Abstract
Background
Noninvasively predicting early response to therapy in recurrent pediatric brain tumors provides a challenge. 3,4-dihydroxy-6-[18F]fluoro-L-phenylalanine (18F-FDOPA) PET/MRI has not been previously studied as a tool to evaluate early response to antiangiogenic therapy in children. The purpose of this study was to evaluate the safety and feasibility of using 18F-FDOPA PET/MRI to assess response to bevacizumab in children with relapsed brain tumors.
Materials and Methods
Six patients with recurrent gliomas (5 low-grade, 1 high-grade) planned to undergo treatment with bevacizumab were enrolled. 18F-FDOPA PET/MRI scans were obtained prior to and 4 weeks following the start of treatment, and these were compared with the clinical response determined at the 3-month MRI. The primary PET measure was metabolic tumor volume (MTV) at 10 to 15 min after 18F-FDOPA injection. For each tumor, the MTV was determined by manually defining initial tumor volumes of interest (VOI) and then applying a 1.5-fold threshold relative to the mean standardized uptake value (SUV) of a VOI in the frontal lobe contralateral to the tumor.
Results
18F-FDOPA PET/MRI was well tolerated by all patients. All tumors were well visualized with 18F-FDOPA on the initial study, with peak tumor uptake occurring approximately 10 min after injection. Maximum and mean SUVs as well as tumor-to-brain ratios were not predictors of response at 3 months. Changes in MTVs after therapy ranged from 23% to 98% (n = 5). There is a trend towards the percent MTV change seen on the 4-week scan correlating with progression-free survival.
Conclusion
18F-FDOPA PET/MRI was well tolerated in pediatric patients and merits further investigation as an early predictor of response to therapy.
Keywords: bevacizumab, FDOPA PET, monitoring response, pediatric brain tumors, recurrent brain tumors
Brain tumors are the most common solid tumor in children and the second-most-common childhood cancer after leukemia. Pediatric brain tumors have heterogeneous histologies, the most common being gliomas. Treatment for pediatric brain tumors may include bevacizumab, a humanized anti-VEGF monoclonal neutralizing antibody. Bevacizumab alone or in combination has shown to be safe and effective in pediatric CNS malignancies, including medulloblastoma1–4 and low-grade glioma,5–8 and improves progression free survival (PFS) in a subset of patients.8–11 A major challenge of treating brain tumors in children and adults is the limited ability to non-invasively predict and monitor response to therapy, particularly early after initiating therapy. Traditionally, brain tumor response to treatment is evaluated at standard intervals by contrast-enhanced MRI, but treatment response can take months to reliably detect with conventional MRI, particularly in nonenhancing tumors. Bevacizumab can decrease contrast enhancement as early as 24 to 48 hours after administration,12–16 but this effect is not correlated to overall survival (OS) or PFS.9,17 Studies evaluating response to bevacizumab using contrast-enhanced, T1-weighted and fluid-attenuated inversion recovery (FLAIR) MR images showed a correlation with PFS to response at weeks 8 and 16 following the start of treatment, but did not correlate with week 2 changes,18 suggesting that MRI is not an effective method for detection of early response. Batchelor et al reported decreased contrast enhancement in patients with glioblastoma treated with AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor; however, decreased enhancement of tumor volume was not associated with survival prolongation.19 These studies emphasize that alternative imaging techniques are needed for evaluating tumor progression and prognosis for patients on anti-angiogenic therapy.
PET using radiolabeled amino acids targeting system L amino acid transport are well established in adult neuro-oncology. This class of PET tracers includes L-[11C]methionine (MET), O-(2-[18F]fluoroethyl)-L-tyrosine (FET), and 3,4-dihyd roxy-6-[18F]fluoro-L-phenylalanine (18F-FDOPA).20–22 Recent articles in the pediatric literature suggest that 18F-FET PET may be helpful in determining newly diagnosed and progressive tumors,23 guiding surgical biopsies,24 and identifying postradiation pseudoprogression from progression.25 A major advantage of these amino acid tracers over many contrast agents is their ability to cross the intact blood-brain barrier through amino acid transport and to visualize the entire tumor volume. Recent guidelines by the Response Assessment in Neuro-Oncology (RANO) working group and European Association for Neuro-Oncology state that amino acid tracers are superior to glucose analogue 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG PET) in differentiating neoplastic from non-neoplastic tissue, delineating extent of tumor, and potentially treatment response, and may be complementary to MRI.2618F-FDG, the uptake of 18F-FDOPA in normal brain is relatively low, improving visualization of low-grade tumors and tumor boundaries in both enhancing and non-enhancing tumors. Tumor cell uptake of 18F-FDOPA utilizes the system L amino acid transporter LAT1, which is upregulated in brain tumors and correlates with microvessel density.27,28 In adults, 18F-FDOPA PET has been shown to detect tumors and recurrence,27,28 predict survival,29 and predict response to therapy, specifically bevacizumab.30,31 Despite the demonstrated utility of 18F-FDOPA in adult neuro-oncology, relatively few studies have evaluated the use of radiolabeled amino acids in pediatric neuro-oncology patients.32–34 The limited data available suggest that radiolabeled amino acids have similar diagnostic utility in pediatric and adult neuro-oncology.
Simultaneous PET/MRI is a relatively new hybrid imaging modality that can provide morphological, functional, and molecular information in a single imaging session. Simultaneous PET/MRI is particularly well-suited to neuro-oncologic imaging with amino acids since peak tumor uptake of these tracers typically occurs within 10 to 20 min after injection, allowing a complete dynamic PET study within the time required to acquire a clinical tumor protocol MRI. Additionally, simultaneous acquisition allows PET and MRI acquisition in the same imaging session and without increasing overall imaging time, which is particularly beneficial to children. For children requiring sedation or anesthesia, simultaneous PET/MRI can reduce the number as well as the duration of sedation/anesthesia sessions, which reduces potential risk to the patient and cost. The purpose of this study was to evaluate the safety and feasibility of using 18F-FDOPA PET/MRI to predict response to antiangiogenic therapy with bevacizumab in children with relapsed brain tumors.
Materials and Methods
Study Population
This study was approved by the Siteman Cancer Center Protocol Review Monitoring Committee and the Washington University School of Medicine institutional review board. Written informed consent was obtained from the medical decision maker for each of our patients, and assent to participate was obtained from each patient when appropriate. We studied a total of 6 patients with relapsed brain tumors between March of 2014 and December of 2015 who were planned to undergo treatment with bevacizumab. Subjects ages 0 to 21 years were included if they had a histologically proven central nervous system tumor (optic pathway gliomas allowed without prior biopsy), had measurable disease on MRI, were planned to be treated with bevacizmab alone or in combination, and had recovered from all toxic effects of prior treatments. Subjects were excluded if they had received myelosuppressive chemotherapy within 3 weeks or nitrosourea within 6 weeks; antineoplastic biological agent within 7 days or within 3 half-lives of agent (whichever is longer) of enrollment; craniospinal radiotherapy within 24 weeks of enrollment; bevacizumab or other antiangiogenic therapy within 3 months of enrollment; or had a contraindication to bevacizumab or irinotecan therapy or MRI. Patients were monitored for adverse events from the time of 18F-FDOPA injection and during the actual imaging period. A follow-up telephone call was made to the patient, parent, or guardian 24 hours following injection (±6 hours) to assess for adverse events. The revised NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was utilized for all toxicity reporting. Adverse events were reported to the Human Research Protection Office (HRPO) and the Quality Assurance and Safety Monitoring Committee (QASMC) at Washington University.
Two patients had a pathologic diagnosis of grade I pilocytic astrocytoma, 2 patients had optic pathway glioma with 1 patient having an addition optical chiasm glioma, assumed to be low-grade, 1 patient had a midbrain ganglioglioma, and 1 patient had a grade IV small cell astrocytoma (Table 1). Four patients underwent at least 1 prior chemotherapy regimen while 2 underwent surgery only. All patients were treated with bevacizumab and irinotecan per standard protocols,5,12,35 and underwent a baseline 18F-FDOPA PET/MRI within 24 hours prior to starting treatment. Bevazicumab 10 mg/kg was given 2 weeks apart for the first 2 doses as a single agent in order to assess imaging response of antiangiogenic therapy alone. The patients then underwent a second 18F-FDOPA PET/MRI 4 weeks after the baseline study and immediately prior to the third dose of chemotherapy. Patient 4 was unevaluable for response to therapy due to technical difficulties with 18F-FDOPA production at the time of the second PET/MRI. After the second 18F-FDOPA PET/MRI study, all patients continued treatment with bevacizumab 10 mg/kg with the addition of irinotecan 125 mg/m2 beginning at the third dose (week 6) and continued for 48 weeks. Two patients (patients 1 and 6) underwent a repeat 18F-FDOPA PET/MRI study at the time of recurrence based on clinical and/or MRI findings.
Table 1.
Characteristics of the 6 Patients Enrolled
| Patient | Age (years) | Sex | Diagnosis | Grade | Prior Treatments |
|---|---|---|---|---|---|
| 1a | 8 | F | Optic nerve glioma | I | Carboplatin/vincristine |
| 1b | 8 | F | Optic chiasm glioma | I | Carboplatin/vincristine |
| 2 | 12 | M | Cerebellar pilocytic astrocytoma | I | Surgical resection × 2 |
| 3 | 8 | F | Thalamic small cell astrocytoma | IV | Biopsy, Radiation + temozolomide/ lomustine |
| 4 | 10 | F | Optic pathway glioma | I* | Carboplatin/vincristine |
| 5 | 10 | M | Optic pathway pilocytic astrocytoma | I | Partial surgical resection, Carboplatin/ vincristine, Lenolidamide |
| 6 | 8 | M | Midbrain ganglioglioma | I | Biopsy |
*Presumed grade I, patient with neurofibromatosis type I.
Imaging Technique
Production of 18F-FDOPA was performed by the Washington University Cyclotron Facility using the standard electrophilic substitution method. The injected mass was less than 0.92 mg/kg for all patients. The PET/MRI studies of the entire brain were performed on a simultaneous 3-Tesla PET/MRI system, Siemens Biograph mMR (Siemens Health Care, Erlangen, Germany) at Washington University in St. Louis, MO. Prior to 18F-FDOPA injection, the patients fasted for at least 4 hours. No administration of carbidopa or other inhibitors of peripheral aromatic amino acid decarboxylase were administered. Baseline vital sign measurements were obtained, and venous access was obtained through a peripheral catheter or a port device. Immediately prior to imaging, patients voided their bladders to increase their comfort during the study.
The PET and MRI data were acquired simultaneously. Dynamic PET data were acquired from tracer injection for at least 45 minutes and up to 60 minutes postinjection of 0.08 mCi (3.0 MBq) per kg of 18F-FDOPA with a minimum dosage of 2 mCi (74 MBq) and maximum dosage of 5 mCi (185 MBq). The emission data were collected with an energy window of 435 to 650 keV. The PET data were corrected for random coincidences, dead time, and scatter. Attenuation correction for PET/MRI studies was performed using the Dixon MRI sequence as recommended by the manufacturer. One patient (patient 5) underwent follow-up 18F-FDOPA PET on a Siemens Biograph 40 PET/CT (Siemens Health Care, Erlangen, Germany) because the PET/MRI system was unavailable. For this patient, a low-dose, non-contrast head CT (25 mAs) was performed for attenuation correction, and the remainder of the PET acquisition and processing was performed as for the PET/MRI studies. In a study with pediatric patients, overall high correlation and good intrapatient reliability between standardized uptake value (SUV) measurements from PET/MRI and PET/CT were noted, although PET/MRI underestimated SUV compared with PET/CT.36 List mode data were collected and framed using the following schedule: 30 × 3 seconds, 12 × 10 seconds, 6 × 20 seconds, 10 × 60 seconds, and 6 to 9 × 5 min. Images were reconstructed using a 3D-OSEM (ordered-subset expectation maximization) iterative reconstruction algorithm. At the conclusion of the imaging, the patients voided again to decrease radiation dose to their bladder. Repeat vital sign measurements were obtained at the end of the imaging session, and patients were evaluated in person or by telephone the following day for any adverse events that could be related to 18F-FDOPA PET/MRI imaging.
Multiplanar, multisequence, contrast-enhanced MR images were acquired simultaneously during PET data acquisition using the standard-of-care protocol for brain tumor imaging at Washington University. In addition, the Dixon sequence for PET data attenuation correction was acquired. The following sequences were acquired prior to the administration of contrast: scout image, Dixon, T1-weighted transaxial, sagittal and coronal spin echo, and FLAIR fat-saturation transaxial. Following the intravenous administration of 0.1 mmol/kg MultiHance (Bracco Imaging) gadolinium-based contrast, the following sequences were performed: dynamic susceptibility contrast-enhanced perfusion, susceptibility weighted imaging, diffusion-weighted transaxial, T2-weighted BLADE transaxial, T1-weighted spin echo transaxial, T1-weighted spin echo fat saturation sagittal, T1-weighted spin echo coronal, and 2.5-mm Stealth axial. The total imaging time for these MR sequences was approximately 45 to 60 min.
For studies with significant head motion during the PET data acquisition, motion correction was performed through automated registration between each reconstructed frame and a reference frame. Image registration was accomplished using elastix, an open source software toolbox for rigid and nonrigid registration of images, using the following parameters: 3 level pyramidal multilevel, and 6 parameter rigid registration by maximizing the Advanced Mattes Mutual Information metric with the Adaptive Stochastic Gradient Descent optimizer.37,38
Image Analysis
The 18F-FDOPA PET/MRI images were reviewed and analyzed using MIM Encore (MIM Software, Inc., Cleveland, Ohio, USA), a commercially available software package. Initial volumes of interests (VOIs) were drawn manually over areas suspected to represent recurrent tumor using both MRI (T1 contrast enhancement, FLAIR/T2 signal hyperintensity) and PET (areas of relatively increased 18F-FDOPA uptake) to define the initial VOI. Thresholding was applied to the initial VOI to define the metabolic tumor volume (MTV). The PET data acquired at 10 to 15 min after 18F-FDOPA injection were summed and used for this analysis. Reference VOIs in normal brain were drawn over the frontal and parietal lobes contralateral to the brain tumor, and these reference VOIs were used to generate SUV thresholds that were applied to the tumor VOIs for each patient. Thresholds from 1.1 to 2.0 were generated in 0.1 increments, and the resulting tumor volumes at each threshold value were calculated based on the volume of tumor at or above the SUV thresholds. A 1.5-fold threshold was selected as higher thresholds made some of the measured MTVs very small on the pre-treatment study. This threshold is similar to those reported for the amino acid [18F]FET.39,40 The maximum SUVs were recorded for each tumor and reference VOI, and the mean tumor SUVs were determined at each threshold value. Tumor-to-brain ratios (TBR) were calculated by dividing the maximum SUV of the tumor by the mean SUV of the reference region to provide TBRmax and the mean SUV of the tumor by the mean SUV of the reference region to provide TBRmean.
Patients in the study received up to eight 6-week cycles of bevacizumab and irinotecan unless discontinued for disease progression. Monitoring while on chemotherapy consisted of clinical and laboratory assessments per standard chemotherapy protocol guidelines. Routine contrast-enhanced MRI of the brain was performed at 3-month intervals for assessment of response to therapy and recurrence/progression of disease upon completion of therapy.
MRI Response Assessment
To assess response to bevacizumab based on MRI, the RANO classification as described by Wen et al41 was applied using the commercial software mint Lesion (Version 3.2.1) (Mint Medical GmbH, Dossenheim, Germany). The perpendicular diameters of lesions were defined manually using FLAIR and T1-weighted postcontrast images with comparison to lesion measurements obtained at baseline and at interval follow-up MRIs.
Results
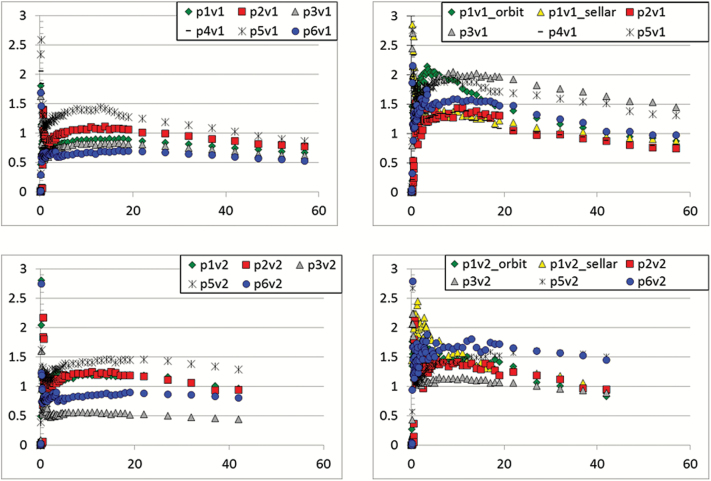
A total of 12 18F-FDOPA PET/MRI and 1 18F-FDOPA PET/CT studies were performed on 6 patients. Of these, 5 underwent baseline and follow-up 18F-FDOPA PET imaging, and 2 underwent a third 18F-FDOPA PET/MRI study for suspected recurrence. 18F-FDOPA PET was well tolerated by all of the patients, and no adverse events related to the imaging studies occurred. Head motion that occurred during the study could be corrected and in all cases did not interfere with assessing the 10 to 15 min post injection time point, even without motion correction. The dynamic PET data acquired at 10 to 15 min after 18F-FDOPA injection were summed and used for this analysis. The summation of frames obtained at 10–15 min were selected since peak tumor 18F-FDOPA uptake occurred in this time range for most patients, and the physiologic 18F-FDOPA uptake in the striatum and other normal brain structures was relatively low. The lack of substantial accumulation of 18F-FDOPA and its metabolites in the striatum at this time point allowed identification of tumor involving the basal ganglia which would be more difficult at later time points. Time-activity curves for tumors and normal brain reference regions before and after bevacizumab therapy are shown in Fig. 1.
Fig. 1 .
Time activity curves for normal brain reference regions and tumors. The time points 10 to 15 min after 18F-FDOPA injection were summed and used for analysis. (a) Normal brain, baseline. (b) Tumor, baseline. (c) Normal brain, after 4 weeks of bevacizumab. (d) Tumor, after 4 weeks of bevacizumab.
In all patients, the tumors were readily visualized with 18F-FDOPA PET/MRI on the baseline study prior to therapy with bevacizumab. Representative images are shown in Figs 2–3. The reference VOIs drawn over the frontal and parietal lobes yielded very similar mean SUVs within individual patients with the absolute difference ranging from 0 to 9.9% with a mean absolute difference of 5.4 ± 3.8% at baseline. The averages of mean SUVs at baseline were 0.93 ± 0.27 for the frontal lobe and 0.95 ± 0.28 for the parietal lobe. The average volumes of the frontal and parietal reference VOIs were 45 ± 16 mls and 74 ± 13 mls, respectively. On the post-treatment study performed following 4 weeks of bevacizumab, the averages of mean SUVs were very similar to baseline, with values of 0.91 ± 0.28 for the frontal lobe and 0.92 ± 0.29 for the parietal lobe. After 4 weeks of therapy, the absolute difference between frontal and parietal reference VOI mean SUVs ranged from 3.4 to 11.1 with a mean absolute difference of 4.8 ± 3.7%. These reference regions were used to generate thresholds for defining MTVs, and the 1.5-fold threshold was selected for further analysis as higher thresholds led to very small tumor volumes in some patients. However, the patient sample size in this study is too small to conclusively define the optimal threshold for defining MTVs. The striatum was not used as this brain region accumulates metabolites of 18F-FDOPA via the dopaminergic metabolic pathway at later time points after injection, and thus is a less reliable reference region for comparing relative concentrations of amino acid uptake based on through system L transport.
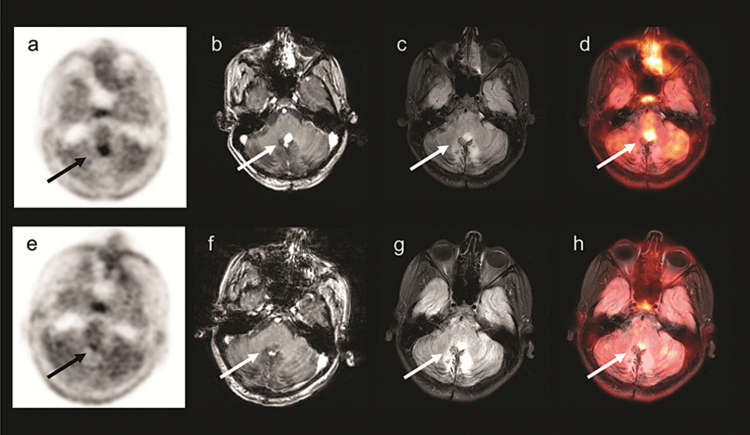
Fig. 2 .
A 12-year-old boy with cerebellar pilocytic astrocytoma (patient 2). Images prior to starting bevacizumab: (a)18F-FDOPA PET (b) T1-weighted MRI with contrast (c) Fluid-attenuated inversion recovery (FLAIR) MRI (d)18F-FDOPA PET/MRI. Images obtained 4 weeks following bevacizumab: (e)18F-FDOPA PET (f) T1-weighted MRI with contrast (g) FLAIR MRI (h)18F-FDOPA PET/MRI, showing near complete resolution of the tumor.
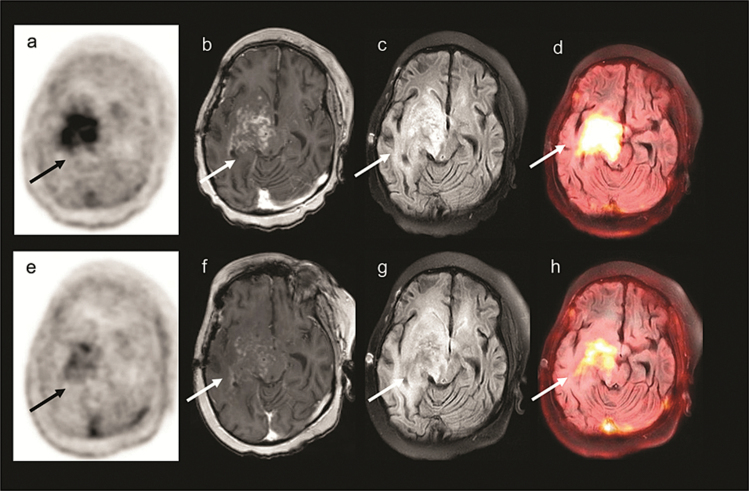
Fig. 3 .
A 8-year-old girl with right thalamic grade IV small cell astrocytoma (patient 3). Images prior to starting bevacizumab: (a)18F-FDOPAPET (b) T1-weighted MRI with contrast (c) Fluid-attenuated inversion recovery (FLAIR) MRI (d)18F-FDOPA PET/MRI. Images obtained 4 weeks following bevacizumab showing modest response: (e)18F-FDOPA PET image (f) T1-weighted MRI with contrast (g) FLAIR MRI (h)18F-FDOPA PET/MRI. Although the standardized uptake value (SUV) of the tumor decreased on the second 18F-FDOPA PET/MRI study, this patient had the smallest decrease in metabolic tumor volume (MTV), 77% of baseline, after 4 weeks of bevacizumab. This patient progressed very rapidly following the start of therapy, receiving only 8 weeks of treatment.
The maximum SUV (SUVmax) for the tumors at baseline ranged from 2.0 to 3.9 with an average value of 2.9 ± 0.6. The mean SUV of the tumors based on the 1.5-fold threshold (SUVmean1.5) ranged from 1.2 to 1.9 with an average value of 1.6 ± 0.3 at baseline. At baseline, the TBRmax ranged from 2.2 to 4.9 with an average value of 3.3 ± 1.0, and the TBRmean ranged from 0.9 to 2.2 with an average value of 1.8 ± 0.5. After 4 weeks of bevacizumab, the SUVmax for the tumors ranged from 1.9 to 3.4 with an average value of 1.4 ± 0.4, and the SUVmean1.5 ranged from 1.5 to 2.2 with an average value of 1.8 ± 0.4. The change between baseline and 4 weeks of therapy ranged from -39% to 16% for SUVmax and from -33% to 48% for SUVmean1.5. After therapy, the TBRmax ranged from 1.5 to 4.2 with an average value of 2.8 ± 1.1, and the TBRmean ranged from 1.5 to 2.7 with an average value of 1.9 ± 0.4. Changes in SUV and TBR measurements did not appear to correspond to response to therapy based on clinical exam and standard MRI evaluations in this small patient cohort. Table S1 in the Supplemental Material available online presents SUVmax and SUVmean1.5 findings relative to the frontal lobe reference region at (a) baseline and (b) after 4 weeks of bevacizumab therapy for individual patient scenarios. Table S2 in the Supplemental Material available online presents TBRmax and TBRmean1.5 findings relative to the frontal lobe reference region at (a) baseline and (b) after 4 weeks of bevacizumab therapy for individual patient scenarios.
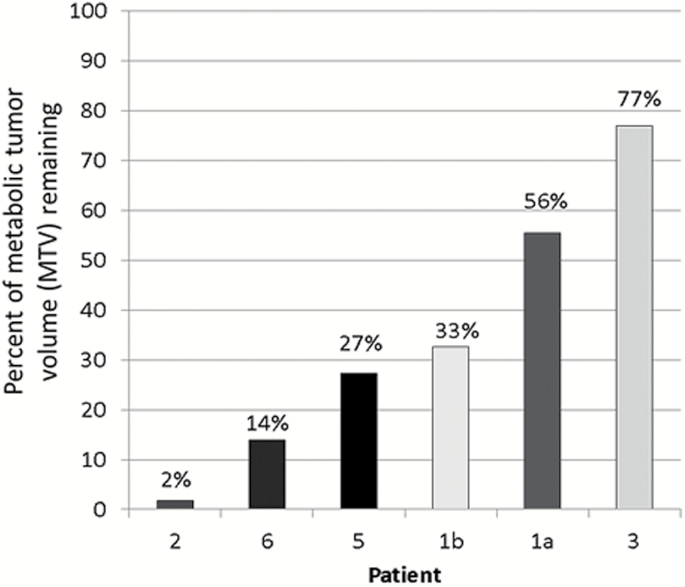
The MTV defined by the 1.5-fold threshold based on the frontal lobe VOI decreased in all patients but by varying magnitudes. The largest decrease was seen in patient 2 with the MTV after 4 weeks of bevacizumab only 2% of the baseline MTV. This result correlated with the 3-month MRI, which showed near complete resolution of his tumor. This patient completed therapy and had stable disease for 10 months when the tumor increased slightly on MRI, and continued to increase in size at 15 months without clinical symptoms. In contrast, patient 3 had the smallest decrease with the MTV after 4 weeks of bevacizumab at 77% of the baseline MTV. This patient progressed very rapidly following the start of therapy, receiving only 8 weeks of treatment. Patient 1 had 2 distinct lesions that had variable response to therapy. The tumor in the optic chiasm (1b chiasm), with an MTV post-therapy that was 33% of the baseline, had a dramatic clinical and radiographic response at 3 months, while the optic nerve glioma (1a orbital) was stable, with an MTV after therapy that was 56% of the baseline. Patient 6 had an MTV on the second study that was 14% of baseline, which correlated well with his dramatic 3-month response on MRI; however, this response was not sustained and he developed tumor progression 7 months into therapy. Percent MTV change for each tumor in each patient is shown in Fig. 4.
Fig. 4.
MTV following 4 weeks of bevacizumab shown as a percent of baseline MTV for each lesion measured in the 6 patients.
Table S3 in the Supplemental Material available online presents the response assessment by PET/MRI and MRI based on RANO criteria was evaluated at baseline (immediately prior to starting bevacizumab), at 4 weeks and 3 months after starting bevacizumab, and at the most recent clinical MRI. The response by the RANO criteria at 4 weeks was available for 4 patients with 4 tumors having stable disease and one tumor having progressive disease. Follow-up MRI demonstrated that 2 of the 4 tumors with stable disease at 4 weeks had partial response based on RANO criteria with sustained response between 4 weeks and 3 months. In terms of the last MRI performed, the response assessment based on RANO criteria was available for all 6 patients, indicating 2 of 6 as responders (33%) with 1 partial response and 1 stable disease, and 4 of 6 (66%) with ultimately progressive disease.
Discussion
This pilot study is the first clinical trial to prospectively assess response to antiangiogenic therapy with bevacizumab using 18F-FDOPA PET/MRI in pediatric brain tumor patients. We found that 18F-FDOPA PET/MRI was feasible and well tolerated in pediatric patients even in the absence of sedation. Given the small number of patients in this pilot study and the heterogeneous grade, pathology, and behavior of the tumors in this study, statistical correlation of SUVmax, SUVmean, TBRmax, TBRmean and MTV to survival was not possible. As seen in previous studies, we did not find an association between changes in SUVmax, SUVmean, TBRmax, TBRmean and clinical or MRI response. We observed a wide range of decreases in MTV (range, 23% to 98%) measured with 18F-FDOPA in pediatric patients after antiangiogenic therapy with bevacizumab. There is a potential trend observed with percent MTV change seen on the 4-week 18F-FDOPA PET/MRI study correlated with response on the MRI-based RANO criteria at 3 months. It is important to note that the RANO criteria require sustained improvement on a follow-up scan at least 4 weeks later to be able to designate complete or partial response. Therefore, for a single time point (ie, 4-week PET/MRI study), the RANO criteria would classify the responders as having stable disease until the follow-up 3-month MRI study was performed. However, it is not clear that 18F-FDOPA PET/MRI predicts longer, sustained response to therapy in pediatric neuro-oncology patients. Larger studies in pediatric patients categorized by specific tumor histology will be needed to determine if 18F-FDOPA PET/MRI can serve as an early predictor of long-term response to bevacizumab.
Amino acid PET imaging with 18F-FDOPA has been studied in adults with gliomas. Schwarzenberg et al evaluated 18F-FDOPA PET prospectively in 30 adult patients with high-grade glioma (glioblastoma or anaplastic astrocytoma) treated with bevacizumab with or without irinotecan.3118F-FDOPA PET imaging was obtained at baseline and at 2 weeks and 6 weeks following start of therapy, and compared to MRI at 6 weeks. Similar to our data, they found that SUVmax and SUVmean were not predictive of response or survival. The absolute MTV value at baseline was not predictive of outcome; however, the MTV at 2 weeks was the strongest predictor of PFS and OS. Responders, defined as a MTV ≤ 18 ml, had a mean survival of 12.1 months versus 3.5 months in nonresponders. In addition, the percent change in MTV was a predictor of survival, with the MTV at 2 weeks being a better predictor of PFS and at 6 weeks of OS. Similar results were obtained using the structurally and pharmacologically similar amino acid 18F-FET in a cohort of 10 patients42 and a separate cohort of 11 patients,43 both of which had recurrent high-grade gliomas.
Morana et al described a case report of a pediatric high-grade glioma patient who had complete resolution of contrast enhancement on 8-week MRI following initiation of bevacizumab and temozolomide.4418F-FDOPA PET at this time point showed high uptake in the nonenhancing component of the tumor. Subsequent 16-week MRI revealed significant progressive disease, indicating that contrast-enhanced MRI is not an adequate early predictor of response to antiangiogenic therapy and that 18F-FDOPA PET may be a better predictor. In a separate report, Morana et al retrospectively evaluated 18F-FDOPA PET/CT and 1H-MRS in 27 pediatric infiltrative lesions, including 21 gliomas (grades I-IV) and 6 noncancerous lesions.3418F-FDOPA PET was positive in 17 patients, defined by tracer uptake above the contralateral normal brain, 1 patient with a false positive had encephalitis, and 5 with false negatives had low-grade infiltrative lesions. Our patient population included 5 low-grade gliomas, all of which showed 18F-FDOPA uptake at baseline but none of which were infiltrative lesions. Additionally, the study performed by Morana et al utilized 18F-FDOPA PET data acquired 20 to 50 min after injection, unlike our study, which utilized 10 to 15 min and corresponded to the time point of peak tumor uptake in our study population.
Although electrophilic fluorination production methods were used for [18F]FDOPA radiosynthesis in this study, nucleophilic no-carrier-added production methods are becoming increasingly prevalent.45 Nucleophilic radiosynthetic methods increase the amount of [18F]FDOPA that can be produced per batch and dramatically reduce the associated mass of nonradioactive FDOPA administered in each study. Future studies using [18F]FDOPA will likely use the nucleophilic method although comparative studies may be needed to confirm that differences in the mass of nonradioactive FDOPA co-administered with [18F]FDOPA dosage not alter tumor imaging properties of this PET tracer.
Simultaneous PET/MRI allowed collection of both PET and MRI data in the same imaging session, which decreased the burden of participation for patients and their families. The duration of a tumor protocol MRI is well matched to the relatively fast kinetics of amino acids like 18F-FDOPA and facilitates the collection of dynamic PET data in both research and clinical settings. We observed variable kinetics in different tumors, and it may be that a single fixed time point for PET acquisition may not be optimal. One limitation of PET/MRI is that accurate attenuation correction for bone remains problematic, and thus PET/MRI may underestimate SUVs adjacent to cortical bone by 5% to 10%.46,47 In our study, we did not attempt to correct for potential errors that might be introduced by MR-based attenuation correction. Our primary measure was relative change in MTV between baseline and after 4 weeks of bevacizumab, and the attenuation correction errors should be constant across studies within each patient. However, absolute SUV measurements and other quantitative assessments could be affected by the use of PET/MRI instead of PET/CT.
Conclusion
This preliminary study demonstrates the safety and feasibility of 18F-FDOPA PET/MRI for monitoring response to bevacizumab early in the course of therapy in pediatric neuro-oncology patients. A range of responses to 4 weeks of bevacizumab as assessed by changes in MTV were observed with 18F-FDOPA PET, as has been reported in adults. Our data suggest that 18F-FDOPA PET/MRI may predict response at 3 months after initiating therapy, but larger studies will be needed before definitive conclusions can be made about the role of this technique in assessing response to therapy.
Supplementary Material
Supplementary data are available at Neuro-Oncology Practice online.
Funding
St. Baldrick’s Foundation
American Cancer Society IRG-58-010-56
Washington University Institute of Clinical and Translational Sciences Grant (UL1TR000448) from the National Center for Advancing Translational Sciences
National Cancer Institute (K08CA154790).
Conflict of interest statement. Dr. Jonathan McConathy has consulted and spoken at PET/MRI user meetings for GE Healthcare and Siemens Healthcare.
Supplementary Material
Acknowledgements
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri for the use of the Imaging and Response Assessment Core. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842 (Eberlein, PI).
References
- 1. Aguilera D, Mazewski C, Fangusaro J, et al. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience. Childs Nerv Syst. 2013;29(4):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonney PA, Santucci JA, Maurer AJ, et al. Dramatic response to temozolomide, irinotecan, and bevacizumab for recurrent medulloblastoma with widespread osseous metastases. J Clin Neurosci. 2016;26:161–163. [DOI] [PubMed] [Google Scholar]
- 3. Piha-Paul SA, Shin SJ, Vats T, et al. Pediatric patients with refractory central nervous system tumors: experiences of a clinical trial combining bevacizumab and temsirolimus. Anticancer Res. 2014;34(4):1939–1945. [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatramani R, Malogolowkin M, Davidson TB, et al. A phase I study of vincristine, irinotecan, temozolomide and bevacizumab (vitb) in pediatric patients with relapsed solid tumors. PLoS One. 2013;8(7):e68416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer. 2013;60(5):776–782. [DOI] [PubMed] [Google Scholar]
- 7. Kalra M, Heath JA, Kellie SJ, et al. Confirmation of bevacizumab activity, and maintenance of efficacy in retreatment after subsequent relapse, in pediatric low-grade glioma. J Pediatr Hematol Oncol. 2015;37(6):e341–e346. [DOI] [PubMed] [Google Scholar]
- 8. Umeda K, Shibata H, Saida S, et al. Long-term efficacy of bevacizumab and irinotecan in recurrent pediatric glioblastoma. Pediatr Int. 2015;57(1):169–171. [DOI] [PubMed] [Google Scholar]
- 9. Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narayana A, Kunnakkat S, Chacko-Mathew J, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parekh C, Jubran R, Erdreich-Epstein A, et al. Treatment of children with recurrent high grade gliomas with a bevacizumab containing regimen. J Neurooncol. 2011;103(3):673–680. [DOI] [PubMed] [Google Scholar]
- 12. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 13. Hutterer M, Hattingen E, Palm C, et al. Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol. 2015;17(6):784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. [DOI] [PubMed] [Google Scholar]
- 16. Hutterer M, Hattingen E, Palm C, et al. Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol. 2015;17(6):784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Artzi M, Bokstein F, Blumenthal DT, et al. Differentiation between vasogenic-edema versus tumor-infiltrative area in patients with glioblastoma during bevacizumab therapy: a longitudinal MRI study. Eur J Radiol. 2014;83(7):1250–1256. [DOI] [PubMed] [Google Scholar]
- 19. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, McConathy J. Radiolabeled amino acids for oncologic imaging. J Nucl Med. 2013;54(7):1007–1010. [DOI] [PubMed] [Google Scholar]
- 21. Kratochwil C, Combs SE, Leotta K, et al. Intra-individual comparison of ¹8F-FET and ¹8F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014;16(3):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galldiks N, Kracht LW, Berthold F, et al. [11C]-L-methionine positron emission tomography in the management of children and young adults with brain tumors. J Neurooncol. 2010;96(2):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunkl V, Cleff C, Stoffels G, et al. The usefulness of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the clinical evaluation of brain tumors in children and adolescents. J Nucl Med. 2015;56(1):88–92. [DOI] [PubMed] [Google Scholar]
- 24. Misch M, Guggemos A, Driever PH, et al. (18)F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Childs Nerv Syst. 2015;31(2):261–267. [DOI] [PubMed] [Google Scholar]
- 25. Korchi AM, Garibotto V, Ansari M, et al. Pseudoprogression after proton beam irradiation for a choroid plexus carcinoma in pediatric patient: MRI and PET imaging patterns. Childs Nerv Syst. 2013;29(3):509–512. [DOI] [PubMed] [Google Scholar]
- 26. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–911. [PubMed] [Google Scholar]
- 28. Herrmann K, Czernin J, Cloughesy T, et al. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 2014;16(4):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karavaeva E, Harris RJ, Leu K, et al. Relationship between [F]FDOPA PET uptake, apparent diffusion coefficient (ADC), and proliferation rate in recurrent malignant gliomas. Mol Imaging Biol. 2015;17(3):434–442. [DOI] [PubMed] [Google Scholar]
- 30. Harris RJ, Cloughesy TF, Pope WB, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwarzenberg J, Czernin J, Cloughesy TF, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Preuss M, Werner P, Barthel H, et al. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Childs Nerv Syst. 2014;30(8):1399–1403. [DOI] [PubMed] [Google Scholar]
- 33. Pirotte BJ, Lubansu A, Massager N, et al. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5(5):486–499. [DOI] [PubMed] [Google Scholar]
- 34. Morana G, Piccardo A, Puntoni M, et al. Diagnostic and prognostic value of 18F-DOPA PET and 1H-MR spectroscopy in pediatric supratentorial infiltrative gliomas: a comparative study. Neuro Oncol. 2015;17(12):1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Couec ML, André N, Thebaud E, et al. ; Comité Pharmacologie of the SFCE. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: toxicity and efficacy trends. Pediatr Blood Cancer. 2012;59(1):34–38. [DOI] [PubMed] [Google Scholar]
- 36. Lyons K, Seghers V, Sorensen JI, et al. Comparison of standardized uptake values in normal structures between PET/CT and PET/MRI in a tertiary pediatric hospital: a prospective study. AJR Am J Roentgenol. 2015;205(5):1094–1101. [DOI] [PubMed] [Google Scholar]
- 37. Shamonin DP, Bron EE, Lelieveldt BP, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform. 2013;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein S, Staring M, Murphy K, et al. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196–205. [DOI] [PubMed] [Google Scholar]
- 39. Lapa C, Linsenmann T, Monoranu CM, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55(10):1611–1616. [DOI] [PubMed] [Google Scholar]
- 40. Pauleit D, Stoffels G, Schaden W, et al. PET with O-(2-18F-Fluoroethyl)-L-Tyrosine in peripheral tumors: first clinical results. J Nucl Med. 2005;46(3):411–416. [PubMed] [Google Scholar]
- 41. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 42. Galldiks N, Rapp M, Stoffels G, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 43. Hutterer M, Nowosielski M, Putzer D, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 44. Morana G, Piccardo A, Garrè ML, et al. Multimodal magnetic resonance imaging and 18F-L-dihydroxyphenylalanine positron emission tomography in early characterization of pseudoresponse and nonenhancing tumor progression in a pediatric patient with malignant transformation of ganglioglioma treated with bevacizumab. J Clin Oncol. 2013;31(1):e1–e5. [DOI] [PubMed] [Google Scholar]
- 45. Libert LC, Franci X, Plenevaux AR, et al. Production at the Curie level of no-carrier-added 6-18F-fluoro-L-dopa. J Nucl Med. 2013;54(7):1154–1161. [DOI] [PubMed] [Google Scholar]
- 46. Su Y, Rubin B, McConathy J, et al. Impact of MR based attenuation correction on neurological PET studies. J Nucl Med. 2016;57(6):913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samarin A, Burger C, Wollenweber SD, et al. PET/MR imaging of bone lesions—implications for PET quantification from imperfect attenuation correction. Eur J Nucl Med Mol Imaging. 2012;39(7):1154–1160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.