Abstract
Introduction
A number of strategies have been attempted to minimize infection risk following transrectal prostate procedures (TRPXs). We report our prospective efforts at augmenting our prophylaxis strategy over time.
Methods
Since 2010, we prospectively monitor post-TRPX infections and changed our prophylaxis regimen twice in an effort to respond to increases in infectious complications. In 2011 we added a single-dose of intramuscular (IM) aminoglycoside to our prophylaxis regimen of fluoroquinolones (FQ) or trimethoprim-sulfamethoxazole. In 2015 we began performing formalin needle-tip disinfection before each biopsy and screening high-risk patients for antibiotic resistance using rectal swab cultures (targeted prophylaxis). We report our rates of infections and antibiotic resistance patterns over this period.
Results
From 2010–2016, we performed 2398 TRPXs; overall, there were 41 cases (1.7%) of infection-related hospitalization, however the rate differed significantly over the study period. The infection-related hospitalization rate declined from 3.8 to 1.1% in the first 3 years following the addition of IM aminoglycoside (2011–2013) – a decrease of 69%. In 2014 our infection rate increased to 2.6% prompting initiation of protocol #3 wherein the addition of target prophylaxis and formalin needle-tip disinfection identified a 29.8% FQ-resistance rate and resulted in another decline in our infection rate to 1.2% - a decrease of 53%.
Conclusions
While the initial addition of IM aminoglycoside appeared to be effective in decreasing post-procedure infections, further augmentation of our prophylaxis regimen through rectal swab screening of high-risk patients and formalin needle-tip disinfection led to an additional decline in rates of infection-related hospitalizations.
Keywords: antibiotic resistance, fiducial marker, infection, prostate biopsy, rectal swab, cost
Introduction
Transrectal ultrasound-guided (TRUS) prostate biopsy is the standard of care for pathologic diagnosis of prostate cancer and is performed over 1 million times per year in United States 1, 2. Additionally, TRUS-guided placement of fiducial markers is common practice in aiding image-guided radiotherapy. While transrectal procedures of the prostate (TRPX) are generally safe, there are complications associated with the procedure including pain, hematuria, hematospermia, hematochezia, urinary tract infection (UTI), and the most serious complication of sepsis.3–7
The utility of antibiotic prophylaxis to minimize infection is well-established however there is no consensus that one particular class of antibiotics is superior.8 The American Urological Association guidelines recommend less than 24 hours of oral fluoroquinolone (FQ) or intravenous (IV) 1st, 2nd, 3rd generation cephalosporin as first-line coverage, and oral trimethoprim-sulfamethoxazole (TMP-SMX) or IV aminoglycoside vs. aztreonam as an alternative regimen.9 However, there is considerable practice variation amongst US urologists.10 Since these recommendations were published in 2008, a number of reports indicate a rising incidence of infectious complications, in particular due to FQ-resistance. 3, 11, 12
We describe our institutional experiences with infectious complications following TRPXs and attempts to combat this trend with fastidious prospective monitoring of complications and changes to our antibiotic prophylaxis regimen, first with the addition of IM aminoglycoside to our empiric regimen of oral FQ or TMP-SMX, and then with the addition of formalin disinfection of the biopsy needle between core sampling and rectal swab culture screening in high-risk patients.
Materials and Methods
Study Overview and Antibiotic Prophylaxis Protocols
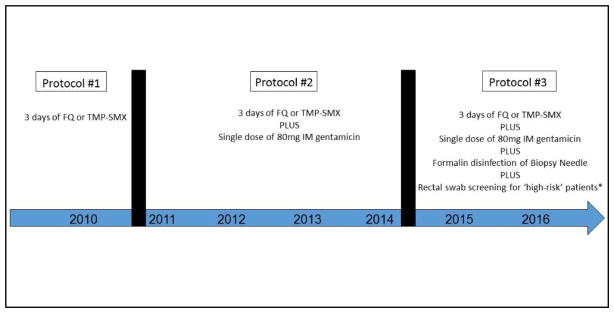
After identifying a high number of post-TRPX infections in 2010, we embarked upon a strategy to optimize outcomes and prospectively collect complication data (Figure 1).13 Prior to 2011 (protocol #1), our standard prophylactic antibiotic regimen consisted of 3 days of FQ (or TMP-SMX if allergic to FQs) starting 1 day before the procedure. Starting in 2011 (protocol #2), we added a single dose of 80mg intramuscular (IM) gentamicin 30 minutes prior to the procedure. Patients with renal insufficiency were administered 250mg of ciprofloxacin and 250mg IM ceftriaxone. Patients allergic to fluoroquinolones or gentamicin were given amoxicillin-clavulanate. Starting in 2015 (protocol #3), we standardized the practice of performing formalin disinfection of the biopsy needle-tip before each biopsy sample, as described by Issa et al.14 Additionally, we identified patients who were high-risk of harboring rectal flora resistant to FQs. High-risk patients included those with previous prostate biopsy-related infection, FQ use in the past 6 months, history of recurrent UTI or prostatitis, recent international travel, and healthcare workers and their families.7, 15–17 Providers were provided a form by our institution which identified criteria for rectal swab culture and instructions on how to perform the test and order it in our electronic medical record. These patients were evaluated two weeks prior to the procedure with a digital rectal exam followed by a cotton swab inserted into the rectum and rotated gently along the anterior rectal wall. The swab was screened for FQ-resistance; if identified, further sensitivities and organism identification was performed to aid in selecting targeted antibiotic prophylaxis.
Figure 1. Prophylaxis Protocols for Transrectal Prostate Procedures.
Abbreviations: FQ – fluoroquinolone, TMP-SMX – trimethoprim-sulfamethoxazole, IM – intramuscular
*High-risk patients defined as those with previous prostate biopsy-related infection, FQ use in the past 6 months, history of recurrent urinary tract infection or prostatitis, recent international travel, and healthcare workers and their families.
Outcomes
The primary outcome for this study was the rate of hospitalization due to post-TRPX infectious complications. Secondary outcomes included urine and blood culture results and antibiotic susceptibilities. In addition, the results of rectal swab culture screening in high-risk patients were examined to determine the incidence of FQ-resistance in this subgroup.
Transrectal Prostate Procedures
All procedures were performed in the outpatient setting. Patient were instructed to self-administer a sodium phosphate enema on the morning of the procedure. A dipstick urinalysis was obtained before the procedure, and patients with suspected UTI underwent urine microscopy and urine culture prior to procedure. TRUS-guided prostate biopsies were typically performed using a 12-core standard template or using magnetic resonance imaging fusion and transrectal fiducial marker placement was performed under ultrasound guidance at right base, right apex and left mid gland. As described above, starting in 2015 we instituted a process of formalin needle tip disinfection between biopsies.
Complication Identification
In 2010, we began a systematic process of following-up with patients one week after their procedure to identify infectious complications, including those that resulted in admission to an outside facility. All patients were instructed to notify his physician with fever or suspicion of infection. Additionally, each patient was contacted at the time of pathology review and asked about infectious complications. A one-week time interval was chosen to identify infections attributable to the TRPX as most post-TRPX infections will occur by this time-point.18 According to the Centers for Disease Control and Prevention, a febrile UTI was defined as fever ≥ 38°C (100.4°F) accompanied by lower urinary tract symptoms (i.e. urgency, frequency, dysuria, or suprapubic tenderness), with or without a positive urine culture. Patients with these symptoms in addition to other signs indicating a systemic inflammatory response syndrome along with laboratory markers of systemic infection were hospitalized for suspected septicemia.19 On admission for infection, all patients received urine culture via clean-catch voided or catheterized specimen, and blood cultures from two separate venipuncture sites.
Statistical Analysis
Patient demographics, clinical characteristics, and Charlson Comorbidity Index (CCI) are reported20. Rates of infectious-related hospitalizations are compared across the different protocols utilized using chi-square analysis. Analysis was performed using SPSS Version 22.0 (IBM, Armonk, NY), Statistical significance was defined by p-value < 0.05.
Results
Infection-Related Hospitalizations
From the beginning of 2010 through the first-half of 2016, we performed 2398 TRPXs and identified 41 patients (1.7%) who required hospitalization for infection after the procedure; 34 patients following prostate biopsy and 7 patients following fiducial marker placement.
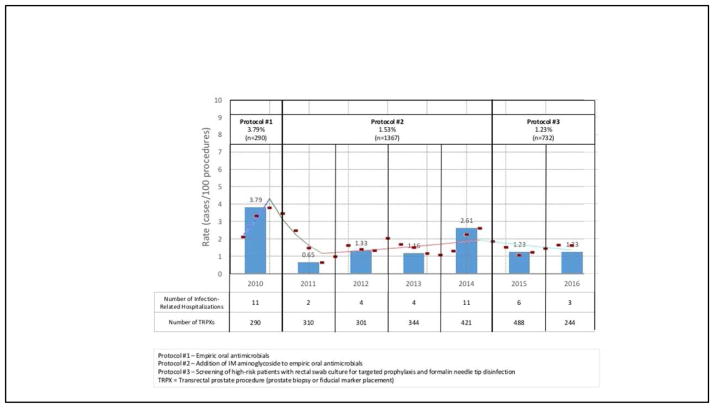
Figure 2 depicts the infection-related hospitalization rates over the study period. Eleven cases (3.8%) of infection-related hospitalization occurred out of 290 TRPXs performed during the original phase of the study period (protocol #1). After initiating protocol #2 (addition of IM aminoglycoside), the infection-related hospitalization rate declined to 1.5% (21 patients out of 1376 TRPXs, p=0.01). In the first 3 years of protocol #2 (2011–2013), the infection-related hospitalization rate was 1.1% – representing a 69% decrease in infectious complications (p<0.01). In the last year of protocol #2 (2014), the infection rate increased to 2.6%, representing a 149% increase over the previous 3 years on the same protocol (p=0.03). After initiating protocol #3 (targeted prophylaxis and formalin needle-tip disinfection) in 2015–2016, the infectious complication rate dropped to 1.2% (9 patients of 732 TRPXs), representing a 53% decrease in the infection rate compared to the prior year (p=0.08).
Figure 2.
Infection-Related Hospitalization Rate Following Transrectal Prostate Procedures and Joinpoint Regression Analysis
Of the 41 patients who were hospitalized for infection, the mean ± standard deviation age of the patients was 65.41 ± 7.98 years, the mean serum prostate specific antigen (PSA) level was 6.6 ± 3.0 ng/dL, and mean prostate volume was 50.2 ± 25.3 cc. The mean CCI with age was 2.6 ± 1.4. Five patients (12.2%) had a previous history of UTI, 4 (9.3%) patients had a history of prostatitis, and 13 patients (31.7%) had a history of lower urinary tract symptoms. Other comorbidities included diabetes (n=10, 24.4%) and immunosuppression (n=1, 2.4%). Previous biopsy had been performed on 20 patients (48.8%) and the mean number of cores taken was 12.7 ± 1.5.
The mean time to onset of symptoms following procedure was 2.2 ± 2.1 days and mean hospitalization length 4.2 ± 1.4 days. Patients presented with the following symptoms: fever (85.4%), chills (71.8%), malaise (39.5%), dysuria (29.7%), urinary retention (13.5%), and urosepsis (26.3%). No patients required intensive care or died.
Microbiology Results
Of the 36 patients with full records available (Table 1), 27 patients (75.0%) had a specific organism isolated during work-up. Bacteriuria was present in 25 patients (69.4%) and bacteremia was present in 12 patients (33.3%). Urine culture results were as follows: 10 patients (27.8%) had a negative urine culture despite fever or other symptoms consistent with UTI. In 23 patients (63.9%), E. coli was isolated and there were 2 patients (5.6%) with E. faecalis, 1 case (2.8%) of P. aeruginosa, and 1 case (2.8%) of B. fragilis isolated after infectious work-up.
Table 1.
Pathogenic Bacteria Isolated Following Transrectal Prostate Procedures
| Protocol #1 | Protocol #2 | Protocol #3 | Overall | |
|---|---|---|---|---|
| Number of Infection-Related Hospitalizations | 11 | 21 | 9 | 41 |
| Number of Bacteria Isolated* | 10 | 16 | 1 | 27 |
| Organisms | ||||
| E. coli | 7 | 16 | - | 23 |
| E. faecalis | 1 | - | 1 | 2 |
| P. aeruoginosa | 1 | - | - | 1 |
| B. fragilis | 1 | - | 1 | |
| Antibiotic Susceptibility | ||||
| Ampicillin | 25% (n=8) | 20.0% (n=15) | - | 25.0% (n=24) |
| Ampicillin-Sulbactam | - | 35.7% (n=14) | 100% (n=1) | 35.7% (n=14) |
| Amoxicillin-Clavulanate | 60.0% (n=5) | 66.7% (n=3) | - | 62.5% (n=8) |
| 1st Generation Cephalosporins | 66.7% (n=6) | 42.9% (n=14) | - | 50% (n=10) |
| 2nd Generation Cephalosporins | 100% (n=3) | 63.6% (n=11) | - | 71.4% (n=14) |
| 3rd Generation Cephalosporins | 80% (n=5) | 85.1% (n=14) | - | 84.1% (n=19) |
| 4th Generation Cephalosporins | 100% (n=5) | 66.7% (n=6) | - | 81.8% (n=9) |
| Aminoglycoside | 75% (n=8) | 73.3% (n=15) | - | 73.9% (n=23) |
| Fluoroquinolones | 22.2% (n=9) | 26.7% (n=15) | - | 25.0% (n=24) |
| Nitrofurantoin | 100% (n=8) | 100% (n=14) | - | 100% (n=22) |
| Trimethoprim-Sulfamethaxazole | 28.6% (n=7) | 13.3% (n=15) | - | 18.2% (n=22) |
| Carbapenems | 100% (n=2) | 100% (n=14) | - | 100% (n=16) |
| Monobactams | - | 100% (n=3) | - | 100% (n=3) |
| Piperacillin-Tazobactam | 100% (n=4) | 100% (n=11) | - | 100% (n=15) |
of 36 patients with full records available who did not present to outside facility
Protocol #1 – Empiric oral antimicrobials
Protocol #2 – Addition of intramuscular aminoglycoside to empiric oral antimicrobials
Protocol #3 – Screening of high-risk patients with rectal swab culture for targeted prophylaxis and formalin needle disinfection
Susceptibility of organisms isolated demonstrated no cases of in vitro resistance to carbapenems, piperacillin-tazobactam, or monobactams. Resistance to FQs and TMP-SMX was found in 75% and 81.8% of isolates, respectively. Isolates were resistant to both FQs and TMP-SMX 48.1% of the time. Sensitivity to 3rd and 4th generation cephalosporins was generally high (>80%), and sensitivity to aminoglycosides was 75% before the initiation of IM aminoglycoside (protocol #1), and remained similar at 73.3% after the addition of IM aminoglycoside (protocol #2). We did not note any significant differences in the antibiotic susceptibility profiles of bacteria isolated during protocol #1 and #2.
Under protocol #3, we identified 84 patients (11.5%) out of a total of 732 TRPXs as being at ‘high-risk’ of harboring FQ-resistant rectal flora. Of those patients screened, 25 patients (29.8%) were identified as harboring FQ-resistance. Four patients developed infections despite being identified as high-risk and given targeted prophylaxis. In 2 cases there was no bacteria isolated during work-up, 1 patient was given targeted prophylaxis after rectal swab culture revealed resistant E. coli but subsequently developed E. faecalis bacteremia, and in 1 case culture data was not available as the patient presented to an outside facility.
Discussion
Our experience represents the attempt to stay ahead of the infectious curve during transrectal prostate procedures. After noting an unacceptably high infection-related hospitalization rate in 2010 of 3.8%, we instituted a policy of adding IM aminoglycoside to our standard prophylaxis regimen resulting in a significant decrease in infectious complications (0.7%) in 2011. We previously reported on the effectiveness of this strategy 13 and in the first 3 years of instituting this prophylaxis regimen the infection-related hospitalization rate remained low (1.1%). However, in 2014 we noted a significant increase in complications (2.6%) prompting a re-evaluation of our regimen. We identified reports purporting the benefits of targeted prophylaxis in high-risk patients as most promising and logical. Additionally, we noted the report on the potential utility of formalin needle-tip disinfection between prostate biopsies.14 Starting in 2015, we added these strategies to our prophylaxis regimen and noted another decline in infectious complications (1.2%).
Although empiric antibiotic prophylaxis has proven efficacy in decreasing the risk of infection following TRPX, increasing bacterial resistance patterns are resulting in a rise of infectious complications.3, 6, 8, 12 A number of strategies have been employed to mitigate this risk. The addition of IM aminoglycoside to empiric prophylaxis has been reported as effective in a number of studies 21, 22, however other studies have failed to demonstrate a reduction in infectious complications.23 Recently, Miyazaki et al. reported on a prospective randomized study comparing oral FQ alone versus oral FQ plus intravenous amikacin. They found no difference in febrile UTI’s between the groups, however they were underpowered to detect a difference.24
Interestingly, the majority of bacteria isolated after IM aminoglycoside administration demonstrated in vitro susceptibility to aminoglycosides. This is not to say that the addition of IM aminoglycosides was not helpful as we do not know how many patients would have experienced infectious without it, but it raises questions about the pharmacokinetics of this method of drug delivery, dosing, and prostatic tissue penetration.
Issa et al. described a technique of disinfecting the tip of the biopsy needle with formalin before prostate biopsy. While they found no statistically significant difference in rates of infectious complications before (0.8%) and after adopting the technique (0.3%, p=0.13), they did demonstrate ex vivo experiments that showed a lack of growth of FQ-resistant E. coli after inoculation with the formalin disinfected needle.14 Although there is a lack of data on long-term side effects (e.g. prostatitis) that could result from placing formalin on a biopsy needle, we felt this was a simple, cost-effective method that might minimize the risk of infection and incorporated it into our practice in 2015.
Empiric prophylaxis is inherently problematic given the rising incidence of antibiotic resistance. There is geographic and temporal variation in resistance patterns, so one institution’s empiric prophylaxis regimen may have little relevance to the local antibiogram of another. 25 Cussans et al. performed a meta-analysis of 9 studies and found an overall FQ-resistance of 22.8% and infection rates were significantly lower in the group that received targeted antibiotics (2.2%) compared to those that received empiric FQ (4.6%).26
Although we felt screening high-risk patients for antibiotic resistance was effective, this strategy depends on properly identifying high-risk patients.7, 15–17 However, 5 out of the 9 patients who developed infectious complications during protocol #3 were not screened for FQ-resistance. Increasing antibiotic resistance patterns will likely further complicate matters, requiring periodic re-evaluation of screening criteria. Furthermore, rectal swab culture is not foolproof; 4 patients in our cohort developed infectious complications despite screening and targeted therapy, in 3 cases there was no organism identified but in 1 case E. faecalis was isolated from serum after rectal swab identified resistant E. coli, demonstrating a discordance between an isolate from rectal culture (which hosts multiple bacteria strains) and the pathogenic bacteria. Further complicating matters and perhaps explaining the negative work-up in some cases is the possibility of colonization from fastidious organisms, including anaerobic bacteria.27 This finding warrants further examination.
The cost of a rectal swab screen for FQ-resistance was $35.00 and when identified, the cost of a reflex culture and organism identification was $55.31. Assuming a 10–20% rate of FQ-resistance in our patient population28, expansion of screening to all patients would cost an average of $40.53–46.06 per patient. Based on our screening of only high-risk patients, we screened 11.5% of patients and our incidence of FQ-resistance in that population was 29.8%. This means that the cost of instituting such a policy averaged out to $5.92 per patient.
Strengths of the study include large sample size and prospective evaluation of infectious complications, including those that resulted in care at an outside facility. Limitations of the study are those inherent in non-randomized analysis of a low-event rate outcome. Additionally, some infectious complications may have occurred later than one week and not been captured in our analysis. We note that our initial prophylaxis regimen consisted of 3 days of antibiotics, a commonly used regimen, although a departure from AUA guidelines of <24 hours of treatment.9 Based on a meta-analysis, a longer course of antibiotics has been reported to decrease risk of bacteriuria, and rates of other infectious complications favored a longer course despite not reaching statistical significance, possibly due underpowering.8 The potential benefit of this strategy should be weighed against the recent U.S. Food and Drug Administration’s ‘black-box’ warning against FQs.29 Additionally, we added two modifications to our prophylaxis regimen in protocol #3. Although this practice change was made to improve patient care based on best-available data, this makes it impossible to determine the impact of each modification separately.
Conclusions
While empiric prophylaxis is effective for most patients, documented uptrends in infection rates and significant geographic variations in antibiotic resistance patterns suggest that this strategy is suboptimal in high-risk patients. Identification of these high-risk patients, performing rectal culture screening, and offering these patients targeted prophylaxis can result in a significant decrease in infectious complications. Quality control measures adjusted through fastidious real-time monitoring can lead to care improvement.
Abbreviations
- TRUS
transrectal ultrasound
- TRPX
transrectal prostate procedures
- UTI
urinary tract infection
- FQ
fluoroquinolone
- IV
intravenous
- TMP-SMX
trimethoprim-sulfamethoxazole
- IM
intramuscular
- CCI
Charlson Comorbidity Index
- PSA
prostate specific antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst. 2007;99:1395. doi: 10.1093/jnci/djm119. [DOI] [PubMed] [Google Scholar]
- 2.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carignan A, Roussy JF, Lapointe V, et al. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62:453. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 5.Thompson PM, Pryor JP, Williams JP, et al. The problem of infection after prostatic biopsy: the case for the transperineal approach. Br J Urol. 1982;54:736. doi: 10.1111/j.1464-410x.1982.tb13637.x. [DOI] [PubMed] [Google Scholar]
- 6.Loh J, Baker K, Sridharan S, et al. Infections after fiducial marker implantation for prostate radiotherapy: are we underestimating the risks? Radiat Oncol. 2015;10:38. doi: 10.1186/s13014-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Association, A. U. The Prevention and Treatment of the More Common Complications Related to Prostate Biopsy Update. Americal Urological Association Education and Research, Inc; 2016. [Google Scholar]
- 8.Zani EL, Clark OA, Rodrigues Netto N., Jr Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011:CD006576. doi: 10.1002/14651858.CD006576.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Wolf JS, Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Hillelsohn JH, Duty B, Blute ML, Jr, et al. Variability of transrectal ultrasound-guided prostate biopsy prophylactic measures. Can J Urol. 2012;19:6573. [PubMed] [Google Scholar]
- 11.Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189:S12. doi: 10.1016/j.juro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adibi M, Hornberger B, Bhat D, et al. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol. 2013;189:535. doi: 10.1016/j.juro.2012.08.194. [DOI] [PubMed] [Google Scholar]
- 14.Issa MM, Al-Qassab UA, Hall J, et al. Formalin disinfection of biopsy needle minimizes the risk of sepsis following prostate biopsy. J Urol. 2013;190:1769. doi: 10.1016/j.juro.2013.04.134. [DOI] [PubMed] [Google Scholar]
- 15.Patel U, Dasgupta P, Amoroso P, et al. Infection after transrectal ultrasonography-guided prostate biopsy: increased relative risks after recent international travel or antibiotic use. BJU Int. 2012;109:1781. doi: 10.1111/j.1464-410X.2011.10561.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson E, Leahy O, Cheng AC, et al. Risk factors for infection following prostate biopsy - a case control study. BMC Infect Dis. 2015;15:580. doi: 10.1186/s12879-015-1328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamdar C, Mooppan UM, Gulmi FA, et al. Multi-drug-resistant bacteremia after transrectal ultrasound guided prostate biopsies in hospital employees and their relatives. Urology. 2008;72:34. doi: 10.1016/j.urology.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 18.Lundstrom KJ, Drevin L, Carlsson S, et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014;192:1116. doi: 10.1016/j.juro.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 19.Zaytoun OM, Vargo EH, Rajan R, et al. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77:1035. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Lorber G, Benenson S, Rosenberg S, et al. A single dose of 240 mg gentamicin during transrectal prostate biopsy significantly reduces septic complications. Urology. 2013;82:998. doi: 10.1016/j.urology.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 22.Ho HS, Ng LG, Tan YH, et al. Intramuscular gentamicin improves the efficacy of ciprofloxacin as an antibiotic prophylaxis for transrectal prostate biopsy. Ann Acad Med Singapore. 2009;38:212. [PubMed] [Google Scholar]
- 23.Raman JD, Rjepaj C, Otteni C. A single 80 mg intravenous gentamicin dose prior to prostate needle biopsy does not reduce procedural infectious complications. Cent European J Urol. 2015;68:229. doi: 10.5173/ceju.2015.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki Y, Akamatsu S, Kanamaru S, et al. A Prospective Randomized Trial Comparing a Combined Regimen of Amikacin and Levofloxacin to Levofloxacin Alone as Prophylaxis in Transrectal Prostate Needle Biopsy. Urol J. 2016;13:2533. [PubMed] [Google Scholar]
- 25.Erb A, Sturmer T, Marre R, et al. Prevalence of antibiotic resistance in Escherichia coli: overview of geographical, temporal, and methodological variations. Eur J Clin Microbiol Infect Dis. 2007;26:83. doi: 10.1007/s10096-006-0248-2. [DOI] [PubMed] [Google Scholar]
- 26.Cussans A, Somani BK, Basarab A, et al. The role of targeted prophylactic antimicrobial therapy before transrectal ultrasonography-guided prostate biopsy in reducing infection rates: a systematic review. BJU Int. 2016;117:725. doi: 10.1111/bju.13402. [DOI] [PubMed] [Google Scholar]
- 27.Miura T, Tanaka K, Shigemura K, et al. Levofloxacin resistant Escherichia coli sepsis following an ultrasound-guided transrectal prostate biopsy: report of four cases and review of the literature. Int J Urol. 2008;15:457. doi: 10.1111/j.1442-2042.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 28.Boyd LB, Atmar RL, Randall GL, et al. Increased fluoroquinolone resistance with time in Escherichia coli from >17,000 patients at a large county hospital as a function of culture site, age, sex, and location. BMC Infect Dis. 2008;8:4. doi: 10.1186/1471-2334-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Administration, U. S. F. a. D. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. Silver Spring, MD: U.S. Food and Drug Administration; 2016. [Google Scholar]