Abstract
Background
Magnetic resonance spectroscopy (MRS) aids noninvasive diagnosis of pediatric brain tumors, but use in clinical practice is not well documented. We aimed to review clinical use of MRS, establish added value in noninvasive diagnosis, and investigate potential impact on patient care.
Methods
Sixty-nine children with lesions imaged using MRS and reviewed by the tumor board from 2014 to 2016 met inclusion criteria. Contemporaneous MRI diagnosis, spectroscopy analysis, histopathology, and clinical information were reviewed. Final diagnosis was agreed on by the tumor board at study end.
Results
Five cases were excluded for lack of documented MRI diagnosis. The principal MRI diagnosis by pediatric radiologists was correct in 59%, increasing to 73% with addition of MRS. Of the 73%, 19.1% (95% CI, 9.1%-33.3%) were incorrectly diagnosed with MRI alone. MRS led to a significant improvement in correct diagnosis over all tumor types (P = .012). Of diagnoses correctly made with MRI, confidence increased by 37% when adding MRS, with no patients incorrectly re-diagnosed. Indolent lesions were diagnosed noninvasively in 85% of cases, with MRS a major contributor to 91% of these diagnoses. Of all patients, 39% were managed without histopathological diagnosis. MRS contributed to diagnosis in 68% of this group, modifying it in 12%. MRS influenced management in 33% of cases, mainly through avoiding and guiding biopsy and aiding tumor characterization.
Conclusion
MRS can improve accuracy and confidence in noninvasive diagnosis of pediatric brain lesions in clinical practice. There is potential to improve outcomes through avoiding biopsy of indolent lesions, aiding tumor characterization, and facilitating earlier family discussions and treatment planning.
Keywords: 1H magnetic resonance spectroscopy (MRS), magnetic resonance imaging (MRI), metabolite profiles, noninvasive diagnosis, pediatric brain tumors
Brain tumors are the most prevalent type of solid cancer in childhood, the most common cause of pediatric cancer death,1 and a significant cause of long-term disability. Treatment options and outcomes are dependent on tumor type, grade, location, and patient age. The current gold standard for diagnosis is histopathology following biopsy or surgical resection,2 with associated risk of morbidity or sampling error.3 Definitive histopathological diagnosis is not available until several days after tissue acquisition and thus cannot be used to guide surgical decision-making, early planning of adjuvant treatments such as chemotherapy or radiotherapy, or timely family discussions.
Imaging is central to the diagnosis and management of brain tumors in children. Childhood brain tumors are diverse in terms of their pathology,2 and different histological tumor types can display overlapping imaging characteristics.4,5 Studies in adults and children have shown that, although conventional MRI provides images with excellent structural detail, it cannot always be used alone to accurately identify specific tumor type or grade,5–10 conclusively differentiate neoplastic from non-neoplastic lesions,4,5,11 or determine the optimal biopsy site of heterogeneous tumors.12
1H-magnetic resonance spectroscopy (MRS) is an advanced MRI technique that provides noninvasive measurements of tissue metabolite profiles. As different tumor types display different key metabolic features,5,13,14 this additional information has potential to aid diagnosis and improve characterization in both adults and children.7,14–19 Although MRS is becoming widely available, few studies have evaluated how it can enhance conventional radiological reporting by adding value to information obtained through MRI alone10,20–22 or assessed impact on patient management and outcome in clinical practice.11,23
Despite evidence for technical feasibility and diagnostic accuracy of MRS in children,6,18,24,25 there has been little systematic comparison of the technique with conventional MRI alone in pediatrics. One recent pediatric study based on retrospective review of MRI and MRS found including visual interpretation of MRS in preoperative diagnosis significantly improved accuracy of radiological diagnosis over MRI alone (87% and 63% accuracy, respectively).10 This indicates MRS can be a useful adjunct to MRI to improve diagnostic accuracy without sophisticated decision support software. However, the design of the study did not allow an assessment of the use of MRS in clinical practice.
Studies are needed to quantify the extent to which MRS facilitates diagnosis and changes patient management with improved outcomes in the pediatric population. Further research is needed to optimally integrate MRS into the pediatric diagnostic pathway to improve clinical management of this vulnerable patient group. We aimed to review the clinical use of MRS in a single center, establish its added value in noninvasive diagnosis and clinical decision-making, and investigate potential impact on patient care.
Patients and Methods
Patients
The study was conducted at the Birmingham Children’s Hospital after being granted ethical approval for functional imaging research. All 69 children referred to the pediatric neuro-oncology tumor board with a suspected brain tumor following imaging with MRI and MRS between January 1, 2014 and January 31, 2016 were included. Informed parental consent was obtained.
MRI and MRS
Single-voxel MRS was performed routinely in children presenting with CNS lesions as part of diagnostic clinical imaging prior to surgical intervention or treatment. MRS was acquired on one of two 1.5T MR scanners (GE Signa Excite, Siemens Avanto) or a 3T MR scanner (Phillips Interna Achieva) after conventional MRI, which included T1-weighted, T2-weighted, diffusion-weighted, and T1-weighted postcontrast sequences. At 1.5T, a single-voxel MRS protocol was used with point-resolved spectroscopy localization, a short echo time of 30 ms, and a repetition time of 1500 ms. Cubic voxels were used with 2-cm or 1.5-cm side length, acquiring 128 or 256 repetitions, respectively. At 3T, a short echo time of around 35 ms and a repetition time of 2000 ms was used. Voxels were generally cubic with side length 2 cm, 1.5 cm, or 1.3 cm with 68, 128, and 196 repetitions being used, respectively. Voxel placement was entirely within the tumor where possible as delineated by conventional MRI. Accurate voxel positioning was confirmed by verifying placement was >3 mm away from bone, scalp, and air and did not predominantly contain normal brain. Spectroscopy processing was undertaken using standard scanner software exported to the hospital PACS (picture archive and communications system; Agfa IMPAX 6.5.2.2016), as routine. Raw spectroscopy data were also processed using TARQUIN (Totally Automatic Robust Quantitation in NMR) v3.2.226 and made available as part of a research study. Spectra were inspected visually for baseline abnormalities, acceptable linewidth, signal-to-noise ratio, and major artifacts.
Tumor Board Diagnosis
The pediatric neuro-oncology tumor board, consisting of experienced pediatric oncologists, pediatric radiologists specializing in neuroradiology, clinical oncologists, neurosurgeons, and histopathologists, reviewed all patients. The structure of our pediatric neuro-oncology tumor board involves review of conventional imaging, followed by MRS, followed by presentation of histopathology where available, ending with consensus diagnosis and management planning. Discussions as each new information modality is presented are documented in detail contemporaneously by an experienced medical secretarial team and reviewed by clinicians prior to storage, enabling deduction of the relative contribution of each information source to the decision-making process.
Diagnostic reference standard was taken as diagnosis agreed by the tumor board at study end, verified through clinical course. Tumors with histopathological confirmation were classified according to the WHO Classification of Tumors of the Central Nervous System 2007, and those lacking histopathological confirmation were given a diagnosis from a predefined list (see Supplementary Data 1).
Radiologists
Reporting radiologists were consultant pediatric radiologists (n = 10) fully trained and qualified in the discipline. Neuroradiology forms an integral part of their training: 7 of the 10 have a special interest in the area through involvement in a relevant clinical study board, and 3 of them are responsible for the neuro-oncology tumor board. It is common for radiologists to discuss cases with colleagues prior to issuing formal reports.
The majority of diagnostic MRI scans in children presenting with CNS lesions were reported by pediatric radiologists with an interest in pediatric neuroradiology. Imaging from patients presenting out of hours were preliminarily reported by the consultant pediatric radiologist on duty, but were usually reviewed by a radiologist with an interest in neuroradiology prior to release of the official imaging report. All MRI and MRS included in this study were reviewed by the pediatric radiologists with an interest in neuroradiology as part of the tumor board.
Conventional MRI Interpretation
Conventional MR images were reported by consultant pediatric radiologists prior to availability of histopathology. Official imaging reports of patients presenting with CNS lesions were retrospectively reviewed to obtain the primary contemporaneous radiological diagnosis. Radiological diagnosis was defined as “correct” (certain) if the only documented diagnosis on the imaging report exactly corresponded to that determined by the tumor board at study end, and “partially correct” (uncertain) if this was the first in a list of differentials or reported as a query. An “incorrect” MRI diagnosis was a report that listed the tumor board diagnosis in a list of differentials but not first, or did not list it at all. Cases with no documented radiological diagnosis were excluded.
MRS Interpretation
Contemporaneous MRS interpretation prior to availability of histopathology was taken from official imaging reports, interclinician correspondence, medical records, and tumor board reports. Documented tumor board discussions and clinical correspondence were reviewed to identify clinical questions answered using information from spectroscopy. Cases in which MRS facilitated noninvasive diagnosis (defined as “provided information documented as contributing to the final correct tumor board consensus diagnosis”), changed presumed diagnosis, helped answer a specific clinical question (defined as “provided information documented as contributing to the decision-making process”), or influenced management or outcome (defined as “provided information documented to have altered clinical management”) were examined in detail and recorded.
The MRS data processed using scanner software and added to PACS were available to radiologists at the time of initial MRI reporting. The accepted system for reporting at Birmingham Children’s Hospital is to view and interpret the conventional MRI first, then follow this by adding information from visual interpretation of MRS, enabling distinction of the contribution made by MRS to the diagnostic process.
The interpretation of MRS used clinically was through visualization of the spectra in the great majority of cases. Spectroscopy processing was undertaken using standard scanner software and exported to hospital PACS in all cases described. Raw spectroscopy data processed using TARQUIN were available in a subset of patients as part of a research study. Contemporaneous MRS interpretation in clinical practice was mainly via visual interpretation by radiologists of the scanner-processed software via PACS, with some support provided by the visual inspection of the TARQUIN processed spectra. Clinicians did not tend to use TARQUIN for metabolite quantitation but anecdotally did find the individual metabolite fits provided by TARQUIN useful. Detailed interpretation of the MRS by expert clinicians and researchers was requested on a subset of 14 patients.
Histopathology
Histopathology reports were obtained from the official hospital results reporting system (Sunquest ICE Desktop Live, version 541). Results of first authorized histopathology reports, central review, and alterations in initial histopathological diagnosis were documented. Time from initial imaging and biopsy or surgery to definitive histopathological diagnosis was recorded and evaluated in light of time to treatment. Unbiopsied cases without histopathology reports and those with inconclusive histopathology reports were reviewed and reasons documented.
Statistical Analysis
“Correct” and “partially correct” MRI reports were combined to determine the percentage of cases with accurate radiological diagnosis. “Correct” MRI reports were interpreted as implying diagnostic certainty, whereas “partially correct” reports implied uncertainty. Noninvasive diagnoses corresponding to those determined by the tumor board following addition of MRS were counted to determine accuracy of MRI combined with MRS. The number of “partially correct” radiology reports confirmed by MRS was taken to reflect increase in diagnostic certainty.
Comparison of post-MRI and post-MRS diagnosis with the reference diagnosis determined how many more cases were diagnosed correctly at each stage. Proportions were reported with 95% confidence intervals calculated from the binomial distribution. McNemar’s test was used to determine if MRS had a statistically significant effect on correct diagnosis compared with MRI alone. The accuracy of MRI +/- MRS for each category of brain tumor (all locations, supratentorial, infratentorial, and brainstem) was made through estimates of sensitivity.
Results
Demographics
Five cases were excluded for lack of documented MRI diagnosis, and 64 patients included in the analysis. The patients’ ages ranged from 1 month to 16 years (median = 9 years); 40 were male and 24 female. All patients had their diagnosis reviewed by the tumor board. The breakdown of cases by tumor board diagnosis can be seen in Supplementary Data 1.
Histopathology
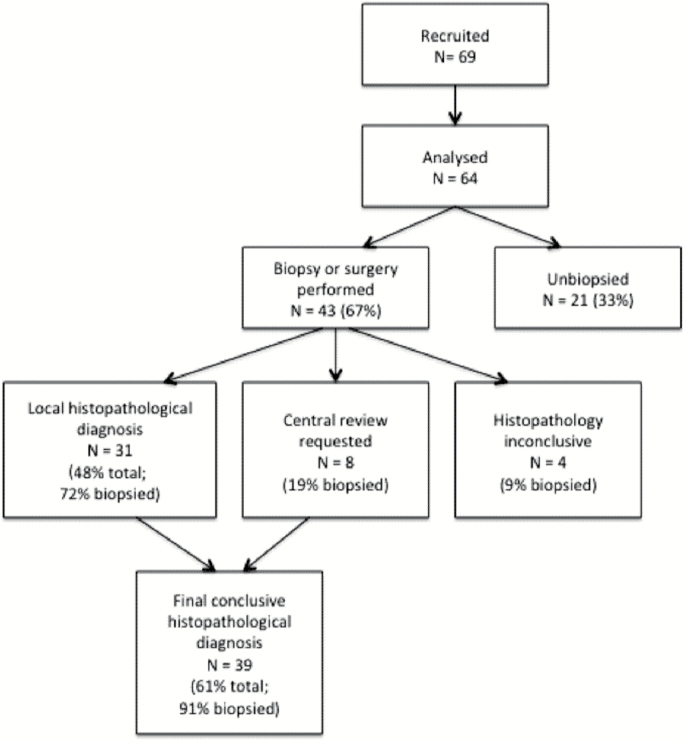
Of the 64 patients, 43 (67%) had a biopsy or surgical resection. Conclusive histopathological diagnosis was available in 39 (61% of all patients, 91% of biopsied patients) a median of 12 (range, 4–40) days following imaging or 9 (range, 0–16) days following biopsy. Central pathology review was undertaken in 8 samples (19%), leading to minor change in diagnoses in 7 (16%). Central pathology review was only undertaken in cases for which there was diagnostic uncertainty. Histopathology was inconclusive in 4 biopsied patients (15%), 1 due to sampling error and 3 due to atypical histopathological appearance. Of all patients, 25 (39%) were managed without histopathological diagnosis. Availability of histopathological diagnosis for CNS lesions is shown in Fig. 1.
Fig. 1.
Availability of histopathology diagnosis for central nervous system lesions.
MRS Quality Assurance
Of the 64 MRS studies performed, 11 (17%) failed quality assurance. Of these, 5 had not met requirements for voxel placement and contained predominantly normal brain or necrotic tumor, 1 was uninterpretable due to presence of blood products, 2 were incorrectly configured, 2 had poor signal:noise ratio, and 1 had poor linewidth probably secondary to intratumoral calcification.
Noninvasive Diagnosis Using MRI Alone and Following Addition of MRS
The principal MRI diagnosis was accurate in terms of exact accordance with diagnosis agreed on by the tumor board at study end in 38 of 64 patients (59% sensitivity), increasing to 47 (73% sensitivity) with addition of MRS (Table 1). Of the 47, 9 cases (19.1%; 95% CI, 9.1%-33.3%) had been incorrectly diagnosed with MRI alone. McNemar’s test showed MRS had a statistically significant effect on correct diagnosis for all tumor types (P = .012). None of the 17 cases incorrectly diagnosed after combining MRS and MRI had been correctly diagnosed after MRI alone. Histological diagnosis was available in 39 of 64 patients (61%). Of this subset, 22 (56%) patients were correctly diagnosed noninvasively using MRI alone, increasing to 26 (67%) with addition of MRS.
Table 1.
Diagnosis of CNS lesions by location using MRI alone, MRI+MRS, and histopathology
| Supratentorial | Posterior Fossa | Brainstem | Total | |
|---|---|---|---|---|
| N | 40 | 17 | 7 | 64 |
| Accurate MRI diagnosisa | 17 (43%) | 15 (88%) | 6 (86%) | 38 (59%) |
| MRI diagnosis “correct” (certain) | 6 (15%) | 9 (53%) | 3 (43%) | 18 (28%) |
| Accurate MRI+MRS diagnosis | 24 (60%) | 16 (94%) | 7 (100%) | 47 (73%) |
| Accurate MRI diagnoses with increased certainty following MRS | 7/17 (41%) | 4/15 (27%) | 3/6 (50%) | 14/38 (37%) |
| MRI diagnosis “incorrect” or “inconclusive” correctly diagnosed by MRS | 8/23 (35%) | 1/2 (50%) | 1/1 (100%) | 10/26 (38%) |
| MRS diagnosis “incorrect” | 2 (5%) | 0 | 0 | 2 (3%) |
| Appropriate change in management following MRS | 17 (43%) | 2 (12%) | 4 (57%) | 23 (36%) |
| Biopsy/resection | 26 (65%) | 16 (94%) | 1 (14%) | 43 (67%) |
| Histopathological diagnosis available | 24 (60%) | 14 (82%) | 1 (14%) | 39 (61%) |
| Central histopathology review requested | 8 (31% of samples) | 0 | 0 | 8 (19% of samples) |
| Histopathology inconclusive | 4 (15% of samples) | 0 | 0 | 4 (9% of samples) |
aAccurate MRI diagnosis = total “correct” (certain) and “partially correct” (uncertain).
Of the 9 cases misdiagnosed by MRI but correctly diagnosed with addition of MRS, 4 had histopathological confirmation. These 9 cases were initially described as dysembryoplastic neuroepithelial tumor (DNET), teratoma, supratentorial ependymoma, epidermoid cyst, low-grade glioma, pineocytoma, atypical teratoid/rhabdoid tumor, atypical germ cell tumor, and indeterminate malignant lesion, based on MRI alone. The remaining 5 cases included 1 brainstem low-grade glioma, 1 DNET, and 3 indolent lesions, all diagnosed following tumor board consensus and verified through clinical course. MRI diagnoses were malignancy for the DNET and indolent lesions, and diffuse intrinsic pontine glioma for the brainstem low-grade glioma.
For 40 supratentorial cases, 17 cases (43% sensitivity) were correctly diagnosed with MRI alone with 24 (60% sensitivity) correctly diagnosed after MRS and MRI. Of the 24 cases, 7 (30.4%; 95% CI, 13.2%-52.9%) were incorrectly diagnosed with MRI alone. None of the 16 supratentorial cases incorrectly diagnosed after MRS and MRI had previously been diagnosed correctly on MRI. McNemar’s test shows a significant effect of including MRS (P = .016). The other tumor groups were too small for separate statistical consideration. Radiological diagnosis using MRI alone was accurate in 15 of 17 posterior fossa (88%) and 6 of 7 brainstem (86%) tumors, increasing to 16 (94%) and 17 (100%), respectively, following addition of MRS.
Of the 38 accurate diagnoses made using MRI, 18 (28%) were certain (“correct”) and 20 (31%) uncertain (“partially correct”). Following addition of MRS, confidence increased in 14 (37%) accurate MRI diagnoses with none incorrectly re-diagnosed. Addition of MRS resulted in an increase in confidence in correct MRI diagnosis in 41%, 27%, and 50% of supratentorial, cerebellar, and brainstem lesions, respectively. MRS identified both ependymomas preoperatively, neither of which were diagnosed on conventional MRI.
Variation in Accuracy of Conventional MRI Report and at Tumor Board
Comparing conventional MRI interpretation on official reports and at tumor board revealed that in only 1 case were both diagnoses incorrect (a diffuse astrocytoma reported initially as a pilocytic astrocytoma and described at tumor as consistent with “neoplasia of unknown cause”).
Lesions without Histopathological Diagnosis
Of all patients, 25 (39%) lacked histopathological diagnosis. Of these, 21 (84%; 33% all patients) were not biopsied and 4 (16%) had histopathology reported as inconclusive (1 due to sampling error, 3 due to atypical histopathological appearance). Tumor board consensus diagnosis was reached in 21 (84%) of these 25 patients, with diagnostic uncertainty remaining in 4 (16%). MRS contributed to diagnosis in 17 (68%) patients in this group, modifying it in 3 (12%). Management was influenced by MRS in 13 (52%) of these patients through avoiding biopsy (n = 10), revision of diagnosis with subsequent appropriate management (n = 1), and alerting to high-grade behavior of lesions initially thought to be low grade (n = 2). For diagnosis of CNS lesions managed without histopathology, see Supplementary Data 2.
Indolent Lesions
A diagnosis of indolent lesion was made in 13 patients (20%). Incidental tumors and cortical dysplasia were diagnosed noninvasively in 11 patients (85%), with MRS being a major contributor to 10 (91%) of these diagnoses. A major contribution describes a case in which diagnosis was unclear prior to addition of MRS interpretation, but diagnosis was made and a management plan decided once information from MRS was available. In these 11 cases, the diagnosis of whether the tumor was indolent or cortical dysplasia was present was not clear from MRI interpretation alone, but when MRS was interpreted and taken into account, the diagnosis was made and subsequently confirmed by clinical course. No malignant lesions were misclassified as indolent using MRS.
Histopathology was available in 2 patients, 1 found to have an arteriovenous malformation and the other a pigmented epidermoid cyst. The former case involved a hyperdense lesion on the dorsal aspect of the pons with a ring of T1 enhancement and visible patchy hemosiderin. Although imaging was suggestive of pontine cavernoma, concerns regarding possible underlying pathology led to national multidisciplinary review with the recommendation for biopsy. The epidermoid cyst had histopathological confirmation following surgical resection of a posterior fossa lesion with associated gross symptomatic hydropcephalus. MRI alone suggested a diagnosis of nonmalignant lesion in 6 cases (46%), none of which were certain (all “partially correct”). Diagnosis was verified through clinical course in all 13 cases. See Supplementary Data 3 for details of diagnosis of indolent lesions.
Treatment
Treatment was initiated a median of 28 (range, 1–364) days following initial imaging. In 10 patients (7 medulloblastoma; 2 diffuse intrinsic pontine gliomas; one glioblastoma multiforme) first-line treatment with radiotherapy commenced a median of 45.5 (range, 13–53) days after imaging, or 43.5 (range, 31–49) days after surgery. In medulloblastoma, the median time from surgery to radiotherapy was 45 days (range, 31–49).
MRS and Clinical Management
MRS influenced clinical management in 33% of cases (21 patients) through avoiding and guiding biopsy, aiding tumor characterization, facilitating diagnosis in cases without histopathology, surgical planning, and identifying unusual tumor types. Cases of interest are given in Figs 2 to 5. Two incorrect diagnoses were made using MRS (atypical teratoid/rhabdoid tumor and supratentorial primitive neuroectodermal tumor, both misdiagnosed as ependymoma). Neither was correctly diagnosed on MRI and management was not altered. MRS diagnosis was not documented in 18 cases (28%).
Fig. 2.
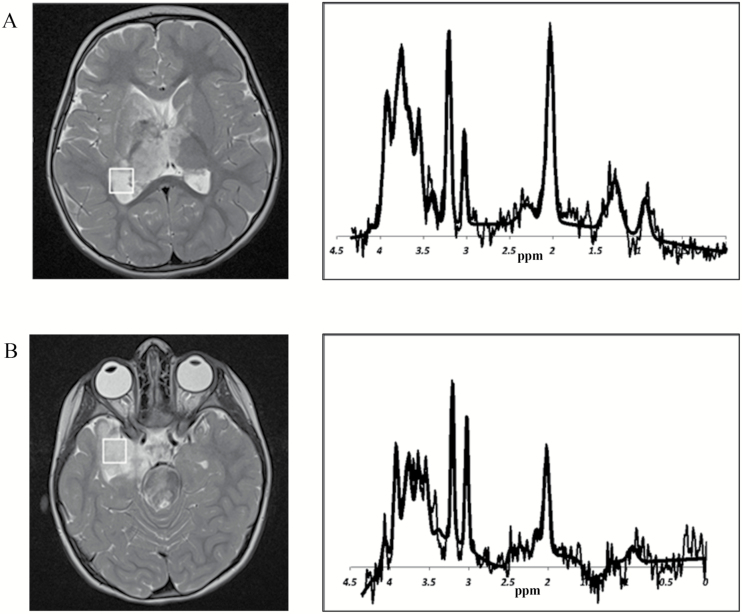
Determining biopsy site in a heterogeneous lesion. A) Right thalamus and trigone. B) Medial temporal lobe. MRI of a 2-year-old girl revealed a diffuse, heterogeneous central nervous system lesion affecting the right thalamus and trigone (A) and medial temporal lobe (B). Although the right trigone was a more surgically accessible biopsy site, it was unclear if this contained representative tumor. Accessing the medial temporal lobe necessitated a more invasive procedure with higher risk of morbidity. To aid decision-making, spectroscopy was performed over potential biopsy sites, revealing increased choline:creatine ratio over the right thalamus and trigone, indicative of tumor tissue. The overall MRS was suggestive of childhood low-grade glioma. Successful biopsy of this area with minimal morbidity yielded histopathological diagnosis of low-grade glioma.
Fig. 5.
Identification of high-grade, unusual tumor type in a case with inconclusive histopathology. MRI of a 13-year-old girl revealed a supratentorial tumor of uncertain diagnosis. Although a definitive diagnosis was not made following biopsy, the lesion demonstrated histological features consistent with a low-grade tumor. MRS demonstrated high-grade features of high choline, low NAA, and lipids. This unusual MRS profile alerted clinicians to the possibility of rare tumor type and possible sampling error on biopsy, which was later confirmed. Close monitoring allowed early detection of rapid growth and metastatic spread. This tumor followed an aggressive course and was diagnosed as a probable astroblastoma following central review.
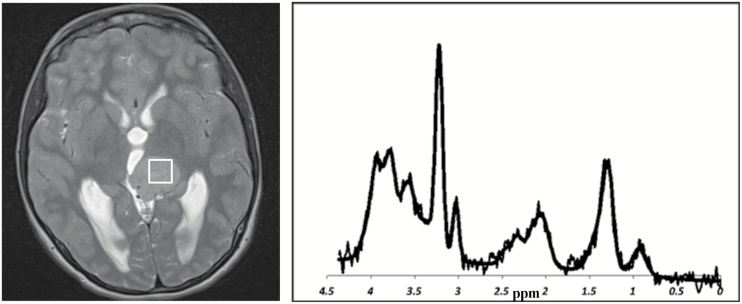
Fig. 3.
Exclusion of metastatic disease in a bifocal, mixed germ cell tumor. A) Pineal component. B) Suprasellar component. MRI of a 9-year-old boy revealed 2 midline tumors radiologically consistent with a mixed germ cell tumor with either bifocal or metastatic disease. MRS profiles of 2 components this tumor appeared very different. The homogeneous pineal component (A) had a spectrum typical of germinoma, whereas the heterogeneous suprasellar component (B) had a profile suggestive of the secreting component of a germ cell tumor. MRS thus facilitated exclusion of metastatic disease in favor of bifocal germ cell tumor without the need for biopsy of the pineal lesion. Metastatic germ cell tumor would have required craniospinal irradiation whereas the diagnosis of bifocal germ cell tumor allowed this to be avoided, and he was subsequently referred for focal proton beam radiotherapy.
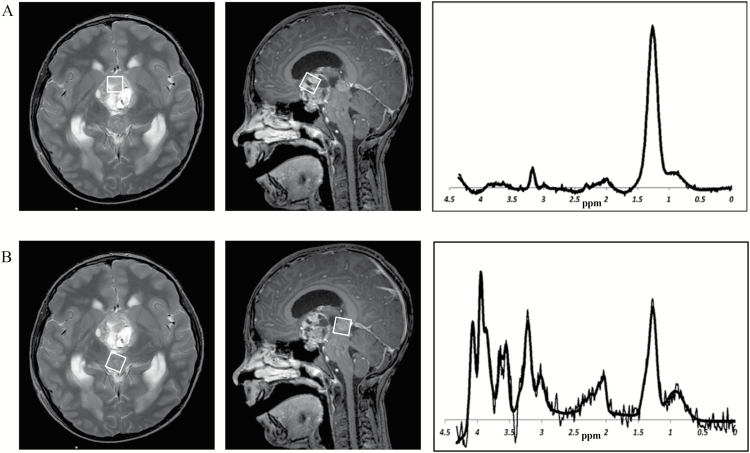
Fig. 4.
Preoperative diagnosis facilitating surgical planning. MRI of a 9-year-old boy with an underlying diagnosis of neurofibromatosis type 2 revealed an infratentorial lesion radiologically consistent with pilocytic astrocytoma or ependymoma. This was diagnosed preoperatively as ependymoma using information from MRS, allowing surgical planning for complete resection. In this case, high myoinositol supported an ependymoma diagnosis, whereas intraoperative histopathology did not confirm the diagnosis. The final histopathological diagnosis confirmed ependymoma.
Discussion
This study indicates that 1H-MRS can guide clinical decision-making in pediatric neuro-oncology through providing information unavailable from conventional imaging or histopathology. Visual interpretation of MRS resulted in improved accuracy and confidence in noninvasive diagnosis of untreated pediatric brain tumors in our clinical practice. Importantly, MRS allowed confident identification of indolent lesions, avoiding biopsy with its associated comorbidities. Clinicians have successfully used MRS to influence clinical management through guiding biopsy of heterogeneous tumors, aiding tumor characterization, facilitating diagnosis in cases without histopathological confirmation, and identifying unusual tumor types. These findings are important, as early noninvasive diagnosis could allow timely family counseling and potentially improve outcomes through facilitating surgical planning and allowing earlier initiation of adjuvant treatment.
This is, to our knowledge, the first study evaluating the impact of MRS on clinical decision-making in pediatric practice. Our findings for levels of diagnostic accuracy of MRI alone and with addition of MRS are similar to those reported by other clinical studies.6,10 A recent report suggested radiologists could accurately diagnose 63% of untreated pediatric brain tumors correctly using MRI, increasing to 87% with addition of MRS.10 This study involved blinded retrospective expert review of optimized MR images and MRS profiles acquired over a 4-year period, whereas our work investigated accuracy of diagnosis and effect on decision-making in real time.
Our study design was intended to reflect actual clinical practice rather than demonstrate the potential of MRS for accurate diagnosis of pediatric brain tumors. We aimed to describe our routine use of MRS through evaluating added value of visual interpretation in a clinical setting in a wide range of unselected CNS lesions, incorporating common challenges such as variations in neuroradiology experience and problems with data quality. Our radiologists usually perform visual interpretation of MRS profiles generated through scanner software prior to availability of TARQUIN analysis. This has the advantage that information is available to the clinical team in real time, but is a limitation in terms of optimizing potential diagnostic accuracy from improved analysis methods.
This study shows the added value of visual interpretation of MRS. A number of studies have shown improved accuracy of MRS using more sophisticated data analysis techniques and automated classifiers.6,18,24,25 Classifiers can give better diagnostic accuracy and can be particularly useful in difficult cases for which the diagnosis is not clear from conventional MRI or visual MRS interpretation. These methods are not, however, currently available for clinical use, and supporting clinical practice is a key objective to allow routine implementation of MRS to improve patient care. As radiologists are not trained to quantitatively evaluate MRS, it is important to present information that is easy to understand and minimize need for complex data analysis. The skill of visual interpretation of MRS has been readily adopted by radiologists in our center and is amenable to clinical use elsewhere. Making classifiers and decision support systems available to radiologists would most likely improve their interpretation of the MRS and is an important aim for the future.
Although histopathology of tumor tissue is the current gold standard for brain tumor diagnosis, its limitations should be considered. In our cohort, 15% of samples were inconclusive and 19% sent for central review, following which 16% of diagnoses were modified. Providing representative tissue samples through biopsy is difficult, particularly as many pediatric tumors are in central vital structures. In view of this, and the large proportion of patients managed without histopathology, the decision was made to take tumor board diagnosis at study end as our diagnostic reference standard.
Histopathological confirmation of diagnosis can take several days, with results in our cohort available 12 (range, 4-40) days post-imaging and central review taking up to 87 days. Delay in reaching definitive diagnosis may translate to delay in initiating treatment. Median time from surgery to radiotherapy in medulloblastoma was 45 days (range, 31-49), longer than the recommended standard of 28 to 41 days necessary to confer good prognosis in standard risk patients. It is important to consider the potential for reducing time to treatment through accurate noninvasive diagnosis, particularly with the emergence of proton therapy where times from decision to treatment initiation may be longer.
This retrospective study was limited by completeness of contemporaneous documentation of both MRI and MRS interpretation and the clinical decisions that ensued. This may have resulted in under-reporting of both diagnostic accuracy and impact on management decisions. Unfortunately, there is no current standardization in either radiological reporting or recording the decisions of the tumor board, and so in a retrospective study such as this in which recording standards were not preset, there is an inevitable lack of precision in the information. A future prospective study should use specific recording standards to overcome this limitation. It is not uncommon for a radiologist to give a broad differential even in light of a more certain suspicion, possibly resulting in a greater number of “partially correct” outcomes. It is, however, difficult to make management decisions based on broad differentials, and it could be argued that MRS added value to the clinical decision-making process through narrowing the broad differential list made from conventional imaging and confirming the correct diagnosis. Follow-up time is relatively short, the longest being 2 years postdiagnosis, and it is possible that tumor board diagnosis may be reviewed in some cases at a later date.
A further limitation is inclusion of a small number of cases managed at a single center, cautioning against generalization of results to centers with different levels of expertise and processes. Our results reflect practice in our institution, one of the largest in the United Kingdom with a well-established, specific neuro-oncology tumor board, an active imaging research program, and well-established interest in advanced MRI techniques such as MRS. We also have the opportunity for support with MRS interpretation from an active research team and expert clinicians. This may limit the ease with which our results can be replicated by institutions with less spectroscopy experience. This study should be repeated in other institutions, preferably in a prospective manner, where definitions and recoding standards can be agreed prior to acquiring data.
A number of patients in this study lacked histopathological diagnosis, reflecting clinical practice. Although it is more challenging to make a definitive diagnosis on cases without histology, tumor board diagnoses were verified through clinical course rather than simply being accepted as correct following MRI and MRS. Clinical course on follow-up is an important element of confirming the diagnosis for unbiopsied lesions. For example, no lesion diagnosed as indolent by the tumor board subsequently demonstrated aggressive behavior.
Benefits of MRS in our center included guidance of management for lesions lacking histopathology, and support of decisions for observation rather than biopsy following MRS suggestive of indolence. A limitation of an observational study is that we can not be certain that patients would not have been managed in the same way without MRS. Contemporaneous documentation did, however, reveal MRS to provide additional useful clinical information contributing to the clinical decisions made, and it could be argued that inclusion of a modality providing reassurance adds value to clinical decision-making regarding observation of benign lesions.
Multiparametric imaging will not replace tumor tissue analysis in the majority of cases, but having a more accurate diagnosis early in the patient pathway can have some major advantages. Surgical strategies can be influenced, plans can be made in a more timely manner even if they need refining later on, and discussions with the family can proceed. Multiparametric imaging provides information additional to, rather than instead of, histopathology.
MRS is widely available clinically and could easily be incorporated into standard MRI protocols during initial diagnostic imaging. Single-voxel MRS can be performed during routine MRI scans often adding no more than 5 minutes to total examination time. Centers without spectroscopy support can use single-voxel MRS, as data are relatively easy to acquire, assess, and interpret visually. More complicated techniques such as multivoxel MRS are difficult to integrate into mainstream practice due to problems acquiring robust data and presenting it easily for radiological interpretation. Multivoxel MRS does, however, have undoubted advantages for large heterogeneous lesions and is used in those situations. Implementation of MRS to date has been hampered by lack of agreed quality control measures, acquisition protocols, and analysis techniques for specific clinical scenarios. Further challenges include processing and presenting information, assessing quality of spectra, and accurately interpreting data.
There is a paucity of research into how to implement information from MRS into the diagnostic pathway in routine clinical practice. Added value of MRS over conventional imaging techniques and histopathology has not been evaluated prospectively in the pediatric population, and studies are needed in this area. The STARD (Standards for Reporting of Diagnostic Accuracy) Guidelines for reporting diagnostic accuracy27 should be adhered to ensure studies of sufficient quality are conducted to provide high-quality evidence for the added value of this technique. Research is also needed to evaluate the impact MRS has on patient management and outcomes.
Conclusion
1H-MRS provides information to facilitate clinical decision-making additional to that available through conventional radiological methods. There is potential to improve patient outcomes through accurate, early, noninvasive diagnosis, avoiding biopsy of indolent lesions, aiding tumor characterization, and facilitating both earlier family discussions and treatment planning. Considerable progress has been made in the development and evaluation of MRS in pediatric oncology. Integration into clinical practice could help guide important management decisions and tailor treatment to the individual patient. Further research is needed to define the optimum use of MRS in a clinical setting and integrate this technique into routine clinical practice to improve our care of children with cancer.
Supplementary Material
Supplementary data are available at Neuro-Oncology Practice online.
Funding
National Institute for Health Research (13–0053).
Conflict of interest statement.
The authors have no conflicts of interest to disclose.
Supplementary Material
Acknowledgements
The authors thank Shaheen Latif for her contribution in facilitating diagnostic MRS. Material described in this paper has been presented orally at the International Society of Paediatric Neuro-Oncology (ISPNO) Meeting, 2016.
References
- 1. Childhood Cancer Research Group CCRG. National Registry of Childhood Tumours/Childhood Cancer Research Group. http://www.ccrg.ox.ac.uk. Accessed March 11, 2016. [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng WH, Lim T. Targeting regions with highest lipid content on MR spectroscopy may improve diagnostic yield in stereotactic biopsy. J Clin Neurosci. 2008;15(5):502–506. [DOI] [PubMed] [Google Scholar]
- 4. Panigrahy A, Blüml S. Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol. 2009;24(11):1343–1365. [DOI] [PubMed] [Google Scholar]
- 5. Panigrahy A, Nelson MD, Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol. 2010;40(1):3–30. [DOI] [PubMed] [Google Scholar]
- 6. Arle JE, Morriss C, Wang ZJ, et al. Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg. 1997;86(5):755–761. [DOI] [PubMed] [Google Scholar]
- 7. Panigrahy A, Krieger MD, Gonzalez-Gomez I, et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol. 2006;27(3):560–572. [PMC free article] [PubMed] [Google Scholar]
- 8. Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 9. Orphanidou-Vlachou E, Auer D, Brundler MA, et al. (1)H magnetic resonance spectroscopy in the diagnosis of paediatric low grade brain tumours. Eur J Radiol. 2013;82(6):e295–e301. [DOI] [PubMed] [Google Scholar]
- 10. Shiroishi MS, Panigrahy A, Moore KR, et al. Combined MRI and MRS improves pre-therapeutic diagnoses of pediatric brain tumors over MRI alone. Neuroradiology. 2015;57(9):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44(5):371–381. [DOI] [PubMed] [Google Scholar]
- 12. Martin AJ, Liu H, Hall WA, et al. Preliminary assessment of turbo spectroscopic imaging for targeting in brain biopsy. AJNR Am J Neuroradiol. 2001;22(5):959–968. [PMC free article] [PubMed] [Google Scholar]
- 13. Hollingworth W, Medina LS, Lenkinski RE, et al. A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. AJNR Am J Neuroradiol. 2006;27(7):1404–1411. [PMC free article] [PubMed] [Google Scholar]
- 14. Preul MC, Caramanos Z, Collins DL, et al. Accurate, noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nat Med. 1996;2(3):323–325. [DOI] [PubMed] [Google Scholar]
- 15. Galanaud D, Nicoli F, Chinot O, et al. Noninvasive diagnostic assessment of brain tumors using combined in vivo MR imaging and spectroscopy. Magn Reson Med. 2006;55(6):1236–1245. [DOI] [PubMed] [Google Scholar]
- 16. Server A, Josefsen R, Kulle B, et al. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010;51(3):316–325. [DOI] [PubMed] [Google Scholar]
- 17. Astrakas LG, Zurakowski D, Tzika AA, et al. Noninvasive magnetic resonance spectroscopic imaging biomarkers to predict the clinical grade of pediatric brain tumors. Clin Cancer Res. 2004;10(24):8220–8228. [DOI] [PubMed] [Google Scholar]
- 18. Davies NP, Wilson M, Harris LM, et al. Identification and characterisation of childhood cerebellar tumours by in vivo proton MRS. NMR Biomed. 2008;21(8):908–918. [DOI] [PubMed] [Google Scholar]
- 19. Wilson M, Cummins CL, MacPherson L. et al. Magnetic resonance spectroscopy metabolite profiles predict survival in paediatric brain tumours. Eur J Cancer. 2013; 49(2):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy M, Loosemore A, Clifton AG, et al. The contribution of proton magnetic resonance spectroscopy (1HMRS) to clinical brain tumour diagnosis. Br J Neurosurg. 2002;16(4):329–334. [DOI] [PubMed] [Google Scholar]
- 21. Julià-Sapé M, Coronel I, Majós C, et al. Prospective diagnostic performance evaluation of single-voxel 1H MRS for typing and grading of brain tumours. NMR Biomed. 2012;25(4):661–673. [DOI] [PubMed] [Google Scholar]
- 22. Galanaud D, Nicoli F, Chinot O, et al. Noninvasive diagnostic assessment of brain tumors using combined in vivo MR imaging and spectroscopy. Magn Reson Med. 2006;55(6):1236–1245. [DOI] [PubMed] [Google Scholar]
- 23. Lin A, Bluml S, Mamelak AN. Efficacy of proton magnetic resonance spectroscopy in clinical decision making for patients with suspected malignant brain tumors. J Neurooncol. 1999;45(1):69–81. [DOI] [PubMed] [Google Scholar]
- 24. Wang Z, Sutton LN, Cnaan A, et al. Proton MR spectroscopy of pediatric cerebellar tumors. AJNR Am J Neuroradiol. 1995;16(9):1821–1833. [PMC free article] [PubMed] [Google Scholar]
- 25. Vicente J, Fuster-Garcia E, Tortajada S, et al. Accurate classification of childhood brain tumours by in vivo ¹H MRS - a multi-centre study. Eur J Cancer. 2013;49(3):658–667. [DOI] [PubMed] [Google Scholar]
- 26. Wilson M, Reynolds G, Kauppinen RA, et al. A constrained least-squares approach to the automated quantitation of in vivo ¹H magnetic resonance spectroscopy data. Magn Reson Med. 2011;65(1):1–12. [DOI] [PubMed] [Google Scholar]
- 27. Bossuyt PM, Reitsma JB, Bruns DE, et al. ; Standards for Reporting of Diagnostic Accuracy Group. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44(5):639–650. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.