Abstract
Intertrigo is a common inflammatory dermatosis of opposing skin surfaces that can be caused by a variety of infectious agents, most notably candida, under the effect of mechanical and environmental factors. Symptoms such as pain and itching significantly decrease quality of life, leading to high morbidity. A multitude of predisposing factors, particularly obesity, diabetes mellitus, and immunosuppressive conditions facilitate both the occurrence and recurrence of the disease. The diagnosis of candidal intertrigo is usually based on clinical appearance. However, a range of laboratory studies from simple tests to advanced methods can be carried out to confirm the diagnosis. Such tests are especially useful in treatment-resistant or recurrent cases for establishing a differential diagnosis. The first and key step of management is identification and correction of predisposing factors. Patients should be encouraged to lose weight, followed up properly after endocrinologic treatment and intestinal colonization or periorificial infections should be medically managed, especially in recurrent and resistant cases. Medical treatment of candidal intertrigo usually requires topical administration of nystatin and azole group antifungals. In this context, it is also possible to use magistral remedies safely and effectively. In case of predisposing immunosuppressive conditions or generalized infections, novel systemic agents with higher potency may be required.
Keywords: Candida, intertrigo, recurrent candidal intertrigo, candidiasis, candidosis, candidal predisposals
Background
Intertrigo (intertriginous dermatitis) is a clinical inflammatory condition that develops in opposing skin surfaces in response to friction, humidity, maceration, or reduced air circulation.1 This common skin disorder may be localized in a small area or involve larger surfaces. Lesions mostly develop in the neck, axilla, sub-mammary fold, and perineum, while other sites may also be involved including antecubital, umbilical, perianal, and interdigital areas as well as abdominal folds, eyelids, and the retroauricular area.1–3
The main factor in the development of the lesions is the mechanical friction on the skin that initially appears as a minimal erythema of the folds. Heat, reduced aeration, humidity, and maceration facilitate intertrigo. Although the condition may occur in both genders and all races, it is more common in diabetic obese individuals residing in hot and humid climates and in bed-ridden or elderly subjects. Urinary or fecal incontinence, inadequate personal hygiene, malnutrition, immunosuppression, and occlusive clothing are among other predisposing factors. Infants are also more likely to develop intertrigo due to drooling and short neck structure with prominent skin folds and a flexed position.2 Many yeasts (particularly Candida) and molds, bacteria, and viral infectious agents may aggravate intertrigo by colonizing on the skin (Table 1).
Table 1.
Infectious agents commonly found in intertrigo
| Microorganisms | Species | References |
|---|---|---|
| Candida | C. albicans, C. glabrata, C. tropicalis, C. krusei, C. parapsilosis, C. dubliniensis, C. famata | 4–11 |
| Fusarium | F. oxysporum, F. solani | 12–18 |
| Dermatophytes | Trichophyton spp., Microsporum spp., and Epidermophyton floccosum | 2,8,9,15,19,20 |
| Malassezia | Mostly M. furfur, M. globosa or M. sympodialis | 9,21–23 |
| Bacteria | S. aureus, S. agalactiae, S. haemolyticus, S. pyogenes, or other streptococcal species Pseudomonas spp., Proteus spp., E. coli, Peptostreptococcus spp., Corynebacterium spp., Acinetobacter spp., etc. | 2,11,24–26 |
| Viruses | Poxviridae, Papillomaviridea (HPVs), Picornaviridae, Retroviridae (HIV), Herpesvirdae, Togaviridae, Parvoviridae, | 27–30 |
Abbreviation: HPV, human papillomavirus.
Intertrigo may transform into a life-long chronic condition. It generally has an insidious onset with symptoms such as itching, pain, burning, or prickling sensations in skin fold areas.7 Initially it presents itself as mildly erythematous papillae or plaques, quickly developing into an exudative erosion, fissures, macerations, and crusts. Erythema due to secondary infections, increased inflammation, papullo-pustules, and bad odor may develop.1,2,7
Diagnosis of intertrigo and its complications are generally based on clinical manifestations and basic microbiological investigations. Microbiological cultures, potassium hydroxide (KOH) preparation, and Gram’s staining may guide the therapy when used for differentiating primary and secondary infections. Wood’s light examination can be used to identify a Pseudomonas, Malassezia, or erythrasma infection more quickly than would a culture. Despite the absence of a characteristic histopathological appearance, biopsy may be required in treatment-resistant cases of intertrigo in order to exclude other skin disorders such as psoriasis or lichen planus.1,2
Treatment of intertrigo should generally focus on the removal of predisposing factors, followed by appropriate use of topical or systemic antimicrobial agents as well as low-potency corticosteroids, if required.
Candida species
Taxonomically, Candida belongs to the phylum Ascomycetes, class Blastomycetes, order Cryptococcales, family Cryptococcaceae, and genus Candida.31 These microorganisms have a diameter of 3–5 μm with a two-layered cell wall. Among more than 200 Candida species identified, only 15 may be associated with primary Candida infections.32 Yeasts associated with Candida species can be found in the normal flora of human skin as well as in the mucosal covering of the gastrointestinal system, genito-urinary system, and respiratory system, in addition to the soil and a variety of foods.31 Human colonization starts on the first day after birth and continues throughout the life-cycle as an opportunistic pathogen. Candida albicans is responsible for the majority of Candida-related noninvasive skin and mucosal candidiasis. However, a more than 50% increase in the incidence of non-albicans Candida species have recently been reported including C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, C. lusitaniae, C. dubliniensis, and C. guilliermondii.33 Each of these organisms exhibits characteristic virulence potential, antifungal susceptibility, and epidemiology.34
Pathogenesis of candidal infection
C. albicans is a part of the normal flora in skin and genital and/or intestinal mucosa in 70% of healthy individuals.35 Similar to many other opportunistic microorganisms of the skin, it exists as a commensal yeast in individuals with an intact immune system. It may lead to mucocutaneous or systemic infections under appropriate conditions.
Many Candida species are known to produce virulence factors like proteases. Species lacking these virulence factors are considered less pathogenic.36–39 Mechanisms of pathogenicity for Candida albicans may be summarized as below: secretion of hydrolases, molecules that mediate adhesion to with concomitant invasion into host cells, the yeast-to-hypha transition, biofilm formation, contact sensing and thigmotropism, phenotypic switching, and a variety of fitness attributes.37
As is the case with all pathogens, the innate immunity of the skin represents the first step of the host defense against Candida.40 Pathogenic invasion is a rather complex process and is initiated through disruption of the physical barrier by the transformation of Candida on the skin from yeast to hypha form. The capability of the yeasts to adhere to epithelium is a strong stimulant for the hyphal transformation and represents the most important step in tissue penetration.37,39–41 Hyphae of C. albicans exhibit stronger epithelial adhesion than yeasts. More aggressive C. albicans species that have no ability to produce hyphae cannot attach to epithelium. Breakdown of the physical barrier with fungal invasion allows the spread of C. albicans to underlying vascular tissues, and then to distant organs. While transformation into the hypha form is a critical virulence factor both for epithelial penetration and phagocyte attachment of C. albicans, the yeast form is required for the development of systemic infection and dissemination.38,41
The contest between the host and Candida involves more specific and complex molecular mechanisms; the recognition of fungal cell wall components, activation of the immune cell signal pathways of the host, and release of cytokines and chemokines.41 Formation of hyphae by C. albicans is also known to represent a very important factor that induces cytokine responses from epithelial cells.42 The importance of cytokine and chemokine production has been underlined almost universally in all studies investigating the epithelial responses to C. albicans. Infected epithelial cells have been found to produce IL-lα/β, IL-6, G-CSF, GM-CSF, and TNFα, in addition to chemokines and cytokines such as RANTES, IL-8, and CCL20.38,43–45
An examination of the immune mechanisms of the skin against C. albicans reveals that the defense barrier initiated with the stromal cells such as keratinocytes and melanocytes as well as the defense proteins released by these cells continues with the pattern recognizing receptors such as Dectin-1 and Toll-like receptor. Individuals with mutations or gene polymorphisms in pathways of these receptors have been found to be more susceptible to Candida infections.46 The major mechanisms of innate immunity against candida infections include neuropeptides such as calcitonin gene-related peptide (CGRP) released in areas where the physical barrier is disrupted, IL-23 release from the dendritic cells, and activation of neutrophils recruited via IL-17 release from γδ T cells that is stimulated by the release of IL-23. On the other hand, IL-17 pathways represent an important component of the adaptive immunity against Candida infections through induction of effector and cytotoxic T lymphocytes.35
Predisposing factors for candidal infections
The main determinant of the non-pathogenic commensal colonization versus pathogenic behavior is the balance between fungal proliferation and the innate and adaptive defenses of the host.41 This balance is disturbed in favor of Candida as a result of various factors that predispose the individual to intertrigo (Table 2).
Table 2.
Predisposing factors for Candida infections.
| Factor group | Factors | |
|---|---|---|
| Dermatoses | Psoriasis | Contact dermatitis |
| Endocrine disease | Diabetes mellitus | Hypoadrenalism |
| Cushing disease | Hypothyroidism | |
| Hypoparathyroidism | ||
| Iatrogenic | Catheters and intravenous lines | Radiation therapy |
| Immunosuppressive agents | Colchicine | |
| Broad spectrum antibiotics | Phenylbutazone | |
| Estrogen containing oral contraceptives | Tranquilizers | |
| Glucocorticoids | ||
| Immunodeficiency | HIV infection | Chronic granulomatous disease |
| SCIDS | Chediak-Higashi syndrome | |
| Myeloperoxidase deficiency | DiGeorge syndrome | |
| Hyper IgE syndrome | Nezelof syndrome | |
| Neutropenia | Other immunosuppressive diseases | |
| Mechanical and environmental | Trauma (burns, abrasions) | Occlusive wearings |
| Occlusion, humidity, maceration | Obesity | |
| Dentures | Tropical environment | |
| Nutritional | Vitamin deficiencies (B6, B12) | Generalized malnutrition |
| Iron deficiency (CMC) | High carbohydrate content | |
| Physiological | Extremes of age (infants, elderly) | Pregnancy |
| Menses | Low vaginal pH | |
| Sialorrhea | Debilitating | |
| Systemic illnesses | Down syndrome | Uremia |
| Acrodermatitis enteropathica | Sjögren syndrome | |
| Other | Uncircumcised penis | Infected sexual partner |
| Poor hygiene | Prolonged hospitalization | |
| Prolonged exposure to water | Finger sucking | |
| Malignancies | Smoking | |
| Severe sweating | Occupational factors |
Clinical forms of candidal skin infection
C. albicans is responsible for approximately 80–90% of all skin infections caused by Candida species. It is an oval-shaped thermal dimorphic yeast with a diameter of 2–6 × 3–9 μm that can produce budding cells, pseudo-hyphae, and true hyphae. Skin infections encompass numerous forms with varying clinical terminology used to describe them. Although the clinical variants of skin infections have been clearly defined in the literature, currently no consensus regarding a standard classification system exists. In a 1996 classification by the American Academy of Dermatology’s Guidelines/Outcomes Committee,47 the infections have been defined on the basis of their location and appearance as follows: cutaneous (intertriginous agents), oral (intra-oral mucosa), genital (vagina and penis), nail unit, and chronic mucocutaneous. However, different clinical classification systems have been proposed in many dermatological or other textbooks, or reviews.31,49–51,57,58,62–65
Regardless of the size of the lesion, Candida infections involving skin folds should be classified under the candidal intertrigo heading, based on the definition intertriginous dermatitis (Table 3).
Table 3.
Clinical presentations and locations of intertriginous candidal infections
| Terminology | Clinical presentation | Location | References |
|---|---|---|---|
| Intertriginous candidiasis (intertrigo) | Erythema, maceration, hydration, crusting, fissuring, folliculitis, papules, pustules, satellite lesions, plaques, foul-smelling, itching, stinging | Abdominal folds | 7,9,11,32,33,51,62,63,66 |
| Axilla and inguinal folds | 2,7,9,11,47,51,54,60,62,63,66–75 | ||
| Cervical or neck creases | 2,7,9,32,41,45,51,60,62,63,71,73,76,77 | ||
| Diaper areas | 2,7,9,31,47,51,63,66,78–80 | ||
| Finger or toe webs | 2,7–9,12,26,47,48,51,61–63,76,81,82 | ||
| Folds of the eyelids | 2,9,51,63,83 | ||
| Intergluteal area | 2,7,9,67,84,85 | ||
| Perianal | 9,11,47,51,62,66,73,75,79,83 | ||
| Perineum | 31,51,62,70,75 | ||
| Retroauricular folds | 51,60,67,73,83 | ||
| Submammary creases | 9,11,66,72,86 | ||
| Umbilicus | 2,9,44,51,70 |
Candidal intertrigo
C. albicans has a predilection for moist and macerated skin folds. The most frequent type of clinical presentation in hairless skin is intertrigo. Pruritic, erythematous, macerated skin areas are observed in intertriginous areas with satellite vesicopustules. The characteristic pustulae rapidly rupture, leading to the formation of collaret type erythematous surface, from which the necrotic epidermis may be easily removed.9,87
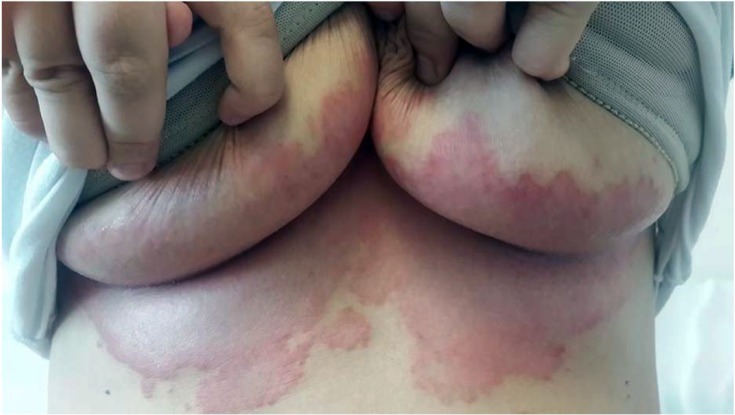
Candidal intertrigo of larger skin folds usually involves the axilla, gluteal, infra-mammary, and genito-crural fold (Figure 1). The moisture and increased temperature on the surface of opposing skin folds provide a suitable medium for the growth of Candida and bacteria. Humid and hot weather, tight underclothes, poor hygiene, and inflammatory skin conditions such as psoriasis may increase the risk of candidal infections.9 Diabetes mellitus and obesity represent the leading predisposing factor. Xerostomia, hyperhidrosis, occlusive wearings, occupational factors, use of corticosteroids or wide spectrum antibiotics, and immunosuppression including HIV infection may also increase the risk.3,51,68,88,89
Figure 1.
Candida intertrigo on the infra-mammary folds of a middle-aged woman.
Diaper candidiasis
Diaper dermatitis is an acute and inflammatory skin reaction in the diaper area (Figure 2). It is generally caused by the yeast colonizing in the gastrointestinal system. Chronic occlusion with wet clothes facilitates the infection. With prevalence ranges between 7 and 35%, it most commonly occurs in infants between 9 and 12 months of age, and may also be seen in adults requiring incontinence pads.10 Infants with Candida diaper dermatitis generally have colonization in their gastrointestinal system with positive stool cultures for Candida. In infants with very low birth weight ≤ 1500 g, candidal colonization of the rectum and stools can be detected in 21–62.5%.90
Figure 2.
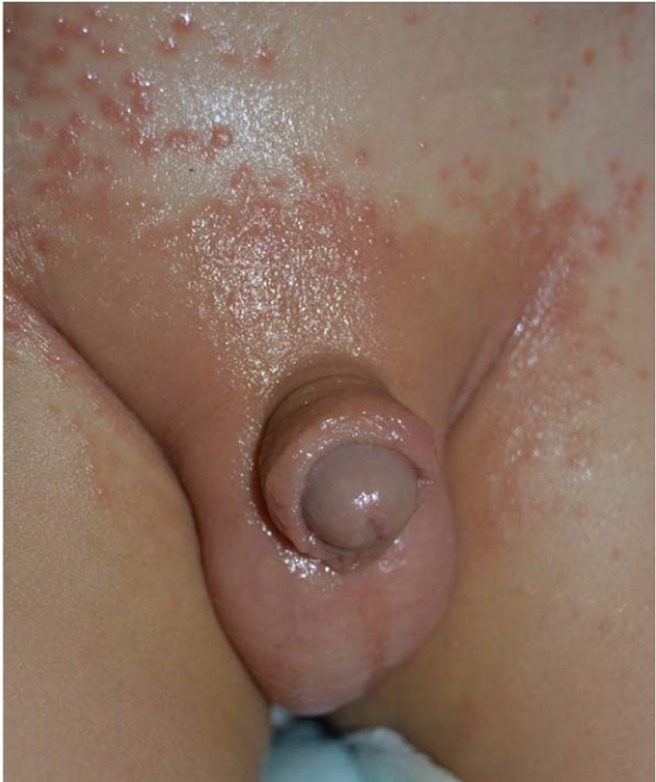
Diaper candidial infection of a child.
Lesions typically start in the perianal region, and spread over the perineum and inguinal area. Not all cases of diaper dermatitis may be caused by Candida, but diaper dermatitis due to candidiasis involves the skin folds. Multiple small erythematous desquamated pustules and satellite lesions extending along the borders of large maculae represent significant findings for diagnosis.51,88,90
Granuloma gluteale infantum is a reaction developing Candida that causes opaque, reddish, irregular papules and/or nodules on the background of an erythematous surface in the diaper area. This is a reactive condition developing due to chronic irritant contact dermatitis caused by urinary incontinence or chronic diarrhea.63,89,91 Diagnosis is generally straightforward, and biopsy may be required to rule out mast cell tumors, pseudolymphoma, lymphoma, and leukemic infiltration.92
Angular cheilitis (perleche)
This condition is characterized by erythema, maceration, transverse fissures, and pain in the corners of the mouth. Although it is localized in the skin folds on the lips, it is classified within the group of oral candidal infections. Recurrent oral candidiasis is a common finding in HIV-infected subjects and is an important prognostic marker.78 In HIV-positive patients, it may occur without other signs when the CD4+ lymphocyte count declines below 200/μL.93 Frequently, it occurs due to use of lip liners in younger individuals, while skin sagging may be a causative factor in the elderly. Tooth loss, ill-fitting dental fixtures, and malocclusion represent other predisposing factors.78,88,89 It may occur concomitantly with submental and cervical intertrigo, particularly in infants and debilitated patients with salivary discharge.
Erosio interdigitalis blastomycetica
Candidal intertrigo settling between the fingers, also termed as erosio interdigitalis blastomycetica (EIB), is an infectious condition that may develop by a candidal or polymicrobial infection. It usually affects the third and fourth fingers or toes due to physical inactivity, moisture, soap, water retention, or disruption of the skin barrier. The moisture under a ring may cause maceration and irritation, facilitating secondary infections with C. albicans. Lesions may cause oval, macerated, whitish lesions that may extend to the lateral borders. Generally, one or more fissures with a reddish-base are present in the middle of the lesions. As the disease progresses the macerated skin is peeled off, leaving an eroded area in which the protruding epidermis is surrounded by a white collar.51 Microbiological cultures suggest that Candida and gram negative bacilli play a role in the development of this condition.94 Very often, it develops as an occupational disease due to chronic maceration in individuals with chronic contact with water such as cooks, barmen, barmaids, dishwasher, housewives, or dentists. Diabetes mellitus is a predisposing disease for EIB, and EIB is an important cutaneous manifestation of diabetes.9,82 Thus, in patients diagnosed with EIB, a diagnosis of de novo or uncontrolled diabetes should be considered.81 The differential diagnosis includes erythrasma and irritant contact dermatitis.9,82
Toe web candidiasis
It is an EIB-like intertriginous Candida infection, commonly occurring in the fourth interdigital space of the toes. It may be asymptomatic or cause mild symptoms. Moist working conditions and use of tight and closed shoes for prolonged periods of time may induce this condition.2,51 The skin exhibits white, macerated, and thickened epidermis. Its appearance is very similar to that of tinea pedis, and significant erythema and desquamation may occur as well.2,51
Perianal, perineal and intergluteal candidosis
Perianal, perineal, and genitocrural areas are naturally moist areas of the skin.9 Intertrigo may develop as an extension of vulvovaginal or intestinal candidiasis or due to spreading from one area to another.9,49,64 Initially, it may present as severe perineal and anal pruritus accompanied by severe itching and burning sensation. An erythematous, oozy dermatitis together with maceration is observed in involved areas (Figure 3). Also satellite lesions in the form of papules or pustules may be observed in the margins of erythematous-macerated plaques and eroded areas.50,84 Absence of satellite lesions does not rule out a diagnosis of candidiasis.51
Figure 3.
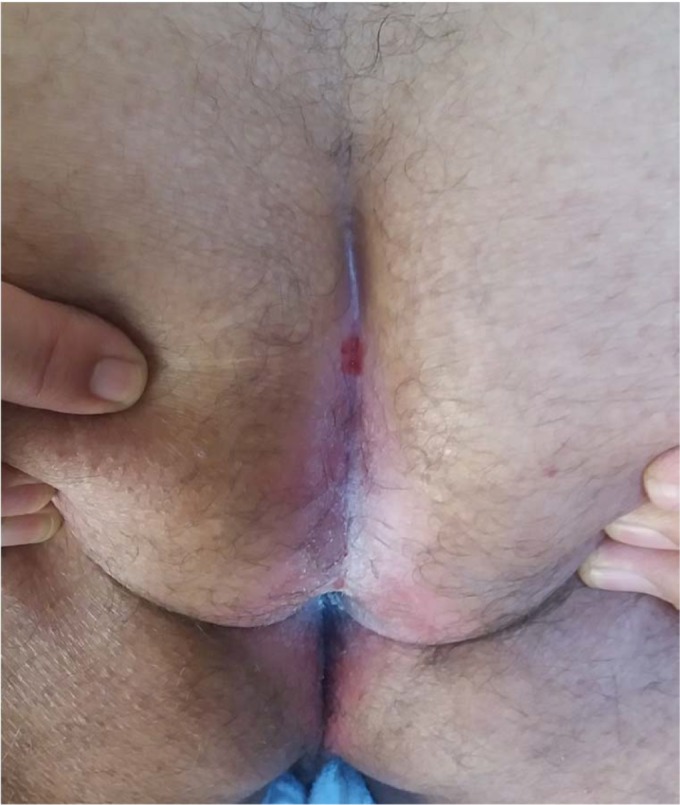
Perianal and intergluteal candidal intertrio of a man.
In cases where the cause of the condition is vulvovaginal or intestinal candidiasis, the disease may exhibit a recurrent and chronic course. Acute genitocrural intertrigo may also develop as a maculopapular eruption in HIV-infected subjects.2
Differential diagnosis of candidal intertrigo
Numerous infectious agents, mainly bacteria and dermatophytes, may lead to similar clinical presentations in the areas affected by Candida intertrigo in addition to a variety of mucocutaneous disorders that can mimic the inflammation in the lesions. Some of these conditions have been presented in Table 4 according to the involved site in intertrigo.
Table 4.
Most common differential diagnoses for intertriginous dermatitis on skin-fold areas
| Differential diagnosis | Anatomical sites of candidal infection
|
||||||
|---|---|---|---|---|---|---|---|
| Large skin folds (axilla, inframammary, umbilical, intergluteal, and genitocrural) | Diaper intertrigo | EIB | Toe web intertrigo | Perianal and perineal intertrigo | intertrigo of the neck folds | Eyelids and retroauricular | |
| Atopic dermatitis | √ | ||||||
| Bacterial intertrigo | √ | √ | √ | √ | √ | √ | √ |
| Bullous impetigo | √ | ||||||
| Contact dermatitis | √ | √ | √ | √ | √ | √ | √ |
| Dermatophyte infections | √ | √ | √ | √ | √ | √ | |
| Drug interaction | √ | ||||||
| Erythrasma | √ | √ | √ | √ | √ | ||
| Extramammary Paget disease | √ | √ | |||||
| Flexural Darier disease | √ | √ | |||||
| Fusarium spp. infections | √ | ||||||
| Glucagonoma | √ | √ | |||||
| Granular parakeratosis | √ | ||||||
| Hailey disease | √ | √ | √ | ||||
| Herpes infections | √ | √ | √ | √ | |||
| HPV infections | √ | √ | √ | ||||
| Langerhans cell histiocytosis | √ | √ | |||||
| Leiner disease | √ | √ | √ | √ | √ | ||
| Lichen planus inversus | √ | ||||||
| Multiple carboxylase deficiency | √ | ||||||
| Psoriasis | √ | √ | √ | √ | √ | ||
| Seborrheic dermatitis | √ | √ | √ | √ | √ | ||
| Syphilis | √ | √ | √ | √ | |||
| Verrucous carcinoma | √ | √ | |||||
| Zinc deficiency/acrodermatitis enteropathica | √ | √ | √ | √ | √ | ||
Preventing recurrent infections
Preventive measures for recurrent intertrigo are used to support the therapy and represent the first step in management. The affected area(s) should be kept dry, clean, and cool with good airing and minimization of skin friction at the fold site. Good hygiene should be maintained in the infected area. Patients should be advised to wear cotton underwear, light clothing in hot and humid weather conditions, and should be warned regarding outdoor activities. Open shoes may help to prevent intertrigo of the toes.2,95
Maceration or irritation due to incontinence should be minimized or eliminated totally if possible. Cleansers, driers, emollients, and skin barrier creams may prove to be useful in such cases.7
Laboratory diagnosis of intertrigo
The clinical appearance of candidal intertrigo usually suffices for a diagnosis. However, laboratory investigations and confirmatory tests may be required, particularly in chronic, resistant, and recurrent cases.51 The simplest examination technique involves identification of the presence of pseudo-hyphae or yeast forms under direct microscopic examination of the samples obtained through scraping and smears that have been prepared with KOH and calcofluor white staining. Also, fluorescent microscopy and trypan blue examination may be used for that purpose. Differentiation between the species, assimilation and fermentation tests are applied on Candidal cultures.58 More advanced techniques rarely required in the clinical practice include PCR, electron microscopy, and microchip diagnostic tests. Biopsy may be performed for the differential diagnosis from psoriasis as well as from dermatoses and dermatophytoses such as tinea. Identification of septa-free hyphae and yeast forms in PAS-stained histopathological samples is diagnostic for Candida.
Treatment of candidal intertrigo
Specific treatment of candidal intertrigo depends on the location, severity, and depth of the infection. Also, the treatment may be guided by the stage of the infection, i.e. acute, subacute, or chronic.58 Initially, the active Candida infection should be medically managed, followed by skin drying measures to reduce the risk of recurrence, and finally by the correction of predisposing factors (Table 3).62,84
Topical anti-fungal agents are the mainstay of treatment in Candidal intertrigo. Topical anti-fungal agents represent the first step in management in mild cases of candidiasis. Nystatin and azole topical antifungals including miconazole, ketoconazole, or clotrimazole may be used twice daily for 2–4 weeks.84 Time-tested magistral preparations may also aid in treatment. In acute lesions, Domeboro® solution (Moberg Pharma North America LLC, Cedar Knolls, NJ, USA), Castellani paint (ICM Pharma, Singapore), or vinegar–water solutions may be applied twice daily for 5–10 minutes. After drying, a mixture of zinc oxide, talc, and glycerin may be administered twice daily. In subacute lesions, after cleansing with benzoyl peroxide, Castellani stain, or vinegar, topical antifungals may be administered. In chronic lesions, rinsing lotion containing zinc-talk applied twice daily may be beneficial. Also, night-time application of antifungal/corticosteroid combinations may be recommended.58 For itchy and painful lesions, an antifungal agent combined with corticosteroids (mostly hydrocortisone) may also be added to the treatment. In cases with local hyperhidrosis, anti-perspiration agents such as 20% aluminum chloride can be used in the long term. If maceration or moisture is present, astringent and antiperspirant solutions may be applied following antifungal creams.
In extensive, severe, and resistant intertrigo, systemic anti-fungal treatment is required. Oral fluconazole at a dose of 50–100 mg/day or itraconazole at a dose of 200 mg/day may be recommended for a total duration of 2–6 weeks until symptoms resolve. For pediatric cases, the recommended fluconazole and itraconazole doses are 6 mg/kg/day and 5–10 mg/kg/day, respectively.84
Diaper candidiasis
Diaper candidiasis can be generally managed with topical antifungal agents. Nystatin ointment or powder is commonly used, with a clinical cure rate of approximately 85%.90 Treatment with other azoles such as clotrimazole and miconazole may also give successful results. Despite similar mycological cure rates, miconazole is more effective than nystatin for symptomatic relief.79,80
For concomitant bacterial infections or irritation, combination of 1% hydrocortisone with antimicrobial agents such as sodium fusidate or clioquinole may be used. If recurrent diaper candidiasis is related to oral and intestinal colonization, addition of oral nystatin suspension may elicit a clinical response.51
Angular cheilitis (perleche)
Angular cheilitis (perleche), when secondary to a Candida infection of the oral mucosa, should be brushed regularly, together with twice daily administration of an antiseptic oral rinse solution such as chlorhexidine gluconate (0.12%, suspension) or Gentian violet 0.5% solution.59,104 Patients with xerostomia should be encouraged to increase water consumption, and sugar-free lozenges should be advised to increase salivation.59
Interdigital candidiasis (EIB and toe web candidiasis)
Special applicators may be recommended for drying the inter-toe spaces in interdigital candidiasis (EIB and toe web candidiasis).9 Also, triggering factors should be avoided. For treatment, topical antifungal agents (azole antifungals) are generally adequate. Good outcomes have been reported with filtering paper adsorbed with Castellani stain.51,105 In recurrent or resistant cases, systemic itraconazole, terbinafine, or amorolfine may be used.
Correction of predisposing factors
Obese patients should be encouraged to lose weight, and diabetes should be under good control.7,81 Patients with large and sagging breasts may benefit from breast reduction surgery.2,106 For excessive sweating between the breasts, sweat-absorbing towels may be utilized. If present, predisposing factors (malocclusion, teeth loss, etc.) should be corrected in patients with angular cheilitis. For anatomical problems, the depth of skin folds may be reduced by injection of cosmetic filling material.9 Topical or systemic administration of corticosteroids may also lead to chronic or recurrent candidiasis via immune suppression.47,62,78 Wide spectrum antibiotics may also lead to Candida colonization and pathogenicity by disrupting the saprophytic flora of the skin and mucosal membranes. A detailed history of medication should be obtained to avoid unnecessary use of antibiotics and corticosteroids.47,50,62,64,107 If high doses are involved, oral contraceptives with lower estrogen content should be preferred. For the recurrent intertrigo of the perianal area and its surroundings due to intestinal colonization, nystatin may be given.32 Nutritional deficiencies such as iron and B2 deficiency may facilitate mucocutaneous candidiasis.58,59,53 Patients wearing rings should be recommended to keep the skin under the ring dry and clean. Good aeration with open shoes may be recommended for toe web intertrigo. In cases with chronic incontinence, regular and absorbing hygienic products should be utilized for skin care.
Prognosis
Candidal intertrigo has a good prognosis in healthy immunocompetent individuals with no co-morbidites, and complete resolution of symptoms may be achieved with correct diagnosis and appropriate topical treatment. Ideally, in all cases with intertriginous candidiasis, all predisposing and provoking factors should be totally eliminated; if that is not possible, then these factors may be reduced. In more severe and recurrent cases of vaginal, oral, or chronic mucocutaneous candidiasis, systemic antifungals generally yield good results.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vakharia P. Intertrigo. 2017. [Accessed August 1, 2017]. http://emedicine.medscape.com/article/1087691.
- 2.Janniger CK, Schwartz RA, Szepietowski JC, Reich A. Intertrigo and common secondary skin infections. Am Fam Physician. 2005;72(5):833–838. [PubMed] [Google Scholar]
- 3.Wolf R, Oumeish OY, Parish LC. Intertriginous eruption. Clin Dermatol. 2011;29(2):173–179. doi: 10.1016/j.clindermatol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Beurey J, Weber M, Percebois G. Etude clinique et mycologique des intertrigos des pieds [Clinical and mycologic study of intertrigo of the feet] Phlebologie. 1969;22(1):73–79. French. [PubMed] [Google Scholar]
- 5.Coldiron BM, Manders SM. Persistent Candida intertrigo treated with fluconazole. Arch Dermatol. 1991;127(2):165–166. [PubMed] [Google Scholar]
- 6.de Andrade MF, Nishinari K, Puech-Leão P. Intertrigo em pacientes com linfedema de membro inferior. Correlacao clinico-laboratorial [Intertrigo in patients with lower limb lymphedema. Clinical and laboratory correlation] Rev Hosp Clin Fac Med Sao Paulo. 1998;53(1):3–5. Portuguese. [PubMed] [Google Scholar]
- 7.Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89(7):569–573. [PubMed] [Google Scholar]
- 8.Katoh T. Guidelines for diagnosis and treatment of mucocutaneous candidiasis. Nihon Ishinkin Gakkai zasshi [Jap J Med Mycol.] 2009;50(4):207–212. doi: 10.3314/jjmm.50.207. Japanese. [DOI] [PubMed] [Google Scholar]
- 9.Metin A, Dilek N, Demirseren DD. Fungal infections of the folds (intertriginous areas) Clin Dermatol. 2015;33(4):437–447. doi: 10.1016/j.clindermatol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Tüzün Y, Wolf R, Bağlam S, Engin B. Diaper (napkin) dermatitis: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33(4):477–482. doi: 10.1016/j.clindermatol.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Veraldi S. Rapid relief of intertrigo-associated pruritus due to Candida albicans with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(Suppl 1):41–43. doi: 10.1111/myc.12058. [DOI] [PubMed] [Google Scholar]
- 12.Bahmaei M, Dehghan P, Kachuei R, Babaei H, Mohammadi R. Interdigital intertrigo due to Fusarium oxysporum. Curr Med Mycol. 2016;2(1):43–46. doi: 10.18869/acadpub.cmm.2.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bissan AT, Iken M, Doumbia M, Ou-Khedda N, El Alaoui M, Lmimouni B. Fusarioses superficielles a Fusarium solani chez un immunocompetent et un immunodeprime diagnostiquees a l’Hopital militaire de Rabat [Fusarioses to Fusarium solani in an immunocompetent and immunocompromised diagnosed in military hospital of Rabat] J Mycol Med. 2017;27(3):382–386. doi: 10.1016/j.mycmed.2017.03.005. French. [DOI] [PubMed] [Google Scholar]
- 14.Diongue K, Ndiaye M, Badiane AS, et al. Intertrigo interorteils a Fusarium solani a Dakar [Tinea pedis due to Fusarium solani in Dakar] J Mycol Med. 2015;25(2):155–158. doi: 10.1016/j.mycmed.2015.02.044. French. [DOI] [PubMed] [Google Scholar]
- 15.Diongue K, Ndiaye M, Diallo MA, et al. Fungal interdigital tinea pedis in Dakar (Senegal) J Mycol Med. 2016;26(4):312–316. doi: 10.1016/j.mycmed.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Néji S, Trabelsi H, Cheikhrouhou F, et al. Fusarioses diagnostiquees au laboratoire d’un CHU en Tunisie: etude epidemiologique, clinique et mycologique [Fusariosis diagnosed in the laboratory of an UH in Tunisia: epidemiological, clinical and mycological study] J Mycol Med. 2013;23(2):130–135. doi: 10.1016/j.mycmed.2013.04.003. French.aw. [DOI] [PubMed] [Google Scholar]
- 17.Romano C, Presenti L, Massai L. Interdigital intertrigo of the feet due to therapy-resistant Fusarium solani. Dermatology. 1999;199(2):177–179. doi: 10.1159/000018233. [DOI] [PubMed] [Google Scholar]
- 18.Varon AG, Nouer SA, Barreiros G, et al. Superficial skin lesions positive for Fusarium are associated with subsequent development of invasive fusariosis. J Infect. 2014;68(1):85–89. doi: 10.1016/j.jinf.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Bazin JC, Hutinel B. Intertrigos mycosiques et lymphangites [Fungal intertrigo and lymphangitis (author’s transl)] J Mal Vasc. 1980;5(2):107–108. French. [PubMed] [Google Scholar]
- 20.Karaca S, Kulac M, Cetinkaya Z, Demirel R. Etiology of foot intertrigo in the District of Afyonkarahisar, Turkey: a bacteriologic and mycologic study. J Am Podiatr Med Assoc. 2008;98(1):42–44. [PubMed] [Google Scholar]
- 21.de González MI, Mendoza M, Bastardo de Albornoz M, Apitz-Castro R. Efectos del ajoeno sobre dermatofitos, Candida albicans y Malassezia furfur [Activity of ajoene on dermatophytes, Candida albicans and Malassezia furfur.] Rev Iberoam Micol. 1998;15(4):277–281. Spanish. [PubMed] [Google Scholar]
- 22.Katoh T, Kagawa S, Ishimoto M. Malassezia intertrigo, a new clinical entity. Mycoses. 1988;31(11):558–562. doi: 10.1111/j.1439-0507.1988.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 23.Gorani A, Oriani A, Klein EF, Veraldi S. Case report. Erythrasmoid pityriasis versicolor. Mycoses. 2001;44(11–12):516–517. doi: 10.1046/j.1439-0507.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- 24.Beaulieu P, Le Guyadec T, Ponties-Leroux B, Boutchnei S, Grossetete G, Millet P. Cas pour diagnostic: intertrigo a Pseudomonas aeruginoasa [A case for diagnosis: Pseudomonas aeruginosa intertrigo] Ann Dermatol Venereol. 1992;119(3):223–225. French. [PubMed] [Google Scholar]
- 25.Block SL. Tricky triggers of intertrigo. Pediatr Ann. 2014;43(5):171–176. doi: 10.3928/00904481-20140417-04. [DOI] [PubMed] [Google Scholar]
- 26.Dekio I, Matsuki S, Morita E. High carriage rate of Staphylococcus aureus and Streptococcus agalactiae in nine cases of fungus-free intertrigo of the toe cleft. Int J Dermatol. 2014;53(4):484–486. doi: 10.1111/j.1365-4632.2012.05537.x. [DOI] [PubMed] [Google Scholar]
- 27.Adisen E, Onder M. Viral infections of the folds (intertriginous areas) Clin Dermatol. 2015;33(4):429–436. doi: 10.1016/j.clindermatol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay D, Ghosh SK. Mucocutaneous features of Chikungunya fever: a study from an outbreak in West Bengal, India. Int J Dermatol. 2008;47(11):1148–1152. doi: 10.1111/j.1365-4632.2008.03817.x. [DOI] [PubMed] [Google Scholar]
- 29.Calikoglu E, Soravia-Dunand VA, Perriard J, Saurat JH, Borradori L. Acute genitocrural intertrigo: a sign of primary human immunodeficiency virus type 1 infection. Dermatology. 2001;203(2):171–173. doi: 10.1159/000051736. [DOI] [PubMed] [Google Scholar]
- 30.Yell JA, Sinclair R, Mann S, Fleming K, Ryan TJ. Human papillomavirus type 6-induced condylomata: an unusual complication of intertrigo. Br J Dermatol. 1993;128(5):575–577. doi: 10.1111/j.1365-2133.1993.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 31.López-Martinez R. Candidosis, a new challenge. Clin Dermatol. 2010;28(2):178–184. doi: 10.1016/j.clindermatol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;26(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller MA, Andes DR, Diekema DJ, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) Registry 2004–2008. PLoS ONE. 2014;9(7):e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan DJ, Westerneng TJ, Haynes KA, Bennett DE, Coleman DC. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141(Pt 7):1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 35.Kashem SW, Kaplan DH. Skin immunity to Candida albicans. Trends Immunol. 2016;37(7):440–450. doi: 10.1016/j.it.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kühbacher A, Burger-Kentischer A, Rupp S. Interaction of Candida species with the skin. Microorganisms. 2017;5(2):32. doi: 10.3390/microorganisms5020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2014;4(2):119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naglik JR, Moyes DL, Wachtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011;13(12–13):963–976. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Höfs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol. 2016;54(3):149–169. doi: 10.1007/s12275-016-5514-0. [DOI] [PubMed] [Google Scholar]
- 40.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Y, Zhang L, Xu Z, et al. Innate immune cell response upon Candida albicans infection. Virulence. 2016;7(5):512–526. doi: 10.1080/21505594.2016.1138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyes DL, Runglall M, Murciano C, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8(3):225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaller M, Mailhammer R, Grassl G, Sander CA, Hube B, Korting HC. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118(4):652–657. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 44.Villar CC, Kashleva H, Mitchell AP, Dongari-Bagtzoglou A. Invasive phenotype of Candida albicans affects the host proinflammatory response to infection. Infect Immun. 2005;73(8):4588–4595. doi: 10.1128/IAI.73.8.4588-4595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weindl G, Naglik JR, Kaesler S, et al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J Clin Invest. 2007;117(12):3664–3672. doi: 10.1172/JCI28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, van de Veerdonk FL, Netea MG. Basic genetics and immunology of Candida infections. Infect Dis Clin North Am. 2016;30(1):85–102. doi: 10.1016/j.idc.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Guidelines of care for superficial mycotic infections of the skin: mucocutaneous candidiasis. Guidelines/Outcome Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34(1):110–115. doi: 10.1016/s0190-9622(96)90842-7. [DOI] [PubMed] [Google Scholar]
- 48.De Britto LJ, Yuvaraj J, Kamaraj P, Poopathy S, Vijayalakshmi G. Risk factors for chronic intertrigo of the lymphedema leg in southern India: a case-control study. Int J Low Extrem Wounds. 2015;14(4):377–383. doi: 10.1177/1534734615604289. [DOI] [PubMed] [Google Scholar]
- 49.Hay RJ, Ashbee HR. Fungal infections. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s Textbook of Dermatology. 9th. Chichester, West Sussex: John Wiley & Sons Inc; 2016. pp. 32.56–32.70. [Google Scholar]
- 50.Hessen MT, Walsh SR, Ferri FF. Mucocutaneous candidiasis. 2010. [Accessed August 8, 2017]. https://www.clinicalkey.com/-!/content/medical_topic/21-s2.0-1014515.
- 51.James WD, Andrews GC, Berger TG, Elston DM. Diseases resulting from fungi and yeasts. In: James WD, Berger T, Elston D, editors. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th. Philadelphia, PA: Saunders Elsevier; 2016. pp. 285–318. [Google Scholar]
- 52.Lisboa C, Santos A, Dias C, Azevedo F, Pina-Vaz C, Rodrigues A. Candida balanitis: risk factors. J Eur Acad Dermatol Venereol. 2010;24(7):820–826. doi: 10.1111/j.1468-3083.2009.03533.x. [DOI] [PubMed] [Google Scholar]
- 53.Lu SY. Perception of iron deficiency from oral mucosa alterations that show a high prevalence of Candida infection. J Formos Med Assoc. 2016;115(8):619–627. doi: 10.1016/j.jfma.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 54.Mamatha KV, Shubha U, Jain CM. Clinical evaluation of the efficacy of Khadiradi yoga avachoornana in Kachchu with special reference to genitoinguinal intertrigo. Ayu. 2010;31(4):461–465. doi: 10.4103/0974-8520.82043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ndiaye M, Taleb M, Diatta BA, et al. Etiology of intertrigo in adults: a prospective study of 103 cases. J Mycol Med. 2017;27(1):28–32. doi: 10.1016/j.mycmed.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Rockwell PG. Acute and chronic paronychia. Am Fam Physician. 2001;63(6):1113–1116. [PubMed] [Google Scholar]
- 57.Ryan KJ, Ray CG. Candida, Aspergillus, Pneumocystis, and other opportunistic fungi. In: Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. 6th. New York: McGraw-Hill Education Medical; 2014. pp. 729–743. [Google Scholar]
- 58.Scheinfeld NS. Cutaneous candidiasis. 2017. [Accessed August 8, 2017]. http://emedicine.medscape.com/article/1090632-overview.
- 59.Sharon V, Fazel N. Oral candidiasis and angular cheilitis. Dermatol Ther. 2010;23(3):230–242. doi: 10.1111/j.1529-8019.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- 60.Silverman RA, Schwartz RH. Streptococcal intertrigo of the cervical folds in a five-month-old infant. Pediatr Infect Dis J. 2012;31(8):872–873. doi: 10.1097/INF.0b013e31825ba674. [DOI] [PubMed] [Google Scholar]
- 61.Sparber F, LeibundGut-Landmann S. Interleukin 17-mediated host defense against Candida albicans. Pathogens. 2015;4(3):606–619. doi: 10.3390/pathogens4030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asiedu DK. Candidiasis, cutaneous. In: Ferri FF, editor. Ferri’s Clinical Advisor 2018: 5 Books in 1. Amsterdam: Elsevier Science Health Science; 2017. pp. 234–235.e231. [Google Scholar]
- 63.Habif TP. Superficial fungal infections. In: Habif TP, editor. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th. Philadelphia, PA: Saunders; 2016. pp. 487–533. [Google Scholar]
- 64.Hay RJ. Fungal infections of the skin. In: Olafsson JH, Hay RJ, editors. Antibiotic and Antifungal Therapies in Dermatology. Switzerland: Springer; 2016. pp. 157–186. [Google Scholar]
- 65.Kauffman CA. Candidiasis. In: Goldman L, Schafer AI, editors. Goldman-Cecil Medicine. 25th. Philadelphia, PA: Elsevier/Saunders; 2016. pp. 2079–2083.e2072. [Google Scholar]
- 66.Mistiaen P, van Halm-Walters M. Prevention and treatment of intertrigo in large skin folds of adults: a systematic review. BMC Nurs. 2010;9:12. doi: 10.1186/1472-6955-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilmer EN, Hatch RL. Resistant “candidal intertrigo”: could inverse psoriasis be the true culprit? J Am Board Fam Med. 2013;26(2):211–214. doi: 10.3122/jabfm.2013.02.120210. [DOI] [PubMed] [Google Scholar]
- 68.Valenti L. Topical treatment of intertriginous candidal infection. Mycoses. 2008;51(Suppl 4):44–45. doi: 10.1111/j.1439-0507.2008.01617.x. [DOI] [PubMed] [Google Scholar]
- 69.Terzieva K, Elsner P. A case of intertrigo resistant to treatment – what is your diagnosis? J Dtsch Dermatol Ges. 2015;13(2):169–171. doi: 10.1111/ddg.12434. [DOI] [PubMed] [Google Scholar]
- 70.Smith SM, Milam PB, Fabbro SK, Gru AA, Kaffenberger BH. Malignant intertrigo: a subset of toxic erythema of chemotherapy requiring recognition. JAAD Case Rep. 2016;2(6):476–481. doi: 10.1016/j.jdcr.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santiago-et-Sanchez-Mateos JL, Beà S, Fernández M, Pérez B, Harto A, Jaén P. Botulinum toxin type A for the preventive treatment of intertrigo in a patient with Darier’s disease and inguinal hyperhidrosis. Dermatol Surg. 2008;34(12):1733–1737. doi: 10.1111/j.1524-4725.2008.34361.x. [DOI] [PubMed] [Google Scholar]
- 72.Nazzaro G, Vaira F, Coggi A, Gianotti R. A 42-year-old woman with a submammary intertrigo. Int J Dermatol. 2013;52(9):1035–1036. doi: 10.1111/ijd.12079. [DOI] [PubMed] [Google Scholar]
- 73.López-Corominas V, Yagüe F, Knöpfel N, et al. Streptococcus pyogenes cervical intertrigo with secondary bacteremia. Pediatr Dermatol. 2014;31(2):e71–e72. doi: 10.1111/pde.12256. [DOI] [PubMed] [Google Scholar]
- 74.Imam TH, Cassarino D, Patail H, Khan N. Refractory intertrigo in the right inguinal crease: challenge. Am J Dermatopathol. 2017;39(8):629. doi: 10.1097/DAD.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 75.Dowd ML, Ansell LH, Husain S, Grossman ME. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2(2):132–134. doi: 10.1016/j.jdcr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53(2):e20–e22. doi: 10.1111/j.1440-0960.2010.00721.x. [DOI] [PubMed] [Google Scholar]
- 77.Butragueño Laiseca L, Toledo Del Castillo B, Marañón Pardillo R. Cervical intertrigo: think beyond fungi. Rev Chil Pediatr. 2016;87(4):293–294. doi: 10.1016/j.rchipe.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Kirkpatrick CH. Chronic mucocutaneous candidiasis. Pediatr Infect Dis J. 2001;20(2):197–206. doi: 10.1097/00006454-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 79.Hoeger PH, Stark S, Jost G. Efficacy and safety of two different antifungal pastes in infants with diaper dermatitis: a randomized, controlled study. J Eur Acad Dermatol Venereol. 2010;24(9):1094–1098. doi: 10.1111/j.1468-3083.2010.03735.x. [DOI] [PubMed] [Google Scholar]
- 80.Blanco D, van Rossem K. A prospective two-year assessment of miconazole resistance in Candida spp with repeated treatment with 025% miconazole nitrate ointment in neonates and infants with moderate to severe diaper dermatitis complicated by cutaneous candidiasis. Pediatr Dermatol. 2013;30(6):717–724. doi: 10.1111/pde.12107. [DOI] [PubMed] [Google Scholar]
- 81.Chiriac A, Chiriac AE, Pinteala T, Foia L, Brzezinski P. Erosio blastomycetica interdigitale sign of Candidiasis and diabetes! Bangladesh J Med Sci. 2014;13(1):105–106. [Google Scholar]
- 82.Adams SP. Dermacase. Erosio interdigitalis blastomycetica. Can Fam Physician. 2002;48:271–277. [PMC free article] [PubMed] [Google Scholar]
- 83.Gjessing HC. Intertrigo; saerlig omtale av perianal, (retro) aurikulaer og periokulaer intertrigo [Intertrigo, with special consideration on perianal, retroauricular and periocular intertrigo] Tidsskr Nor Laegeforen. 1953;73(12):488–490. Norwegian. [PubMed] [Google Scholar]
- 84.Parker ER, Dellavalle RP, Rosen T, Ofori AO. Candidal intertrigo. 2013. [Accessed August 12, 2017]. last updated Jul 2017; https://www.uptodate.com/contents/candidal-intertrigo/
- 85.Tulipan L. Intertrigo (chafing) treated with tannic acid and brilliant green. J Am Med Ass. 1941;116(14):1518–1519. [Google Scholar]
- 86.Dogan B, Karabudak O. Treatment of candidal intertrigo with a topical combination of isoconazole nitrate and diflucortolone valerate. Mycoses. 2008;51(Suppl 4):42–43. doi: 10.1111/j.1439-0507.2008.01605.x. [DOI] [PubMed] [Google Scholar]
- 87.Janik MP, Heffernan MP. Yeast infections: candidiasis and tinea (pityriasis) versicolor. In: Wolf K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7th. New York: McGraw-Hill Medical; 2008. pp. 1822–1830. [Google Scholar]
- 88.Verma S, Heffernan M. Fungal infections. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Leffell D, editors. Fitzpatrick’s Dermatology in General Medicine. 7th. New York: McGraw-Hill Medical; 2008. pp. 1807–1821. [Google Scholar]
- 89.Elewski BE, Hughey LC, Sobera JO, Hay R. Fungal diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, editors. Dermatology. 3rd. [Edinburgh]: Elsevier/Saunders; 2012. pp. 1251–1284. [Google Scholar]
- 90.Rowen JL. Mucocutaneous candidiasis. Semin Perinatol. 2003;27(5):406–413. doi: 10.1016/s0146-0005(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 91.Rashid A. Arthroconidia as vectors of dermatophytosis. Cutis. 2001;67(5 Suppl):23–23. [PubMed] [Google Scholar]
- 92.Pryzbilla B, Rueff F. Contact dermatitis. In: Burgdorf WHC, Braun-Falco O, editors. Braun-Falco’s Dermatology. 3rd. Heidelberg: Springer; 2009. pp. 491–540. [Google Scholar]
- 93.Reiss E, Shadomy HJ, Lyon GM. Fundamental Medical Mycology. Hoboken, NJ: John Wiley & Sons; 2012. pp. 250–301. [Google Scholar]
- 94.Loo DS. Cutaneous fungal infections in the elderly. Dermatol Clin. 2004;22(1):33–50. doi: 10.1016/s0733-8635(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 95.Draijer LW, Folmer H. NHG-Farmacotherapeutische richtlijn Intertrigo. Huisarts en Wetenschap. 2007;50(1):33–35. [Google Scholar]
- 96.Bazex J. Intertrigo. Diagnostic orientation. Rev Prat. 1992;42(13):1689–1692. [PubMed] [Google Scholar]
- 97.Benkalfate L, Zein K, le Gall F, Chevrant-Breton J, Rivalan J, le Pogamp P. Calcified intertrigo, a rare cause of cutaneous calcinosis. Ann Dermatol Venereol. 1995;122(11–12):789–792. [PubMed] [Google Scholar]
- 98.Carleton A. A case of pseudomembiunous intertrigo. Br J Dermatol. 1943;55(6):154–158. [Google Scholar]
- 99.Collier C. Within the fold: treatments of intertrigo. JAAD. 2007;56(2):AB126. [Google Scholar]
- 100.Honig PJ, Frieden IJ, Kim HJ, Yan AC. Streptococcal intertrigo: an underrecognized condition in children. Pediatrics. 2003;112(6 Pt 1):1427–1429. doi: 10.1542/peds.112.6.1427. [DOI] [PubMed] [Google Scholar]
- 101.Ke CL, Chen CC, Lin CT, Chen GS, Chai CY, Cheng ST. Fluvoxamine-induced bullous eruption mimicking hand-foot syndrome and intertrigo-like eruption: rare cutaneous presentations and elusive pathogenesis. J Am Acad Dermatol. 2006;55(2):355–356. doi: 10.1016/j.jaad.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5(9):901–902. [PubMed] [Google Scholar]
- 103.Plaza AI, Sancho MI, Millet PU, et al. Erythematous, vesicular, and circinate lesions in a 78-year-old female – benign familial pemphigus. An Bras Dermatol. 2017;92(3):439–440. doi: 10.1590/abd1806-4841.20176711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Millsop JW, Fazel N. Oral candidiasis. Clin Dermatol. 2016;34(4):487–494. doi: 10.1016/j.clindermatol.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 105.Sundaram SV, Srinivas CR, Thirumurthy M. Candidal intertrigo: treatment with filter paper soaked in Castellani’s paint. Indian J Dermatol Venereol Leprol. 2006;72(5):386–387. doi: 10.4103/0378-6323.27763. [DOI] [PubMed] [Google Scholar]
- 106.Chadbourne EB, Zhang S, Gordon MJ, et al. Clinical outcomes in reduction mammaplasty: a systematic review and meta-analysis of published studies. Mayo Clin Proc. 2001;76(5):503–510. doi: 10.4065/76.5.503. [DOI] [PubMed] [Google Scholar]
- 107.Ding X, Yan D, Sun W, Zeng Z, Su R, Su J. Epidemiology and risk factors for nosocomial non-Candida albicans candidemia in adult patients at a tertiary care hospital in North China. Med Mycol. 2015;53(7):684–690. doi: 10.1093/mmy/myv060. [DOI] [PubMed] [Google Scholar]