Abstract
Purpose
Anastomotic leakage is a major surgical complication following esophagectomy and gastric pull-up. Specific risk factors such as celiac trunk (TC) stenosis and high calcification score of the aorta have been identified, but no data are available on their relative prognostic values. This retrospective study aimed to compare and evaluate calcification score versus stenosis quantification with regards to prognostic impact on anastomotic leakage.
Patients and methods
Preoperative contrast-enhanced computed tomography scans of 164 consecutive patients with primary esophageal cancer were evaluated by two radiologists to apply a calcification score (0–3 scale) assessing the aorta, the celiac axis and the right and left postceliac arteries. Concurrently, the presence and degree of stenosis of TC and superior mesenteric artery were recorded for stenosis quantification.
Results
Anastomotic leakage was noted in 14/164 patients and 12/14 showed stenosis of TC (n=11). The presence of TC stenosis was found to have a significant impact on anastomotic healing (p=0.004). The odds ratio for the prediction of anastomotic leakage by the degree of stenosis was 1.04 (95% CI, 1.02–1.07). Ten of 14 patients had aortic calcification scores of 1 or 2, but calcification scores of the aorta, the celiac axis and the right and left postceliac arteries did not correlate with the corresponding TC stenosis values and showed no influence on patient outcome as defined by the occurrence of anastomotic insufficiency (p=0.565, 0.855, 0.518 and 1.000, respectively). Inter-reader reliability of computed tomography analysis and absolute agreement on calcium scoring was mostly over 90%. No significant differences in preoperative comorbidities and patient characteristics were found between those with and without anastomotic leakage.
Conclusion
Measurement of TC stenosis in preoperative contrast-enhanced computed tomography scans proved to be more reliable than calcification scores in predicting anastomotic leakage and should, therefore, be used in the risk assessment of patients undergoing esophagectomy and gastric pull-up.
Keywords: TC stenosis, calcification score, anastomotic leakage, stenosis quantification, Ivor Lewis esophagectomy, graft perfusion
Plain language summary
Why was this study done? Surgical treatment for esophageal cancer is a complex procedure during which the esophagus is removed and then reconstructed using the stomach. Blood flow to certain areas of the stomach is compromised by the formation of a gastric tube as substitute for the esophagus, the pull-up and connection to the remaining esophageal stump. Over time, gastric tissue without sufficient blood supply can become necrotic, causing the reconstructed esophagus to leak. Risk factors for developing this major complication include a narrowing of certain blood vessels and deposits of calcium in these vessels, both of which can be detected on computed tomography scans taken before surgery. The goal of this study is to find out whether determination of blood vessel narrowing or measurement of calcium deposits is the more effective method in identifying risk factors for developing anastomotic leaks after surgery.
What did the researchers do and find? Our research found that while the measurement of calcium deposits in blood vessels is quick and easy to perform, more deposits did not necessarily lead to a higher risk for leakage. Narrow blood vessels, however, were highly accurate in predicting the occurrence of leaks.
What do these results mean? Our results suggest that routine screening for narrowing of blood vessels of the stomach before surgery could greatly help in predicting risk for postoperative leakage of the reconstructed esophagus.
Introduction
Esophagectomy and gastric pull-up combined with neo-adjuvant chemoradiation therapy are the gold standard for curative treatment of esophageal cancer in an operable stage of the disease.1–3 Despite major advances in surgical and perioperative management over the past decades, it is still accompanied by significant morbidity (40%–60%) and mortality rates (5%).4–6 One of the causes of relatively high perioperative morbidity is anastomotic leakage due to compromised tissue perfusion of the proximal gastric tube.5,7,8
Arteries supplying the stomach mainly arise from the celiac trunk (TC); only small additional branches may arise from the superior mesenteric artery (SMA).9,10 In the course of gastric tube preparation and mobilization, partial devascularization of the stomach is necessary, which leaves the right gastroepiploic and the right gastric arteries the only remaining arteries supplying the graft.8,9
This exclusive perfusion from the right gastroepiploic and the right gastric arteries may lead to impaired microvascularization of its boundary zone, which is the proximal gastric tube at the site of esophagogastric anastomosis. Although several different surgical techniques have been described in the past with the aim to achieve better or additional blood flow to the reconstructed site (eg, microvascular anastomosis, whole stomach replacement), relative ischemia of the proximal gastric tube remains a problem.4,11–14
Few attempts have been made to evaluate the vascular risk factors for gastric tube ischemia in patients undergoing esophagectomy and gastric pull-up. So far, no significant impact of TC or SMA stenosis on gastric tube circulation has been proven,15 but van Rossum et al recently discovered calcification of the aorta and the right postceliac arteries (comprising the common hepatic artery, the gastroduodenal artery and the right gastroepiploic artery) as a risk factor for anastomotic leakage after esophagogastrostomy.9
Additionally, computed tomography angiography (CTA) has been shown to be a very reliable tool for stenosis grading of the TC and the SMA.16 In this context, a cut-off at ≥50% stenosis has been reported to have a significant impact on patient outcome involving the mesenteric vessels,16 whereas stenosis ≥70% represents a significant prognostic factor in the outcome of carotid artery stenosis.17 Thus far, however, no clear cut-off has been established to identify patients at risk of developing anastomotic leakage after gastric pull-up.
The aim of this study is to compare the predictive value of a calcification scoring system as established by van Rossum et al versus CTA stenosis grading of TC and SMA with regards to the reliability, consistency and prognostic impact on anastomotic insufficiency after gastric pull-up.
Patients and methods
We conducted a retrospective, single-center study and collected data on consecutive patients who underwent esophagectomy and gastric pull-up in the Department of Abdominal Surgery of the University Hospital, Cologne in the period from January to December 2014.
Patients with primary esophageal cancer who underwent curative operative therapy and the availability of a preoperative contrast-enhanced computed tomography (CE-CT) scan of the abdomen with a section thickness of 1 mm were the inclusion criteria of the study. Patients who underwent esophagectomy and gastric pull-up due to other underlying pathology were excluded.
Diagnosis of anastomotic leakage was defined as anastomotic dehiscence confirmed during endoscopy or operation.
In addition, we documented preoperative comorbidities, mortality and histopathologic parameters in all patients with anastomotic insufficiency and 28 randomly selected patients who did not have anastomotic insufficiency from the medical records (ratio: 2 to 1). This study was approved by the institutional review board of the medical faculty of the University of Cologne (16-057), which waived the requirement for written informed consent because of the retrospective, observational nature of the study. All accessed patient data were de-identified.
Surgical technique
The surgical procedure consisted of a right-sided anterolateral thoracotomy with en bloc esophageal resection and extended two-field lymphadenectomy of mediastinal and abdominal nodes. Subsequently, gastric pull-up was performed by high intrathoracic esophagogastrostomy (Ivor Lewis esophagectomy) with a 25 or 28 mm circular stapler (DST Series™ EEA™ Stapler, Medtronic, Minneapolis, MN, USA). The complete technique of laparoscopic or open mobilization of the stomach and transthoracic esophagectomy with two-field lymphadenectomy is described in detail elsewhere.18 In all patients, specimens were removed en bloc and lymph nodes were dissected in accordance with a standardized protocol.
Image acquisition
At our institution, preoperative staging examinations were performed on a 64- or 128-slice computed tomography (CT) scanner (Brilliance iCT; Philips Healthcare, Cleveland, OH, USA) with 64×0.625 or 128×0.625 mm section collimation and a pitch factor of 0.64 or 0.49, respectively. All scans were performed with a tube voltage of 120 kV and an automatic mAs modulation. A bolus of 100 mL of contrast medium was injected intravenously at a rate of 3 or 4 mL/s, followed by a 30 mL saline solution chaser. Scans in the arterial phase (CTA) of the upper abdomen and in the venous phase of the thorax and abdomen were acquired with bolus tracking. A statistical iterative reconstruction algorithm (iDose, Philips Healthcare, Cleveland, OH, USA) with a soft tissue convolution kernel and window setting was used to reconstruct all images from the acquired raw data with the iteration level 4. All reconstructed images were archived in the hospital’s picture archive and communication system for further image analysis and documentation purposes. External scans (n=26) included in our study were contrast enhanced in the arterial phase with a slice thickness of 1 mm.
CT analysis
All preoperative CT scans in the arterial phase were independently evaluated by one consultant with 8 years of experience in gastrointestinal imaging and another resident with 3 years of experience.
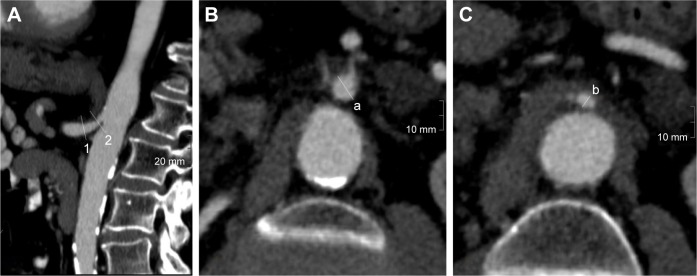
Both radiologists were blinded to the outcome of the patients as well as to each other. Stenoses of the TC and the SMA were evaluated separately using multiplanar reconstructions. The diameters of the vessels were measured in the normal (a) and narrowest (b) lumen. Percent stenosis (s) was calculated using the North American Symptomatic Carotid Endarterectomy Trial formula (Figure 1) as follows:19
Figure 1.
Evaluation of vessel diameter in CE-CT.
Notes: (A) Parasagittal curved MPR of a proximal TC stenosis with location of the orthogonal cuts at the normal (1) and maximally stenosized (2) lumen. Orthogonal planes where the measurements are performed are presented in (B and C). Degree of stenosis (s) in (%) is calculated using the formula s = (a−b)/a × 100.
Abbreviations: CE-CT, contrast enhanced-computed tomography; MPR, multiplanar reconstruction; TC, celiac trunk.
After the evaluation of TC and SMA stenosis, each preoperative CT scan was additionally assessed for calcification by the same readers. Therefore the aorta, the TC and the left and right postceliac axis were analyzed according to the scoring system developed by van Rossum et al9 (Table 1). Individual adjustments of window settings were performed by the readers if required.
Table 1.
Definitions used to grade calcifications of the supplying arteries of the gastric tube seen on preoperative CT images by van Rossum et al9
| Artery | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Aortaa | Absent | Minor calcifications: <9 foci and ≤3 foci extending over ≥3 sections | Major calcifications: >9 foci or >3 foci extending over ≥3 sections |
| Celiac axis | Absent | Minor calcifications: extending over <3 sections or MCSD of single focus ≤10 mm | Major calcifications: extending over ≥3 sections and MCSD of single focus >10 mm or involving both the proximal (aortoceliac) and distal (hepatosplenic bifurcation) parts |
| Right postceliac arteriesb | Absent | ≥1 calcifications | Not applicable |
| Left postceliac arteriesc | Absent | ≥1 calcifications | Not applicable |
Notes:
Aorta defined as descending part of thoracic aorta and abdominal part of aorta above celiac level.
Right postceliac arteries defined as common hepatic artery, gastroduodenal artery and right gastroepiploic artery.
Left postceliac arteries defined as splenic artery and left gastroepiploic artery. Adapted with permission from van Rossum PS, Haverkamp L, Verkooijen HM, et al. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 2015;274(1):124–132.9
Abbreviations: CT, computed tomography; MCSD, maximum cross-sectional diameter.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 23. Fisher’s exact test was used to evaluate qualitative data, whereas Mann–Whitney U test was used for quantitative data. Differences between patients with and without anastomotic leak were evaluated using χ2 tests for categorical variables and Student’s t-tests for independent groups. A p-value <0.05 was considered to be significant. Univariate logistic regression was used to calculate the odds ratio for the predictive value of the degree of stenosis, describing the alteration of the odds to get an anastomotic leakage per increase of 1% stenosis. Interobserver reliability was assessed by absolute agreement. The presented data are the results of an experienced radiologist with 8 years of experience in gastrointestinal imaging.
Results
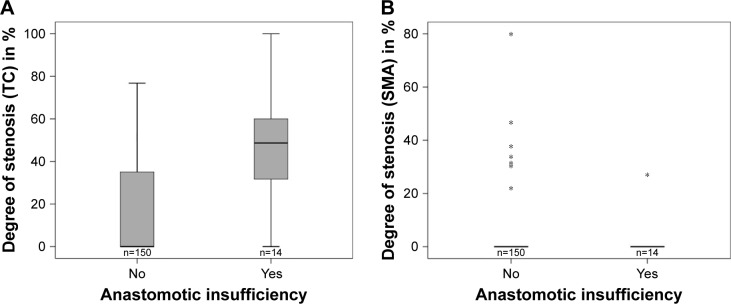
Out of the 164 patients (female: n=42, mean age 61 years, range 38–84 years; men: n=122, mean age 62 years, range 30–86 years) who met the inclusion criteria, 14 developed anastomotic leakage (8.5%). Twelve of the 14 patients with anastomotic insufficiency showed a stenosis of TC (n=11) or SMA (n=1) in the preoperative CT scan. Among the patients without anastomotic leakage, 58 showed stenosis in TC (n=56) and/or SMA (n=9). The mean degree of stenosis in patients with anastomotic leakage was 44.4% (range 0%–100%, SD 29.5%) for TC and 27.0% (n=1) for isolated SMA stenosis. In patients with proper anastomotic healing, the mean degree of stenosis was 15.9% (0%–76.8%, SD 22.6%) in TC and 39.9% (22.0%–80.0%, SD 17.0%) in SMA (Figure 2).
Figure 2.
Degree of (A) TC and (B) SMA stenosis in patients with and without anastomotic insufficiency after esophagectomy and gastric pull-up.
Note: *Outliers with a distance from the box of more than 3xIQR.
Abbreviations: SMA, superior mesenteric artery; TC, celiac trunk.
Ten of the 14 patients who developed anastomotic insufficiency had an aortic calcification score of 1 or 2. For anastomotic leakage and calcification score of 1 or 2, the corresponding numbers were n=6 for the celiac axis, n=1 for the right post-celiac artery and n=5 for the left postceliac artery. Among the patients without anastomotic leakage, 86 had an aortic and 61 had TC calcification scores of 1 or higher. Score 1 calcification of the right postceliac artery was found in the scans of 7 patients and of the left postceliac artery in 52 patients. Results of the corresponding calcification scores are presented in Table 2.
Table 2.
Frequency of calcification score of patients with/without AI
| Score | Aorta
|
Celiac trunk
|
Right postceliac axis
|
Left postceliac axis
|
||||
|---|---|---|---|---|---|---|---|---|
| AI
|
AI
|
AI
|
AI
|
|||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| 0 | 4 (28.6) | 64 (42.7) | 8 (57.1) | 89 (59.3) | 13 (92.9) | 143 (95.3) | 9 (64.3) | 97 (65.1) |
| 1 | 4 (28.6) | 38 (25.3) | 3 (21.4) | 37 (24.7) | 1 (7.1) | 7 (4.7) | 5 (35.7) | 52 (34.9) |
| 2 | 6 (42.9) | 48 (32.0) | 3 (21.4) | 24 (16.0) | – | – | – | – |
Note: Data are n (%).
Abbreviation: AI, anastomotic insufficiency.
The presence of TC stenosis had a significant impact on anastomotic healing (p=0.004), whereas no correlation between stenosis of the SMA and anastomotic leakage could be shown (p=0.601). The degree of TC stenosis was significantly higher when anastomotic leakage was present (p<0.001). The odds ratio for the prediction of anastomotic leakage by the degree of stenosis was 1.04 (95% CI, 1.02–1.07). Although the influence was significant (p<0.001) in logistic regression, it was not possible to define a reliable cut-off to preoperatively predict anastomotic insufficiency.
Calcifications of the aorta, the celiac axis and the right and left postceliac arteries showed no influence on patient outcome as defined by the occurrence of anastomotic insufficiency (p=0.565, 0.855, 0.518 and 1.000, respectively).
Inter-reader reliability for the CT analysis was mostly over 90%. Absolute agreement on calcium scoring between the two raters was 90.8% (aorta), 95.7% (celiac axis), 94.5% (right postceliac axis), 96.9% (left postceliac axis), 79.8% (TC stenosis), 89.0% (SMA stenosis) and 99.3% (high-grade vascular stenosis of ≥70%).
No significant difference in tumor entity and preoperative comorbidities was found in univariable analysis in the two groups with and without anastomotic insufficiency (Table 3).
Table 3.
Histopathologic results and preoperative comorbidities in association with anastomotic leak
| Common comorbidities | Total (N=42) | Without anastomotic leak (n=28) | With anastomotic leak (n=14) | p-value |
|---|---|---|---|---|
| Tumor entity | ||||
| Adenocarcinoma | 29 | 21 (72.4) | 8 (27.6) | 0.238a |
| Squamous cell carcinoma | 13 | 7 (53.8) | 6 (46.2) | |
| BMI, in kg/m2 (mean, SD) | 26 (4.8) | 26 (4.8) | 26 (4.8) | 1.000b |
| BMI ≥25 | 26 (61.9) | 16 (57.1) | 10 (71.4) | 0.369a |
| Heart failure | 8 (19.0) | 5 (17.9) | 3 (21.4) | 0.781a |
| Diabetes mellitus | 6 (14.3) | 2 (7.1) | 4 (28.6) | 0.061a |
| Smoker | 24 (57.1) | 17 (60.7) | 7 (50.0) | 0.508a |
| Peripheral vascular disease | 2 (4.8) | 1 (3.6) | 1 (7.1) | 1.000a |
Notes: Values in parentheses are percentages unless indicated otherwise.
χ2 test,
Student’s t-test.
Abbreviation: BMI, body mass index.
In-hospital mortality was 2.4%. Two patients with anastomotic leak died following sepsis 27 and 26 days after surgery, respectively. One patient in the group without anastomotic insufficiency (n=150) died because of luminal bleeding 45 days after esophagectomy. A second patient developed sepsis of unknown cause with rapidly progressive multiorgan failure 13 days after esophagectomy.
Discussion
Anastomotic leakage is the main cause of postoperative morbidity and mortality in patients undergoing esophagectomy and gastric pull-up. Identification of prognostic factors to predict leakage at the anastomotic site is essential for foreseeing and minimizing complications, which ultimately improves outcome in patients with curable esophageal cancer. Gastric pull-up following esophagectomy is associated with considerable impairment of microcirculation at the anastomotic site in the gastric fundus. This is due to the gastric devascularization with dissection of the left gastric artery, the gastric tube formation with disturbance of the intramural capillary network, as well as the gastric pull-up. Since the gastric tube is mainly, if not exclusively, perfused by the gastroepiploic artery, the cranial part of the tube graft is at high risk of suffering from ischemia if TC or SMA perfusion is compromised. Ischemia, in turn, impairs anastomotic healing and consequently causes leakage.
Multiple techniques for preoperative assessment of gastric perfusion have been described in the literature, including digital subtraction angiography from a transfemoral arterial access and noninvasive imaging (eg, duplex sonography, magnetic resonance imaging and CT).9,15,20,21 Few studies, however, have been performed to analyze the effect of vascular pathology on anastomotic insufficiency after gastric pull-up. This study compares the reliability, consistency and prognostic impact of two different radiologic methods for identifying vascular pathology, namely, calcification scores and stenosis quantification.
Arterial calcification of the blood vessels supplying the gastric tube has been identified as an independent risk factor for leakage after esophagectomy with cervical anastomosis.22 Calcifications of the aorta and the right postceliac arteries were found to be independent risk factors for anastomotic insufficiency.9 The calcification scoring system evaluated in this study was developed by van Rossum et al by adapting a validated visual grading system for arterial abnormalities found on CT scans to predict cardiovascular events.23,24 As previous findings have shown that additional parameters such as irregularities, plaques and elongation of the aortic wall had no influence on the prediction model,23 this study was constrained to the definitions used to grade calcifications listed in Table 1. Application of the calcification score needs some level of training and habituation to the classification system. Once internalized, it is easy to apply and, hence, shows an almost perfect inter-reader agreement in van Rossum et al’s study as well as in our study; yet, we were not able to confirm the predictive value of the calcification score system. This might be due to the fact that the number and length of arterial calcifications do not necessarily correlate with the severity of perfusion impairment. Another limitation of the calcification score system is the disregard for non-calcified vascular stenoses such as soft plaques, median arcuate ligament syndrome and external vascular compression. In this retrospective study, a total of 164 consecutive patients treated at a high-volume center were analyzed for correlation between preoperative CT scan and postoperative leakage, and the leakage rate was <10% (14/164). The extent of statistical analysis was, therefore, limited by sample size in consideration of the fact that only 14 of the 164 patients presented with anastomotic leakage.
In a previous study by Schröder et al, stenosis quantification of the TC and SMA could not be correlated to incidents of anastomotic insufficiency following esophagectomy.15 It is worth noting of the fact that the Schröder study included only a small number of selected patients (n=23) and radiologic assessment was based on preoperative arterial angiography instead of CE-CT.
We chose CE-CT in the arterial phase for evaluation of vascular stenosis because these scans are routinely obtained during staging examinations, which avoids the need for additional radiation exposure and reapplication of contrast agents. Furthermore, CTA yields the best image quality, diagnostic accuracy and reaches the highest level of agreement and significance in correlation to stenosis grading of mesenteric arteries among the noninvasive imaging modalities.16 Arterial stenosis is easily and intuitively evaluable on CE-CT scans by measurement of the minimal and normal vascular diameter. This evaluation technique factors in all underlying pathologies of vascular stenosis independent of the presence of calcifications, and has been established as a valid prognostic tool for ischemic conditions such as ischemic stroke in patients with carotid artery stenosis.19
Our inter-reader variability was considerably lower in arterial stenosis measurement than in the application of the calcification score. This is, on one hand, explained by the subjectivity over whether a stenosis is present or not in patients with only slight proximal vessel tapering, which might be an anatomic variant (eg, due to median arcuate ligament) instead of stenosis. On the other hand, the lumina of TC and SMA are relatively small; hence, measurement deviations in the submillimeter range, which are unavoidable, have a relatively high impact on interobserver agreement. Nevertheless, inter-reader variability for arterial stenosis measurement was still 80% at least and almost 100% for the detection of vascular stenosis of 70% or more.
It is worth highlighting that there are many other factors that might contribute to deficient wound healing and describing them in detail would go beyond the scope of this study. Predictors of anastomotic leak after esophagectomy were presented in an analysis by the Society of Thoracic Surgeons General Thoracic Database including 7,595 esophagectomies, with 804 (10.6%) leaks.13 In their study, factors associated with leakage on univariate analysis included obesity, heart failure, coronary disease, vascular disease, hypertension, steroids, diabetes, renal insufficiency, tobacco use, procedure duration >5 hours and type of procedure (p<0.05). Multivariable regression analysis associated heart failure, hypertension, renal insufficiency and type of procedure as risk factors for the development of leak (p<0.05). On the contrary, we did not observe any difference between patients with and without anastomotic leak with regards to the assessed preoperative comorbidities, with the exception of TC stenosis. To sum up, what most of the above-mentioned factors have in common is the potential to compromise the blood supply to the healing anastomosis site. Conversely, it should be a consistent practice to optimize the initial conditions of these patients who are at risk of anastomosis leakage before planning on esophagectomy. For patients with asymptomatic TC stenosis identified by CT, no individualized treatment has been analyzed so far. In principle, two different approaches seem to be promising: TC recanalization by stent angioplasty or ischemic conditioning of the conduit.14,18,26 With both options, however, the lack of a reliable threshold for therapeutic intervention based on the degree of stenosis makes it a difficult decision in terms of clinical management prior to esophagectomy. Further prospective studies are clearly needed to establish an agreement on the reliable “cut-off value” of stenosis that necessitates therapeutic intervention.
Conclusion
Preoperative screening of CE-CT scans for stenosis quantification and calcification scores as part of an individual risk assessment for anastomotic leakage after esophagectomy are quick and simple practical methods. Both methods demonstrate a high interobserver reliability without incurring further costs or subjecting the patient to more diagnostic tests. Within the limitations of this study, we are able to show that TC stenosis is positively correlated with anastomotic leakage, the likelihood of anastomotic insufficiency increases with the degree of stenosis, and that high-grade stenoses are reliably detectable with CE-CT analysis. No correlation, however, could be found between arterial calcification scores and anastomotic leakage despite contrary findings in the literature.9,22,25 Larger prospective studies are clearly needed to further explore and substantiate our findings. With regards to clinical implication, we recommend that patients scheduled for esophagectomy undergo routine preoperative screening for TC stenosis in order to identify those with an increased risk for anastomotic leakage.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 2.Kumagai K, Rouvelas I, Tsai JA, et al. Survival benefit and additional value of preoperative chemoradiotherapy in resectable gastric and gastro-oesophageal junction cancer: a direct and adjusted indirect comparison meta-analysis. Eur J Surg Oncol. 2015;41(3):282–294. doi: 10.1016/j.ejso.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 3.D’Journo XB, Thomas PA. Current management of esophageal cancer. J Thorac Dis. 2014;6(Suppl 2):S253–S264. doi: 10.3978/j.issn.2072-1439.2014.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alldinger I, Sisic L, Hochreiter M, et al. Outcome, complications, and mortality of an intrathoracic anastomosis in esophageal cancer in patients without a preoperative selection with a risk score. Langenbecks Arch Surg. 2015;400(1):9–18. doi: 10.1007/s00423-014-1257-8. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol. 2014;110(5):599–610. doi: 10.1002/jso.23759. [DOI] [PubMed] [Google Scholar]
- 6.Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012;19(1):99–103. doi: 10.1245/s10434-011-1926-6. [DOI] [PubMed] [Google Scholar]
- 7.Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198(4):536–541. doi: 10.1016/j.jamcollsurg.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Zehetner J, DeMeester SR, Alicuben ET, et al. Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg. 2015;262(1):74–78. doi: 10.1097/SLA.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossum PS, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology. 2015;274(1):124–132. doi: 10.1148/radiol.14140410. [DOI] [PubMed] [Google Scholar]
- 10.Liebermann-Meffert D. Anatomical basis for the approach and extent of surgical treatment of esophageal cancer. Dis Esophagus. 2001;14(2):81–84. doi: 10.1046/j.1442-2050.2001.00160.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagawa H, Seto Y, Nakatsuka T, Kaizaki S, Muto T. Microvascular anastomosis for additional blood flow in reconstruction after intrathoracic esophageal carcinoma surgery. Am J Surg. 1997;173(2):131–133. doi: 10.1016/S0002-9610(96)00410-2. [DOI] [PubMed] [Google Scholar]
- 12.Collard JM, Tinton N, Malaise J, Romagnoli R, Otte JB, Kestens PJ. Esophageal replacement: gastric tube or whole stomach? Ann Thorac Surg. 1995;60(2):261–266. doi: 10.1016/0003-4975(95)00411-d. [DOI] [PubMed] [Google Scholar]
- 13.Kassis ES, Kosinski AS, Ross P, Jr, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96(6):1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 14.Schröder W, Hölscher AH, Bludau M, Vallböhmer D, Bollschweiler E, Gutschow C. Ivor-lewis esophagectomy with and without laparoscopic conditioning of the gastric conduit. World J Surg. 2010;34(4):738–743. doi: 10.1007/s00268-010-0403-x. [DOI] [PubMed] [Google Scholar]
- 15.Schröder W, Zähringer M, Stippel D, et al. Does celiac trunk stenosis correlate with anastomotic leakage of esophagogastrostomy after esophagectomy? Dis Esophagus. 2002;15(3):232–236. doi: 10.1046/j.1442-2050.2002.00252.x. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer PJ, Pfarr J, Trentmann J, et al. Comparison of noninvasive imaging modalities for stenosis grading in mesenteric arteries. Rofo. 2013;185(7):628–634. doi: 10.1055/s-0033-1335212. [DOI] [PubMed] [Google Scholar]
- 17.North American Symptomatic Carotid Endarterectomy Trial (NASCET) Investigators Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. Stroke. 1991;22(6):816–817. doi: 10.1161/01.str.22.6.816. [DOI] [PubMed] [Google Scholar]
- 18.Hölscher AH, Schneider PM, Gutschow C, Schröder W. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg. 2007;245(2):241–246. doi: 10.1097/01.sla.0000245847.40779.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett HJM, Taylor DW. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 20.Volteas N, Labropoulos N, Leon M, Kalodiki E, Chan P, Nicolaides AN. Detection of superior mesenteric and coeliac artery stenosis with colour flow Duplex imaging. Eur J Vasc Surg. 1993;7(6):616–620. doi: 10.1016/s0950-821x(05)80705-4. [DOI] [PubMed] [Google Scholar]
- 21.Fleischmann D. Multiple detector-row CT angiography of the renal and mesenteric vessels. Eur J Radiol. 2003;45(Suppl 1):S79–S87. doi: 10.1016/s0720-048x(02)00364-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Zhao G, Li J, et al. Calcification of arteries supplying the gastric tube increases the risk of anastomotic leakage after esophagectomy with cervical anastomsis. J Thorac Dis. 2016;8(12):3551–3562. doi: 10.21037/jtd.2016.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gondrie MJ, Mali WP, Jacobs PC, Oen AL, van der Graaf Y, PROVIDI Study Group Cardiovascular disease: prediction with ancillary aortic findings on chest CT scans in routine practice. Radiology. 2010;257(2):549–559. doi: 10.1148/radiol.10100054. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs PC, Prokop M, Oen AL, van der Graaf Y, Grobbee DE, Mali WP. Semiquantitative assessment of cardiovascular disease markers in multislice computed tomography of the chest: interobserver and intraobserver agreements. J Comput Assist Tomogr. 2010;34(2):279–284. doi: 10.1097/RCT.0b013e3181bbcff6. [DOI] [PubMed] [Google Scholar]
- 25.Goense L, van Rossum PS, Weijs TJ, et al. Aortic calcificatin increases the risk of anastomotic leakage after Ivor-Lewis Esophagectomy. Ann Thorac Surg. 2016;102(1):247–252. doi: 10.1016/j.athoracsur.2016.01.093. [DOI] [PubMed] [Google Scholar]
- 26.Gaujoux S, Sauvanet A, Vullierme MP, et al. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249(1):111–117. doi: 10.1097/SLA.0b013e3181930249. [DOI] [PubMed] [Google Scholar]