Abstract
In this crossover study of ezetimibe monotherapy in 48 antiretroviral-treated patients with human immunodeficiency virus infection, the mean changes in low-density lipoprotein cholesterol were −5.3% (−11 mg/dL) and +5.5% (+4 mg/dL) with ezetimibe treatment and placebo, respectively (P = .04). Ezetimibe was safe and effective in reducing low-density lipoprotein cholesterol and is an option for patients who cannot tolerate treatment with a statin.
Cohort studies suggest that patients with HIV infection are at increased risk of cardiovascular diseases (CVDs) and that antiretrovirals may contribute to this excess risk [1–5]. Elevation of low-density lipoprotein (LDL) cholesterol has been observed during therapy with preferred antiretroviral regimens for HIV infection—potentially heightening CVD risk [6–12]. Subsequently, the use of 3-hydroxy-3-methyl-glutaryl-CoA–reductase inhibitors (statins) has dramatically increased in HIV clinics in the United States [13]. However, therapy with statins can be problematic for HIV-infected patients receiving antiretrovirals, because of drug interactions and overlapping toxicity.
Ezetimibe reduces cholesterol absorption and is indicated as adjunctive therapy to diet and/or statins for the reduction of LDL cholesterol in patients with primary hypercholesterolemia [14]. The drug is metabolized independent of the cytochrome P3A4 pathway and is glucoronidated by UGT1A1, UGT1A3, and UGT2B15; therefore, a significant interaction with anti-retroviral agents is unlikely. However, there are limited data regarding the use of ezetimibe in HIV-infected patients [15–17].
To explore the tolerability of ezetimibe and its effect on LDL cholesterol in HIV-infected individuals, we conducted a randomized double-blind, placebo-controlled, 2-period crossover study of ezetimibe as monotherapy in patients receiving combination antiretroviral therapies.
Patients and methods
Participants were HIV-infected adults who were receiving a triple-antiretroviral regimen for ⩾3 months, had an LDL cholesterol level ⩾75 mg/dL, a fasting triglyceride level ≤800 mg/dL, a CD4+ cell count >100 cells/μL, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels ≤1.5 times the upper limit of normal, and a serum creatinine level ≤1.5 times the upper limit of normal; they were not receiving a lipid-lowering medication (statins, fibrates, niacin, or fish oil) during the 3 months before study entry and had HIV RNA levels ≤10,000 copies/mL. All patients were referred from the University of North Carolina Infectious Diseases Clinic or the HIV and Cardiology Clinics at the University of California–San Francisco.
The study was approved by the institutional review boards at the University of North Carolina and the University of California–San Francisco. Participants were randomized to treatment with 10 mg ezetimibe daily or to placebo for 6 weeks, after which the study drug was stopped for a 2-week washout phase. Participants then restarted study treatment with the opposite therapy from their original assignment (figure 1). All subjects were asked to maintain their usual diet and exercise habits during the trial.
Figure 1.
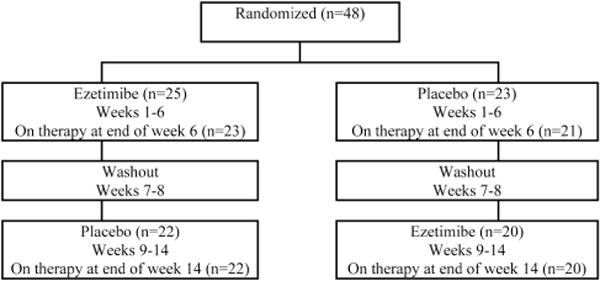
Disposition of patients
At entry and at weeks 6, 8, and 14, blood was collected after patient fasting (>8 h) for direct measurements of LDL-cholesterol (β-quant plasma ultracentrifugation), total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels (PPD Global Central Labs); blood chemistry; and CD4+ cell count. Self-reported study-drug adherence was assessed at each visit through use of a modified AIDS Clinical Trials Group Self-Reported Adherence Survey.
Adverse clinical and laboratory events were graded according to the Division of AIDS toxicity grading table. The study was monitored by the University of North Carolina School of Medicine Data and Safety Monitoring Board.
The primary objective was to compare the mean percentage change in directly measured LDL cholesterol with ezetimibe treatment versus placebo. In an a priori calculation that assumed a power of 80%, a 2-tailed α < .05, a treatment effect of a 17% reduction in LDL cholesterol following 6 weeks of active drug but no change with placebo, and an SD of 10, a sample size of 35 patients was needed. To account for loss to follow-up, the target enrollment was set at 50 subjects.
Differences in percentage change of lipid levels from pre- to postintervention between ezetimibe treatment and placebo were compared using generalized linear models with a normal probability distribution fit, with generalized estimation equations to account for repeated measures. All assessments were intention-to-treat analyses with the last observation carried forward. The proportion of subjects with a new grade 2 or greater adverse effect was compared between the study arms, as were mean percentage changes in HDL cholesterol and triglyceride levels.
Results
From February 2005 through March 2006, 48 patients were enrolled; 25 were randomized to receive ezetimibe first and then were switched to placebo, and 23 patients started with placebo and then were switched to ezetimibe (figure 1). Patient characteristics are shown in table 1.
Table 1.
Patient baseline characteristics, by treatment.
| Variable | Ezetimibe/placebo (n = 25) |
Placebo/ezetimibe (n = 23) |
Overall (n = 48) |
|---|---|---|---|
| Male sex | 19 (76) | 18 (78) | 37 (77) |
| Ethnicity | |||
| Black, non-Hispanic | 12 (48) | 11 (48) | 23 (48) |
| White, non-Hispanic | 12 (48) | 10 (43) | 22 (46) |
| Other | 1 (4) | 2 (9) | 3 (6) |
| Age, mean years ± SD | 47 ± 5.8 | 46 ± 7.3 | 46 ± 6.5 |
| Cholesterol level, mean mg/dL ± SD | |||
| Direct LDL | 131 ± 38.2 | 125 ± 29.7 | 128 ± 34.2 |
| HDL | 51 ± 15.3 | 47 ± 8.0 | 49 ± 12.4 |
| Triglycerides after fasting | 166 ± 80.0 | 163 ± 75.7 | 165 ± 77.2 |
| Body mass index,a mean ± SD | 32 ± 23.4 | 31 ± 8.1 | 32 ± 17.5 |
| CD4 count, mean cells/uL | 592 ± 230 | 596 ± 311 | 594 ± 269 |
| HIV RNA level <400 copies/mL | 23 (92) | 20 (87) | 43 (90) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Ezetimibe/placebo, the group that received ezetimibe in the first 6-week period and placebo in the second 6-week period; placebo/ezetimibe, the group receiving therapy in the reverse order.
Calculated as the weight in kilograms divided by square of the height in meters.
The mean direct LDL cholesterol level (±SD) at entry was 128 ± 34.2 mg/dL and did not differ by study arm (P = .54). After 6 weeks of therapy, the mean percentage changes in LDL cholesterol were −5.3% ± 27% and 5.5% ± 19% with ezetimibe treatment and placebo, respectively (P = .04) (figure 2A).
Figure 2.
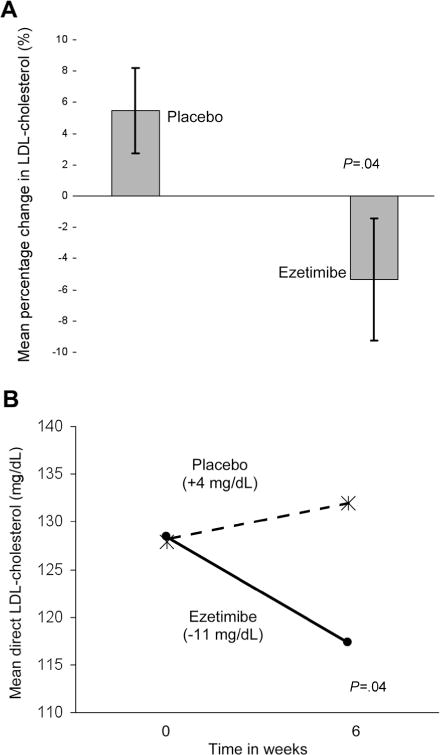
A, Mean 6-week percentage change (and SD) in low-density lipoprotein (LDL) cholesterol levels of patients receiving ezetimibe versus placebo. B, Mean direct LDL-cholesterol levels before and after 6 weeks of treatment with ezetimibe (blackened dot) versus placebo (*).
The difference in mean percentage of LDL-cholesterol change attributable to ezetimibe, as compared with placebo, was −10.8% (95% CI, −21.3 to −0.3; P = .04). The 6-week mean (±SD) of the absolute change in LDL cholesterol was −11 ± 33 mg/dL with ezetimibe treatment, compared with 4 ± 26.8 mg/dL with placebo (P = .04) (figure 2B and table 2). The difference in mean absolute change of LDL cholesterol attributable to ezetimibe, as compared with placebo, was −15 mg/dL (95% CI, −29 to −1; P = .04). Treatment order did not influence these results.
Table 2.
Direct low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride levels at the start and end of the 6-week therapy study period.
| Variable | Lipid level after fasting, mean mg/dL ± SD | P | |
|---|---|---|---|
| Start of therapy | End of therapy | ||
| Direct LDL-cholesterol level | |||
| With ezetimibe treatment | 128 ± 35.3 | 117 ± 30.2 | .04 |
| With placebo | 128 ± 32.3 | 132 ± 30.1 | |
| HDL-cholesterol level | |||
| With ezetimibe treatment | 48 ± 13.2 | 48 ± 11.9 | NS |
| With placebo | 48 ± 10.9 | 46 ± 12.3 | |
| Triglyceride level | |||
| With ezetimibe treatment | 177 ± 90.9 | 173 ± 93.9 | NS |
| With placebo | 183 ± 133.1 | 184 ± 101.1 | |
NOTE. NS, not significant.
HDL-cholesterol and triglyceride levels were comparable at study entry and at the end of each study period for both study arms (P = .21 for the HDL comparison and P = .90 for the triglyceride comparison) (table 1). There was no statistically significant difference in the means of percentage change or absolute change in HDL-cholesterol or triglyceride levels in comparison of ezetimibe and placebo (table 2).
Six subjects discontinued the study prematurely: 3 during the placebo or washout period and 3 during the active-drug phase. Among those receiving ezetimibe, 1 experienced a new grade-2 increase in ALT and AST levels at week 6, 1 experienced grade-2 abdominal pain after a single dose of ezetimibe, and 1 moved from the trial-site area at week 3. Of note, the subject who developed the grade-2 ALT and AST levels entered the study with an ALT level that was grade 1 but <1.5 times the upper limit of normal and had hepatitis C virus coinfection. Of the placebo-receiving individuals who prematurely discontinued study medication, 1 developed a grade-2 increase in AST level at week 8 (end of washout), 1 reported a grade-2 headache and nosebleed on day 3, and 1 experienced worsening of pre-existing peripheral neuropathy at week 4. CD4+ cell counts were stable across time, with no effect of study treatment (data not shown).
Three patients modified antiretroviral therapy—all were receiving placebo at the time of modification and all received ezetimibe first in the crossover design—with the following changes: zidovudine-lamivudine to tenofovir-emtricitabine, amprenavir to fosamprenavir, and ritonavir-boosted atazanavir to unboosted atazanavir.
Perfect treatment adherence was reported by 87%, 93%, 98%, and 98% of all patients at weeks 3, 6, 11, and 14, respectively. Perfect adherence was reported at 94% of study visits (weeks 3, 6, 11, and 14), although adherence was slightly different by receipt of ezetimibe versus placebo (91% vs. 97%; P = .05).
Discussion
Ezetimibe monotherapy significantly reduced LDL-cholesterol levels among HIV-infected patients receiving antiretroviral therapy and was well tolerated. Six weeks of therapy with ezetimibe reduced LDL cholesterol by a mean of 11%. Data from epidemiological cohorts and trials of LDL cholesterol–lowering therapies demonstrate a log-linear relationship between LDL cholesterol and risk of coronary heart disease, such that every 1% decrease in LDL cholesterol is associated with a 1% reduction in the relative risk of heart disease [18, 19]. Therefore, a decrease in LDL cholesterol of 11% would be meaningful to many HIV-infected patients, with the assumption that ezetimibe-induced reductions in LDL cholesterol are equivalent to lifestyle or other lipid-lowering therapies in attenuating CVD risk. Recent comparative clinical trials of antiretroviral regimens that are preferred for initial treatment of HIV infection have observed on-study increases in LDL cholesterol of 4%–29% [5–12]. Therefore, monotherapy with ezetimibe may negate much of the LDL-cholesterol increase that accompanies potent antiretroviral therapy.
The major limitation of this investigation was the short duration of treatment. A study of longer administration of ezetimibe would provide additional data on the durability of the LDL-cholesterol reductions observed. However, clinical trials of lipid-lowering therapies, including those of ezetimibe, suggest a peak effect 4–6 weeks after treatment initiation, and the National Cholesterol Education Program recommends re-evaluation of lipid levels 4–6 weeks after initiation of therapeutic interventions [18]. We intentionally enrolled patients with LDL-cholesterol levels that were not considered to be abnormally elevated. Because the relationship between LDL cholesterol and CVD is linear, the effect of the study intervention across a spectrum of LDL-cholesterol levels is of importance; however, the effects of the drug among those with much higher LDL-cholesterol levels may be different from what was observed. Other, smaller studies of ezetimibe among HIV-infected individuals have observed beneficial changes in HDL-cholesterol and triglyceride levels with this agent, whereas we did not observe any change [15–17]. Lastly, the ENHANCE Trial comparing ezetimibe in conjunction with simvastatin versus simvastatin alone in patients with heterozygous familial hypercholesterolemia showed no improvement in carotid intimal thickening with the addition of ezetimibe [20]. The results of ongoing large clinical trials of ezetimibe with clinical CVD outcomes among HIV-uninfected people may provide additional data to inform the use of this agent in HIV-infected individuals.
In summary, we found ezetimibe to be well tolerated in HIV-infected patients and to be capable of significant reductions in LDL cholesterol levels. Ezetimibe monotherapy should be considered to be a lipid-lowering option for HIV-infected patients requiring modest LDL-cholesterol reduction and may be particularly useful in patients who are unable to take or do not reach their treatment goals with statins.
Acknowledgments
We thank the study participants, for their generous contribution; the SCOPE Study from the University of California–San Francisco, for participant referrals; and Angela Kashuba, for pharmacological advice.
Financial support. Merck provided an unrestricted, investigator-initiated grant but had no role in the analysis of the data or the preparation of the manuscript. University of North Carolina Center for AIDS Research (National Institutes of Health [NIH] grant AI 50410–04); University of North Carolina General Clinical Research Center (National Center for Research Resources NIH grant RR00046); grant support through the University of North Carolina at Chapel Hill from Merck (to D.A.W., R.J.S., and J.J.E.), Abbott Laboratories (to D.A.W. and J.J.E.), Roche (to D.A.W.), Schering-Plough (to R.J.S.), and Panacos (to J.J.E.).
Footnotes
Potential conflicts of interest. D.A.W. serves as a speaker for Abbott Laboratories, Bristol Myers Squibb, GlaxoSmithKline, Tibotec, Gilead Sciences, and Roche; D.W. has received consulting fees from Pfizer and Merck– Schering-Plough and lecture fees from Pfizer. R.J.S. has served as a speaker for Merck, Schering-Plough, and Pfizer and as a consultant for Merck, Schering-Plough, Pfizer, and Immtech; J.J.E. has received speaker honoraria from Monogram Biosciences, Roche, and TiboTec and is an ad hoc consultant to Abbott Laboratories, Bristol Myers Squibb, GlaxoSmithKline, and Virco. All other authors: no conflicts.
References
- 1.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 2.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with HIV disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein D, Hurley L, Silverberg M, et al. Surveillance data for myocardial infarction hospitalizations among HIV+ and HIV Northern Californians: 1994-2006 [abstract 807]; Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 5.Iloeje UH, Yuan Y, L’Italien G, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 6.Eron J, Jr, Yeni P, Gathe J, Jr, et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368:476–82. doi: 10.1016/S0140-6736(06)69155-1. [DOI] [PubMed] [Google Scholar]
- 7.Haubrich RH, Riddler S, DiRienzo G, et al. the AIDS Clinical Trials Group 5142 Study Team Metabolic outcomes of ACTG 5142: a prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection [abstract 38]; Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 8.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, DeJesus E, Arribas JR, et al. for the Study 934 Group Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 10.Malan N, Krantz E, David N, et al. the 089 Study Group Efficacy and safety of atazanavir-based therapy in antiretroviral naive HIV-1 infected subjects, both with and without ritonavir: 48-week results from AI424-089 [abstract 107LB]; Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [Google Scholar]
- 11.Walmsley S, Bredeek U, Avihingsanon A, et al. Evaluation of the impact of highly active antiretroviral therapy (HAART) on lipid profiles—data from the 24-week interim analysis of the Gemini Study: saquinavir/r (SQV/r) BID vs lopinavir/r (LPV/r) BID plus emtricitabine/tenofovir (FTC/TDF) QD in ARV-naive HIV-1–infected patients [abstract TUPEB069]; Program and abstracts of the 4th International AIDS Society Conference; Sydney, Australia. 2007. [Google Scholar]
- 12.van Leth F, Phanuphak P, Stroes E, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy–naive patients infected with HIV-1. PLoS Med. 2004;1:e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein K, Armon C, Buchacz K, Moorman A, Wood K, Brooks J, the HIV Outpatient Study Group Analysis of cardiovascular risk factors in the HIV outpatient study cohort [abstract 735]; Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006. [Google Scholar]
- 14.Zetia [package insert] North Wales, PA: Merck/Schering-Plough; 2007. [Google Scholar]
- 15.Negredo E, Moltó J, Puig J, et al. Ezetimibe, a promising lipid-lowering agent for the treatment of dyslipidaemia in HIV-infected patients with poor response to statins. AIDS. 2006;20:2159–64. doi: 10.1097/01.aids.0000247573.95880.db. [DOI] [PubMed] [Google Scholar]
- 16.Coll B, Aragonés G, Parra S, Alonso-Villaverde C, Masana L. Ezetimibe effectively decreases LDL-cholesterol in HIV-infected patients. AIDS. 2006;20:1675–7. doi: 10.1097/01.aids.0000238418.43937.3b. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MT, Johns KW, Bondy GP. Ezetimibe is effective when added to maximally tolerated lipid lowering therapy in patients with HIV. Lipids Health Dis. 2007;6:15. doi: 10.1186/1476-511X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]


