Abstract
The marine-derived Aspergillus protuberus MF297-2 and the terrestrial A. amoenus NRRL 35600 produce enantiomeric prenylated indole alkaloids. Investigation of biological activities of the natural and synthetic derivatives revealed that (−)-enantiomers of notoamides A and B, 6-epi-notoamide T, and stephacidin A inhibited receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL)–induced osteoclastogenic differentiation of murine RAW264 cells more strongly than their respective (+)-enantiomers. Among them, (−)-6-epi-notoamide T was the most potent inhibitor with an IC50 value of 1.7 μM.
Keywords: Notoamide, Enantiomer, Osteoclastogenesis, Aspergillus, Fungus
Notoamides A–D were isolated from the fungus Aspergillus protuberus MF297-2,1 and successively fifteen new notoamides were obtained from the same strain.2–6 During the biosynthetic studies of notoamides, two antipodes, (−)-stephacidin A and (+)-notoamide B, were isolated from A. amoenus (formerly A. versicolor) NRRL 35600 along with (+)-versicolamide B, a diastereomer of (+)-notoamide B,7 and recently five antipodal congeners were isolated.8 We have been interested in the question if the antipodes showed enantiomerically-specific biological activities, and we tested notoamides of natural as well as synthetic origin in our in-house screening. We here report that the (−)-enantiomers of notoamides A and B, 6-epi-notoamide T,9 and stephacidin A inhibited receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL)–induced osteoclastogenic differentiation of murine RAW264 cells more strongly than their respective (+)-enantiomers. Among them, (−)-6-epi-notoamide T was the most potent inhibitor with an IC50 value of 1.7 μM.
Osteoporosis is associated with the deregulation of osteoclast function, and therefore agents that affect osteoclastogenesis have been attracted much attentions.10,11 Bone homeostasis is maintained by the balance between bone formation by osteoblasts and bone resorption by osteoclasts.12 Stimulation of a monocyte/macrophage lineage by RANKL leads to its differentiation into multinuclear osteoclasts.12–14 RANKL stimulus activates downstream signaling pathways including the NF-κB and MAPK signaling pathways, which up-regulate the expression of osteoclast-specific genes, such as those encoding tartrate-resistant acid phosphatase (TRAP) and enzymes involved in cell fusion.
Notoamide derivatives, used in this study, were isolated from A. protuberus,1 A. amoenus,7 and A. taichungensis IBT 1940415 or synthesized9 (Figure 1). In order to test the inhibition by notoamides of RANKL-induced formation of multinuclear osteoclasts, the murine RAW264 cells were incubated with RANKL in the presence of samples at a concentration of 10 μg/mL.16 With respect to three pairs of natural enantiomers, notoamides A and B and stephacidin A, the (−)-enantiomers inhibited the osteoclastogenesis, detected by TRAP assay,16 more strongly than their respective (+)-enantiomers (Figure 2). Although synthetic (±)-notoamide T showed no inhibition at 10 μg/mL, whereas the C6-epimers, (±)-6-epi-notoamide T, completely inhibited at the same concentrations. We then separated the racemate, (±)-6-epi-notoamide T, into enantiomers by HPLC with a chiral-phase column17 and found that the separated (+)- and (−)-enantiomers showed inhibitory activities with IC50 values of 4.4 and 1.7 μM, respectively, also indicating enantio-specific inhibitory activity. Next, we examined the inhibitory effect of (−)-6-epi-notoamide T against the differentiation of RAW264 cells induced by treatment with RANKL, detected by tartrate-resistant acid phosphatase (TRAP) staining.16 In an experimental control, the cells differentiated into multinuclear osteoclasts by stimulus of RANKL, but (−)-6-epi-notoamide T at 5 μM inhibited the differentiation completely (Figure 3).
Figure 1.
Structures of notoamide derivatives isolated from (a) A. protuberus, (b) A. amoenus, and (c) A. taichungensis, and (d) synthesized notoamides.
Figure 2.

Inhibitory effects of notoamide congeners on RANKL-induced TRAP activity. RAW264 cells were treated with RANKL (250 ng/mL) in the presence or absence of notoamide derivatives (10 μg/mL) or quercetin (a positive control; 3.1 μg/mL) and allowed to differentiate for four days. TRAP activity was measured as absorbance at 405 nm. Quadruplicate experiments were carried out and the error bars represent the standard deviation.
Figure 3.
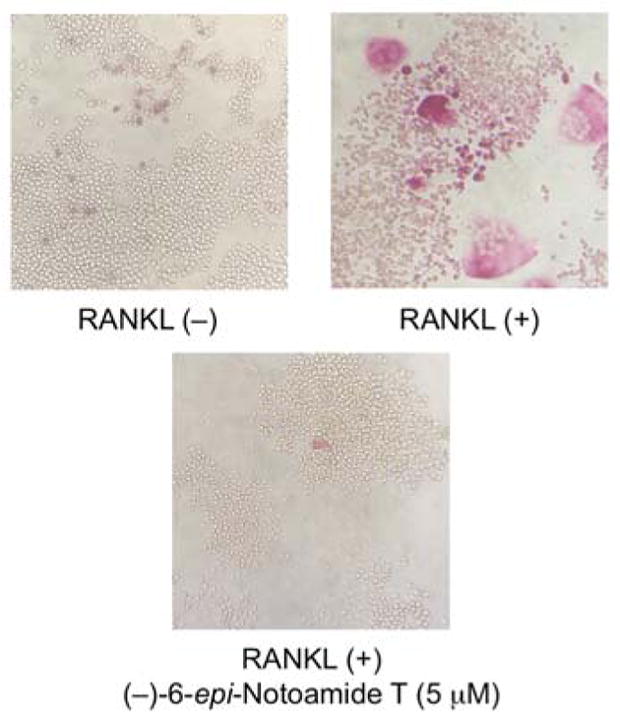
Inhibitory effect of (−)-6-epi-notoamide T on RANKL-induced multinuclear osteoclastogenesis. RAW264 cells were allowed to differentiate by treatment with RANKL (250 ng/mL) in the presence or absence of (−)-6-epi-notoamide T (5 μM) for four days and were then stained with TRAP-staining solution. TRAP-positive cells stained red.
RANKL-induced osteoclastogenic differentiation is associated with up-regulation of specific genes. In order to examine if the inhibitory effect of (−)-6-epi-notoamide T relates to the expression of osteoclastogenic-specific genes, total RNA was prepared and analyzed by real-time RT-PCR.18 Although RANKL (50 ng/mL) induced the expression of Nfatc1 (Nuclear factor of activated T cells c1; NFATc1) in RAW264 cells as well as osteoclastgenesis-specific genes including Acp5 (tartrate-resistant acid phosphatase 5; TRAP), Ctsk (cathepsin K; CTSK), Atp6v0d2 (ATPase, H+ transporting, lysosomal V0 subunit D2), Dcstamp (dendrocyte expressed seven transmembrane protein; DC-STAMP), and Ocstamp (Osteoclast stimulatory transmembrane protein; OC-STAMP), (−)-6-epi-notoamide T (5 μM) suppressed the mRNA expression levels of these genes by 20–50% (Figure 4).
Figure 4.
Inhibitory effect of (−)-6-epi-notoamide T on the expression levels of osteoclast marker genes. RAW264 cells were treated with RANKL (50 ng/mL) and (−)-6-epi-notoamide T (5 μM) for five days. Gene expression levels were evaluated by real-time RT-PCR and calculated from the data of duplicate experiments.
Regarding other enantiomeric compounds containing the bicyclo[2.2.2]diazaoctane ring system, (−)-versicolamides B and C have never been isolated from fungi and only a trace amount of (−)-6-epi-stephacidin A has been isolated from A. amoenus. Therefore, we could not compare their effects with (+)-enantiomers. Notoamides C and D, containing a dioxopiperazine ring, scarcely inhibited RANKL-induced osteoclastogenesis (see Figure 2).
To date, we have been studying the structures,1–8,15 syntheses,9,19–24 and biosyntheses6,9,23,25,26 of prenylated indole alkaloids from three fungi, A. protuberus, A. amoenus, and A. taichungensis. We have, for a long time, been interested in the subject if the enantiomers of notoamide derivatives showed the enantiomerically distinct biological activities. This is the first report describing the enantioselective biological activities of notoamide enantiomers. Interestingly, among the natural and synthetic notoamide congeners tested in this study, the (−)-enantiomers of notoamides A and B, 6-epi-notoamide T, and stephacidin A showed more potent inhibition of RANKL–induced osteoclastgenesis than their respective (+)-enantiomers. Among them, (−)-6-epi-notoamide T was the most potent inhibitor (IC50, 1.7 μM). Expression of osteoclast-specific genes, encoding TRAP and other enzymes involved in cell fusion, is up-regulated by the NF-κB and MAPK signaling pathways, which are activated by RANKL stimulus. Efforts to clarify the inhibitory mechanisms of notoamides are under investigation in our laboratory.
Acknowledgments
This work was financially supported in part by Grants-in-Aid for Scientific Research (No. 17H0399400 to ST) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Financial support from the National Institutes of Health (Grant CA 070375 to RMW) is gratefully acknowledged.
References and notes
- 1.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Notoamides A–D: new prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 2.Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Isolation of notoamides E, a key precursor in the biosynthesis of prenylated indole alkaloids in a marine-derived fungus, Aspergillus sp. J Am Chem Soc. 2009;131:3834–3835. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukamoto S, Kato H, Samizo M, Nojiri Y, Ohnuki H, Hirota H, Ohta T. Notoamides F–K: prenylated indole alkaloids isolated from a marine–derived fungus, Aspergillus sp. J Nat Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Isolation of antipodal (−)-versicolamide B and notoamides L–N from a marine–derived fungus, Aspergillus sp. Org Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P–R from a marine–derived fungus, Aspergillus sp. J Nat Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Nakahara T, Sugimoto K, Matsuo K, Kagiyama I, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S. Isolation of notoamide S and enantiomeric 6-epi-stephacidin A from the fungus Aspergillus amoenus: biogenetic implications. Org Lett. 2015;17:700–703. doi: 10.1021/ol5037198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew Chem Int Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimoto K, Sadahiro Y, Kagiyama I, Kato H, Sherman DH, Williams RM, Tsukamoto S. Isolation of amoenamide A and five antipodal prenylated alkaloids from Aspergillus amoenus NRRL 35600. Tetrahedron Lett. 2017;58:2797–2800. doi: 10.1016/j.tetlet.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunderhaus JD, McAfoos TJ, Finefield JM, Kato H, Li S, Tsukamoto S, Sherman DH, Williams RM. Synthesis and bioconversions of notoamide T: a biosynthetic precursor to stephacidin A and notoamide B. Org Lett. 2013;15:22–25. doi: 10.1021/ol302901p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawatani M, Osada H. Osteoclast–targeting small molecules for the treatment of neoplastic bone metastases. Cancer Sci. 2009;100:1999–2005. doi: 10.1111/j.1349-7006.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 13.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 14.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagiyama I, Kato H, Nehira T, Frisvad JC, Sherman DH, Williams RM, Tsukamoto S. Taichunamides: prenylated indole alkaloids from Aspergillus taichungensis (IBT 19404) Angew Chem Int Ed. 2016;55:1128–1132. doi: 10.1002/anie.201509462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The murine RAW264 cells were cultured in MEMα medium (Wako) containing 10% heat-inactivated fetal bovine serum (CORNING) and 1 × penicillin/streptomycin (Nacalai Tesque) under a humidified atmosphere containing 5% CO2 at 37°C. TRAP assay: RAW264 cells were cultured in a 96-well plate for one day and treated with samples (10 μg/mL) in the presence of sRANKL (a soluble form of RANKL, Oriental Yeast; 250 ng/mL) for four days. After washing with phosphate-buffered saline (PBS), the cells were lysed with TRAP buffer (50 mM sodium tartrate, 50 mM sodium acetate, 150 mM KCl, 0.1% TritonX-100, 1 mM sodium ascorbate, and 0.1 mM FeCl3, pH 5.7; 100 μL/well) on ice for 10 min. An aliquot (20 μL) of the resulting cell extract was added to 100 μL of TRAP buffer containing 2.5 mM p-nitrophenyl phosphate (Thermo Fisher Scientific). After incubation at 37°C for four hours, 50 μL of 0.9 M NaOH was added to the reaction mixture and the absorbance of the solution was measured at 405 nm. TRAP activity was calculated from the data of quadruplicate experiments. TRAP staining: RAW264 cells were treated with or without (−)-6-epi-notoamide T (5 μM) in the presence of sRANKL (250 ng/mL) in a 96-well plate for four days. The differentiated cells were washed with PBS and treated with 4% paraformaldehyde solution for 10 min at room temperature. After washing with PBS, the cells were stained with TRAP-staining solution (50 mM sodium tartrate, 45 mM sodium acetate, 0.1 mg/mL naphthol AS-MX phosphate (Sigma–Aldrich), and 0.6 mg/mL fast red violet LB salt (Sigma–Aldrich), pH 5.2) for 1 hour at room temperature. TRAP-positive cells that contained three or more nuclei were determined to be multinuclear osteoclasts.
- 17.Separation of (±)-6-epi-notoamide T by HPLC with a chiral-phase column: A synthesized racemic mixture of 6-epi-notoamide T (5.0 mg) was separated into (−)- (0.89 mg; TR, 26 min) and (+)-enantiomers (0.73 mg; TR, 51 min) by HPLC with a chiral-phase column (CHIRALCEL OJ-H (4.6 × 250 mm, Daicel Corporation), 75 to 65% n-hexane–2-PrOH in 110 min, 1.0 mL/min, UV 283 nm).
- 18.Real-time RT-PCR analysis: Total RNAs were isolated from cultured cells using RNeasy Plus Micro Kit® (QIAGEN, Hilden, Germany and cDNA synthesis was performed with PrimeScript™ RT Master Mix (Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s protocol. PCR primers were purchased from Hokkaido System Science (Sapporo, Japan). The following primer sets were used: Nfatc1, forward 5′-CTCGAAAGACAGCACTGGAGCAT and reverse 5′-CGGCTGCCTTCCGTCTCATAG; Acp5, forward 5′-AAATCACTCTTTAAGACCAG and reverse 5′-TTATTGAATAGCAGTGACAG; Ctsk, forward 5′-CCAGTGGGAGCTATGGAAGA and reverse 5′-AAGTGGTTCATGGCCAGTTC; Atp6v0d2, forward 5′-TCAGATCTCTTCAAGGCTGTGCTG and reverse 5′-GTGCCAAATGAGTTCAGAGTGATG; Dcstamp, forward 5′-ATGCGAAGCTCCTTGAGAAA and reverse 5′-ATTTGCAGGGATTGTCTGC; Ocstamp, forward 5′-CCTTGGGCCTCCATATGACC and reverse 5′-GAGGAGTGCCGAGGTGAATC. After initial denaturation at 95 °C for 10 min, PCR was performed for various cycles (10 sec at 95 °C, 5 sec at annealing temperature and 14 sec at 72 °C) using LightCycler® FastStart DNA Master SYBR Green I mix (Roche Diagnostics, Indianapolis, USA).
- 19.Grubbs AW, Artman GD, III, Tsukamoto S, Williams RM. A concise total synthesis of the notoamides C and D. Angew Chem Int Ed. 2007;46:2257–2261. doi: 10.1002/anie.200604377. [DOI] [PubMed] [Google Scholar]
- 20.Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM. A concise, biomimetic total synthesis of stephacidin A and notoamide B. Angew Chem Int Ed. 2007;46:2262–2265. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]
- 21.Miller KA, Tsukamoto S, Williams RM. Asymmetric total syntheses of (+)- and (−)-versicolamide B and biosynthetic implications. Nat Chem. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAfoos TJ, Li S, Tsukamoto S, Sherman DH, Williams RM. Studies on the biosynthesis of the stephacidins and notoamides. Total synthesis of notoamide S. Heterocycles. 2010;82:461–472. doi: 10.3987/COM-10-S(E)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finefield JM, Kato H, Greshock TJ, Sherman DH, Tsukamoto S, Williams RM. Biosynthetic studies of the notoamides: isotopic synthesis of stephacidin A and incorporation into notoamide B and sclerotiamide. Org Lett. 2011;13:3802–3805. doi: 10.1021/ol201284y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finefield JM, Sherman DH, Tsukamoto S, Williams RM. Studies on the biosynthesis of the notoamides: synthesis of an isotopomer of 6-hydroxydeoxybrevianamide E and biosynthetic incorporation into notoamide J. J Org Chem. 2011;76:5954–5958. doi: 10.1021/jo200218a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato H, Nakamura Y, Finefield JM, Umaoka H, Nakahara T, Williams RM, Tsukamoto S. Study on the biosynthesis of the notoamides: pinacol-type rearrangement of isoprenyl unit in deoxybrevianamide E and 6-hydroxydeoxybrevianamide E. Tetrahedron Lett. 2011;52:6923–6926. doi: 10.1016/j.tetlet.2011.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, Nakahara T, Yamaguchi M, Kagiyama I, Finefield JM, Sunderhaus JD, Sherman DH, Williams RM, Tsukamoto S. Bioconversion of 6-epi-notoamide T produces metabolites of unprecedented structures in a marine-derived Aspergillus sp. Tetrahedron Lett. 2015;56:247–251. doi: 10.1016/j.tetlet.2014.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]




