Abstract
Objective
To investigate the impact of moderate wine consumption on the risk of prostate cancer (PCa). We focused on the differential effect of moderate consumption of red versus white wine.
Design
This study was a meta-analysis that includes data from case–control and cohort studies.
Materials and methods
A systematic search of Web of Science, Medline/PubMed, and Cochrane library was performed on December 1, 2017. Studies were deemed eligible if they assessed the risk of PCa due to red, white, or any wine using multivariable logistic regression analysis. We performed a formal meta-analysis for the risk of PCa according to moderate wine and wine type consumption (white or red). Heterogeneity between studies was assessed using Cochrane’s Q test and I2 statistics. Publication bias was assessed using Egger’s regression test.
Results
A total of 930 abstracts and titles were initially identified. After removal of duplicates, reviews, and conference abstracts, 83 full-text original articles were screened. Seventeen studies (611,169 subjects) were included for final evaluation and fulfilled the inclusion criteria. In the case of moderate wine consumption: the pooled risk ratio (RR) for the risk of PCa was 0.98 (95% CI 0.92–1.05, p=0.57) in the multivariable analysis. Moderate white wine consumption increased the risk of PCa with a pooled RR of 1.26 (95% CI 1.10–1.43, p=0.001) in the multi-variable analysis. Meanwhile, moderate red wine consumption had a protective role reducing the risk by 12% (RR 0.88, 95% CI 0.78–0.999, p=0.047) in the multivariable analysis that comprised 222,447 subjects.
Conclusions
In this meta-analysis, moderate wine consumption did not impact the risk of PCa. Interestingly, regarding the type of wine, moderate consumption of white wine increased the risk of PCa, whereas moderate consumption of red wine had a protective effect. Further analyses are needed to assess the differential molecular effect of white and red wine conferring their impact on PCa risk.
Keywords: wine, prostate cancer, alcohol, risk of cancer, meta-analysis
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer among men in the USA with an estimated 161,360 new cases in 2017.1 Worldwide, it is the second most common cancer and the sixth cause of cancer death among men,2 with an estimated 1.1 million cases and 307,000 deaths in 2012.3 There are well-established risk factors for PCa, such as family history,4,5 hereditary genes,6 racial/ethnic background (eg, African ethnicity),7 and age. Also, a wide variety of exogenous/environmental/lifestyle factors have been shown to impact the risk of PCa development and progression.8 For example, alcohol intake has been recently suggested as a risk factor for PCa development in a meta-analysis that included 27 studies showing a significant dose–response relationship between the level of alcohol intake and the risk of PCa.9 On the other hand, a large prospective European cohort study failed to observe an association between alcohol consumption and PCa risk.10 Both studies did not assess the type of alcohol consumption. Despite association between alcohol intake and PCa risk, the effect of wine consumption on PCa risk is not yet fully understood. Furthermore, association between wine consumption and risk of PCa demands further investigation as several studies have suggested that polyphenols from red wine have a chemoprotective role in PCa cell lines.11,12
Therefore, we hypothesized that wine, specifically red wine, has a protective effect on PCa development. To test this hypothesis, we performed a meta-analysis assessing the effect of moderate wine consumption on PCa in a first step and then that of red and white wine differentially.
Materials and methods
A systematic search of Web of Science, Medline/PubMed, and Cochrane library was performed using the terms “wine” and “prostate cancer” on December 1, 2017. All original articles that fulfilled the inclusion criteria were included. We performed additional cross-checking of reference lists, including those of previous meta-analyses and “hand-searched” for additional references in the selected articles, reviews, and meta-analyses reporting on the topic.
Informed consent was not required for this type of study.
Inclusion and exclusion criteria
The PICOS (Population, Intervention, Comparator, Outcome and Study design approach was utilized to define study eligibility according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria (www.prisma-statement.org).13 Studies were considered eligible if they assessed the risk of PCa due to red and white wine and wine in general using multivariable logistic regression analysis in the general population or compared with a control group of individuals without PCa. For each selected study, the following items were recorded: first author’s name, year of publication, country, number of patients, age, and variables used in the multivariable analysis, risk ratios (RRs) of PCa in multivariable analysis, dose of wine and followup in case of cohort studies. Two independent investigators (MDV and SK) assessed study quality using the Newcastle-Ottawa Scale (NOS)14 for cohort studies. A total score of 5 or less was considered low; 6–7 was considered intermediate, and 8–9 was considered high quality. Most included studies had intermediate and high quality score according to NOS (Figure S1).
Statistical analysis
We performed a formal meta-analysis for the risk of PCa according to moderate wine consumption and moderate consumption of type of wine (white or red). RRs with their 95% CIs from each study were used to calculate pooled RRs. Pooled estimates were calculated with the fixed effect model, if no significant heterogeneity was identified; alternatively, the random effect model was used when significant heterogeneity was detected. Statistical heterogeneity was defined based on Cochrane’s Q p-value or I2 statistics. We performed “leave-one-out” sensitivity analysis. To evaluate publication bias, Egger linear regression and funnel plots were examined. In case of reporting only RRs for low- and high-risk PCa, we included in the meta-analysis the RRs for high-risk PCa. Statistical analyses were performed using Stata 11.0 statistical software (Stata Corp., College Station, TX, USA).
Results
A total of 930 abstracts and titles were initially identified. After removal of duplicates, reviews, and conference abstracts, 83 full-text original articles were screened. Finally, 17 studies (a total of 611,169 subjects) were included for final evaluation fulfilling the inclusion criteria.15–31 The PRISMA flow chart summarizing the process of study selection is shown in Figure 1. Potential publication bias was examined by both a funnel plot and an Egger’s test and we did not find any publication bias (Figure 2). Assessment of the main studies biases are shown in the risk bias table (Figure S2).
Figure 1.
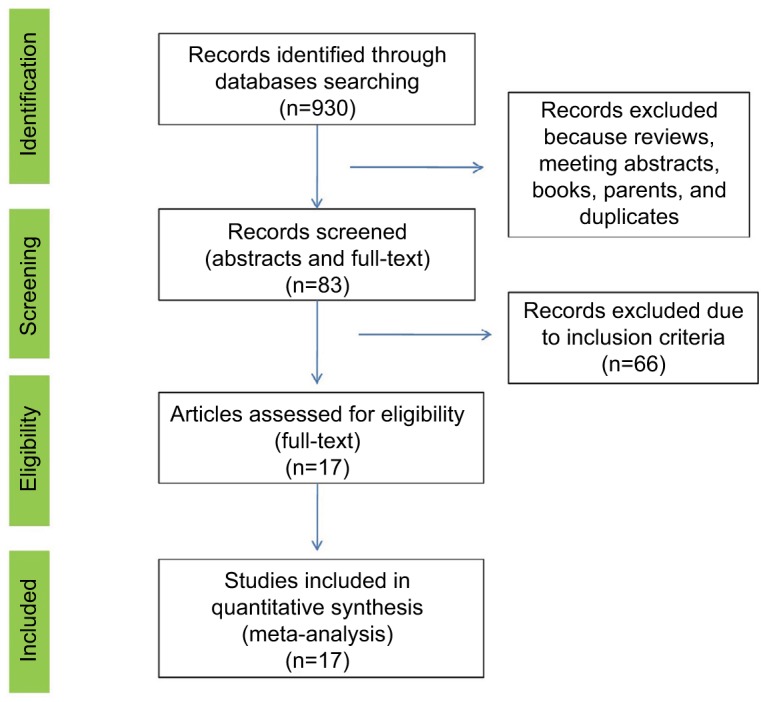
PRISMA flow chart of the study selection process.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
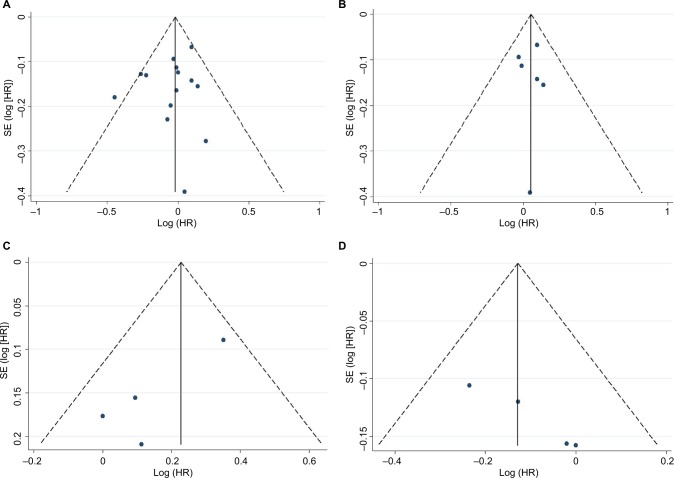
Figure 2.
Funnel plots with pseudo 95% confidence limits for the association between moderate wine intake and the risk of PCa in all the included studies (A), only in cohort-studies (B), association between moderate white wine intake and the risk of PCa (C), and association between moderate red wine intake and the risk of PCa (D).
Abbreviations: PCa, prostate cancer; SE, standard error; HR, hazard ratio.
Effect of moderate consumption of wine on PCa risk
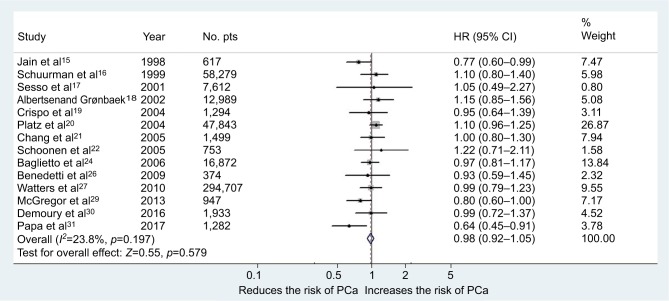
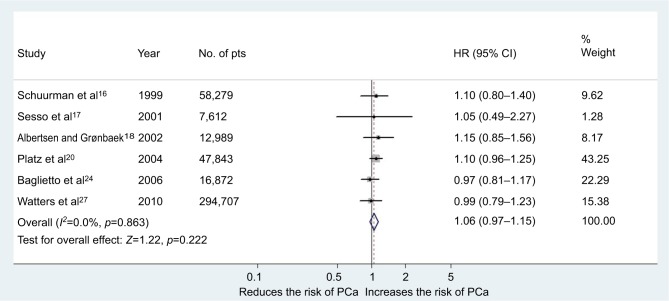
Overall, 14 studies (455,413 subjects) fulfilled the inclusion criteria regarding moderate wine consumption and risk of PCa (6 cohort and 8 case–control studies).15–22,24,26,27,29–31 The main characteristics of the studies, as well as dose of wine consumption, are shown in Table 1. In the first meta-analysis, we included all the studies regardless of design. The pooled RR for the risk of PCa was 0.98 (95% CI 0.92–1.05, p=0.57) in the multivariable analysis (Figure 3). The Cochrane’s Q test (χ2=17.6; p=0.19) and I2 test (I2=23.8%) did not show a significant heterogeneity. The funnel plots identified one study over the pseudo 95% CI (Figure 2A). Furthermore, we performed a second meta-analysis in which we included only cohort studies (438,302 subjects from which 19,238 developed PCa during observation/follow-up). The results were confirmed with a pooled RR of 1.06 (95% CI 0.96–1.15, p=0.22) in the multivariable analysis (Figure 4). The Cochrane’s Q test (χ2=1.9; p=0.86) and I2 test (I2=0%) did not show a significant heterogeneity. The funnel plots identified all studies in the pseudo 95% CI (Figure 2B). The results did not differ when we performed a sensitivity analysis “leave-one-out.”
Table 1.
Studies investigating the impact of wine consumption on the risk of PCa
| No. | Study | Year | Country | Study type | No. of pts | Age (years) | Variables | RRs wine and risk of PCa | Dose | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jain et al15 | 1998 | Canada | Case–control | 637/617 PCa | 69.8 | Age, smoking status, total energy intakes, and consumption of other types of alcohol | 0.77 (0.60–0.99) | 0–9 g/day | – |
| 2 | Schuurman et al16 | 1999 | the Netherlands | Case–cohort | 58,279/680 Pca | 55–69 | Age, socioeconomic status and, family history of PCa | 1.1 (0.8–1.4) | 0.1–4 g/day | 6.3 |
| 3 | Sesso et al17 | 2001 | USA | Case–cohort | 7,612/366 PCa | 66.6 | Age, BMI, physical activity, smoking status, and family history of PCa | 1.05 (0.49–2.27) | ≥3 drinks/day | 6 |
| 4 | Albertsen and Grønbaek18 | 2002 | Denmark | Case–cohort | 12,989/233 | NA | Age, education, physical activity, BMI, smoking status, and study of origin | 1.15 (0.85–1.56) | 1–13 drinks/week | 12.3 |
| 5 | Crispo et al19 | 2004 | Italy | Case–control | 1,451/1,294 PCa | 45–75 | Age, center, education, BMI, physical activity, and family history of PCa | 0.95 (0.64–1.39) | 3–4 drinks/day | – |
| 6 | Platz et al20 | 2004 | USA | Case–cohort | 47,843/2,479 PCa | 40–75 | Age, BMI at age 21 years, height, pack-years of smoking in the previous decade, family history of PCa, major ancestry, diabetes, vasectomy, vigorous physical activity, and intakes of total energy, calcium, fructose, tomato sauce, red meat, fish, vitamin E (>15 mg/day), and α-linolenic acid | 1.10 (0.96–1.25) | 2–5.9 g/day | 12 |
| 7 | Chang et al21 | 2005 | Sweden | Case–control | 1,130/1,499 PCa | 45–79 | Age, smoking history, BMI, family history of PCa, intake of other alcohol types, dairy products, red meat, and fruits and vegetables | 1.0 (0.8–1.3) | 0–15 g/day | – |
| 8 | Schoonen et al22 | 2005 | King County, WA, USA | Case–control | 703/753 PCa | 40–64 | Age, PSA screening, total lifetime number of female sexual partners, smoking status, and consumption of other types of alcohol | 1.22 (0.71–2.11) | ≥8 drinks/week | – |
| 9 | Baglietto et al24 | 2006 | Melbourne, Australia | Case–cohort | 16,872/732 PCa | 27–75 | Age, education, BMI, smoking, total energy intake, and previous medical conditions | 0.97 (0.81–1.17) 1.02 (0.83–1.26) Low-grade PCa 0.76 (0.51–1.14) High-grade PCa |
1–19 g/day | >10 |
| 10 | Benedetti et al26 | 2009 | Canada | Case–control | 507/374 PCa | Mean 59.7/62.9 PCa | Age, smoking status, cigarette-year, respondent status, ethnicity, census tract income, years of schooling, and time since quitting | 0.93 (0.59, 1.45) | >7 drinks/week | – |
| 11 | Watters et al27 | 2010 | 11 States from USA | Case–cohort | 294,707/17,227 PCa | 50–71 | Age, race, education, marital status, height, BMI, physical activity, family history of PCa, diabetes, self-reported health status, cigarette smoking, prostate- specific antigen screening and digital rectal examination, total energy excluding alcohol, a-tocopherol, calcium, red meat, fish, tomato, a-linolenic acid, and selenium | 1.05 (1–1.09) Low-grade PCa 0.99 (0.79–1.23) High-grade PCa |
<1 drink/day | >8 |
| 12 | McGregor et al29 | 2013 | Alberta, Canada | Case–control | 1,039/947 PCa ≥Stage II | Mean 68.5/69.8 | Age, residence region, education, family history of PCa, BPH, number of DRE tests, number of PSA tests | 0.8 (0.6–1) | 1–7 drinks/week | – |
| 13 | Demoury et al30 | 2016 | Montreal, Canada | Case–control | 1,994/1,933 PCa | Mean 65/64 | Age, ancestry, family history of PCa, education, smoking, physical activity, BMI, fruit and vegetables consumption, history of diabetes, and other types of beverages | 1.12 (0.88–1.43) Low-grade PCa 0.99 (0.72–1.37) High-grade PCa |
>35 drinks/year | – |
| 14 | Papa et al31 | 2017 | Victoria, Australia | Case–control | 951/1,282 agg. PCa | Median 62.9/66.8 | Age, family history of PCa, smoking status, BMI, socioeconomic status, ethnicity and country of birth, and intakes of the other beverage types | 0.64 (0.45–0.91) | 5–7 days/week | – |
Abbreviations: PCa, prostate cancer; BMI, body mass index; PSA, prostate-specific antigen; BPH, benign prostatic hyperplasia; DRE, digital rectal examination; agg, aggressive (high-grade or advanced disease); pts, patients; RRs, risk ratios.
Figure 3.
Forest plot for risk of PCa in the case of moderate consumption of wine (all studies).
Abbreviations: PCa, prostate cancer; pts, patients.
Figure 4.
Forest plot for risk of PCa in the case of moderate consumption of wine (cohort studies).
Abbreviations: PCa, prostate cancer; pts, patients.
Effect of the type of wine consumed on PCa risk
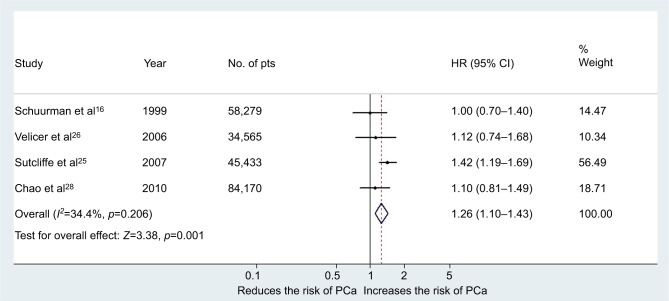
Five studies investigated the risk of PCa according to consumption of white or red wine. We used RRs reported for moderate consumption (the same dose for white and red wine was considered). Four were cohort studies (222,447 subjects, from which 6,184 developed PCa during observation/follow-up)16,23,25,28 and one was a case–control study.22 The main characteristics of the included studies, as well as dose of wine consumption, are shown in Table 2 for white wine and in Table 3 for red wine. Moderate white wine consumption increased significantly the risk of PCa with a pooled RR of 1.26 (95% CI 1.10–1.43, p=0.001) in the multivariable analysis (Figure 5). The Cochrane's Q test (χ2=4.6; p=0.2) and I2 test (I2=34.4%) did not show a significant heterogeneity. The funnel plots identified all studies in the pseudo 95% CI (Figure 2C). When we excluded from the analysis the results reported by Sutcliffe et al,25 moderate white wine consumption was not associated with an increased risk of PCa: pooled RR 1.05 (95% CI 0.87–1.28, p=0.56). In all the other cases of exclusion, one study from the analysis showed that white wine was associated with an increased risk of Pca.
Table 2.
Studies investigating the impact of white wine consumption on the risk of PCa
| No. | Study | Year | Country | Study type | No. of pts | Age (years) | Variables | RRs white wine and risk of PCa | Dose | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Schuurman et al16 | 1999 | the Netherlands | Case– cohort | 58,279/680 Pca | 55–69 | Age, socioeconomic status, family history of PCa | 1.0 (0.7–1.4) | 0.1–4 g/day | 6.3 |
| 2 | Schoonen et al22 | 2005 | King County, WA, USA | Case– control | 703/753 PCa | 40–64 | Age, PSA screening, total lifetime number of female sexual partners, smoking status, and consumption of other types of alcohol | 0.91 (0.44–1.86) | ≥8 drinks/week | – |
| 3 | Velicer et al23 | 2006 | Washington, USA | Case– cohort | 34,565/816 PCa | 50–76 | Age, PSA, other types of alcohol consumed | 1.12 (0.74–1.68) | 5 drinks/week to <2/day | 4 |
| 4 | Sutcliffe et al25 | 2007 | USA | Case– cohort | 45,433/3,348 PCa | 40–75 | Age, race/ethnicity, BMI, cumulative family history of PCa, height, cigarette smoking in the past 10 years, baseline intakes of total energy, tomato sauce, red meat, fish, calcium and vitamin E, baseline energy-adjusted intakes of fructose and a-linolenic acid, physical activity and updated diabetes mellitus type 2 and vasectomy status, and all other specific alcoholic beverage types | 1.42 (1.19–1.69) | 2–4 drinks/week | 16 |
| 5 | Chao et al28 | 2010 | California, USA | Case– cohort | 84,170/1,340 PCa | 45–69 | Age, race/ethnicity, income, BMI, intake of other alcoholic beverage, meat consumption, family history of PCa, person history of PSA testing, STI, BPH, BPH surgery, prostatitis, and diabetes mellitus | 1.10 (0.81–1.49) | >1 drink/day | 5 |
Abbreviations: PCa, prostate cancer; pts, patients; RRs, risk ratios; BMI, body mass index; PSA, prostate-specific antigen; STIs, sexually transmitted infections; BPH, benign prostatic hyperplasia.
Table 3.
Studies investigating the impact of red wine consumption on the risk of PCa
| No. | Study | Year | Country | Study type | No. of pts | Age (years) | Variables | RRs Red wine and risk of PCa | Dose | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Schuurman et al16 | 1999 | the Netherlands | Case– cohort | 58,279/680 PCa | 55–69 | Age, socioeconomic status, family history of PCa | 1.0 (0.7–1.3) | 0.1–4 g/day | 6.3 |
| 2 | Schoonen et al22 | 2005 | King County, WA, USA | Case– control | 703/753 PCa | 40–64 | Age, PSA screening, total lifetime number of female sexual partners, smoking status, and consumption of other types of alcohol | 0.45 (0.23–0.85) | ≥8 drinks/week | – |
| 3 | Velicer et al23 | 2006 | Washington, USA | Case– cohort | 34,565/816 PCa | 50–76 | Age, PSA, other types of alcohol consumed | 0.98 (0.72–1.33) | 5 drinks/week to <2/day | 4 |
| 4 | Sutcliffe et al25 | 2007 | USA | Case– cohort | 45,433/3,348 PCa | 40–75 | Age, race/ethnicity, BMI, cumulative family history of PCa, height, cigarette smoking in the past 10 years, baseline intakes of total energy, tomato sauce, red meat, fish, calcium and vitamin E, baseline energy- adjusted intakes of fructose and a-linolenic acid, physical activity and updated diabetes mellitus type 2 and vasectomy status, and all other specific alcoholic beverage types | 0.79 (0.64–0.97) | 2–4 drinks/week | 16 |
| 5 | Chao et al28 | 2010 | California, USA | Case– cohort | 84,170/1,340 PCa | 45–69 | Age, race/ethnicity, income, BMI, intake of other alcoholic beverage, meat consumption, family history of PCa, person history of PSA testing, STI, BPH, BPH surgery, prostatitis, and diabetes mellitus | 0.88 (0.70–1.12) | >1 drink/day | 5 |
Abbreviations: PCa, prostate cancer; pts, patients; RRs, risk ratios; BMI, body mass index; PSA, prostate specific antigen; BPH, benign prostatic hyperplasia; STIs, sexually transmitted infections.
Figure 5.
Forest plot for the risk of PCa in the case of moderate consumption of white wine (cohort studies).
Abbreviations: PCa, prostate cancer; pts, patients.
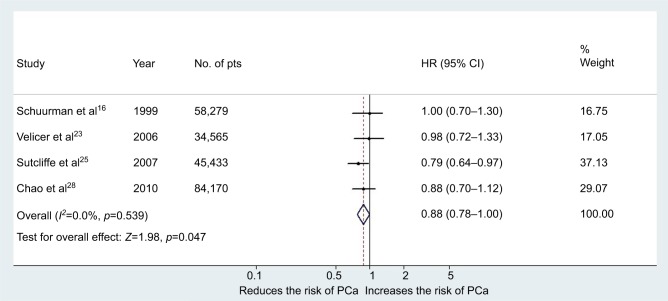
Moderate red wine consumption was associated with a decreased risk of PCa with a pooled RR of 0.88 (95% CI 0.78–0.999, p=0.047) in the multivariable analysis (Figure 6). The Cochrane's Q test (χ2=2.16; p=0.53) and I2 test (I2=0%) did not show a significant heterogeneity. The funnel plots identified all studies in the pseudo 95% CI (Figure 2D). The results remain significant also after the addition of the RRs from the case–control study of Schoonen et al22 with a pooled RR of 0.86 (95% CI 0.76–0.97, p=0.01). However, in this case the heterogeneity increased, but not to a significant level, Cochrane's Q test (χ2=6.06; p=0.19) and I2 test (I2=34%). When we excluded from the analysis the results reported by Sutcliffe et al,25 moderate red wine consumption was not associated with a decreased risk of PCa: pooled RR 0.88 (95% CI 0.71–1.09, p=0.24). In all the other cases of exclusion, only one study from the analysis showed that red wine was associated with a decreased risk of Pca.
Figure 6.
Forest plot for risk of PCa in the case of moderate consumption of red wine (cohort studies).
Abbreviations: PCa, prostate cancer; pts, patients.
Discussion
This study is to our knowledge the first meta-analysis to investigate the impact of moderate wine consumption and the risk of developing PCa, including 611,169 subjects from 17 studies. According to this meta-analysis moderate wine consumption is not a risk factor for PCa development. Interestingly, the analysis regarding type of wine consumed sustains the fact that moderate wine consumption does not impact PCa risk. We found an antagonist effect as moderate white wine consumption increases the risk of PCa, whereas moderate red wine consumption had a protective role against PCa. However, when we excluded the results reported by Sutcliffe et al25 (45,433 subjects with 16 years follow-up), there was not a significant association between type of wine consumed and risk of PCa.
Nevertheless, our meta-analysis has several limitations. First, there is a selection bias in the studies as all were nonrandomized observational or case–control studies. Second, the definition of moderate consumption is imprecise with differences between studies introducing heterogeneous results. Still, all studies had a maximum of one glass of wine per day as moderate consumption. Third, despite a large number of patients in our analyses, the number of studies in our meta-analysis was limited to 17. Fourth, most studies were done in western countries with a likely preponderance of Caucasians. Metabolization and polymorphisms are highly variable between races and habits. Fifth, we decided to restrict the present meta-analysis to “moderate consumption” of wine alone, thus precluding analyses of dose–response relationship. We analyzed only the effect of moderate consumption; as in many countries, there are dietary habits that include one glass of wine per day during meals and the scope of the meta-analysis was not to encourage alcohol/wine consumption, but instead to point out the effects of responsible wine drinking on the risk of PCa.
The relationship between alcohol consumption and the risk of PCa remains a controversial issue.32 Middleton Fillmore et al demonstrated in a meta-analysis that heavy alcohol consumption is associated with a higher risk of developing PCa.33 Similarly, Zhao et al’s meta-analysis showed a significant dose–response relationship between level of alcohol intake and risk of PCa.9 On the contrary, a large prospective European study that included 142,607 male participants found no association between alcohol consumption and PCa risk.10 Regarding types of alcohol consumption, in a large cohort of 3,927 subjects, Demoury et al showed that beer was associated with a 37% increase risk of high-grade PCa.30 In the present study, we found that wine is not associated with an increased risk of PCa as other alcohol or beer consumption is. This could be based on several factors that make wine less harmful than other types of alcohols. One of the factors might be the chemical composition of wine, which is a hydroalcoholic solution (~78% water) with a wide range of bioactive chemical components, including aldehydes, esters, ketones, lipids, minerals, organic acids, phenolics, soluble proteins, sugars, and vitamins.34 Second, the anticarcinogenic effect of polyphenols mainly contained by red wine may balance any other harmful effects of wine consumption.35 Third, in the case of beer, the bioavailability of the phenolic compounds is very low, thus decreasing their potential anticarcinogenic effects.36
Furthermore, the mechanism between alcohol consumption and carcinogenesis is not fully understood. It seems to be based on acetaldehyde, the first metabolite of ethanol that has been suggested to be carcinogenic by promoting cancer development though various mechanisms, such as interference with DNA replication, induction of DNA damage, and formation of DNA adducts.37 However, wine consumption, especially red wine, has been associated with decreased inflammation and overall mortality as well as moderate alcohol consumption.10,38 Schoonen et al22 and Sutcliffe et al25 found, in large cohort studies, that red wine consumption decreases the risk of PCa, whereas white wine does not. Red wine’s protective role against PCa development could be due to the bioactivity of polyphenols that are a complex mixture of flavonoids (such as anthocyanins and flavan-3-ols) and nonflavonoids (such as resveratrol, cinnamates, and gallic acid). Resveratrol is the most studied compound and its concentration is 10-fold higher than in white wine.39 Resveratrol is added from the skin of red grapes during the creation process. Its concentration in red wine ranges from 1.2 to 2.0 g/L.39 It has been studied regarding its anticarcinogenesis including PCa, and many studies showed that resveratrol causes cell growth, proliferation inhibition, and activation of apoptosis in human PCa cell lines including PC3, DU145, and LNCaP.40–43 Sgambato et al found that resveratrol not only inhibits cell proliferation but also prevents the accumulation of reactive oxygen species production and oxidative DNA damage in cells exposed to oxidative agents.44 On the other hand, white wine contains also a small amount of resveratrol, but despite an experimental study that showed an association between white wine and antiproliferative effect, clinical studies do not support this finding.43 Nevertheless, the beneficial effects of moderate red wine consumption might be due to all its compounds and not only due to resveratrol.39 Although we focused only on “moderate” consumption of red wine in this study, it is unclear whether effectiveness of red wine polyphenols depends on the amount of consumption or not. To better assess the relationship between wine consumption, especially the appropriate amount of red wine and PCa risk in the general population, further studies are needed.
Conclusion
In this meta-analysis, moderate wine consumption did not influence the risk of PCa. However, moderate consumption of white wine increased the risk of PCa, whereas moderate consumption of red wine had a protective role. This hypothesis-generating data should serve as a rationale for uncovering the molecular underpinnings of this differential effect in order to potentially devise prevention strategies in the at-risk population.
Supplementary materials
Newcastle-Ottawa Scale.
Notes: Each study was judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the ascertainment of the exposure of interest for cohort studies. There was a maximum of 4 stars for the selection, 2 stars for the comparability, and 3 stars for exposure components. The highest quality studies are awarded up to 9 stars.
(A) Risk of bias summary of the studies that analyses the association between moderate wine consumption and PCa risk (red: high risk; green: low risk). (B) Risk of bias summary of the studies that analyses the association between moderate white and red wine consumption and PCa risk (red: high risk; green: low risk).
Abbreviation: NOS, Newcastle-Ottawa Scale.
References
- 1.Jain MG, Hislop GT, Howe GR, Burch JD, Ghadirian P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int J Cancer. 1998;78(6):707–711. doi: 10.1002/(sici)1097-0215(19981209)78:6<707::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Schuurman AG, Goldbohm RA, van den Brandt PA. A prospective cohort study on consumption of alcoholic beverages in relation to prostate cancer incidence (The Netherlands) Cancer Causes Control CCC. 1999;10(6):597–605. doi: 10.1023/a:1008925103542. [DOI] [PubMed] [Google Scholar]
- 3.Sesso HD, Paffenbarger RS, Lee IM. Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. Int J Epidemiol. 2001;30(4):749–755. doi: 10.1093/ije/30.4.749. [DOI] [PubMed] [Google Scholar]
- 4.Albertsen K, Grønbaek M. Does amount or type of alcohol influence the risk of prostate cancer? Prostate. 2002;52(4):297–304. doi: 10.1002/pros.10120. [DOI] [PubMed] [Google Scholar]
- 5.Crispo A, Talamini R, Gallus S, et al. Alcohol and the risk of prostate cancer and benign prostatic hyperplasia. Urology. 2004;64(4):717–722. doi: 10.1016/j.urology.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Platz EA, Leitzmann MF, Rimm EB, Willett WC, Giovannucci E. Alcohol intake, drinking patterns, and risk of prostate cancer in a large prospective cohort study. Am J Epidemiol. 2004;159(5):444–453. doi: 10.1093/aje/kwh062. [DOI] [PubMed] [Google Scholar]
- 7.Chang ET, Hedelin M, Adami H-O, Grönberg H, Bälter KA. Alcohol drinking and risk of localized versus advanced and sporadic versus familial prostate cancer in Sweden. Cancer Causes Control. 2005;16(3):275–284. doi: 10.1007/s10552-004-3364-2. [DOI] [PubMed] [Google Scholar]
- 8.Schoonen WM, Salinas CA, Kiemeney LALM, Stanford JL. Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer. 2005;113(1):133–140. doi: 10.1002/ijc.20528. [DOI] [PubMed] [Google Scholar]
- 9.Baglietto L, Severi G, English DR, Hopper JL, Giles GG. Alcohol consumption and prostate cancer risk: results from the Melbourne collaborative cohort study. Int J Cancer. 2006;119(6):1501–1504. doi: 10.1002/ijc.21983. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti A, Parent M-E, Siemiatycki J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prev. 2009;32(5–6):352–362. doi: 10.1016/j.canep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am J Epidemiol. 2010;172(7):773–780. doi: 10.1093/aje/kwq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGregor SE, Courneya KS, Kopciuk KA, Tosevski C, Friedenreich CM. Case-control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control. 2013;24(3):451–461. doi: 10.1007/s10552-012-0131-7. [DOI] [PubMed] [Google Scholar]
- 13.Demoury C, Karakiewicz P, Parent M-E. Association between lifetime alcohol consumption and prostate cancer risk: a case-control study in Montreal, Canada. Cancer Epidemiol. 2016;45:11–17. doi: 10.1016/j.canep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Papa NP, MacInnis RJ, Jayasekara H, et al. Total and beverage-specific alcohol intake and the risk of aggressive prostate cancer: a case-control study. Prostate Cancer Prostatic Dis. 2017;20(3):305–310. doi: 10.1038/pcan.2017.12. [DOI] [PubMed] [Google Scholar]
- 15.Velicer CM, Kristal A, White E. Alcohol use and the risk of prostate cancer: results from the VITAL cohort study. Nutr Cancer. 2006;56(1):50–56. doi: 10.1207/s15327914nc5601_7. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe S, Giovannucci E, Leitzmann MF, et al. A prospective cohort study of red wine consumption and risk of prostate cancer. Int J Cancer. 2007;120(7):1529–1535. doi: 10.1002/ijc.22498. [DOI] [PubMed] [Google Scholar]
- 17.Chao C, Haque R, Van Den Eeden SK, Caan BJ, Poon K-YT, Quinn VP. Red wine consumption and risk of prostate cancer: the California Men’s Health Study. Int J Cancer. 2010;126(1):171–179. doi: 10.1002/ijc.24637. [DOI] [PubMed] [Google Scholar]
Acknowledgments
MDV had an EUSP (European Urological Scholarship Programme) lab/clinical fellowship awarded by EAU (European Association of Urology) and an Ernst Mach Grant awarded by OeAD, Austria.
Footnotes
Author Contributions
All authors contributed towards data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Lynch HT, Kosoko-Lasaki O, Leslie SW, et al. Screening for familial and hereditary prostate cancer. Int J Cancer. 2016;138(11):2579–2591. doi: 10.1002/ijc.29949. [DOI] [PubMed] [Google Scholar]
- 5.Randazzo M, Müller A, Carlsson S, et al. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau) BJU Int. 2016;117(4):576–583. doi: 10.1111/bju.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eeles RA, Olama AAA, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45(4):385–391. 391e1–e2. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan DSW, Mok TSK, Rebbeck TR. Cancer genomics: diversity and disparity across ethnicity and geography. J Clin Oncol. 2016;34(1):91–101. doi: 10.1200/JCO.2015.62.0096. [DOI] [PubMed] [Google Scholar]
- 8.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Stockwell T, Roemer A, Chikritzhs T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer. 2016;16(1):845. doi: 10.1186/s12885-016-2891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrmann S, Linseisen J, Key TJ, et al. Alcohol consumption and the risk for prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomark Prev. 2008;17(5):1282–1287. doi: 10.1158/1055-9965.EPI-07-2888. [DOI] [PubMed] [Google Scholar]
- 11.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23(1):17–28. [PubMed] [Google Scholar]
- 12.Jasiński M, Jasińska L, Ogrodowczyk M. Resveratrol in prostate diseases: a short review. Cent Eur J Urol. 2013;66(2):144–149. doi: 10.5173/ceju.2013.02.art8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. [Accessed October 30, 2017]. Available from: http://www.Ohri.ca/Programs/Clinical_epidemiology/Oxford.Htm.
- 15.Jain MG, Hislop GT, Howe GR, Burch JD, Ghadirian P. Alcohol and other beverage use and prostate cancer risk among Canadian men. Int J Cancer. 1998;78(6):707–711. doi: 10.1002/(sici)1097-0215(19981209)78:6<707::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Schuurman AG, Goldbohm RA, van den Brandt PA. A prospective cohort study on consumption of alcoholic beverages in relation to prostate cancer incidence (The Netherlands) Cancer Causes Control. 1999;10(6):597–605. doi: 10.1023/a:1008925103542. [DOI] [PubMed] [Google Scholar]
- 17.Sesso HD, Paffenbarger RS, Lee IM. Alcohol consumption and risk of prostate cancer: The Harvard Alumni Health Study. Int J Epidemiol. 2001;30(4):749–755. doi: 10.1093/ije/30.4.749. [DOI] [PubMed] [Google Scholar]
- 18.Albertsen K, Grønbaek M. Does amount or type of alcohol influence the risk of prostate cancer? Prostate. 2002;52(4):297–304. doi: 10.1002/pros.10120. [DOI] [PubMed] [Google Scholar]
- 19.Crispo A, Talamini R, Gallus S, et al. Alcohol and the risk of prostate cancer and benign prostatic hyperplasia. Urology. 2004;64(4):717–722. doi: 10.1016/j.urology.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Platz EA, Leitzmann MF, Rimm EB, Willett WC, Giovannucci E. Alcohol intake, drinking patterns, and risk of prostate cancer in a large prospective cohort study. Am J Epidemiol. 2004;159(5):444–453. doi: 10.1093/aje/kwh062. [DOI] [PubMed] [Google Scholar]
- 21.Chang ET, Hedelin M, Adami H-O, Grönberg H, Bälter KA. Alcohol drinking and risk of localized versus advanced and sporadic versus familial prostate cancer in Sweden. Cancer Causes Control. 2005;16(3):275–284. doi: 10.1007/s10552-004-3364-2. [DOI] [PubMed] [Google Scholar]
- 22.Schoonen WM, Salinas CA, Kiemeney LALM, Stanford JL. Alcohol consumption and risk of prostate cancer in middle-aged men. Int J Cancer. 2005;113(1):133–140. doi: 10.1002/ijc.20528. [DOI] [PubMed] [Google Scholar]
- 23.Velicer CM, Kristal A, White E. Alcohol use and the risk of prostate cancer: results from the VITAL cohort study. Nutr Cancer. 2006;56(1):50–56. doi: 10.1207/s15327914nc5601_7. [DOI] [PubMed] [Google Scholar]
- 24.Baglietto L, Severi G, English DR, Hopper JL, Giles GG. Alcohol consumption and prostate cancer risk: results from the Melbourne collaborative cohort study. Int J Cancer. 2006;119(6):1501–1504. doi: 10.1002/ijc.21983. [DOI] [PubMed] [Google Scholar]
- 25.Sutcliffe S, Giovannucci E, Leitzmann MF, et al. A prospective cohort study of red wine consumption and risk of prostate cancer. Int J Cancer. 2007;120(7):1529–1535. doi: 10.1002/ijc.22498. [DOI] [PubMed] [Google Scholar]
- 26.Benedetti A, Parent M-E, Siemiatycki J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prev. 2009;32(5–6):352–362. doi: 10.1016/j.canep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Watters JL, Park Y, Hollenbeck A, Schatzkin A, Albanes D. Alcoholic beverages and prostate cancer in a prospective US cohort study. Am J Epidemiol. 2010;172(7):773–780. doi: 10.1093/aje/kwq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao C, Haque R, Van Den Eeden SK, Caan BJ, Poon K-YT, Quinn VP. Red wine consumption and risk of prostate cancer: the California Men’s Health Study. Int J Cancer. 2010;126(1):171–179. doi: 10.1002/ijc.24637. [DOI] [PubMed] [Google Scholar]
- 29.McGregor SE, Courneya KS, Kopciuk KA, Tosevski C, Friedenreich CM. Case-control study of lifetime alcohol intake and prostate cancer risk. Cancer Causes Control. 2013;24(3):451–461. doi: 10.1007/s10552-012-0131-7. [DOI] [PubMed] [Google Scholar]
- 30.Demoury C, Karakiewicz P, Parent M-E. Association between lifetime alcohol consumption and prostate cancer risk: a case-control study in Montreal, Canada. Cancer Epidemiol. 2016;45:11–17. doi: 10.1016/j.canep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Papa NP, MacInnis RJ, Jayasekara H, et al. Total and beverage-specific alcohol intake and the risk of aggressive prostate cancer: a case-control study. Prostate Cancer Prostatic Dis. 2017;20(3):305–310. doi: 10.1038/pcan.2017.12. [DOI] [PubMed] [Google Scholar]
- 32.Fowke JH, Howard L, Andriole GL, Freedland SJ. Alcohol intake increases high-grade prostate cancer risk among men taking dutasteride in the REDUCE trial. Eur Urol. 2014;66(6):1133–1138. doi: 10.1016/j.eururo.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton Fillmore K, Chikritzhs T, Stockwell T, Bostrom A, Pascal R. Alcohol use and prostate cancer: a meta-analysis. Mol Nutr Food Res. 2009;53(2):240–255. doi: 10.1002/mnfr.200800122. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes I, Pérez-Gregorio R, Soares S, Mateus N, de Freitas V. Wine flavonoids in health and disease prevention. Molecules. 2017;22(2):E292. doi: 10.3390/molecules22020292. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sancho M, Mach N. Effects of wine polyphenols on cancer prevention. Nutr Hosp. 2014;31(2):535–551. doi: 10.3305/nh.2015.31.2.8091. Spanish. [DOI] [PubMed] [Google Scholar]
- 36.Grønbaek M, Mortensen EL, Mygind K, et al. Beer, wine, spirits and subjective health. J Epidemiol Community Health. 1999;53(11):721–724. doi: 10.1136/jech.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health J. 2007;30(1):38–41. 44–47. [PMC free article] [PubMed] [Google Scholar]
- 38.Holman CD, English DR, Milne E, Winter MG. Meta-analysis of alcohol and all-cause mortality: a validation of NHMRC recommendations. Med J Aust. 1996;164(3):141–145. doi: 10.5694/j.1326-5377.1996.tb122011.x. [DOI] [PubMed] [Google Scholar]
- 39.Arranz S, Chiva-Blanch G, Valderas-Martínez P, Medina-Remón A, Lamuela-Raventós RM, Estruch R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4(7):759–781. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kampa M, Hatzoglou A, Notas G, et al. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37(2):223–233. doi: 10.1207/S15327914NC372_16. [DOI] [PubMed] [Google Scholar]
- 41.Brizuela L, Dayon A, Doumerc N, et al. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010;24(10):3882–3894. doi: 10.1096/fj.10-160838. [DOI] [PubMed] [Google Scholar]
- 42.Burton LJ, Rivera M, Hawsawi O, et al. Muscadine grape skin extract induces an unfolded protein response-mediated autophagy in prostate cancer cells: a TMT-based quantitative proteomic analysis. PloS One. 2016;11(10):e016411. doi: 10.1371/journal.pone.0164115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenta R, Fragopoulou E, Tsoukala M, et al. Antiproliferative effects of red and white wine extracts in PC-3 prostate cancer cells. Nutr Cancer. 2017;69(6):952–961. doi: 10.1080/01635581.2017.1340489. [DOI] [PubMed] [Google Scholar]
- 44.Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittadini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res. 2001;496(1–2):171–180. doi: 10.1016/s1383-5718(01)00232-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Newcastle-Ottawa Scale.
Notes: Each study was judged on eight items, categorized into three groups: the selection of the study groups; the comparability of the groups; and the ascertainment of the exposure of interest for cohort studies. There was a maximum of 4 stars for the selection, 2 stars for the comparability, and 3 stars for exposure components. The highest quality studies are awarded up to 9 stars.
(A) Risk of bias summary of the studies that analyses the association between moderate wine consumption and PCa risk (red: high risk; green: low risk). (B) Risk of bias summary of the studies that analyses the association between moderate white and red wine consumption and PCa risk (red: high risk; green: low risk).
Abbreviation: NOS, Newcastle-Ottawa Scale.