Abstract
Beta-blockers are a potential option to manage peri-operative atrial fibrillation. Landiolol is a new ultra-short beta-blocker with a half-life of only 4 minutes and very high beta-1 selectivity which has been used for treatment and prevention of atrial fibrillation in pulmonary surgery and gastro-intestinal surgery. Due to its limited negative inotropic effect and high beta-1 selectivity landiolol allows for control of heart rate with minimal impact on blood pressure. Landiolol is well tolerated by the respiratory system. Additional benefits are related to the regulation of the inflammatory response and blunting of the adrenergic pathway. There is a limited number of trials with total of 61 patients undergoing lung resection or oesophagectomy who developed post-operative atrial fibrillation and were treated with landiolol. The experience with landiolol for prevention is more documented than landiolol application for treatment of post-operative atrial fibrillation. There are 9 comparative studies with a total of 450 patients administered landiolol for prevention of post-operative atrial fibrillation. The use of low dosage (5-10mcg/kg/min) is usually sufficient to rapidly control heart rate which is associated with earlier and higher rate of conversion to sinus rhythm as compared to the controls. The excellent tolerance of landiolol at lower dosage (3-5mcg/kg/min) allows to initiate prophylactic use during surgery and postoperatively. Landiolol prophylaxis is associated with reduced incidence of post-operative atrial fibrillation without triggering adverse events related to a beta-blockade.
Keywords: Landiolol, Atrial fibrillation, Supraventricular arrhythmia, Cardioversion, Antiarrhythmic therapy, Diastolic dysfunction
Introduction
Post-operative atrial fibrillation (POAF) is a frequent complication of cardiac surgery. Nevertheless, the atrial fibrillation (AF) incidence following non-cardiac surgery such pulmonary surgery or oesophagectomy is also high, ranging from 10% to 20%1–12 and up to 40% in some series.13,14 Although some prevention strategies have enabled a reduction from 25% to 15%, AF remains a frequent complication.15,16
Some risk factors are similar to those identified for cardiac surgery, like age, male gender, sympathovagal instability, and oxidative and inflammation stress. The cardiac-related risk factors include ischaemic heart disease,2 left systolic dysfunction, increased left atrial volume and atrial dysfunction2,3 or high brain natriuretic peptide (BNP).4,14 Other risks are more specific to pulmonary and oesophageal surgery and include gastric dilatation,5 extent of surgical resection,4 preoperative chemotherapy,2 the chronic obstructive pulmonary disease (COPD), and poor pulmonary function.15 The lower preoperative fluctuation of heart rate in response to various stimuli is considered as a risk factor too, similarly to observed lower heart rate variability in the critically ill.6,7
Right atrial volume overload and stretch accompanying increased pulmonary venous pressure or a vessel dissection following pulmonary resection are also believed to induce AF.17
Post-operative atrial fibrillation may be transient and uncomplicated;2 however, as for the cardiac surgery, it is associated with post-operative complications, longer hospital stay, higher morbidity, and increased long-term mortality.8–10
Beta-blockers are a potential option to manage POAF, both for prevention and treatment. In addition, patients under chronic beta-blockade who undergo major operations such as thoracotomy, lung resection, or oesophagectomy represent a challenge for prolonged impairment of oral intake of their medications in the post-operative period. A history of beta-blocker therapy conveys a risk of arrhythmia when the medication is interrupted in the critically ill.18,19 Unreliable oral intake in the perioperative period may induce a beta-blockade withdrawal syndrome which is a risk factor for AF.20 Therefore, intravenous administration is preferable to oral route, and the strategy to manage POAF should consider a chronic beta-blockade.
Landiolol is a new ultra-short beta-blocker with a half-life of only 4 min and a high beta-1 selectivity which has been used for treatment and prevention of AF in pulmonary surgery17,21–27 and gastro-intestinal surgery.28–32
Landiolol has also been shown to be well tolerated perioperatively and in intensive care units in patients with airway hyper-reactivity.33
Patients undergoing pulmonary resection accompanied by POAF are frequently affected with respiratory complications such as pneumonia and acute respiratory failure. Hence, good tolerance and minimal impact of landiolol on respiratory function make it an ideal therapy of tachycardia in patients undergoing thoracotomy and requiring post-operative ventilatory support.21
Experience with landiolol for prevention of AF in non-cardiac surgery is more documented than landiolol application for treatment of POAF. There are only limited trials21,22,28,29 with total of 61 patients undergoing lung resection or oesophagectomy who developed POAF and were treated with landiolol to control heart rate. Conversely, there are nine comparative studies22–27,30–32 with a total of 450 patients treated with landiolol for prevention of POAF.
Landiolol use to control heart rate in patients with post-operative atrial fibrillation
In two retrospective trials, landiolol was compared to standard of care with matching patient status and comorbidities between the groups of patients. Heart rate decrease was significantly lower, and the time to restore sinus rhythm was shorter in landiolol group compared to the controls (see Table 1). The effect on blood pressure was minimal and the overall tolerance was good with a trend for less adverse events in the landiolol group. In both studies, landiolol was well tolerated with comparable incidence of adverse events in the study and control groups.
Table 1.
Comparative studies for treatment of post-operative atrial fibrillation
| Type of surgery |
Lung surgery (Nojiri et al.21) |
|
|---|---|---|
| Number of patients | Landiolol n = 15 | Control n = 15 |
| Rate of conversion to SR | at 2 h 8/15 (53%) | at 2 h 3/15 (20%) |
| at 12 h 11/15 (73%) | at 12 h 8/15 (53%) | |
| Time to convert to SR | 8.1 ± 11.0 h | 23.0 ± 26.0 h, |
| Landiolol dose used | Ten patients at 5 mcg/kg/min | 0.25 mg digoxin |
| Five patients at 10 mcg/kg/min | +5 mg verapamil on day 1 | |
|
Adverse events |
Pneumonia (n = 1) |
Pneumonia (n = 4) |
| hypotension (n = 2) | ||
|
acute respiratory distress syndrome (n = 1) | ||
| Type of surgery |
Oesophagectomy (Niwa et al.28) |
|
|
Number of patients |
Landiolol n = 8 |
Control n = 13 |
| Rate of conversion to SR | at 2 h 5/8 (62.5%) | at 2 h 1/13 (7.7%) |
| at 12 h 8/8 (100%) | at 12 h 7/13 (53.8%) | |
| Time to convert to SR | 3.6 ± 6.6 h | 23.3 ± 5.2 h |
| Landiolol dose used | 6.5 ± 3.4 mcg/kg/min | Digoxin (n = 11) |
| subsequently increased to | Verapamil (n = 6) | |
| 7.7 ± 4.4 mcg/kg/min | Disopyramide (n = 3) | |
| Adverse events | AF recurrence (n = 1) | AF recurrence (n = 3) |
| SBP<90 mmHg/HR<50 b.p.m. (n = 1) | ||
A single digoxin dose of 0.25 mg may have been too low as compared to usual dose that allow titration up to two times 0.5 mg per day.
SR, sinus rhythm.
A similar efficacy and good tolerance was revealed in two additional series of patients treated with landiolol. Control of heart rate was effective in 85%29 and 92%22 of patients. This was associated with a cardioversion to sinus rhythm in 56% (14/25)29 and 77% (10/13).22
The landiolol dosages used in these series of patients were higher, applying loading dose of 60 mcg/kg/min followed by infusion of 10 mcg/kg/min titrated upwards to 40 mcg/kg/min22,29
This regimen was further adjusted starting with decreasing loading dose of 60–20 mcg/kg/min followed by infusion of 1–5 mcg/kg/min, 21 which was close to the dosage used in comparative studies.21,28
Some patients (13/25) necessitated infusion longer than 24 h with no adverse events related to the circulatory or respiratory system.22
The blood pressure was reduced on average by 10%.22,29 Mori et al.29 identified two cases with decrease in systolic blood pressure below 80 mmHg, and two cases showing a 30% decrease which resolved without requiring discontinuation of landiolol nor support with catecholamines.
There was no report of bronchospasm or serious adverse events such as ischaemia or congestive heart failure when using landiolol in this population.21,22,28,29
Landiolol use to prevent post-operative atrial fibrillation
Landiolol has also been successfully used to control heart rate during surgery and post-operatively for preventing the occurrence of POAF.
Landiolol was well tolerated with minimal haemodynamic impact. In the larger randomized controlled trial (RCT) by Ojima et al.,32 landiolol reduced heart rate and induced a drop of blood pressure on the 1st post-operative day (POD1) which normalized on POD2. One study reported a low incidence of hypotension (3%) which resolved quickly upon reducing dose.24 In general, most studies reported no bradycardia and no hypotension17,24,30,31 or respiratory symptoms26 with no need to decrease dose or stop the infusion.
Overall, low dose (from 2 to 5 mcg/kg/min) of landiolol infused during surgery17,23,24,26–31 or immediately after surgery25,32 consistently reduced significantly incidence of POAF by three-fold on average.
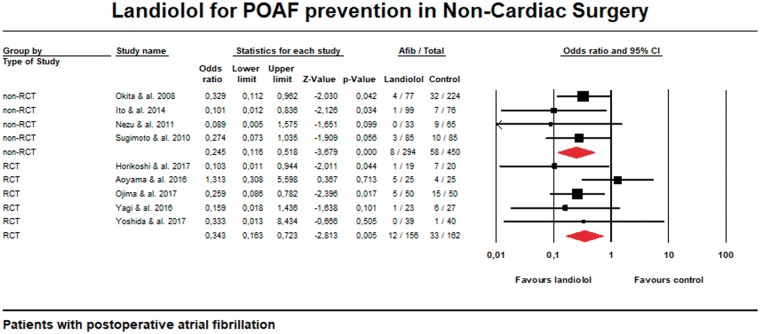
Nevertheless, there are yet few prospective RCT available in non-cardiac surgery. Figure 1 shows that non-randomized comparative trials demonstrate a similar trend towards reduction of POAF incidence with landiolol. Some RCT are associated with too low incidence of POAF and thus underpowered to show a significant difference.30 This low incidence may be also due to less complex surgical procedures.30 On the other hand, other case series reported high incidence probably due to recruiting elderly population which is associated with a higher risk of POAF (mean age of included patients developing POAF was 82 years).17
Figure 1.
Meta-analysis of comparative trials.
Interestingly, one RCT reported a lower rate of non-haemodynamic complications in the group treated with landiolol in addition to prevent POAF.32 In this study, landiolol was found to decrease complications related to oesophagectomy (>Grade II morbidity) with a rate of 40% (20/50) vs. 60% (30/50) in the control group. The relation between POAF and other complications related to oesophagectomy was previously shown by the same team who identified a rate of other complications of 63.2% in the group of patients associated with POAF while the patient free of POAF displayed only 16.0% of complications.
Ojima et al.32 have shown that sinus tachycardia with a rate above 100 b.p.m. on POD1 was the most significant risk factor for POAF. Their patients, undergoing complex procedures and benefitting from the early mobilization at POD1 and POD2 displayed more frequently sinus tachycardia and were prone to develop POAF. In this setting, landiolol was used as the first line therapy for controlling and manage POAF.5
Perspectives
In addition to heart rate control and blunting adrenergic stress, landiolol has been shown to regulate production of cytokines, and other inflammatory regulators that may also contribute to prevention of POAF and reduction of the post-operative complications.29 The suppression of inflammatory systemic response could be beneficial for patients with post-operative complications accompanied by the arrhythmia.29
In non-cardiac surgery, landiolol infusion was associated with lower IL-6 levels. This effect may be related to the time course of landiolol infusion. Indeed, when landiolol infusion was limited to intraoperative period, plama levels of IL-6 were different at the end of surgery; however, this difference disappeared in the post-operative period.27
This significant difference at end of surgery was also observed in another study with infusion covering intraoperative period and continued until the next morning, but with no difference in IL-6 levels at POD1 and POD2.31 Conversely, when landiolol infusion was started on POD1, IL-6 levels were significantly lower when measured on POD3 and POD5, but not at POD1.
Other cytokines (IL-10, IL-8, TNF-alpha)32 or inflammatory markers like CRP which are increased post-operatively were not significantly different between landiolol and control group.26,27,31
Conclusion
In conclusion, landiolol is a promising drug to manage POAF in non-cardiac surgery with a profile that allow for control of heart rate with minimal impact on blood pressure. Landiolol has limited negative inotropic effect and is well tolerated by the respiratory system. Additional benefits related to the regulation of inflammatory response and blunting of the adrenergic pathway probably contribute to the decreased incidence of POAF.
The use of low dosage (5–10 mcg/kg/min) is usually sufficient to rapidly control heart rate which is associated with earlier and higher rate of conversion to sinus rhythm as compared to the controls.
The excellent tolerance of landiolol at lower dosage (3–5 mcg/kg/min) allows to initiate prophylactic use during surgery and post-operatively. Landiolol prophylaxis is associated with reduced incidence of POAF without triggering adverse events related to a beta-blockade. Optimized infusion scheme with continuing landiolol infusion in the post-operative period seems to be associated with better response, while infusion limited to the intraoperative period may not be sufficient.27
Conflict of interest: Supported by the educational grant of Amomed Co.
References
- 1. Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L.. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg 2012;7:4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ivanovic J, Maziak DE, Ramzan S, McGuire AL, Villeneuve PJ, Gilbert S, Sundaresan RS, Shamji FM, Seely AJ.. Incidence, severity and perioperative risk factors for atrial fibrillation following pulmonary resection. Interact Cardiovasc Thorac Surg 2014;18:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raman T, Roistacher N, Liu J, Zhang H, Shi W, Thaler HT, Amar D.. Preoperative left atrial dysfunction and risk of postoperative atrial fibrillation complicating thoracic surgery. J Thorac Cardiovasc Surg 2012;143:482–487. [DOI] [PubMed] [Google Scholar]
- 4. Amar D, Zhang H, Shi W, Downey RJ, Bains MS, Park BJ, Flores R, Rizk N, Thaler HT, Rusch VW.. Brain natriuretic peptide and risk of atrial fibrillation after thoracic surgery. J Thorac Cardiovasc Surg 2012;144:1249–1253. [DOI] [PubMed] [Google Scholar]
- 5. Ojima T, Iwahashi M, Nakamori M, Nakamura M, Katsuda M, Iida T, Hayata K, Yamaue H.. Atrial fibrillation after esophageal cancer surgery: an analysis of 207 consecutive patients. Surg Today 2014;44:839–847. [DOI] [PubMed] [Google Scholar]
- 6. Ciszewski P, Tyczka J, Nadolski J, Roszak M, Dyszkiewicz W.. Lower preoperative fluctuation of heart rate variability is an independent risk factor for postoperative atrial fibrillation in patients undergoing major pulmonary resection. Interact Cardiovasc Thorac Surg 2013;17:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt HB, Werdan K, Muller-Werdan U.. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care 2001;7:314–322. 10.1097/00075198-200110000-00002 [DOI] [PubMed] [Google Scholar]
- 8. Murthy SC, Law S, Whooley BP, Alexandrou A, Chu KM, Wong J.. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg 2003;126:1162–1167. [DOI] [PubMed] [Google Scholar]
- 9. Chin J-H, Moon Y-J, Jo J-Y, Han YA, Kim HR, Lee E-H, Choi I-C, Lee H-S.. Association between postoperatively developed atrial fibrillation and long-term mortality after esophagectomy in esophageal cancer patients: an observational study. PLoS One 2016;11:e0154931.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stawicki SP, Prosciak MP, Gerlach AT, Bloomston M, Davido HT, Lindsey DE, Dillhoff ME, Evans DC, Steinberg SM, Cook CH.. Atrial fibrillation after esophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg 2011;59:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SH, Ahn HJ, Yeon SM, Yang M, Kim JA, Jung DM, Park JH.. Potentially modifiable risk factors for atrial fibrillation following lung resection surgery: a retrospective cohort study. Anaesthesia 2016;71:1424–1430. [DOI] [PubMed] [Google Scholar]
- 12. Ng EP, Velez-Cubian FO, Rodriguez KL, Thau MR, Moodie CC, Garrett JR, Fontaine JP, Toloza EM.. Surgical outcomes associated with postoperative atrial fibrillation after robotic-assisted pulmonary lobectomy: retrospective review of 208 consecutive cases. J Thorac Dis 2016;8:2079–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tisdale JE, Wroblewski HA, Wall DS, Rieger KM, Hammoud ZT, Young JV, Kesler KA.. A randomized, controlled study of amiodarone for prevention of atrial fibrillation after transthoracic esophagectomy. J Thorac Cardiovasc Surg 2010;140:45–51. [DOI] [PubMed] [Google Scholar]
- 14. Cardinale D, Sandri MT, Colombo A, Salvatici M, Tedeschi I, Bacchiani G, Beggiato M, Meroni CA, Civelli M, Lamantia G, Colombo N, Veglia F, Casiraghi M, Spaggiari L, Venturino M, Cipolla CM.. Prevention of atrial fibrillation in high-risk patients undergoing lung cancer surgery: the PRESAGE trial. Ann Surg 2016;264:244–251. [DOI] [PubMed] [Google Scholar]
- 15. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Salvatici M, Veronesi G, Veglia F, Fiorentini C, Spaggiari L, Cipolla CM.. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. Circulation 2007;115:1339–1344. [DOI] [PubMed] [Google Scholar]
- 16. Ma JY, Wang Y, Zhao YF, Wu Z, Liu LX, Kou YL, Yang JJ.. Atrial fibrillation after surgery for esophageal carcinoma: clinical and prognostic significance. World J Gastroenterol 2006;12:449–452. 10.3748/wjg.v12.i3.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yagi K, Usuda J, Sakamoto A.. Perioperative landiolol infusion reduces the incidence of atrial fibrillation after pulmonary lobectomy: postoperative randomized controlled Study. Open J Anesthesiol 2016;6:119–123. 10.4236/ojanes.2016.68020 [DOI] [Google Scholar]
- 18. Macchia A, Romero M, Comignani PD, Mariani J, D’Ettorre A, Prini N.. Previous prescription of beta-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis. Crit Care Med 2012;40:2768–2772. [DOI] [PubMed] [Google Scholar]
- 19. Balik M, Kolnikova I, Maly M, Waldauf P, Tavazzi G, Kristof J.. Propafenone for supraventricular arrhythmias in septic shock-Comparison to amiodarone and metoprolol. J Crit Care 2017;41:16–23. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 21. Nojiri T, Yamamoto K, Maeda H, Takeuchi Y, Funakoshi Y, Maekura R, Okumura M.. Efficacy of low-dose landiolol, an ultrashort-acting β-blocker, on postoperative atrial fibrillation in patients undergoing pulmonary resection for lung cancer. Gen Thorac Cardiovasc Surg 2011;59:799–805. [DOI] [PubMed] [Google Scholar]
- 22. Nakano T, Shimizu K, Kawashima O, Kamiyoshihara M, Nagashima T, Ibe T, Takeyoshi I.. Effect of landiolol hydrochloride, an ultra-short-acting beta1-selective blocker, on supraventricular tachycardia, atrial fibrillation and flutter after pulmonary resection. J Clin Pharm Ther 2012;37:431–435. [DOI] [PubMed] [Google Scholar]
- 23. Okita T, Uji M, Shinjo T, Morioka M, Kumano H, Ishimura N, Nishiwada M.. [Use of landiolol hydrochloride for the prevention of atrial fibrillation after lung resection]. Masui 2008;57:953–958. [PubMed] [Google Scholar]
- 24. Sugimoto R, Nakayama Y, Nawa Y, Orimo K, Yamakage M.. Administration of landiolol decreases the incidence of atrial fibrillation after lung lobectomy. Anesthesiology 2010; 18 October, A1141 (abstract). [Google Scholar]
- 25. Nezu N, Ogawa F, Matsui Y, Amano H, Hara H, Kurouzu N, Iyoda A, Satoh Y.. Effect of postoperative low-dose landiolol hydrochloride for the prevention of tachyarrhythmia after primary lung cancer surgery. J Jpn Assoc Chest Surg 2011;25:472–478. [Google Scholar]
- 26. Ito K, Nozaki M, Sakamoto R, Suzuki T, Masuda R, Iwazaki M.. Safety of landiolol infusion in patients undergoing lung resection. Open J Anesthesiol 2014;4:183–190. [Google Scholar]
- 27. Aoyama H, Otsuka Y, Aoyama Y.. Landiolol infusion during general anesthesia does not prevent postoperative atrial fibrillation in patients undergoing lung resection. Gen Thorac Cardiovasc Surg 2016;64:735–741. 10.1007/s11748-016-0707-3 [DOI] [PubMed] [Google Scholar]
- 28. Niwa Y, Koike M, Iwata N, Kobayashi D, Tanaka C, Fujii T, Nakayama G, Sugimoto H, Fujiwara M, Kodera Y.. Effect of landiolol hydrochloride on tachyarrhythmia after esophagectomy. Hepatogastroenterology 2014;61:1546–1551. [PubMed] [Google Scholar]
- 29. Mori K, Yamada K, Fukuda T, Mitsui T, Kitamura T, Yamaguchi D, Ando J, Wada I, Nomura S, Shimizu N, Seto Y.. Landiolol hydrochloride for early postoperative tachycardia after transthoracic esophagectomy. Surg Today 2014;44:848–854. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida T, Furukita Y, Yamamoto Y, Nishino T, Inoue S, Morimoto M, Okumura K, Toba H, Yoshida M, Takizawa H, Tangoku A.. A randomized, open label study of the efficacy of prophylactic 24-h low-dose landiolol for atrial fibrillation in transthoracic esophagectomy. Esophagus 2017;14:97–103. [Google Scholar]
- 31. Horikoshi Y, Goyagi T, Kudo R, Kodama S, Horiguchi T, Nishikawa T.. The suppressive effects of landiolol administration on the occurrence of postoperative atrial fibrillation and tachycardia, and plasma IL-6 elevation in patients undergoing esophageal surgery: a randomized controlled clinical trial. J Clin Anesth 2017;38:111–116. [DOI] [PubMed] [Google Scholar]
- 32. Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Kato T, Kitadani J, Tabata H, Takeuchi A, Yamaue H.. Randomized clinical trial of landiolol hydrochloride for the prevention of atrial fibrillation and postoperative complications after oesophagectomy for cancer. Br J Surg 2017;104:1003–1009. [DOI] [PubMed] [Google Scholar]
- 33. Yamakage M, Iwasaki S, Jeong SW, Satoh J, Namiki A.. Beta-1 selective adrenergic antagonist landiolol and esmolol can be safely used in patients with airway hyperreactivity. Heart Lung 2009;38:48–55. [DOI] [PubMed] [Google Scholar]