Abstract
Medulloblastoma is the most common malignant brain tumor affecting children. These tumors are high grade with propensity to metastasize within the central nervous system and, less frequently, outside the neuraxis. Recent advancements in molecular subgrouping of medulloblastoma refine diagnosis and improve counseling in regards to overall prognosis. Both are predicated on the molecular drivers of each subgroup—WNT-activated, SHH-activated, group 3, and group 4. The traditional therapeutic mainstay for medulloblastoma includes a multimodal approach with surgery, radiation, and multiagent chemotherapy. As we discover more about the molecular basis of medulloblastoma, efforts to adjust treatment approaches based on molecular risk stratification are under active investigation. Certainly, the known neurological, developmental, endocrine, and psychosocial injury related to medulloblastoma and its associated therapies motivate ongoing research towards improving treatment for this life-threatening tumor while at the same time minimizing long-term side effects.
Keywords: chemotherapy, medulloblastoma, radiation, risk stratification, targeted therapy
Clinical Case Presentation
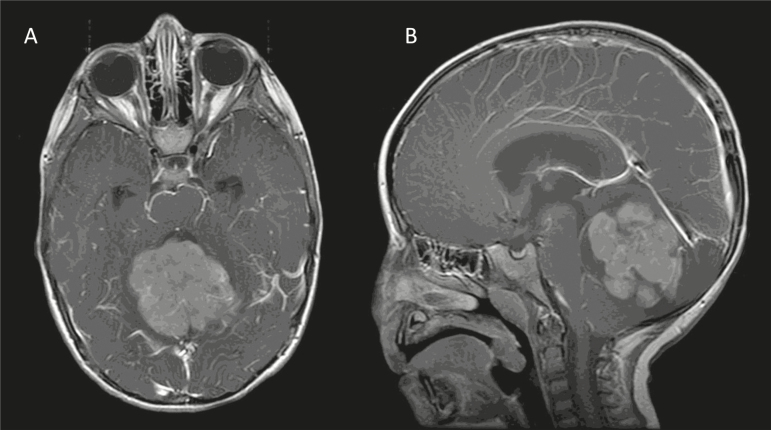
A 2-year-old boy with gross motor delays presented with wide-based, unsteady gait and headaches over 1 month. A brain MRI reveals a large posterior fossa mass (Fig. 1). He underwent gross total resection and pathology showed medulloblastoma, World Health Organization (WHO) grade IV.
Fig. 1.
Axial (A) and sagittal (B) postgadolinium, T1-weighted images at time of diagnosis.
Epidemiology
Medulloblastoma is the most common malignant brain tumor of childhood, comprising about 20% of all pediatric brain tumors.1 They can occur in both children and adults, although greater than 70% of cases are found in children less than 18 years old.2 Medulloblastomas are considered high-grade embryonal tumors based on histology and cell of origin, and have historically been grouped with other embryonal tumors under the category of primitive neuroectodermal tumors (PNET). However, medulloblastomas are now recognized as their own entity and, based on molecular pathways that drive their tumorigenesis, are subclassified into WNT-activated, SHH-activated, group 3, and group 4.3
The incidence of medulloblastoma is estimated to be 0.7 per 100000 children per year with a male predominance; the relative risk for males is 1.5 times that of females.1 The male predominance appears to be greatest among patients older than 3 years with a near 1:1 male-to-female ratio occurring in children less than 3 years.2 Sex may also have a prognostic role, with one recent review of over 1200 patients showing a median survival in females of 152 months as compared to only 90 months in males.2
There are several genetic predisposition syndromes linked to the development of medulloblastoma. Gorlin syndrome involves a germline mutation in the PTCH1 or SUFU genes and leads to heightened risk of basal cell carcinomas and medulloblastoma, with SUFU mutations carrying the highest risk for medulloblastoma.4,5 Other germline mutations associated with medulloblastoma include APC mutations in Turcot syndrome type 2, TP53 mutations in Li-Fraumeni Syndrome, as well as BRCA2 and PALB2.5–7
Clinical Case Relevance
Our case exhibited the usual epidemiology patterns—he was a male who presented at 2 years of age. On presentation, he had no evidence of familial predisposition syndrome, no notable family history of cancer, and no signs suggestive for a genetic syndrome.
Initial Supportive Care
Medulloblastoma most frequently arises at the level of the fourth ventricle within the posterior fossa. Accordingly, common presenting symptoms are consistent with obstructive hydrocephalus or cerebellar dysfunction including headache, vomiting, gait disturbance, and imbalance.8,9 Cranial nerve palsies can be seen and in infants, macrocephaly can be prominent.9 Average time from symptom onset to diagnosis ranges from 2 to 6 months.8,9
Corticosteroids, Emergent Radiation, and Surgery
It is important to recognize patients with medulloblastoma are at risk of increased intracranial pressure. For patients showing evidence of obstruction and in whom immediate surgical resection is not possible, initiation of corticosteroids to decrease tumor-associated edema and/or surgical placement of an external ventricular drain or ventriculoperitoneal shunt may be considered. Patients with bulky, metastatic leptomeningeal disease may present with spinal cord compression and lower extremity weakness or bladder/bowel incontinence. In this setting, corticosteroids should be initiated quickly along with possible emergent radiation or debulking surgery to prevent permanent spinal cord injury. Seizures are a less commonly seen presenting symptom, but do occur.
Clinical Case Relevance
The patient was found to have mild hydrocephalus without evidence of metastatic disease at time of initial diagnosis. Dexamethasone was initiated prior to resection.
Initial Diagnostic Imaging
The majority of medulloblastomas occur in a midline location, involving the vermis or fourth ventricle, but can be seen more laterally in the cerebellar hemispheres.10,11 On CT imaging, medulloblastoma appears hyperdense, but tumor extension into the fourth ventricular foramina may be poorly characterized.10,11 Compared to CT, MRI is a more powerful method to evaluate the primary tumor, leptomeningeal spread, and extent of disease within the fourth ventricular foramen or cerebellopontine angle.11 On T1-weighted imaging, medulloblastoma can be isointense or hypointense; while on T2/FLAIR (fluid attenuated inversion recovery) imaging, tumors are hyperintense.10,11 The distinguishing characteristic of medulloblastoma on MRI is reduced diffusion on diffusion-weighted imaging.12 This feature is attributable to high cellularity and nuclear-to-cytoplasmic ratio. Large cell and anaplastic variants often show increased apparent diffusion coefficient values.13
Spine imaging is critical for evaluation of metastatic disease and contrast-enhanced MRI is more sensitive in detecting metastatic spread than CSF analysis alone.14 Disease in the spine usually manifests as nodular enhancement of leptomeninges with nerve root thickening.14 MR spectroscopy typically demonstrates elevated choline, reduced N-acetyl aspartate, and occasional lactate peaks.10 PET imaging is not routinely indicated for medulloblastoma staging, but 18F-fluorodeoxyglucose ([18F]FDG) uptake has been variably correlated with survival and leptomeningeal dissemination.15,16
MRI characteristics such as tumor location and enhancement correlate with molecular subtypes of medulloblastomas.13,17 WNT-activated tumors are predominantly located within the cerebral peduncle/cerebellopontine angle. SHH-activated tumors are commonly found in cerebellar hemispheres. Group 3 and group 4 tumors are found along the midline with extension into the fourth ventricle.18,19 Lack of contrast enhancement in group 4 tumors can sometimes differentiate them from group 3 tumors.17
Surgery
Goals of Surgery
The goals for operative intervention are: 1) relieve mass effect, 2) re-establish CSF circulation, 3) obtain diagnostic tissue, and 4) reduce tumor burden. At the time of diagnosis, gross total resection of the primary tumor is standard of care. However, survival difference between gross total resection and near total resection (> 90% of tumor removed) has not been proven.20
Treatment of Hydrocephalus
Although hydrocephalus is noted in the majority of patients presenting with medulloblastoma, placement of a ventriculoperitoneal shunt or external ventricular drain prior to resection is rarely indicated.21,22 In addition to infectious risk, upward transtentorial herniation is a rare but serious complication of preoperative ventricular drainage.23 Earlier tumor detection and the use of corticosteroids have also made preoperative drainage less necessary than in the past. In cases where symptoms of hydrocephalus are refractory to maximal medical therapy, an external ventricular drain should be placed and CSF drained in a controlled manner. Most patients have resolution of hydrocephalus following tumor resection, but approximately 40% will require a ventriculoperitoneal shunt within 4 weeks of resection.22 Lee and others identified factors associated with permanent shunting include young age, extensive preoperative ventricular dilatation, and large tumors.22 Patients with postoperative lethargy, especially if worsening, or with a large pseudomeningocele should be screened for hydrocephalus.
Surgical Technique
Medulloblastomas frequently push the vermis in the posterior direction leading to division or interruption of the vermis with tumor removal. Two key approaches, telovelar and transvermian, are used to minimize damage to the vermis and deep cerebellar nuclei.
Telovelar vs Transvermian Approach
The telovelar approach uses the cerebellomedullary fissure, defined as the cleft between the anterior surface of the tonsils and the posterior aspect of the caudal medulla. No known functional neural tissue exists within these structures, making this approach the choice of many surgeons. For larger tumors, extension through the foramen of Magendie and thinning of the vermis and cerebellar peduncles may be present. In these cases, the tumor should be debulked to allow for partial restoration of normal anatomy.24 For the transvermian approach, the inferior vermis is identified and split vertically. Generally, the telovelar approach is preferred given the proposed relationship between splitting the vermis and development of cerebellar mutism.25,26
Tumor Resection—Both Approaches
During resection, an attempt to locate a plane between the tumor and surrounding tissue should be made. This allows for resection without damage to underlying structures. Neuronavigation and neuromonitoring may be useful and identifying the fourth ventricle above and below the tumor is essential to prevent entry into the brainstem. From a vascular standpoint, medulloblastomas may have large draining veins and preservation of these can prevent significant bleeding.
Complications
A dreaded complication following medulloblastoma resection is cerebellar mutism syndrome, also referred to as posterior fossa syndrome.25 These terms describe a syndrome of speech apraxia, hypotonia, ataxia, emotional lability, and cranial nerve deficits. Up to 30% of patients experience cerebellar mutism after undergoing medulloblastoma resection. Multiple theories aim to explain etiology; however, none have been proven. One popular theory suggests splitting of the inferior vermis plays a role, yet avoiding splitting fails to improve the rate of mutism.25,26 The dentatothalamic pathway may play a role, but is also not the sole determinant. Most patients regain at least some speech but may experience persistent lack of full speech, abnormal tone, and balance problems.25,27 Additional postoperative complications include infection, pseudomeningocele, and CSF leak.
Clinical Case Relevance
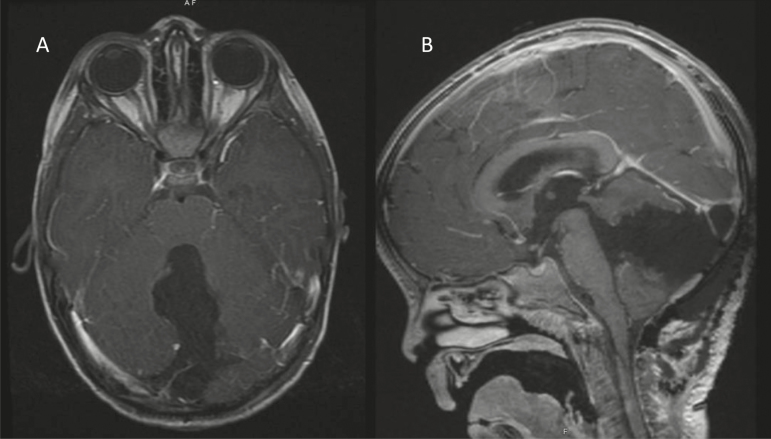
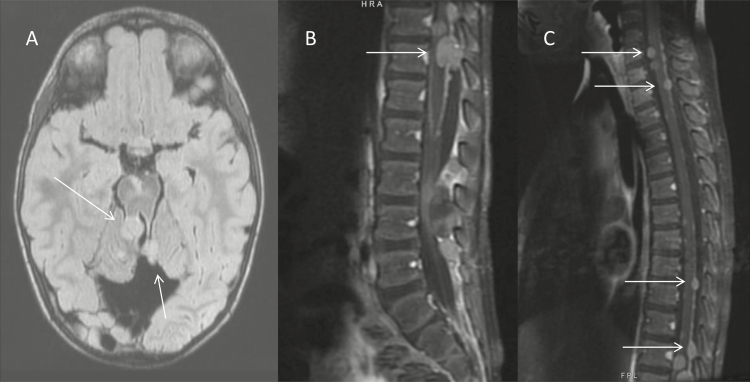
Our patient underwent gross total resection at time of initial diagnosis (Fig. 2). The patient had no intraoperative or postoperative complications. At the time of recurrence, the patient was not deemed a surgical candidate given the extent of metastatic disease and evidence of spinal cord compression (Fig. 3).
Fig. 2.
Axial (A) and sagittal (B) postgadolinium, T1-weighted images postsurgical resection.
Fig. 3.
Axial fluid-attenuated inversion recovery (FLAIR) imaging of brain (A), postgadolinium, T1-weighted lumbar spine (B), and cervical and thoracic spine (C) at time of recurrence. Arrows indicate sites of recurrent, metastatic disease.
Staging
Medulloblastoma has a high likelihood of spreading throughout the central nervous system and is one of the few central nervous system tumors with potential for extraneural metastasis. Thus, standard of care includes brain and spine imaging and, if clinically indicated, imaging for extraneural metastases. Staging should include full spine MRI and CSF cytology to assess for leptomeningeal dissemination. Because the combination of full spine imaging and CSF analysis is more sensitive than each modality alone, both should be done to most reliably identify spinal metastatic disease.28 If spine imaging is not done prior to surgery, it can be done postoperatively but not before 10 days after surgery due to potential for false positives from postsurgical blood products within the subarachnoid space. If there is concern for extraneural metastases such as in bone marrow (blood count abnormalities), lymph nodes (palpable, firm nodes), or bone (pain or presence of solid masses), bone marrow aspiration and biopsy and whole body PET or bone scans should be done.
Prior to the more recent molecular risk stratification, patients older than 3 years were staged according to degree of resection combined with amount of metastases. The Chang staging system identified tumors as M0 to M4 ranging from no evidence of gross metastasis to extraneural metastasis.29 This score was combined with degree of surgical resection (< or ≥ 1.5 cm2 postoperative residual tumor) to determine low-risk (M0 and < 1.5 cm2 residual tumor) or high-risk (M1-4 and ≥ 1.5 cm2 residual tumor) disease. Current staging continues to use broad categories of focal or metastatic residual disease, but now incorporates histologic and molecular markers.
Clinical Case Relevance
The patient had negative spine imaging and CSF cytology at time of initial diagnosis, but was found to have clear evidence of bulky metastatic leptomeningeal disease at recurrence.
Pathology
Medulloblastoma is a primitive, small round blue cell tumor of the neuronal lineage. It is a high-grade embryonal neoplasm that demonstrates brisk mitotic activity, scattered apoptotic cells, and foci of necrosis. Neuronal differentiation is evidenced by diffuse synaptophysin positivity in most tumors, although focal glial, melanotic, or myogenic differentiation can be observed. Histologic subtypes of medulloblastoma include classic, large cell, anaplastic, nodular/desmoplastic, and extensive nodularity. Medulloblastomas with large cell or anaplastic features typically show a mixture of these 2 variants, and such tumors are classified as combined large cell/anaplastic histologic subtype. Tumors with classic histology show cells with minimal cytoplasm and dense basophilic nuclei present in diffuse sheets. Homer Wright (neuroblastic) rosettes may be seen. Large cell medulloblastoma is comprised of cells with increased cytoplasm, large nuclei with vesicular chromatin, and conspicuous nucleoli. Anaplastic medulloblastoma is composed of cells with marked nuclear pleomorphism, atypical mitotic figures, nuclear molding, and cell-cell wrapping. Nodular/desmoplastic medulloblastoma demonstrate pale islands or nodules of neurocytic differentiation with more cellular and mitotically active internodular zones that contain a dense intercellular reticulin network. Medulloblastomas with extensive nodularity are composed of numerous back-to-back nodules with thin internodular zones that are relatively devoid of primitive elements.30,31
Beyond histology, medulloblastoma classification is based on molecular differences and signaling pathways driving tumor development. In 2010, a consensus statement identified four predominant subgroups: WNT-activated, SHH-activated, group 3, and group 4.32 Immunohistochemical markers help stratify medulloblastomas into each of the molecular subgroups: WNT-activated tumors typically show classic histology and immunostaining positive for β-catenin aberrantly located in cell nuclei; SHH-activated frequently show nodular/desmoplastic histology and immunostaining positive for GAB1; Group 3 and Group 4 tumors commonly have either classic or large cell/anaplastic histologic features and negative GAB1 and nuclear β-catenin immunostaining.32
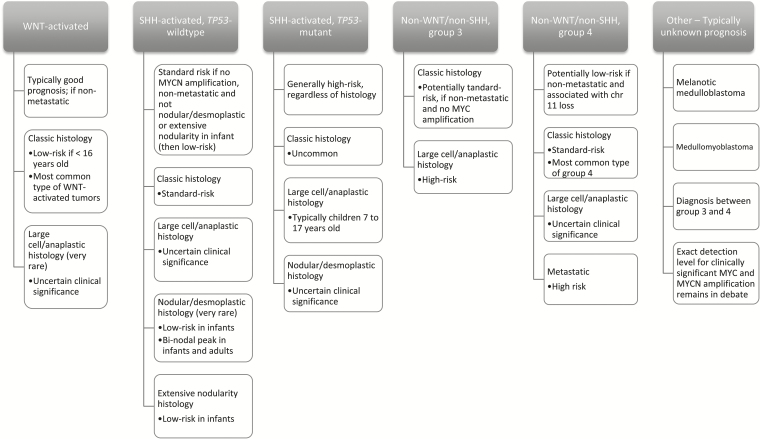
In 2016, the revised fourth edition of the WHO Classification of Tumours of the Central Nervous System recommended integrated medulloblastoma classification, including both histologic and molecular features into the diagnosis.33 Each subgroup potentially portends important prognostic indications; however, rather than individually delineating separate prognoses for every possible histologic and genetic combination, the guidelines encourage integrated interpretation based on findings unique to each tumor.33 The guidelines highlight five commonly identified histologically and molecularly integrated subgroups with associated prognoses (Fig. 5, derived from Louis et al and Ramaswamy et al).33,34
Fig. 5.
Example of World Health Organization (WHO) 2016 molecularly integrated classification for medulloblastoma, with anticipated corresponding clinical and prognostic characteristics.30,34
Clinical Case Relevance
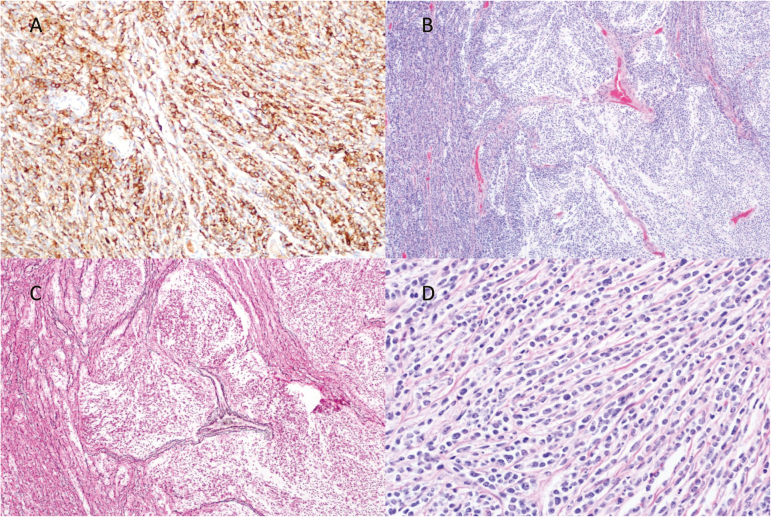
The patient’s tumor was nodular/desmoplastic subtype with SHH-activation. Histology revealed a primitive small round blue cell tumor with intervening pale nodules composed of cells with neurocytic differentiation. There was diffuse synaptophysin positivity with increased staining in the nodules. SMARCB1/INI-1 was intact in tumor cells, and no nuclear staining for β -catenin was seen. Diffuse strong immunostaining for GAB1 was present. Fluorescence in situ hybridization revealed absence of MYC or MYCN amplification and diploid status of chromosome 17 (Fig. 4). At time of relapse, targeted next-generation sequencing was performed on tumor tissue and peripheral blood. The patient was found to have a germline inactivating frameshift mutation in the SUFU gene with loss of remaining wild-type allele in the tumor. The presence of a germline SUFU mutation combined with the patient’s known medulloblastoma met criteria for diagnosis of Gorlin syndrome.35
Fig. 4.
Pathology from initial diagnosis showing diffuse positivity for GAB1 staining (A), scattered nodules (B), reticulin (C), and desmoplasia (D).
Radiation Therapy
Adjuvant treatment for medulloblastoma often commences with radiotherapy, usually 3 to 4 weeks after surgery. Radiation involves treatment of the entire craniospinal axis, known as craniospinal irradiation (CSI). Patients with standard-risk disease have conventionally received CSI with 23.4 Gy plus a boost to 54 to 59.4 Gy to the posterior fossa. The classic CSI field for medulloblastoma consists of prone positioning with opposed lateral brain portals and posterior spinal axis field, including sacral nerve roots with matching in the region of the cervical spine or at posterior spinal axis junctions if multiple fields are necessary. Daily verification of beam alignment is done with light field matching. Historically, the posterior fossa was treated with at least a 1-cm margin and inferior border set at C2. However, recent investigations demonstrated focal radiation to the surgical bed is comparable to entire posterior fossa radiation with less risk of radiation exposure to surrounding structures.36,37 Advances in image-guided radiotherapy, such as daily cone beam computerized tomography, have facilitated more focal irradiation and many now practice this more limited approach. The randomized phase 3 study ACNS0331 evaluated whether target volume reduction to the primary tumor bed alone could be performed without compromising disease control in standard-risk medulloblastoma. Initial results confirm equivalent 5-year event free and overall survival for focal surgical bed versus entire posterior fossa boost.
Evolving understanding of the genomic drivers and prognostic indicators of medulloblastoma, combined with adverse effects of CSI in young patients, suggests a single approach for all patients is not appropriate.32 Recent studies have demonstrated intensification of chemotherapy with autologous stem cell rescue may allow reduction or delay of CSI without decreasing survival.38,39 CSI dose reduction or complete avoidance is currently being investigated in patients with advantageous features (ie, focal, WNT-activated; NCT 01878617, NCT02017964). In standard-risk patients, CSI to a total dose of 23.4 Gy (reduced from ≥ 36 Gy) remains effective when administered with adjuvant chemotherapy.40 Unfortunately, results of ACNS0331 demonstrated inferior event-free survival and overall survival with further CSI decrease to 18 Gy.41
Alternative radiation modalities such as proton-beam and volumetric arc or intensity-modulated radiation therapy are increasingly employed to minimize dose outside target volumes and mitigate long-term effects of radiation. Outcome data for these modalities are limited; however, comparisons suggest superior sparing of normal structures with the use of protons.42
Re-irradiation
Re-irradiation is often not considered for recurrent medulloblastoma due to potential toxicity and uncertain efficacy. However, several have reported this technique is safe and may prolong survival, with most benefit derived in patients who have no evidence of residual disease after re-resection.43,44 In select cases, re-irradiation for relapsed medulloblastoma has achieved 5-year progression free and overall survival from first relapse of 48% and 65%, respectively.44
Clinical Outcomes After Radiation
Medulloblastoma survivors are affected by intellectual, neurological, and physical disabilities from treatment, with profound impacts on quality of life, neurocognitive dysfunction, strokes, and risk of secondary malignancies.27,45–47 Long-term adverse effects of radiotherapy vary directly with dose, and inversely with patient age at time of radiotherapy.48 Baseline assessments of cognitive and endocrine function should be performed for all patients, as CSI may lead to stunted growth and development due to neurologic, neuroendocrine, and skeletal insult. In terms of neurocognition, processing speed and memory appear most affected, but all neurocognitive parameters should be followed.45
Clinical Case Relevance
The patient was initially treated on study through the radiation-sparing trial ACNS1221. At time of recurrence, he underwent emergent CSI to a total dose of 36 Gy with a posterior fossa boost of 18 Gy and lower spine boost of 9 Gy. The patient went on to show diffuse white matter hyperintensity as well as global volume loss on follow-up imaging, likely due to radiation.
Chemotherapy
A variety of chemotherapy and radiation combinations and, more recently, targeted agents and immunotherapy, have been investigated in the treatment of medulloblastoma. Regardless of the approach, nearly all strategies recommend multiple agents serving as a backbone to decrease or eliminate exposure to radiation and, for the extremely young, delay time to radiation and limit permanent neurocognitive injury.
Traditional Chemotherapies
Chemotherapy regimens, comprising platinum agents (cisplatin or carboplatin), alkylators (lomustine or cyclophosphamide), and vincristine, represent the most common backbone of medulloblastoma therapy.40,49–52 The order and combination of these drugs and their relationship to radiation has been the focus of a number of multicenter clinical trials with studies very early on suggesting promise for multimodal therapy.53,54 Early trials in children with high-risk medulloblastoma treated with CSI with concurrent vincristine followed by maintenance chemotherapy including lomustine, cisplatin, and vincristine showed improved overall survival compared to historical controls and provided support for adjuvant chemotherapy in high-risk therapy.50,51,55,56 Adjuvant chemotherapy in standard-risk medulloblastoma was then shown to allow de-escalation of CSI with no reduction in survival.49 The Pediatric Oncology Group also showed delaying radiation in patients less than 3 years was possible through postoperative chemotherapy.57 The International Society of Pediatric Oncology and German Society of Pediatric Oncology went on to investigate the use of chemotherapy before or after radiation. The study showed no therapeutic benefit for preradiation chemotherapy and significantly poorer outcomes in average-risk patients receiving preradiation chemotherapy with reduced radiation.58
Additional follow-up clinical trials have investigated therapy options driven by stratification according to low-risk or high-risk disease (Table 1). Certain medulloblastoma histologies, specifically the nodular/desmoplastic subtype, have shown better responses to chemotherapy alone and potential to avoid or defer radiation.59–65 The HIT SKK 2000 trial illustrated an impressive 5-year event-free survival of 95% and overall survival of 100% for patients with nodular/desmoplastic medulloblastoma.63 Children’s Oncology Group study ACNS1221 follows a modified HIT SKK 2000 protocol without intraventricular methotrexate for patients with ND medulloblastoma; final outcomes are pending in this study.
Table 1.
Therapeutic trials for children with medulloblastoma
| Trial | Target population | Approach/Drugs | Outcomes |
|---|---|---|---|
|
SIOP
PNET-3 85 |
> 3 years of age with low-risk (M1/M0) disease | Vincristine, etoposide, carboplatin, and cyclophosphamide followed by radiation versus radiation alone | Chemotherapy + Radiation - 3-year EFS 79% - 5-year EFS 74% Radiation alone - 3-year EFS 65% - 5-year EFS 60% |
| SFOP 86 | < 5 years of age with newly diagnosed, maximally resected disease | Carboplatin, procarbazine, etoposide, cisplatin, vincristine, and cyclophosphamide over 7 cycles Salvage with busulfan and thiotepa, second-look surgery, and radiation at time of local relapse versus melphalan, busulfan, and thiotepa with autologous stem cell rescue plus focal radiation for recurrent focal disease or melphalan, cisplatin, thiotepa with autologous stem cell rescue and reduced-dose CSI for recurrent, metastatic disease |
Demonstrated cure without radiation possible in patients with focal disease who underwent gross total resection and those with local recurrence could be salvaged by combined chemotherapy and radiation |
| HIT SKK ‘87 60 | < 3 years of age | HIT SKK ‘87 Postoperative arms determined by standard or high-risk disease Low risk: Procarbazine, vincristine, high-dose methotrexate High risk: Procarbazine, ifosfamide, etoposide, high-dose methotrexate, cisplatin, cyclophosphamide Radiation at 3 years of age or time of progression |
Chemotherapy can prolong time to radiation or allows potential complete avoidance of radiation in patients with focal disease who achieved gross total resection Children without macroscopic metastases (complete resection): - 10-year PFS 53% - 10-year OS 59% Children without macroscopic metastases (incomplete resection): - 10-year PFS 56% - 10-year OS 67% Children with metastases: - 10-year PFS 33% - 10-year OS 44% |
| HIT SKK ‘91 84 | Arm 1—Neoadjuvant chemotherapy (Ifosfamide, etoposide, high-dose methotrexate, cisplatin, cytarabine x 2 cycles) before radiation Arm 2—Postoperative radiation with concomitant vincristine followed by cisplatin, lomustine, and vincristine x 8 cycles |
Maintenance chemotherapy most effective in patients 6 years or older with low-risk disease, otherwise no statistically significant differences between arms | |
|
HIT SKK
‘92 pilot 59 |
< 3 years of age | Postoperative cyclophosphamide, methotrexate, vincristine, carboplatin, and etoposide with intraventricular methotrexate Radiation only if residual disease and >18 months of age at end of chemotherapy |
Children without macroscopic metastases (complete resection): - 5-year PFS 93% - 5-year OS 100% Children without macroscopic metastases (incomplete resection): - 5-year PFS 43% - 5-year OS 56% Children with macroscopic metastases: - 5-year PFS 36% - 5-year OS 40% Children with ND MB: - 10-year PF 89% - 10-year OS 89% |
|
Head Start
I & II 65 |
< 3 years of age | Induction with vincristine, cisplatin, cyclophosphamide, and etoposide x 5 cycles Consolidation with myeloablative chemotherapy with carboplatin, thiotepa and autologous stem cell transplant (Doses varied between Head Start I & II) |
Children without macroscopic metastases (complete resection): - 5-year PFS 52% - 5-year OS 79% Children without macroscopic metastases (incomplete resection): - 5-year PFS 64% - 5-year OS 57% Children with ND MB: - 5-year PF 67% - 5-year OS 78% |
|
COG
99703 64 |
6 months to 3 years of age | Induction with cisplatin, vincristine, cyclophosphamide, and etoposide x 3 cycles Consolidation with high-dose carboplatin and thiotepa followed by autologous stem cell transplant x 3 cycles |
- 5-year EFS 44% - 5-year OS 64% Children with ND MB: - 5-year OS 85% |
|
CCG
9921 98,99 |
< 3 years of age | Induction vincristine, cisplatin, cyclophosphamide, and etoposide alternating with vincristine, carboplatin, ifosfamide, and etoposide Maintenance vincristine, etoposide, and cyclophosphamide No radiation unless residual tumor after induction or metastatic disease at diagnosis |
- 5-year EFS 32% - 5-year OS 43% Children with ND MB: - 5-year EFS 77% - 5-year OS 85% |
|
COG
P9934 100 |
8 months to 3 years of age | Induction with cyclophosphamide, vincristine, cisplatin, and etoposide followed by age- and response-adjusted radiation, if no progression Maintenance with cyclophosphamide, vincristine, and oral etoposide |
- 4-year EFS 50% - 4-year OS 69% Children with ND MB: - 4-year EFS 58% |
|
COG
9961 79 |
3 years to 21 years of age | Randomized to postradiation cisplatin and vincristine plus either CCNU or cyclophosphamide | - 5-year EFS 81% - 5-year OS 87% No impact of chemotherapy choice |
Abbreviations: EFS, event-free survival; OS, overall survival; PFS, progression-free survival; ND MB, nodular/desmoplastic medulloblastoma.
Molecular Subgrouping of Medulloblastoma and Therapeutic Implications
The recent insight on the biology of medulloblastoma and clinical outcomes according to WNT-activated, SHH-activated, group 3, and group 4 has led to attempts at treatment stratification and investigation of targeted therapies. WNT-activated medulloblastomas exhibit fenestrated vasculature, altering structure of the blood-brain barrier, and allowing greater penetration of chemotherapy.66 Given the excellent prognosis of children with these tumors, ongoing trials are investigating reduction of treatment intensity (NCT02212574, NCT01878617). There are currently no effective WNT-pathway inhibitors able to penetrate the blood-brain barrier; however, this pathway remains a potential for targeted therapy. SHH-activated medulloblastoma are most typically associated with intermediate outcomes.67 Mutations in PTCH1, SUFU, or SMO, or amplifications of GLI2, are the most frequent genomic aberrations in SHH-activated tumors.3,32,68–70 Accordingly, small molecule inhibitors of SMO, such as vismodegib, have shown efficacy in relapsed SHH-activated medulloblastoma, but drug resistance can develop.71 For instance, genomic alterations downstream of SMO (ie, GLI2 and SUFU) confer resistance to SMO-inhibitors and tumors harboring MYC-amplifications commonly exhibit rampant progression despite multimodal therapy. There is currently a paucity of drugs targeting MYC, but preclinical investigation surrounds BET-bromodomain, HDAC, IGFR1, and PI3K inhibitors as well as cell-cycle modulators.72–76 The least molecularly well-defined is group 4, yet possible enrichment with CDK6 or MYCN amplification raises the possibility of BET-bromodomain or cell-cycle inhibition as promising approaches.
Clinical Case Relevance
The patient was initially enrolled on clinical trial ACNS1221, an ongoing study targeting young children with newly diagnosed, nonmetastatic nodular desmoplastic medulloblastoma. At time of relapse, the child was treated with salvage therapy with CSI followed by repeat multidrug chemotherapy as per ACNS0332. ACNS0332 using combined chemotherapy and radiation upfront was chosen at relapse based on the child’s emergent need for radiation. The child demonstrated bulky relapse throughout the CNS and exhibited symptoms of spinal cord compression at multiple sites. Combination chemotherapy and radiation was given with the goal of decreasing tumor burden as rapidly as possible. Given the patient’s germline and somatic SUFU mutation downstream of SMO, he was not deemed a candidate for targeted SMO inhibition.
Surveillance
There are currently no strict guidelines for post-therapy imaging surveillance of patients with medulloblastoma. Most institutions follow a pattern of more frequent imaging within the first year after therapy, usually every 2 to 3 months, with spacing out of imaging thereafter. The utility of both brain and spine MRI has been brought into question given reports of low numbers of patients presenting with isolated spine relapse. Instead, spine surveillance based on risk for spine recurrence (ie, prior metastatic disease or temporal proximity to initial diagnosis) has been proposed.77 Recent Children’s Oncology Group guidelines recommend spine imaging for only the first 24 to 36 months off therapy if no increased clinical concern for spine recurrence or, for patients with nodular/desmoplastic medulloblastoma, only if metastases present at initial diagnosis. Once patients have been stable without evidence of recurrence for 5 years, annual imaging is reasonable. It is important to consider germline mutations and exposure to radiotherapy during surveillance planning, as both can increase risk for secondary central nervous system malignancy such meningioma or glioma.78–81
Prognosis and Survivorship
The progression-free and overall survival rates for all children with medulloblastoma improved in a step-wise fashion over the preceding thirty-years, but have plateaued since the mid-2000s.32,36,40,58,82–84 As previously noted, children 3 years or older at diagnosis have conventionally been separated into 2 major risk groups. Those with “average-risk disease” (no evidence of dissemination at diagnosis and total or near total resection) have a 5-year progression-free survival between 80% and 85% after CSI and local boost with chemotherapy.36,38,40 The majority of these patients who are disease-free at 5 years following diagnosis maintain remission, although late relapses occur in 5% to 10%.36,40 However, it is likely many “late relapses” are secondary high-grade glial tumors.79 For patients with high-risk disease, primarily those with dissemination at diagnosis, the 5-year survival is between 50% and 65%, with a similar rate of late relapse as for standard-risk disease.38,84,85 The pattern of relapse does not differ greatly between patients with dissemination at diagnosis compared to those without—over 60% of patients have dissemination, with or without primary site failure.36,38,40,84,85
The probability of survival in infants and children less than 3 years of age at diagnosis is lower than older children.57,59,61,62,64,86 It remains unclear whether this is due to biologic differences in the very young or whether it is avoidance of CSI in this population. After chemotherapy with or without primary site irradiation, reported survival rates range from 20% to over 50%.57,61,64,86 Such variation is likely due to patients with nodular/desmoplastic tumors, as 75% of these patients survive up to five years with chemotherapy alone.59,62,64,86 Survival after chemotherapy alone or chemotherapy plus local radiotherapy in infants with nondesmoplastic tumors appears less than 40% and significantly lower with disseminated disease.64,86
Progression-free and overall survival have to be re-evaluated in light of biologic understanding of medulloblastoma.3,32,87 Children with WNT-activated tumors have a near 100% rate of survival after treatment with radiation and chemotherapy, even with dissemination.3,32,87,88 Patients with SHH-activated tumors have more variable progression-free and overall survival depending on the site of the SHH pathway alteration and presence of concomitant TP53 mutations.3,32,67 Those with upstream mutations in SMO or PTCH, primarily infants and young children, have a better prognosis and experience 5-year event-free survival of 70% to 80%.67 Children with downstream alterations such as GLI 2 amplification or with concomitant TP53 mutations experience 5-year overall survival of 30% or less.67 Patients with group 3 and 4 disease have outcomes dependent on specific molecular abnormalities.3,32,87 Patients with MYC amplification experience 5-year event-free and overall survival of less than 50% and carry higher incidence of dissemination. Patients harboring loss of chromosome 11 carry excellent prognoses and those with isochromosome 17q have somewhat poorer prognoses.3 It is important to note the potential need for germline testing in patients with identified SHH-activated tumors with associated SUFU or TP53 somatic mutations. A substantial proportion of patients with SHH-activated tumors carry germline mutations in these genes and this must be considered in surveillance and prognostic counseling, given their negative impact on outcome and indication for genetic counseling.34,89
At time of relapse, especially disseminated relapse, long-term disease control is unlikely with presently available salvage therapy. The exception to this may be isolated primary site relapse, especially in infants who have not received radiation therapy. In such patients, treatment with additional chemotherapy, often high-dose chemotherapy and focal radiation, has resulted in a long-term disease control in 30% to 50%.61,86
Although survival remains the primary goal of treatment for medulloblastoma, survivor quality of life is frequently riddled with neurologic, endocrine, physical, and psychosocial sequelae.46,90 The most common is cognitive impairment, which may be secondary to the tumor, hydrocephalus, radiation, and other poorly understood host factors.47,91–93 Those with a history of posterior fossa syndrome carry higher risk for cognitive impairment.91 CSI and local boost radiotherapy have significant detrimental impact on intelligence, especially in younger children. There is no specific age cutoff for this damaging effect; however, children between 3 and 7 years of age receiving 36 Gy CSI plus local boost radiotherapy experience 20- to 30-point declines in IQ within 3 years of treatment, on top of often impaired baseline intelligence.92,93 Even after reduction of CSI dose to 23 Gy, there is a decline of 10 to 15 IQ points.92
One of the most common forms of long-term sequelae from treatment related to medulloblastoma is neuroendocrine. Endocrine compromise is almost solely due to radiation to the hypothalamus and pituitary, although radiation scatter to the thyroid may result in hypothyroidism. Growth hormone deficiency is the most common deficit and is usually apparent 1 to 3 years after radiation. Decreased growth from growth hormone deficiency is exacerbated by the effect of CSI on vertebral growth, which can result in a 1- to 3-inch reduction in overall height. Growth hormone replacement, especially in children more than 2 years from diagnosis, has not resulted in a higher incidence of disease relapse and should be considered.94
Neurosensory deficits have been well reported in children surviving medulloblastoma, with hearing loss related to cisplatin and, less frequently, radiation being the most common. Other deficits include vertigo, cataracts, and gross motor issues.90 Strokes and secondary tumors are additional devastating consequences in patients with medulloblastoma. The incidence of stroke may affect up to 10% of survivors 10 or more years after diagnosis. Secondary tumors tend to occur 5 or more years from diagnosis, most commonly high-grade gliomas, followed by meningiomas 10 or more years from therapy, and with highest risk in patients with germline SUFU mutations.79,90,95
Lastly, patients with medulloblastoma can exhibit significant psychosocial compromise. Physically, they tend to be shorter and suffer from obesity, which can exacerbate psychosocial challenges.90,96,97 Due to psychosocial deficits, survivors can become isolated with decreased likelihood of independent living.90
Conclusions
Medulloblastoma is the most frequent pediatric malignant CNS tumor. Recent molecular advances are driving clinical trials and development of novel therapies, but there remain questions about how to best stratify treatment based on molecular findings and how to best combine targeted therapies with more traditional therapeutic mainstays. Ongoing and upcoming trials will hopefully provide insight into these questions and ideally lead to better prediction of outcomes and decreased long-term deficits.
Funding
National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-Clinical and Translational Science Institute grant [KL2TR000143 to SM]; National Institute of Health T32 grant [CA128583 to CK]; UCSF-Clinical and Translational Science Institute Strategic Opportunities Support Program [A119683 to CK]; Alex’s Lemonade Stand Foundation [A120729 to CK]; National Institutes of Health Director’s Early Independence Award [DP5 OD021403 to DS]; and Career Development Award from the UCSF Brain Tumor SPORE [P50 CA097257 to DS].
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. McKean-Cowdin R, Razavi P, Barrington-Trimis J, et al. Trends in childhood brain tumor incidence, 1973–2009. J Neurooncol. 2013;115(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Curran EK, Sainani KL, Le GM, et al. Gender affects survival for medulloblastoma only in older children and adults: a study from the surveillance epidemiology and end results registry. Pediatr Blood Cancer. 2009;52(1):60–64. [DOI] [PubMed] [Google Scholar]
- 3. Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith MJ, Beetz C, Williams SG, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. J Clin Oncol. 2014;32(36):4155–4161. [DOI] [PubMed] [Google Scholar]
- 5. Taylor MD, Mainprize TG, Rutka JT. Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: a review. Neurosurgery. 2000;47(4):888–901. [DOI] [PubMed] [Google Scholar]
- 6. Hottinger AF, Khakoo Y. Neurooncology of familial cancer syndromes. J Child Neurol. 2009;24(12):1526–1535. [DOI] [PubMed] [Google Scholar]
- 7. Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332(13):839–847. [DOI] [PubMed] [Google Scholar]
- 8. Dörner L, Fritsch MJ, Stark AM, et al. Posterior fossa tumors in children: how long does it take to establish the diagnosis? Childs Nerv Syst. 2007;23(8):887–890. [DOI] [PubMed] [Google Scholar]
- 9. Molineus A, Boxberger N, Redlich A, et al. Time to diagnosis of brain tumors in children: a single-centre experience. Pediatr Int. 2013;55(3):305–309. [DOI] [PubMed] [Google Scholar]
- 10. Fruehwald-Pallamar J, Puchner SB, Rossi A, et al. Magnetic resonance imaging spectrum of medulloblastoma. Neuroradiology. 2011;53(6):387–396. [DOI] [PubMed] [Google Scholar]
- 11. Koeller KK, Rushing EJ. From the archives of the AFIP: medulloblastoma: a comprehensive review with radiologic-pathologic correlation. Radiographics. 2003;23(6):1613–1637. [DOI] [PubMed] [Google Scholar]
- 12. Rumboldt Z, Camacho DL, Lake D, et al. Apparent diffusion coefficients for differentiation of cerebellar tumors in children. AJNR Am J Neuroradiol. 2006;27(6):1362–1369. [PMC free article] [PubMed] [Google Scholar]
- 13. Yeom KW, Mobley BC, Lober RM, et al. Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol. 2013;200(4):895–903. [DOI] [PubMed] [Google Scholar]
- 14. Meyers SP, Wildenhain SL, Chang JK, et al. Postoperative evaluation for disseminated medulloblastoma involving the spine: contrast-enhanced MR findings, CSF cytologic analysis, timing of disease occurrence, and patient outcomes. AJNR Am J Neuroradiol. 2000;21(9):1757–1765. [PMC free article] [PubMed] [Google Scholar]
- 15. Gururangan S, Hwang E, Herndon JE, 2nd, Fuchs H, George T, Coleman RE. [18F]fluorodeoxyglucose-positron emission tomography in patients with medulloblastoma. Neurosurgery. 2004;55(6):1280–1288; discussion 1288–1289. [DOI] [PubMed] [Google Scholar]
- 16. Tripathi M, Jain N, Jaimini A, et al. Demonstration of diffuse leptomeningeal metastasis in a treated case of medulloblastoma with F-18 FDG PET/CT. Clin Nucl Med. 2009;34(8):530–532. [DOI] [PubMed] [Google Scholar]
- 17. Perreault S, Ramaswamy V, Achrol AS, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibson P, Tong Y, Robinson G, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468(7327):1095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teo WY, Shen J, Su JM, et al. Implications of tumor location on subtypes of medulloblastoma. Pediatr Blood Cancer. 2013;60(9):1408–1410. [DOI] [PubMed] [Google Scholar]
- 20. Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. [DOI] [PubMed] [Google Scholar]
- 21. Albright AL. The value of precraniotomy shunts in children with posterior fossa tumors. Clin Neurosurg. 1983;30:278–285. [DOI] [PubMed] [Google Scholar]
- 22. Lee M, Wisoff JH, Abbott R, et al. Management of hydrocephalus in children with medulloblastoma: prognostic factors for shunting. Pediatr Neurosurg. 1994;20(4):240–247. [DOI] [PubMed] [Google Scholar]
- 23. El-Gaidi MA, El-Nasr AH, Eissa EM. Infratentorial complications following preresection CSF diversion in children with posterior fossa tumors. J Neurosurg Pediatr. 2015;15(1):4–11. [DOI] [PubMed] [Google Scholar]
- 24. Rajesh BJ, Rao BR, Menon G, et al. Telovelar approach: technical issues for large fourth ventricle tumors. Childs Nerv Syst. 2007;23(5):555–558. [DOI] [PubMed] [Google Scholar]
- 25. Gudrunardottir T, Sehested A, Juhler M, et al. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27(3):355–363. [DOI] [PubMed] [Google Scholar]
- 26. Rekate HL, Grubb RL, Aram DM, et al. Muteness of cerebellar origin. Arch Neurol. 1985;42(7):697–698. [DOI] [PubMed] [Google Scholar]
- 27. Palmer SL, Hassall T, Evankovich K, et al. Neurocognitive outcome 12 months following cerebellar mutism syndrome in pediatric patients with medulloblastoma. Neuro Oncol. 2010;12(12):1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fouladi M, Gajjar A, Boyett JM, et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol. 1999;17(10):3234–3237. [DOI] [PubMed] [Google Scholar]
- 29. Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 30. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.World Health Organization Histological Classification of Tumours of the Central Nervous System. France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 31. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 34. Ramaswamy V, Remke M, Bouffet E, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bree AF, Shah MR; BCNS Colloquium Group Consensus statement from the first international colloquium on basal cell nevus syndrome (BCNS). Am J Med Genet A. 2011;155A(9):2091–2097. [DOI] [PubMed] [Google Scholar]
- 36. Merchant TE, Kun LE, Krasin MJ, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70(3):782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolden SL, Dunkel IJ, Souweidane MM, et al. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol. 2003;21(16):3079–3083. [DOI] [PubMed] [Google Scholar]
- 38. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 39. Raleigh DR, Tomlin B, Buono BD, et al. Survival after chemotherapy and stem cell transplant followed by delayed craniospinal irradiation is comparable to upfront craniospinal irradiation in pediatric embryonal brain tumor patients. J Neurooncol. 2017;131(2):359–368. [DOI] [PubMed] [Google Scholar]
- 40. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 41. Wolden S.Molecularly driven radiation decisions: protons, photons or none? ASCO Annual Meeting, Chicago, IL; 2016. [Google Scholar]
- 42. St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(3):727–734. [DOI] [PubMed] [Google Scholar]
- 43. Wetmore C, Herington D, Lin T, et al. Reirradiation of recurrent medulloblastoma: does clinical benefit outweigh risk for toxicity? Cancer. 2014;120(23):3731–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakst RL, Dunkel IJ, Gilheeney S, et al. Reirradiation for recurrent medulloblastoma. Cancer. 2011;117(21):4977–4982. [DOI] [PubMed] [Google Scholar]
- 45. Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31(28):3494–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2016;31(2):237–254. [DOI] [PubMed] [Google Scholar]
- 47. Roddy E, Sear K, Felton E, et al. Presence of cerebral microbleeds is associated with worse executive function in pediatric brain tumor survivors. Neuro Oncol. 2016;18(11):1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mabbott DJ, Spiegler BJ, Greenberg ML, et al. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. [DOI] [PubMed] [Google Scholar]
- 49. Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. [DOI] [PubMed] [Google Scholar]
- 50. Packer RJ, Siegel KR, Sutton LN, et al. Efficacy of adjuvant chemotherapy for patients with poor-risk medulloblastoma: a preliminary report. Ann Neurol. 1988;24(4):503–508. [DOI] [PubMed] [Google Scholar]
- 51. Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81(5):690–698. [DOI] [PubMed] [Google Scholar]
- 52. Packer RJ, Sutton LN, Goldwein JW, et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991;74(3):433–440. [DOI] [PubMed] [Google Scholar]
- 53. Duffner PK, Cohen ME, Thomas PR, et al. Combination chemotherapy in recurrent medulloblastoma. Cancer. 1979;43(1):41–45. [DOI] [PubMed] [Google Scholar]
- 54. Thomas PR, Duffner PK, Cohen ME, et al. Multimodality therapy for medulloblastoma. Cancer. 1980;45(4):666–669. [DOI] [PubMed] [Google Scholar]
- 55. Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72(4):572–582. [DOI] [PubMed] [Google Scholar]
- 56. Tait DM, Thornton-Jones H, Bloom HJ, et al. Adjuvant chemotherapy for medulloblastoma: the first multi-centre control trial of the International Society of Paediatric Oncology (SIOP I). Eur J Cancer. 1990;26(4):464–469. [PubMed] [Google Scholar]
- 57. Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328(24):1725–1731. [DOI] [PubMed] [Google Scholar]
- 58. Bailey CC, Gnekow A, Wellek S, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995;25(3):166–178. [DOI] [PubMed] [Google Scholar]
- 59. Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 60. Rutkowski S, Gerber NU, von Hoff K, et al. ; German Pediatric Brain Tumor Study Group. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grundy RG, Wilne SH, Robinson KJ, et al. ; Children’s Cancer and Leukaemia Group (formerly UKCCSG) Brain Tumour Committee. Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–133. [DOI] [PubMed] [Google Scholar]
- 62. Rutkowski S, von Hoff K, Emser A, et al. Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol. 2010;28(33):4961–4968. [DOI] [PubMed] [Google Scholar]
- 63. von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011;13(6):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cohen BH, Geyer JR, Miller DC, et al. ; Children’s Oncology Group. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children’s Oncology Group. Pediatr Neurol. 2015;53(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50(6):1169–1175. [DOI] [PubMed] [Google Scholar]
- 66. Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29(4):508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kool M, Jones DT, Jäger N, et al. ; ICGC PedBrain Tumor Project. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jones DT, Jäger N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Robinson GW, Orr BA, Wu G, et al. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33(24):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bandopadhayay P, Bergthold G, Nguyen B, et al. BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin Cancer Res. 2014;20(4):912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Henssen A, Thor T, Odersky A, et al. BET bromodomain protein inhibition is a therapeutic option for medulloblastoma. Oncotarget. 2013;4(11):2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pei Y, Liu KW, Wang J, et al. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma. Cancer Cell. 2016;29(3):311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prince EW, Balakrishnan I, Shah M, et al. Checkpoint kinase 1 expression is an adverse prognostic marker and therapeutic target in MYC-driven medulloblastoma. Oncotarget. 2016;7(33):53881–53894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Venkataraman S, Alimova I, Balakrishnan I, et al. Inhibition of BRD4 attenuates tumor cell self-renewal and suppresses stem cell signaling in MYC driven medulloblastoma. Oncotarget. 2014;5(9):2355–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perreault S, Lober RM, Carret AS, et al. Surveillance imaging in children with malignant CNS tumors: low yield of spine MRI. J Neurooncol. 2014;116(3):617–623. [DOI] [PubMed] [Google Scholar]
- 78. Aavikko M, Li SP, Saarinen S, et al. Loss of SUFU function in familial multiple meningioma. Am J Hum Genet. 2012;91(3):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Inskip PD, Sigurdson AJ, Veiga L, et al. Radiation-related new primary solid cancers in the Childhood Cancer Survivor Study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 2016;94(4):800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morgenstern PF, Shah K, Dunkel IJ, et al. Meningioma after radiotherapy for malignancy. J Clin Neurosci. 2016;30:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18(16):3004–3011. [DOI] [PubMed] [Google Scholar]
- 83. Taylor RE, Bailey CC, Robinson K, et al. ; International Society of Paediatric Oncology; United Kingdom Children’s Cancer Study Group. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21(8):1581–1591. [DOI] [PubMed] [Google Scholar]
- 84. Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys. 2000;46(2):269–279. [DOI] [PubMed] [Google Scholar]
- 85. Taylor RE, Bailey CC, Robinson KJ, et al. ; United Kingdom Children’s Cancer Study Group Brain Tumour Committee; International Society of Paediatric Oncology. Impact of radiotherapy parameters on outcome in the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58(4):1184–1193. [DOI] [PubMed] [Google Scholar]
- 86. Grill J, Sainte-Rose C, Jouvet A, et al. ; French Society of Paediatric Oncology. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–580. [DOI] [PubMed] [Google Scholar]
- 87. Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 91. Wells EM, Khademian ZP, Walsh KS, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. [DOI] [PubMed] [Google Scholar]
- 92. Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. [DOI] [PubMed] [Google Scholar]
- 93. Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70(5):707–713. [DOI] [PubMed] [Google Scholar]
- 94. Packer RJ, Boyett JM, Janss AJ, et al. Growth hormone replacement therapy in children with medulloblastoma: use and effect on tumor control. J Clin Oncol. 2001;19(2):480–487. [DOI] [PubMed] [Google Scholar]
- 95. Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys. 2013;86(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wolfe-Christensen C, Mullins LL, Scott JG, et al. Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer. 2007;49(5):723–726. [DOI] [PubMed] [Google Scholar]
- 97. Johnson DL, McCabe MA, Nicholson HS, et al. Quality of long-term survival in young children with medulloblastoma. J Neurosurg. 1994;80(6):1004–1010. [DOI] [PubMed] [Google Scholar]
- 98. Leary SE, Zhou T, Holmes E, et al. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the Children’s Oncology Group. Cancer. 2011;117(14):3262–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Geyer JR, Sposto R, Jennings M, et al. ; Children’s Cancer Group. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23(30):7621–7631. [DOI] [PubMed] [Google Scholar]
- 100. Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol. 2012;30(26):3181–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]