Abstract
The growth of precision medicine has made access to biobanks with high-quality, well-annotated neuro-oncology biospecimens critical. Developing and maintaining neuro-oncology biobanks is best accomplished through multidisciplinary collaboration between clinicians and researchers. Balancing the needs and leveraging the skills of all stakeholders in this multidisciplinary effort is of utmost importance. Collaboration with a multidisciplinary team of clinicians, health care team members, and institutions, as well as patients and their families, is essential for access to participants in order to obtain informed consent, collect samples under strict standard operating procedures, and accurate and relevant clinical annotation. Once a neuro-oncology biobank is established, development and implementation of policies related to governance and distribution of biospecimens (both within and outside the institution) is of critical importance for sustainability. Proper implementation of a governance process helps to ensure that the biospecimens and data can be utilized in research with the largest potential benefit. New NIH and peer-reviewed journal policies related to public sharing of ‘omic’ data generated from stored biospecimens create new ethical challenges that must be addressed in developing informed consents, protocols, and standard operating procedures. In addition, diversification of sources of funding for the biobanks is needed for long-term sustainability.
Keywords: biobanking, biospecimens, brain tumors
The advent and growing use of high-throughput techniques for profiling biospecimens for biomarker discovery and validation for precision medicine has made access to well-annotated biobanks and high-quality biospecimens critical.1 A recent National Cancer Institute (NCI) analysis showed that research using previously established banks had lower costs overall, and resulted in more publications per year than grants proposing to do solely prospective specimen collection.2 In addition, all the tissue contributors to the TCGA effort did so—whether prospectively or retrospectively—from established biobanks. Biobanks are an invaluable resource to a neuro-oncology research program, where low incidence of specific histologies may limit prospective recruitment and collection of specimens.
Developing and maintaining these biobanks is best accomplished through collaboration between clinicians, researchers, and their institutions, and balancing the needs and “value-add” for these stakeholders is of utmost importance. Collaboration with clinicians, health care team members, institutions, and patients and their families is essential to obtaining informed consent, biospecimens with short ischemic time collected under strict standard operating procedures, and accurate and relevant clinical annotation. Neuro-oncology biobanking presents challenges that are unique from other diseases, and it requires additional disease-specific expertise in order to develop and maintain a successful bank.
One initial challenge in establishing a biobank is developing standardized policies related to governance and distribution of biospecimens (both within and outside the institution). Involving both clinical and research stakeholders in the prioritization of projects obtaining access to biospecimens is critical, so that these biospecimens, which are often limited, can be prioritized for the greatest potential benefit to participants. Having pre-established policies for transfer of biospecimens or biobanks between institutions is also a critical component for involvement of new collaborations. Creating a sustainable biobanking program can also be a challenge as the costs of consent, obtaining and processing biospecimens, storing biospecimens, and research on the biospecimens is expensive. New National Institutes of Health (NIH)3,4 and peer-reviewed journal policies related to public sharing of ‘omic’ data generated by these stored biospecimens create new ethical challenges that must be addressed by the biobanker in developing informed consents, protocols and standard operating procedures.
Biobanking 101: The Consenting Process, Participant Information, and Data Sharing
The first step in starting a biobank is to clearly define the population and types of specimens that will be collected. Unlike most other cancers, which typically occur almost entirely in adults, primary brain tumors occur both in adults and children. Moreover, the types of tumors that occur in each age group are different, and even the same types of tumors have very different prognoses in patients depending on age.5–7 It is also important to determine if any histologic types are of particular interest. Some biorepositories may wish to collect only specific types of histologies but others may collect specimens on many subtypes, potentially including both malignant and nonmalignant histologies. Pediatric and adult patients, as well as malignant and nonmalignant patients, are typically treated by different disease teams at most institutions and there is usually very little overlap in the clinical teams, though pathology and radiology teams may overlap (or not). For investigators interested in banking metastatic brain tumors, it is important to coordinate with disease teams covering the most common primaries that metastasize to brain (lung, breast, renal, GI, and melanoma)8,9 in order to obtain matched samples from both brain metastases and primary tumors. Knowing which patient populations will be targeted for the bank is critical to appropriate planning. Including pediatric patients in a population of interest may also require additional attention to protocol and consent development, as there are different ethical guidelines in place for pediatric populations. It is necessary that all critical stakeholders are involved as soon as possible in order to assure optimal bank planning. Both a narrowly defined purpose and casting a wide net have advantages and drawbacks. A narrowly focused bank allows for the concentration of limited resources on a specific tumor type of interest, and may improve the depth and quality of annotation that is able to be collected. A more broadly focused bank will allow for more potential projects in the future, as research programs and questions change, but they will require more resources in order to accrue similar numbers of prioritized histologies.
There are two distinct types of biospecimens that may be collected for biobanking: excess clinical biospecimens (also called “discarded” biospecimens) and biospecimens collected explicitly for research with informed consent. Excess clinical biospecimens comprise a portion of blood or tissue that was originally collected for clinical care, but for which the entire sample is not required for clinical care and hence can be utilized for research purposes.10 The determination of ‘discarded’ and use of these biospecimens for research is often covered under hospital policy and procedure, or an institution-wide protocol. Due to the rarity of many types of brain tumors where even tertiary academic institutions may only see a few cases of some histologies each year, access to these discarded and archival specimens is a critical resource for neuro-oncology biobanking. Access to these clinical archive specimens is often through the pathology department of these institutions, which may have specimens dating back decades. Collaboration with a neuro-pathologist to develop a protocol for access to these specimens, as well as assist in the organization, pathology review, and use of these specimens is critical. Research biospecimens are those collected intentionally for the purposes of research, with informed consent. One of the most fundamental components of establishing a successful biobank is the development of a sustainable and thorough consent process. Many research institutions have relied on ‘opt-out’ consent for use for tissue considered ‘discarded,’ but with the increasing use of these biospecimens for ‘omic’ studies, an ‘opt-in’ approach is needed and may soon be required by federal guidelines and institutional guidelines.11–14 Differentiation between excess clinical and research biospecimens is important for the purposes of biospecimen utilization, as each type of biospecimen may only be appropriately used for specific types of investigations. Regardless of the type of biospecimen collected, it is critical that the biobank itself be established through a protocol with well-defined standard operating procedures that is approved by an Institutional Review Board (IRB).
The ideal consent procedure for a successful biobank is one that is part of a streamlined process that dovetails with a participant’s clinical needs. This can be achieved through collaboration between clinicians and researchers. Neuro-oncology patients often receive multidisciplinary care, and establishing a relationship with neurosurgeons, neuro-oncologists, neuropathologists, neuro-radiologists, and radiation oncologists increases the likelihood of a successful bank. All of these disciplines provide important input into critical data and specimens to collect, as well as assist in the generation of new research questions once the bank has been established. Institutional support for this process is critical; both in terms of making time for the consent process during the participant’s clinical encounter as well as providing research nurses and/or clinical research staff who are trained to carry out the informed consent process. Prior to consent, screening for patients that fit eligibility criteria is heavily dependent on institutional support and research staff expertise. A comprehensive and accurate screening process, performed by professionals with up-to-date clinical knowledge in regards to histologic criteria and clinical care, allows for the most efficient use of resources in targeting the consenting process. Drawing on the expertise of research nurses and clinical research staff who are familiar with neuro-oncology and the experience of brain tumor patients is an additional way to increase the quality of eligibility screening, the consent process, and the clinical annotation.
Changes to NIH rules3,4 and data sharing policies at many peer-reviewed journals15 require public sharing of data. Informing participants of these policies is essential; therefore, it is critical that information related to these policies be introduced during the informed consent process. This may also require providing information to potential participants on the Genetic Information Nondiscrimination Act (GINA)16 as well as detailed information about how tissues will be used and stored. Federal grants have specific requirements for data security and require that information on plans for data storage be submitted with these grants. In addition, institutions may have specific policies and infrastructure for storage of these data.
A critical choice in setting up a biobank is determining whether or not the participants will be individually identifiable to researchers in the future. If they are to be identifiable then it is important to determine who will be allowed to gain access and use the identifiable information, and how this information can be used. There are many benefits to having deidentified biospecimens (where any information that may potentially link the biospecimen to the individual it was obtain from is removed), including the potential for these biospecimens to be used under an exempt IRB protocol. It also removes an additional level of security from biobank record keeping. However, keeping a link to the participant of origin may be preferable, as it allows for continued collection of postsurgical and clinical outcomes data. It also allows for re-review of the medical records and—for purposes including tracking treatments and clinical outcomes—that may be necessary for both hypothesis generation and for development of new research questions. For many projects, especially those focused on personalized medicine, keeping a link to the participant of origin may be critical for further data collection. Many brain tumor projects focused on biomarkers and targeted assays use progression-free survival and overall survival as endpoints, both of which require identifiability. Accurate collection of these data, particularly in regards to full treatment information and accurate progression time points, will require collaboration between researchers and a multidisciplinary clinical team. However, limited individuals should be allowed access to identifiable information and should be appropriately trained to handle these data in order to ensure security and confidentiality.
Generation of ‘omic’ data carries with it several risks that may not be present in other data types. Many federal grants now require that these data be made publically available once they have been generated. These data may reveal risk for other diseases that are not the disease of interest, or other genetic characteristics that may have an effect on individual health that were not the specific target of study. GINA16 provides protection from discrimination by insurance companies and employers (over a certain number of employees) that is based on genetic data, but this represents only one component of risk that may be associated with these samples. With the increasing generation of ‘omic’ data from banked specimens, it is important to consider that patients (and their relatives) may be individually identifiable from these data.17 Previous analyses have demonstrated that it is possible to individually identify persons from only a small number (<100) of genetic markers.18,19 Genotyping arrays and DNA sequencing generate data on >500000 markers, which means that these data are not truly ‘deidentified.’20 It may also be possible to identify individuals using RNA expression data21 or annotation data generated for analyses.22 As a result it is critical that patients are informed of all the potential risks, as well as the protections that will be afforded to them if ‘omic’ data will be generated, particularly if it will be made publically available.
Proposed changes to the Common Rule23 may significantly change the landscape of informed consent in the near future, particularly for biobanking. These proposed Common Rule changes, if instituted, would likely remove the possibility for use of biospecimens from biobanks of discarded tissues without having full informed consent. Current recommendations suggest the use of a ‘broad consent’ for collection of biospecimens. A broad consent is a consent that requests the use of biospecimens and clinical data without a specific purpose in mind, but for a broad range of future studies and identifying information remains associated with their biospecimens and clinical data.24,25 This is in contrast to blanket consent where subjects allow access to their biospecimens without any restriction, but often with the expectation that no identifying information be attached. The ‘broad consent’ would often take the form of an institution-wide informed consent, presented to potential participants upon check in to the hospital system. Because of the expense and burden of obtaining informed consent from all potential participants entering a hospital, investigators have attempted to use different types of consent procedures, including online portals and tablets, which limit the amount of labor by research professionals that is spent on consent and may be preferred by patients.26,27 ‘Broad consent’ would then allow for individual researchers to develop their own protocols—either exempt or nonexempt depending on the type of patient data required—to access these biospecimens and hence enhance translational research.
Biobanking Governance and the Institutional Review Board
A successful biobank requires engagement with the IRB at several points in the process (Figure 1). First, a biobanking protocol must be developed and approved by the IRB. Collaboration with the IRB allows the investigator to leverage the IRBs knowledge for the development of a comprehensive protocol and informed consent process that is accessible to all potential participants. This partnership between investigator and IRB is critical to the success of the biobanking process. The specific culture of each institution’s IRB will drive many of the decisions an investigator will make in setting up a biobank. Once the biobank protocol has been approved, the IRB will review it annually to determine that it is continuing to conform to ethical standards. Annual review is critical to biobanking, as ‘best practices’ continue to change over time.
Fig. 1.
Pathway for successful biobanking.
The IRB is also a critical partner once the biobank is established and has accrued enough samples to be utilized for research projects. IRBs will vary significantly on their determinations about the level of risk for secondary-use projects.28 Engaging the IRB at all stages allows the team to develop an understanding of IRB culture, and tailor the protocol and procedures to match this. Consensus ethical guidelines for these biobanks are still being developed, and as a result many IRB leaders are continuing to modify their policies and procedures about these projects.29
Assuring Quality in Biospecimen and Data Collection
Another component when starting a biobank, is that specific standard operating procedures be developed and adhered to for collection, processing, and storage of biospecimens as well as clinical data. Having a biobank of high-quality biospecimens with clinical annotations is critical for the success of a biobank and for the biobank’s value for the research community. Many large, established biobanking operations have made their standard operating procedures publicly available, including the National Cancer Institute,30 the European Organization for Research and Treatment of Cancer (EORTC),31,32 the UK Biobank,33 and many others.34
In the collection of both fresh frozen, and formalin fixed paraffin embedded (FFPE) tissues, ischemic time can have a significant effect on biospecimen quality.32,35 For some studies, the ischemic time of a tissue may not significantly affect results. DNA can be isolated from tissue that has had over 1 hour of ischemic time without significant degradation. For RNA or protein-based studies, increasing ischemic time can cause significant increases in degradation.36,37 Standard operating procedures for biobanking generally recommends an ischemic time of less than 20 to 30 minutes.31,32,38
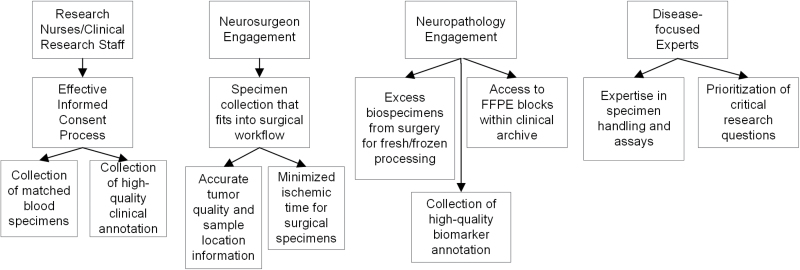
Obtaining high-quality biospecimens requires collaboration with clinicians (Figure 2). Critical to the success of a biobank is collaboration between surgeons and pathologists who specialize in the specific tissue types to be collected by each biobank. Surgeons may sometimes have to determine the optimal tissues to sample and contribute prior to surgery, and may even be willing or able to annotate the precise location of the sample for comparison to various preoperative imaging studies. Surgeons may also have to alter their technique—though never at the risk of patient safety. For example, while cavitating suction/ultrasound devices (eg, CUSA, SONAPET) are FDA-approved and may safely improve surgical efficiency, the process may alter tissue morphology and likely initiate a ‘damage’ or ‘trauma’ signal. Since the tissue is also collected in a filter attached to the device, use of this technique may also delay harvest of the sample, increasing ischemic times, and limit the surgeon’s ability to distinguish which sample came from which region. Although utilization of such specimens has been described by a few centers, the most successful tumor biobanks have typically utilized traditional mechanical resection techniques to harvest tissue for the biorepository, which has the advantage of limiting damage to the tissues and preserving histology. The surgeons must also be willing to provide specimens for research, which may (minimally) disrupt the rhythm of the surgery depending on the collection protocol and personnel involved.
Fig. 2.
Optimizing biospecimen quality through interdisciplinary collaboration.
The engagement of the pathologists is also critical for performing quality control, as well as facilitating access to discarded clinical biospecimens for research. For tissues that are collected specifically for research purposes, it is critical that they collaborate with the surgeons to minimize the time between when biospecimen is removed from the patient’s body and when it is being frozen or fixed in formalin (ischemic time). This can be accomplished by the pathologist or pathology tech coming to the operating room to “snap freeze” specimens, or by allowing the surgeons to do so in the operating room according to strict guidelines (standard operating procedures). Engaging with clinicians requires balancing their clinical duties with their roles as researchers.39,40 Of course, the clinical needs of the participant will always be primary, and as a result may sometimes limit biospecimen quantity and quality.
Engaging with clinicians allows for the collection of more accurate biospecimen annotations. Tracking ischemic time is an important component of any biobanking protocol, and these professionals will be the best sources of knowledge for time points at which a biospecimen is removed and fixed. Many biobanks may also want to collect biospecimens other than tissues, including but not limited to blood, saliva, and urine. For these biospecimens, it is critical that they be handled according to specific protocols in order to preserve the quality of DNA, RNA, and protein. For blood and saliva, these may be collected using tubes specifically designed for this purpose which contain compounds that will fix DNA and RNA for later extraction.41 These allow the biospecimens to be stored at room temperature for a longer period of time, which may work well with the work flow of some biobanks. If these are not used, biospecimens should be stored in a 4°C freezer as soon as possible. Urine should be frozen as soon as possible in order to maintain quality of protein, DNA, and RNA for future molecular analysis.
Once a biospecimen has been obtained and processed for storage, quality control procedures become essential. Many types of malignant brain tumors are particularly heterogeneous, which requires significant attention to quality control procedures. Glioblastoma in particular is highly necrotic, and it is important to make sure that specimens used for molecular assays have as minimal a proportion of necrosis as possible. Quality control criteria established by The Cancer Genome Atlas (TCGA) required a minimum tumor nuclei percentage of 60%, and maximum necrosis level of 50% for these tumors. Other types of gliomas present different quality control challenges. Many lower grade gliomas may be highly diffuse, making samples with a high proportion of tumor nuclei difficult to obtain. Depending on clinical needs and standard operating procedures, biospecimens may be evaluated for quality control prior to being fixed, or after they have been fixed. Biospecimens may be evaluated using hematoxylin and eosin stained, frozen section slides immediately prior to freezing or fixation, which would provide the most accurate structural view of the tissue. For biospecimens that are FFPE, it is a simple process to cut slides off the resulting block in order to verify tissue quality, or to determine specific parts of a biospecimen that may be best suited for an individual project. For frozen biospecimens where a slide is not taken prior to freezing, it may be necessary to evaluate these for quality control after they have already been stored in a freezer but prior to use. This may take the form of performing a frozen section on this tissue in order for a pathologist to evaluate biospecimen quality. Depending on the method of freezing, this may produce artifacts in the tissue that inhibit the quality control process while not being an accurate reflection of tissue quality. Hence recommendations to take slides from the top and bottom of each biospecimen prior to freezing may be implemented to decrease these artifacts. Once tissues have undergone quality control, it may be possible to microdissect these specimens in order to limit the amount of necrosis or normal brain tissue included in the sample. This requires additional annotation from the pathologist, as well as collaboration with persons with the technical expertise to perform this procedure. The rarity of these tumors, as well as the resources required to collect them, make it critical to maximize the ability to utilize all collected specimens.
Disease Team Collaboration for Prioritization of Biobank Resources
Biobanks may take the form of private biobanks or institutional biobanks. In the case of private biobanks, the biospecimens have been collected solely for the use of one investigator. Institutional biobanks typically provided specimen to any investigator within an institution. Biospecimens collected via the private model may also be available to other investigators, but this design gives the investigator who has established the biobank ultimate control over prioritization of projects utilizing these biospecimens. Institutional biobanks typically have the financial support of the institution at large, and may be collected via a biobanking core facility rather than an individual investigator. This type of biobank may be less focused on a specific disease, and may cast a larger net while losing the depth of clinical annotation that may be available in an individual biobank.
For either type of biobank, it is important to keep a well-organized inventory of collected biospecimens. This is a critical component in order to facilitate utilization with up-to-date knowledge of the specific biospecimens available within the biobank. The ability to query this inventory in order to meet the needs of specific projects is also critical, as different research projects may have specific needs in regards to participant demographics, biospecimen size, biospecimen heterogeneity, imaging annotations, type of treatment the participant received, clinical outcomes, etc.
For both types of biobanks, having a well-defined procedure for investigator access to and utilization of biospecimens is as critical. The amount of effort that goes into collecting and annotating these biospecimens is significant, and as a result it is important to make sure that they are being used for the highest priority projects. Individual institutions may have predetermined policies about resource sharing and access to biospecimens. This is usually determined by disease-focused teams that direct the research agenda for each disease, and are thus the best authorities on which projects should take priority. After these determinations have been made, it is the responsibility of the biobanker (whether an individual or a core facility) to facilitate access for individual investigators. In addition to prioritization, this may also include signing a data use agreement, a material transfer agreement, or other paperwork. A fee-for-service payment from the requesting investigator may also be required.
Collaboration Within and Between Institutions
Science is becoming increasingly collaborative, with many investigators having multiple collaborators across multiple institutions. As a result, it is essential to have a standard operating procedure for study prioritization and transfer of biospecimens between investigators and institutions.
Institutions likely have procedures for prioritization of use of stored biospecimens. In addition, it is important to assure that any use of the stored biospecimens is governed by an IRB-approved protocol. Depending on the amount of annotation needed by the investigator, these may be ruled as exempt or nonexempt. Investigators may choose to have collaborators or other utilizers of the biospecimens sign a data use agreement, which may also be required by some institutions. This will specifically detail the data that researchers will have access to, as well as the appropriate uses of this data.
When biospecimens or associated data leave an institution, the standard mechanism is to prepare a material transfer agreement that will be signed by both institutions.42,43 This is a legal contract that governs the use of the exchange materials, as well as protects the interests of all involved institutions and investigators. The development of these agreements involves legal representation from all institutions, who must agree to the language of the agreement and sign on behalf of the institution. The International Society for Biological and Environmental Repositories has specific recommendations44 for what should be included in these agreements, although the final contents are at the discretion of participating institutions.
Biospecimen Longitudinal Data Collection and Detailed Annotation
For many studies, collection of follow-up and clinical outcomes data may be essential, especially for studies of biomarkers of survival or response to treatment. The first step in collecting this type of data is making sure that this is included in the informed consent document, and that each participant individually opts in to this active follow-up (ie, they are contacted over time for status updates) or passive follow-up (ie, their medical records as searched on a regular basis for status updates). Since not all participants who consent to biobanking may consent to this option, it is important to be able to track the detailed consent information for any participant included in the biobank. A database that includes all consent information and is easily searchable is essential. Even with this consent, it may limit investigator access to participant information only at the location where the biospecimen was originally collected. As many patients will at some point utilize health care services at more than one institution, or move out of region, not all follow-up data will be available to the investigator. Once information on a participant is no longer available, it is important to note that this participant has been lost to follow-up. In the case of measuring outcomes data, failing to note this information may result in inflation of the participant’s survival time or time to recurrence as the investigator does not have access to the date of death or recurrence via the institution’s records.
Within the consent, it is important to note specifically what information the investigator will be collecting going forward. This is specifically required under HIPAA, which states that researchers must specifically outline what information would be accessed from the patient’s medical record as well as detailed information about who will be allowed to access the information.45 It may be useful to the investigator to use language that provides blanket access to the entirety of the participant’s record at that institution. Without this type of language, investigators may be limited going forward as research questions change. For example, for a cancer-based study where a participant has consented only to follow-up regarding their specific disease, an investigator may not be able to access information regarding future health conditions that may or may not be associated with their cancer diagnosis.
With biobanks that are institutional and individually identifiable, linkage of the biobank to the electronic medical record can provide cost-effective clinical annotation with relatively high accuracy.46 Regardless of whether this type of infrastructure exists, careful thought should be given to the knowledge level of the person responsible for abstracting the data. For some pieces of data (eg, specific disease diagnosis based on lab results or interpretation of symptoms), it may be necessary for a clinician to review a participant’s record. For other information, such as prescription drugs a participant may be taking, this information could be abstracted from a well-trained, nonclinical research professional. The individual responsible for abstracting this information has a significant effect on the accuracy and reliability of the data collected, and it is important to make sure that both of these features are being maximized. Collecting accurate and complete clinical data requires collaboration with the multidisciplinary neuro-oncology team at the institution. Many factors, such as Karnofsky Performance Status, are routinely collected as part of clinical care but may not always be included in the patient’s clinical chart. Collaborating with clinicians helps to ensure that this data is collected prospectively, and increases the validity of outcomes studies conducted using this data.
As biomarkers become increasingly important in the categorization of brain tumor histologies, most notably with the recent revisions to the WHO grade classification scheme5 that utilize many validated biomarkers in their revised definitions, this information becomes critical to efficiently select patient samples for analyses from the biobank. Though many studies using the samples may perform ‘omic’ testing, many of these markers may already have been evaluated as part of a patient’s clinical care. These may include immunohistochemistry staining for mutations in IDH1, INI1, P53, or ATRX, fluorescence in situ hybridization (FISH) for 1p/19q deletion or EGFR amplification, and methylation analyses for MGMT. The regularity with which any of these tests are ordered will vary depending on the patient population seen by an institution, as well as by the individual neuropathologist. Planning for abstraction of these variables up front will increase the usability and efficiency of banked specimens. This is also a critical point for collaboration with the multidisciplinary clinical team at the institution. Working with the neuropathologists to find what markers they are routinely testing for, and how these markers are being assessed, is critical for planning data collection as well as using this data in the future, particularly for biomarkers that are not Clinical Laboratory Improvement Amendments (CLIA) approved.
Working Towards Long-Term Biobanking Sustainability
Building a successful and useful biobank requires long-term commitment from individual investigators, as well as sustained institutional support (Figure 1). Initial costs for starting a biobank will be much higher than those for regular maintenance, and these may not necessarily be supported by investigators’ grants. Development of these biobanks is an important foundation on which to build grant submissions, so this initial support is in the best interest of both the investigators and institutions. Once established, there are regular costs associated with maintenance of the biobank (eg, staff to screen and consent participants and follow-up with participants, staff to manage freezers, maintenance of databases, freezer maintenance, etc.) that may not necessarily factor into a price-per-biospecimen. Planning for these needs is critical to creating a sustainable biobank.47
It is important to distinguish at the outset of a biobank whether or not the biospecimens collected under the biobanking protocol reside primarily with the investigator or with the institution. Investigators may not necessarily remain affiliated with the institution where the biobank has been established, and it is important to plan for this contingency ahead of time. If the investigator has funding tied to the biobank and is continuing to use these biospecimens in their research initiatives, they may want to take the biospecimens or aliquots of the biospecimens and a copy of any clinical annotations with them when they leave the institution. Due to changes in institutional structure, biobanks may also change institutional affiliation after their establishment. Having a plan in place for these contingencies is essential.
There are many different positions/models and policies on biospecimen ‘ownership,’ and these will vary by individual and institution.48 There is no clearly accepted legal position.49,50 These positions/models could largely be divided into whether the relationship between the investigator/institution and biospecimens are seen as one of ‘ownership’ (where the institution is the legal owner of the biospecimen), vs ‘stewardship’ (where the investigator and institution are acting as steward, while the patient retains ownership of the biospecimen).51,52 These positions/models may also vary depending on whether these biospecimens were collected for the purposes of research or as clinical samples. Even though biospecimens were collected specifically for the purposes of research, if they arose as a result of a patient’s clinical care (eg, collected during a surgery), there may be occasions when all biospecimens need to be utilized for clinical work and will no longer be available for research. A clear ownership/governance structure that balances the needs of researchers and institutions, while also taking into account the desires of participants is also critical to successful and compliant use of the biospecimens for research.
Conclusions
The age of personalized medicine has made the development and sustainability of biobanks containing high-quality and well-annotated biospecimens critical to ongoing neuro-oncology research. Starting and managing a useful and sustainable neuro-oncology biobank requires a significant amount of planning, organization, and management in order to optimize the utility of these resources to maximize opportunities for collaboration. This is critical as research methodologies and scientific questions change quite rapidly. Institutional support for these biobanks is a critical component of their ability to lead to strong science and increase the institutions’ competitiveness for future research funding and support.
Funding
QTO and JSB-S were supported by the National Cancer Institute Case Comprehensive Cancer Center Support Grant (P30CA043703).
References
- 1. Schully SD, Carrick DM, Mechanic LE, et al. Leveraging biospecimen resources for discovery or validation of markers for early cancer detection. J Natl Cancer Inst. 2015;107(4):djv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrick DM, Mette E, Hoyle B, et al. The use of biospecimens in population-based research: a review of the National Cancer Institute’s Division of Cancer Control and Population Sciences grant portfolio. Biopreserv Biobank. 2014;12(4):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institutes of Health. NOT-OD-03-032: Final NIH Statement on Sharing Research Data http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html Published February 26, 2003. Accessed November 3, 2016.
- 4. National Institutes of Health. NOT-OD-14-124: NIH Genomic Data Sharing Policy http://grants.nih.gov/grants/guide/notice-files/NOT-OD-14-124.html Published August 27, 2014. Accessed November 3, 2016.
- 5. Louis DN, Ohgaki H, Wiestler OD, Cavanee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 6. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. The Cancer Genome Atlas Research Network , Brat DJ, Verhaak RG, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 9. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 10. Riegman PH, van Veen EB. Biobanking residual tissues. Hum Genet. 2011;130(3):357–368. [DOI] [PubMed] [Google Scholar]
- 11. Giesbertz NA, Bredenoord AL, van Delden JJ. Inclusion of residual tissue in biobanks: opt-in or opt-out? PLoS Biol. 2012;10(8):e1001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pulley J, Clayton E, Bernard GR, et al. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helgesson G, Dillner J, Carlson J, et al. Ethical framework for previously collected biobank samples. Nat Biotechnol. 2007;25(9):973–976. [DOI] [PubMed] [Google Scholar]
- 14. Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alsheikh-Ali AA, Qureshi W, Al-Mallah MH, et al. Public availability of published research data in high-impact journals. PLoS One. 2011;6(9):e24357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Genetic Information Nondiscrimination Act of 2008, Pub L No. 110–233, 110th Cong, 2nd Sess (2008) http://www.eeoc.gov/laws/statutes/gina.cfm Accessed November 3, 2016.
- 17. Weil CJ, Mechanic LE, Green T, et al. NCI think tank concerning the identifiability of biospecimens and “omic” data. Genet Med. 2013;15(12):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin Z, Owen AB, Altman RB. Genetics. Genomic research and human subject privacy. Science. 2004;305(5681):183. [DOI] [PubMed] [Google Scholar]
- 19. Gymrek M, McGuire AL, Golan D, et al. Identifying personal genomes by surname inference. Science. 2013;339(6117):321–324. [DOI] [PubMed] [Google Scholar]
- 20. McGuire AL, Gibbs RA. Genetics. No longer de-identified. Science. 2006;312(5772):370–371. [DOI] [PubMed] [Google Scholar]
- 21. Schadt EE, Woo S, Hao K. Bayesian method to predict individual SNP genotypes from gene expression data. Nat Genet. 2012;44(5):603–608. [DOI] [PubMed] [Google Scholar]
- 22. Im HK, Gamazon ER, Nicolae DL, et al. On sharing quantitative trait GWAS results in an era of multiple-omics data and the limits of genomic privacy. Am J Hum Genet. 2012;90(4):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. 80 FR 53931: Federal Policy for the Protection of Human Subjects—Notice Of Proposed Rulemaking 2015; https://federalregister.gov/a/2015-21756 Accessed September 21, 2015.
- 24. Grady C, Eckstein L, Berkman B, et al. Broad consent for research with biological samples: workshop conclusions. Am J Bioeth. 2015;15(9):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wendler D. Broad versus blanket consent for research with human biological samples. Hastings Cent Rep. 2013;43(5):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowbotham MC, Astin J, Greene K, et al. Interactive informed consent: randomized comparison with paper consents. PLoS One. 2013;8(3):e58603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thiel DB, Platt J, Platt T, et al. Testing an online, dynamic consent portal for large population biobank research. Public Health Genomics. 2015;18(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldenberg AJ, Maschke KJ, Joffe S, et al. IRB practices and policies regarding the secondary research use of biospecimens. BMC Med Ethics. 2015;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rothwell E, Maschke KJ, Botkin JR, et al. Biobanking research and human subjects protections: perspectives of IRB Leaders. IRB. 2015;37(2):8–13. [PMC free article] [PubMed] [Google Scholar]
- 30. National Cancer Institute, Biorepositories and Biospecimen Research Branch. Biospecimen collection, processing, storage, retrieval, and dissemination. Best Practices—Technical and Operational Best Practices. http://biospecimens.cancer.gov/bestpractices/to/bcpsrd.asp Updated: March 29, 2016. Accessed: November 3, 2016. [Google Scholar]
- 31. Mager SR, Oomen MH, Morente MM, et al. Standard operating procedure for the collection of fresh frozen tissue samples. Eur J Cancer. 2007;43(5):828–834. [DOI] [PubMed] [Google Scholar]
- 32. Morente MM, Mager R, Alonso S, et al. TuBaFrost 2: Standardising tissue collection and quality control procedures for a European virtual frozen tissue bank network. Eur J Cancer. 2006;42(16):2684–2691. [DOI] [PubMed] [Google Scholar]
- 33. Peakman TC, Elliott P. The UK Biobank sample handling and storage validation studies. Int J Epidemiol. 2008;37(Suppl 1):i2–i6. [DOI] [PubMed] [Google Scholar]
- 34. Yong WH, Dry SM, Shabihkhani M. A practical approach to clinical and research biobanking. Methods Mol Biol. 2014;1180:137–162. [DOI] [PubMed] [Google Scholar]
- 35. Turashvili G, Yang W, McKinney S, et al. Nucleic acid quantity and quality from paraffin blocks: defining optimal fixation, processing and DNA/RNA extraction techniques. Exp Mol Pathol. 2012;92(1):33–43. [DOI] [PubMed] [Google Scholar]
- 36. Hong SH, Baek HA, Jang KY, et al. Effects of delay in the snap freezing of colorectal cancer tissues on the quality of DNA and RNA. J Korean Soc Coloproctol. 2010;26(5):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Cecco L, Musella V, Veneroni S, et al. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009;9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shabihkhani M, Lucey GM, Wei B, et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin Biochem. 2014;47(4–5):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thasler WE, Thasler RM, Schelcher C, et al. Biobanking for research in surgery: are surgeons in charge for advancing translational research or mere assistants in biomaterial and data preservation? Langenbecks Arch Surg. 2013;398(4):487–499. [DOI] [PubMed] [Google Scholar]
- 40. Tsikitis VL, Lu KC, Douthit M, Herzig DO. Surgeon leadership enables development of a colorectal cancer biorepository. Am J Surg. 2013;205(5):563–565; discussion 565. [DOI] [PubMed] [Google Scholar]
- 41. Wahlberg K, Huggett J, Sanders R, et al. Quality assessment of biobanked nucleic acid extracts for downstream molecular analysis. Biopreserv Biobank. 2012;10(3):266–275. [DOI] [PubMed] [Google Scholar]
- 42. Parodi B, Visconti P, Ruzzon T, Truini M. Governance of biobanks for cancer research: proposal for a material transfer agreement. In: Pascuzzi G, Izzo U, Macilotti M, eds. Comparative Issues in the Governance of Research Biobanks. Berlin, Germany: Springer-Verlag; 2013:327–332. [Google Scholar]
- 43. Hallmans G, Vaught J. Best practices for establishing a biobank. In: Dillner J, ed. Methods in Biobanking. Vol 675 New York, NY: Humana Press; 2011:241–260. [DOI] [PubMed] [Google Scholar]
- 44. International Society for Biological and Environmental Repositories. 2012 best practices for repositories collection, storage, retrieval, and distribution of biological materials for research international society for biological and environmental repositories. Biopreserv Biobank. 2012;10(2):79–161. [DOI] [PubMed] [Google Scholar]
- 45. Clayton EW. Informed consent and biobanks. J Law Med Ethics. 2005;33(1):15–21. [DOI] [PubMed] [Google Scholar]
- 46. Bowton E, Field JR, Wang S, et al. Biobanks and electronic medical records: enabling cost-effective research. Sci Transl Med. 2014;6(234):234cm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uzarski D, Burke J, Turner B, et al. A Plan for academic biobank solvency-leveraging resources and applying business processes to improve sustainability. Clin Transl Sci. 2015;8(5):553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cadigan RJ, Easter MM, Dobson AW, et al. “That’s a good question”: university researchers’ views on ownership and retention of human genetic specimens. Genet Med. 2011;13(6):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hakimian R, Korn D. Ownership and use of tissue specimens for research. JAMA. 2004;292(20):2500–2505. [DOI] [PubMed] [Google Scholar]
- 50. Hakimian R, Taube S, Bledsoe M, Aamodt R. National Cancer Institute Cancer Diagnosis Program: 50-state Survey of Laws Regulating the Collection, Storage, and Use of Human Tissue Specimens and Associated Data for Research. US Department of Health and Human Services, National Institutes of Health; 2004. [Google Scholar]
- 51. Dressler LG. Biospecimen “ownership”: counterpoint. Cancer Epidemiol Biomarkers Prev. 2007;16(2):190–191. [DOI] [PubMed] [Google Scholar]
- 52. Ness RB; American College of Epidemiology Policy Committee Biospecimen “ownership”: point. Cancer Epidemiol Biomarkers Prev. 2007;16(2):188–189. [DOI] [PubMed] [Google Scholar]