease states. Ann Rheum Dis 1987;46:139–45. 10.1136/ard.46.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollingsworth PN, Cheah PS, Dawkins RL, et al. Observer variation in grading sacroiliac radiographs in HLA-B27 positive individuals. J Rheumatol 1983;10:247–54. [PubMed] [Google Scholar]
- 30. Landewé R, Dougados M, Mielants H, et al. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7. 10.1136/ard.2008.091793 [DOI] [PubMed] [Google Scholar]
- 31. Wanders A, Landewé R, Dougados M, et al. Association between radiographic damage of the spine and spinal mobility for individual patients with ankylosing spondylitis: can assessment of spinal mobility be a proxy for radiographic evaluation? Ann Rheum Dis 2005;64:988–94. 10.1136/ard.2004.029728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Machado P, Landewé R, Braun J, et al. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis 2010;69:1465–70. 10.1136/ard.2009.124206 [DOI] [PubMed] [Google Scholar]
- 33. Poddubnyy D, Fedorova A, Listing J, et al. Physical function and spinal mobility remain stable despite radiographic spinal progression in patients with ankylosing spondylitis treated with TNF-α inhibitors for up to 10 years. J Rheumatol 2016;43:2142–8. 10.3899/jrheum.160594 [DOI] [PubMed] [Google Scholar]
- 34. Baraliakos X, Listing J, Rudwaleit M, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–5. 10.1136/ard.2006.066415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baraliakos X, Listing J, von der Recke A, et al. The natural course of radiographic progression in ankylosing spondylitis--evidence for major individual variations in a large proportion of patients. J Rheumatol 2009;36:997–1002. 10.3899/jrheum.080871 [DOI] [PubMed] [Google Scholar]
- 36. Maas F, Spoorenberg A, Brouwer E, et al. Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS One 2015;10:e0122693 10.1371/journal.pone.0122693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab Demonstrates Low Radiographic Progression and Sustained Efficacy through 4 Years in Patients with Active Ankylosing Spondylitis [abstract]. Arthritis Rheumatol 2017;69 Abstract number: 3L. [Google Scholar]
- 38. Braun J, Baraliakos X, Hermann KG, et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis 2014;73:1107–13. 10.1136/annrheumdis-2012-203075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baraliakos X, Listing J, Fritz C, et al. Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years--early clinical response predicts long-term outcome. Rheumatology 2011;50:1690–9. 10.1093/rheumatology/ker194 [DOI] [PubMed] [Google Scholar]
- 40. Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710–5. 10.1136/annrheumdis-2012-202698 [DOI] [PubMed] [Google Scholar]
- 41. Maas F, Arends S, Brouwer E, et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res 2017;69:1011–9. 10.1002/acr.23097 [DOI] [PubMed] [Google Scholar]
- 42. Molnar C, Scherer A, Baraliakos X, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–9. 10.1136/annrheumdis-2017-211544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramiro S, van Tubergen A, Stolwijk C, et al. Scoring radiographic progression in ankylosing spondylitis: should we use the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) or the Radiographic Ankylosing Spondylitis Spinal Score (RASSS)? Arthritis Res Ther 2013;15:R14 10.1186/ar4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Heijde D, Landewé R. Selection of a method for scoring radiographs for ankylosing spondylitis clinical trials, by the Assessment in Ankylosing Spondylitis Working Group and OMERACT. J Rheumatol 2005;32:2048–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-212377supp001.docx (517.8KB, docx)