Abstract
Advancements in measurement technologies and modeling capabilities continue to result in an abundance of exposure information, adding to that currently in existence. However, fragmentation within the exposure science community acts as an obstacle for realizing the vision set forth in the National Research Council’s report on Exposure Science in the 21st century to consider exposures from source to dose, on multiple levels of integration, and to multiple stressors. The concept of an Aggregate Exposure Pathway (AEP) was proposed as a framework for organizing and integrating diverse exposure information that exists across numerous repositories and among multiple scientific fields. A workshop held in May 2016 followed introduction of the AEP concept, allowing members of the exposure science community to provide extensive evaluation and feedback regarding the framework’s structure, key components, and applications. The current work briefly introduces topics discussed at the workshop and attempts to address key challenges involved in refining this framework. The resulting evolution in the AEP framework’s features allows for facilitating acquisition, integration, organization, and transparent application and communication of exposure knowledge in a manner that is independent of its ultimate use, thereby enabling reuse of such information in many applications.
Recognizing the complexity of exposure studies
For several decades, many fields in the biological sciences have recognized the complex nature of networks that regulate essential biological processes leading to beneficial or adverse health outcomes. In toxicology, the Adverse Outcome Pathway (AOP) framework acts as a means for assembling and organizing biological knowledge to facilitate the prioritization of research and to apply research results in support of human health and ecosystem risk assessment.1–4 The National Academies acknowledge that a similar framework is also necessary for exposure science. For instance, due to the complex nature of exposure data, such as spatial and temporal fragmentation, in addition to challenges associated with generation of data and knowledge across numerous disciplines, an organizational framework that works towards bridging that knowledge and data would greatly strengthen the field of exposure science.5
Fragmentation within exposure science arises from many aspects of its goals and practices. Exposure data are gathered at multiple levels of biological organization (e.g., population, individual, or test system) and in a variety of media (e.g., air, soil, water, food, biological specimens, consumer products, and cell culture) in space and time.5–7 Individuals and research groups involved in collecting exposure data are similarly diverse, spanning the breadth from Tribal Community institutions8 to cities, state and federal agencies, academic laboratories, industries, and non-governmental institutions.9 Finally, these assorted data are used in multiple disciplines, such as environmental chemistry, geographic spatial analyses, dosimetry analysis, and human health risk assessment. The complex nature of fragmentation in exposure data results in this data being scattered across various repositories and institutions,5 thus impeding the ability to make most data “findable, accessible, interoperable, and reproducible (FAIR Guiding Principles)”.10 It is often the case that data are used only once for the specific purpose of the study generating that data, thus limiting the full potential the data might offer if incorporated into other studies. Even when data are readily available to investigators, diverging structural schemas, data dictionaries, and code sets often hinder their practical use in other applications.11
In addition to recognizing the need for a framework for organizing disparate data, the National Academies also refers to a lack of an infrastructure that has the potential to better organize and coordinate the existing and rapidly evolving components of exposure science.5 Currently, numerous tools and databases exist that predict or store specific exposure information, from environmental media (e.g., soil, dust, water) to biological media (e.g., blood, cell, gill surface).12–17 There are also guidance documents on integrating exposure information into risk assessment. However, no infrastructure exists that organizes existing exposure measurements and predictions to allow for a holistic dissemination of exposure information, regardless of the type of stressor. As investigators continue to advance their respective fields through progression of monitoring, analytical, and modeling methodologies, such an infrastructure would enable data collected for a specific purpose to be applied and integrated to other relevant areas of research, enhancing the benefits beyond the original purposes for which those data were collected. Over time, a broader coverage of exposure knowledge will become apparent to investigators across multiple disciplines, allowing them to design new studies that might not otherwise be possible without such a holistic perspective.
A framework for exposure science
Just as ontologies have previously formalized descriptions of biology and toxicology,18–20 development of an exposure ontology has acted as an initial step towards addressing the need for an infrastructure within the exposure science community.21 An exposure ontology allows exposure events to be described in a systematic manner that supports both inter-operability and, eventually, computational reasoning. Also needed is a framework able to explicitly capture both the specific attributes of an exposure event, as a complement to exposure ontology, and the inter-relationships among exposure events.
This exposure science framework, designed to support the public health objectives outlined in the National Academies report,5 is proposed as the Aggregate Exposure Pathway (AEP) concept.22 An AEP is defined as “the assemblage of existing knowledge concerning biologically, chemically, and physically plausible, empirically supported links between the introduction of a chemical or other stressor into the environment and its concentration at asite of action, or target site exposure (TSE)”.22 Within the AEP framework, a specific emphasis has been placed on allowing for dissemination and reuse of measured or predicted data across a variety of scientific disciplines in order to inform a more comprehensive exposure overview for stressors. Additionally, a simple terminology used in the AEP framework allows it to remain generic, consistent, and interoperable for use across multiple disciplines and for all stressors.
At first glance, the AEP framework closely shares many features with existing exposure constructs such as exposure ontology, conceptual site models (CSMs),23 fate and transport models, and exposure models. The AEP framework and its terminology are neither intended to replace existing constructs and traditional exposure science methods, nor to alter current risk assessment practices. Rather, the AEP framework can facilitate integration among existing disciplines, constructs, and models by making fragmented exposure information readily available. One such example of an existing tool is MERLIN-Expo, which is an integrated modeling system that contains a library of exposure models for simulating concentrations of stressors in a wide range of environmental and biological media.13 Each model within this tool is the result of a concerted effort to achieve exposure predictions under a specific scenario, such as simulating the distribution of organic contaminants and metals in abiotic media of river systems. This effort requires an understanding of the mechanisms in advance, as well as an investment in time and resources, to model the system and to generate de nova predictions.
While the AEP framework can and should incorporate more advanced knowledge of the system, its scope is much broader. By systematically capturing exposure information without the effort involved in modeling underlying mechanisms, the AEP framework can highlight emergent properties of the system and facilitate the development and expansion of modeling efforts. As an example, consider two disjointed models that capture different points along an exposure continuum. If these models report input and output exposure data as it relates to an AEP, then additional information found within that AEP could offer clues into how these two models might be connected. While establishing this connection would be possible without the AEP framework in some cases, it may not be as efficient. In cases where the models fall into different fields of study, it is possible no inherent connection between the two would be recognized in the absence of a comprehensive, systematic framework that assembles data and relationships among those data in an organized manner. Therefore, the AEP should serve as this unifying framework that strengthens communication of exposure measurements, predictions, and data-gaps to the public health community in a manner that allows for data generated from one study to be reused and repurposed, and that enables humans and computers to more efficiently organize and analyze these data.
A major motivating factor for the AEP framework involves its complementation to the AOP framework, thereby allowing hazard information to be interpreted at the relevant level of biological organization (e.g., molecular level, external exposures).22 AOPs are chemical agnostic by design, and thus the only link between stressors and an AOP lies at the molecular initiating event (MIE), which is the initial interaction between a molecule and a biomolecule or biosystem.24 With an AEP providing available stressor-specific exposure information for a biological target, and an AOP linking molecular perturbations at that target site with adverse outcomes of regulatory concern, dose–response results generated from toxicity testing can be interpreted from a mechanistically-based context, greatly strengthening confidence in the risk assessment process. For example, the lowest effective dose measured from an in vitro assay, which relates to an MIE, can be compared to cellular or tissue concentrations derived from real world exposure data organized in AEPs. When considering multiple chemicals that induce activity within the same in vitro assay, exposure at the most relevant site of action for each of those chemicals may be estimated from exposure data organized in their individual AEPs, or perhaps even through use of exposure data obtained from AEPs of similar chemicals. Exposures from individual chemicals can then be converted into exposure equivalents of an index chemical based on relative potency, to estimate the cumulative effect from dose addition of those chemicals. Finally, a survey of available exposure data could be used to prioritize chemicals for high throughput toxicity screening, such that the coverage for toxicity information is more extensive where exposures are known to be higher. These examples do not imply that all AEPs should have corresponding AOPs. Rather, the AEP framework facilitates more efficient connections between exposure and hazard information in order to encourage systems-based approaches that generate measurements or estimates of exposure that better inform the source-to-outcome continuum.
Refining the elements of the aggregate exposure pathway framework
Overview
Recognizing that successful refinement of the AEP framework requires continuing dialogue and collaboration amongst experts in exposure science, a two-day workshop (May 9–11, 2016, Durham, NC) was organized to assemble a small group of participants from government, academia, and industry to address several key topics. These topics included definition, development, and applications of AEPs, along with conceptual design of an infrastructure. The workshop consisted of small group breakout sessions, followed by larger group discussions among participants. Reception of the AEP concept has been mixed; some envision great potential for the framework to enhance exposure science, and others question the distinction between AEPs and existing exposure science tools. A summary of the presentations and discussions is available in the workshop proceedings.25 This manuscript attempts to address several concerns and inquiries emerging from the workshop, especially in regards to the definition and development of an AEP. Other topics, including recommendations for infrastructure design, applications of AEPs, and integration of ecological and human exposures, will be included in future publications. Additionally, case studies demonstrating the integration of the AEP and AOP frameworks are underway and will be presented in separate publications.
One critical issue arising from the workshop is whether an AEP is required to be stressor specific or if it can incorporate information from multiple stressors sharing common exposure characteristics (e.g., an AEP specifically for DDT vs. a generic chemical-agnostic AEP that might be applied to all endocrine disruptors). A general consensus among workshop participants is that attempting to define one AEP in terms of more than one stressor is nearly an impossible task. Additionally, reducing AEPs to generic fate, transport, and transformation processes is not the original intent for proposing such a framework. There is considerable difficulty in determining the approach and resolution for grouping together stressors into a single AEP when these stressors share characteristics for one part of the pathway but not others. For example, both DDT and bisphenol A are found in surface water, but the latter is unstable in soil while DDT binds strongly to soil. In this example, it becomes problematic to decide whether a hypothetical AEP for endocrine disruptors should include soil as a medium of interest. Furthermore, a single concentration value cannot be established for multiple stressors within the same grouping (e.g., disinfection by-products). Rather, only concentrations for individual stressors within that grouping can be reported (e.g., chloroform, bromate, chloramine). Thus, an individual AEP continues to be defined as stressor specific, but this definition should not be a barrier to the broader use of the framework, such as applying a network of individual AEPs to investigate co-exposures to multiple chemicals. Also, although examples provided herein focus on chemicals, the ultimate intent is for the framework to encompass the full range of stressors, i.e., those entities that are not only chemical, but also biological, biomechanical, and physical agents.
The workshop participants also suggested that the terminology in the AEP framework, originally adopted from the AOP framework, required revision. Rather than adopting “key event” and “key event relationship” from AOPs for use as the elements in AEPs, the more appropriate terms conforming to the unique characteristics inherent within exposure information are now listed as “key exposure state” (KES) and “key transitional relationship” (KTR). Describing an AEP using this revised terminology provides a uniform and easily understood format for intuitively reporting, sharing, communicating, and applying exposure data. Refinement of these two elements is discussed further below.
Key exposure state
A KES is represented as a node in an AEP framework and is defined as the state of a stressor in space and time. A KES is either a qualitative (i.e., detected) or quantitative (i.e., concentration) state of a specific stressor measured or predicted within an environmental or a biological medium, or within a manufactured product. The state of a stressor within a medium is driven by physicochemical properties of that particular stressor, such as in the case with a highly volatile chemical found primarily in media types pertaining to air. In addition to stressor-specific properties, each KES also reflects general characteristics associated with the respective environmental, biological, or manufactured medium. For example, ozone levels are influenced by the composition of nitrogen oxides, volatile organic compounds, and amount of sunlight, all of which constitute characteristics of the outdoor air in which ozone levels are measured. Finally, the number of KESs within an AEP for a stressor can also be influenced by its commercial use and release or mode of entry to the environment.26
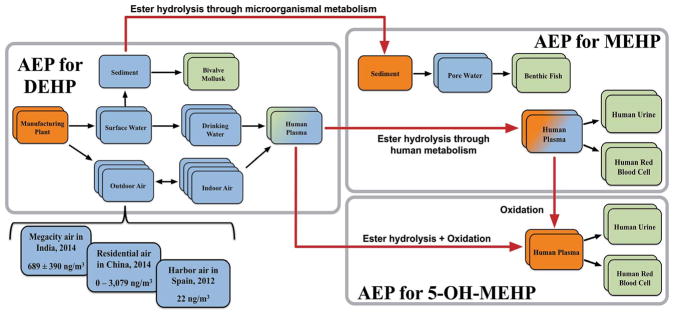
An AEP is anchored by two specialized KESs at both ends: a source and a TSE (Fig. 1). Unlike other KESs that are solely databased, source(s) and TSE(s) are implicitly included in an AEP even when measurements or predictions are not available. As defined in the Agency for Toxic Substances and Disease Registry’s (ATSDR) Public Health Assessment Guidance Manual,27 a source is the state of a stressor at its introduction or conception. For chemicals in commerce, the source could be production volume at a manufacturing plant. The source in an AEP for a metabolite originating from environmental or biological processes (e.g., photolysis, hydrolysis, or biodegradation/biotransformation) is its state in the medium in which the transformation process is occurring (e.g., blood or soil). While source information may not always be obtainable, one can be certain that an origin does exist for any stressor. Additionally, source information, particularly whether the source is far-field (e.g., emissions of waste perfluorinated surfactants into a river) or near-field (e.g., spraying of surfactant-containing cleaners onto a kitchen counter), is critical for risk management or remediation purposes.
Fig. 1.
The two elements of the Aggregate Exposure Pathway (AEP) framework are key exposure states (KESs; rectangles) and key transitional relationships (KTRs; arrows). Each KES captures spatial, temporal, and organism-specific exposure information. There are two specialized KESs: a source (orange rectangle) and a target site of exposure (TSE; green rectangle). In some cases, a TSE for one stressor can also act as an intermediate KES for another stressor depending on a user’s preferred target of interest (shown as blue-green rectangles). In other cases, a source for one stressor can also act as an intermediate KES for another stressor when there are multiple generations of metabolites (shown as blue-orange rectangles). There are also two types of key transitional relationships: processes that describe the movement of a stressor between media within an AEP (black arrows) and processes that describe the conversion of one stressor to another across two AEPs (red arrows). Here, a hypothetical AEP for diethylhexyl phthalate (DEHP) is presented with inclusion of several known media types in which it is found, along with hypothetical AEPs for two metabolites of DEHP, monoethylhexyl phthalate (MEHP) and 5-hydroxy-monoethylhexyl phthalate (5-OH-MEHP). Each media type is represented by stacks of KESs associated with that medium, with each KES representing exposure data from an individual study.
On the other end, the term TSE has been taken from the National Academies report on Exposure Science in the 21st century,6 and is defined here as the state of a stressor at a site of action (e.g., tissue or organ). Within the context of the purpose of a study applying an AEP, any KES has the potential to be considered a TSE, provided that exposure information for that KES is relevant to a specific target or its surrogate. The TSE may be ostensibly external to an organism (e.g., skin, gill, or plant surface), or internal (e.g., organ, tissue, cell, or enzyme).28 A TSE for one organism of interest might also act as an intermediate KES that links to another KES, source, or TSE (Fig. 1 and 2a). For example, a TSE that represents mercury concentrations measured in fish tissue can also be a KES that leads to a TSE representing mercury measured in human blood, through human consumption of contaminated fish tissues. Such a capability also allows for a natural integration among humans and wildlife, as both entities affect each other in a reciprocal manner. It is important to recognize that while both source(s) and TSE(s) are implied in an AEP, it is the specific purpose of the study that determines whether a source or TSE is needed for that study. For example, a study that compares an established guidance level for a given stressor (e.g., annual standard for PM2.5 is 12 μg m−3) to environmental measurements or a study that analyzes the temporal trends of exposures does not require explicit linkage to a biological target. Thus, a TSE is not needed for these types of studies, but source data can be useful for interpreting the study ndings. On the other hand, an epidemiological study, which investigates the association between exposure to a stressor and adisease outcome, rarely use source data as exposure metrics.
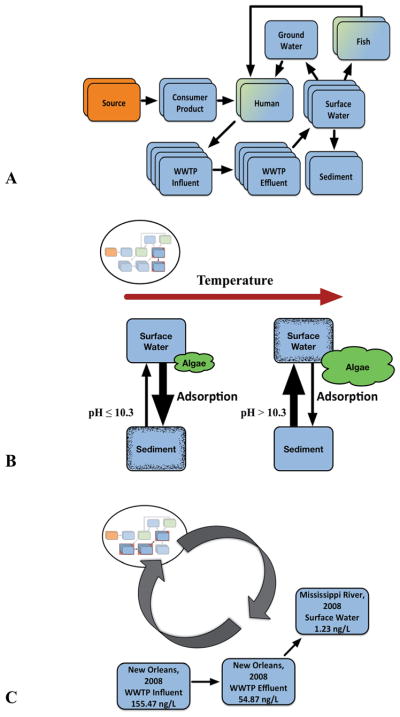
Fig. 2.
(A) The full Aggregate Exposure Pathway (AEP) for estradiol is presented here for two primary biological targets – humans and fish can act as both as a target site of exposure (TSE) as well as an intermediate key exposure state (KES). This full AEP is used as an inset (top-left) for (B) and (C), with the KESs (rectangles) and key transitional relationships (KTRs) of interest highlighted in red and remaining as dark arrows, respectively. (B) Concentrations of estradiol are higher in riverine sediment when adsorption is greater under cool weather conditions, as sparse algal populations during this period result in pH levels below the pKa value for estradiol in water (left panel). During periods of warm temperature, algal blooms can cause pH levels to rise above estradiol’s pKa value and lead to lower adsorption into sediment (right panel) and higher estradiol concentrations in overlying water. (C) The reciprocal nature of AEP development is demonstrated here. A partial AEP for a stressor is provided through data generated from individual studies and can contribute KESs and/or KTRs to a full AEP. A partial AEP can be extracted from a full AEP for specific applications, such as developing an exposure model. In this example, KESs that represent estradiol concentrations in wastewater treatment plant (WWTP) influent and effluent from New Orleans municipal facilities and in Mississippi River Water were obtained from published literature (Wang, et al., 2012).
Key transitional relationship
The connection between two KESs in the AEP framework is described by the term KTR, which represents either the movement of a stressor within one AEP (Fig. 1; black arrows) or transformation from one stressor to another stressor across two different AEPs (Fig. 1; red arrows). Some stressors might only be generated within a biological system through biotransformation of one or more parent compounds, as in the case with 5-hydroxy-monoethylhexyl phthalate (Fig. 1). In this case, the KTR links the parent compound to the source of the metabolite within the same biological medium. In other cases, a stressor may originate from transformation of its precursor in both environmental and biological media, as illustrated with mono(2-ethylhexyl) phthalate (MEHP) (Fig. 1).
While a KES is always associated with a measurement or prediction, a KTR that connects any two KESs is often a general description of a mechanistic process based upon scientific principles or professional judgment. KTRs can also be quantitative (i.e., rate) or qualitative. For example, a predicted perchlorate intake rate of 0.015 μg per kg per day at the 95th percentile among women of reproductive age in the U.S.29 is a quantitative KTR; microsomal degradation of bis(2-ethylhexyl) phthalate (DEHP) into MEHP in soil30 is a qualitative KTR. KTRs are often subjective, in that different investigators may have alternative views on whether two KESs are related or how they are related. Therefore, it is up to the discretion of the investigator to decide which KES(s) and associated KTR(s) are most relevant and should be included within a particular study.
Similar to a KES, a KTR is influenced by the physicochemical properties of stressors in addition to general characteristics of the media, and thus mediates the concentrations of the stressor(s) in two connected KESs. These general characteristics are often expressed as parameters in mathematical models to predict the dynamic transition from one KES to another, e.g., estradiol transport between sediment and overlying riverine surface water (Fig. 2a). Throughout most of the year, estradiol is adsorbed easily onto the sediment due to its high lipophilicity, resulting in lower concentrations of estradiol in overlying water (Fig. 2b). However, during warm summer months, algal blooms can cause a rise in surface water pH above that of the pKa value for estradiol,31 resulting in a significantly reduced adsorption capacity32 and lower estradiol concentrations in sediment (Fig. 2b), and subsequently higher water concentrations. In the above example, algal population growth rate and resulting bloom decay can be incorporated into a mathematical model to predict changes in surface water pH level and the subsequent impact on estradiol concentrations in surface water and in sediment.
Levels of organization
Within the first AEP manuscript22 and during the May 2016 workshop, the diagrams of AEPs were presented with each key event (now KES) grouped within specific compartments, such as environmental media, external exposure, or internal exposure. This earlier diagram of the AEP intentionally adopted a visual representation similar to that of the AOP framework. In the case of an AOP, assigning key events into one of various scales of biological organization (e.g., molecular, organ) is warranted because the biological markers being measured clearly determine the most relevant biological level (e.g., survival rate at the population level, cytotoxicity at the cellular level). In the case of an AEP, the object being measured is always the potential stressor, and the distinction among exposure compartments is not as straightforward as with biological organization. For example, assigning outdoor air to “environmental media” and indoor air to “external exposure” is arbitrary and defeats the purpose of the AEP being an interoperable and universal framework. Consequently, the updated diagram of AEPs (Fig. 1) now shows KESs that are connected by their associated KTRs in no specific compartmentalized format, as the KESs themselves capture spatial, temporal, and organism-specific characteristics in their description. The refined definition of a KES also addresses the need for specificity when recording the concentration of a stressor measured or predicted at a given place and time, which can be visualized by the stacks of individual KESs under a given medium (Fig. 1 and 2). For example, the AEP for estradiol may contain four KESs related to its measurement in waste water treatment plant (WWTP) influent that represent four distinct measurements, which are likely contributed by different studies.
The levels of biological organization originally defined for the AOP can be useful in determining whether a TSE under consideration provides sufficient linkage to an AOP. The TSE coincides with the site of a target whose perturbation can lead to an adverse outcome. As the levels of biological organization range from the molecular to the population level, a TSE can be defined at any level. Internal dosimetry is not required to be addressed if an investigator determines that evaluation of an external TSE is sufficient for the purpose of the study. In other cases, it would be perfectly reasonable to define multiple TSEs in series within a given AEP to highlight the different levels of biological organization at which exposure can be determined for that particular stressor. For example, if effects of interest were based on epidemiological data, then the most appropriate level of resolution for the TSE would likely be at the individual level. In contrast, when effects of interest are based on in vitro assay that ties to a MIE within an AOP, then the most appropriate level of resolution for the TSE would be at the cellular or tissue level. If a stressor is not measurable at the cellular or tissue level (e.g., concentration in brain), then its measurement at a biological level further upstream (e.g., concentration in plasma) could act as a TSE. Once measurements of that stressor within a cell or tissue are made possible, as through technological advancements, then that original upstream TSE becomes an intermediate KES.
Developing aggregate exposure pathways
The advantages of the AEP framework can only be fully realized if a concerted effort is made to facilitate information exchange within and beyond the exposure science community. The broad acceptance of the AOP framework is a result of collaborative effort among an international community of developers and users, as well as a central hub for sharing and storing AOP-related information (http://aopkb.org). While the AEP framework will also benefit from the creation of a data infrastructure that supports a communal effort for developing and populating individual AEPs, it also enjoys the advantage of being able to exploit decades of research leading to the development of environmental and exposure models. For example, multimedia environmental fate models33 can serve as repositories of knowledge and conceptual understanding about the interactions between chemicals and environmental systems; about chemical-specific properties that determine behaviors such as partitioning, mass transfer, and chemical reactions; and about the properties of the systems that enable the behaviors. These numerous exposure tools provide a wealth of data that can complement AEP development. An information exchange platform may be suitable for integrating decentralized information from existing data sources, by indexing these data in a manner that renders them more searchable. The effort to develop such an infrastructure is non-trivial, and this decision will be best made after a thorough review of existing resources and needs of applications and tools most likely to benefit from AEPs.
The concept of incremental development is employed in the AOP framework through incorporation of new information as it is discovered, thus allowing AOPs to be qualitatively or quantitatively applied regardless of their stages of completion. The same holds true with development and application of AEPs. Monitoring studies can contribute to individual KESs or a small number of directly-linked KESs. In addition, non-targeted studies and many targeted monitoring studies analyze multiple stressors collected within the same media.34,35 Not only do these studies contribute individual KESs for their respective AEPs, but they also generate co-occurrence information that can contribute to networks of AEPs. Information on current KESs and KTR mechanisms organized in an AEP can also inform the development or refinement of computational models, for predicting downstream KESs and conducting a priori exposure assessment. As more KESs are generated over time, either through measurements or predictions, existing and new models can be coupled to enable a more robust transition across multiple KESs. For example, estradiol concentrations measured in WWTP influent and effluent from New Orleans municipal facilities and in Mississippi River water can contribute to an AEP for estradiol (Fig. 2c),36 and these data can also inform the development of a wastewater process model to predict estradiol concentrations in other water bodies. As an AEP continues to evolve into an ever-expanding interconnected network of exposure information, data gaps are more easily identified, thus enabling prioritization of research efforts and the emergence of new applications and tools that can advance the current state of exposure knowledge.
Characterization of variability and uncertainty, while critical to each stage of the risk-assessment process, has remained a particular challenge in exposure assessment.6,37,38 Variability refers to the heterogeneity of values in a system and can be better characterized but not reduced through additional measurements. Uncertainty, on the other hand, typically arises from incomplete information on the components of a system (e.g., KESs) or relationships among components (e.g., KTRs). Although not captured as explicit components of the AEP, the addition of a broad range of measurements or predictions for both KESs and KTRs gathered from disparate sources throughout AEP development can aid in addressing variability and uncertainty. As more information is collected for a particular media type or mechanism, AEPs can help capture and characterize variability; moreover, by drawing attention to and helping to elucidate the complexities in the exposure pathways, AEPs can also assist in generating new hypotheses to reduce uncertainty.
Application of the aggregate exposure pathway framework
The AEP framework serves as a mechanism to universally organize and integrate exposure measurements and predictions, which can then subsequently be used in a variety of applications. In the 2016 workshop, the participants introduced several potential applications, some of which are listed below. Additional recommended applications can be found in the workshop proceedings.25
Organizing exposure data to support risk-based screening
Standardizing data reporting and sharing
Describing potential pathways to test new hypotheses
Supporting life-cycle assessment
Informing the exposome
Developing read-across approaches to estimate exposures for data-poor chemicals
Refining and increasing confidence in exposure assessment
Reducing uncertainty in risk assessment
Identifying and prioritizing data gaps for research and funding needs
Improving communications with decision makers and stakeholders
The details regarding use of these and other applications of AEPs will be provided in a later publication.
Finally, AEPs may be used to underscore the interdependence between human and ecosystem health. The methodologies for human health and ecological risk assessment were developed independently,39–41 and it was soon realized that both should be integrated in a holistic approach that views humans as a component of an ecosystem to address real-life (i.e., non-idealized) scenarios of multi-stressor, multimedia, and multi-species exposures. As Suter and colleagues note, exposures to environmental chemicals by human and nonhuman organisms result from the same sources, the same transportation and transformation processes, and often in the same media.42 Thus, a common multimedia fate and transport model can be used to estimate the time course of media-specic concentrations for all organisms. Such a model can be populated using information organized in an AEP that encompasses the relevant terrestrial and aquatic components of the ecosystem. Moreover, an assembly of AEPs into a network can facilitate moving beyond a stressor-by-stressor approach that focuses on a single media, a single source, and a single toxic endpoint, into decision-making that considers the overall risks to human and nonhuman organisms, populations, and ecosystems.
Conclusions
The concept of the AEP framework has seen significant criticism, feedback, and suggestions regarding its refinement since its introduction.22 The content within this current work reflects the perspectives of the authors after considering feedback from participants of the May 2016 workshop, and presents possible avenues on addressing key issues arising from that workshop and initiating continued dialogue among experts in the fields of exposure science, ecological modeling, and risk assessment. As more data are collected within the AEP framework, users will benefit from better-organized and integrated exposure information, regardless of the uses for those data. In addition, future research can be tailored to address known data gaps and modeling needs highlighted by the comprehensive integration of exposure information. Furthermore, the coupling of the AEP and AOP frameworks will support the interpretation of in vitro dose–response data so that health effects can be more efficiently assessed for exposures to multiple chemical and non-chemical stressors. The examples considered herein do not capture the full potential offered by the AEP framework, and additional case studies are underway to further highlight its value. While there are certain similarities between AEPs and existing exposure science resources, the potential applications listed herein and ability to complement existing tools and models stand as a testament to the significant contributions such a framework might provide for 21st century exposure science.
Environmental significance.
The environmental health sciences community has seen numerous benefits arising from the broad applicability of science-based frameworks for organizing toxicological and biological information. The field of exposure science is poised to reap similar benefits from a complementary framework capable of organizing, sharing, and integrating exposure data and knowledge. The Aggregate Exposure Pathway seeks to characterize those chemical and non-chemical stressors that are found throughout the environment and within biological systems. This framework will promote more efficient data integration to support modeling efforts, and to make exposure information more readily accessible for a variety of applications. This framework will also aid in the assessment and minimization of health risks posed by stressors to humans and ecological systems through its data dissemination capabilities.
Acknowledgments
The authors would like to thank several internal U.S. EPA reviewers, as well as Drs Edmund Seto, Rosemary Zaleski, and Judy LaKind, for their comments on a draft of this manuscript. Special thanks to the 2016 AEP workshop attendees. Funding for Dr Leonard was provided by the Oak Ridge Institute for Science and Education Research Participation Program at the US EPA. This work (JGT) was supported by P30 ES000210 (Oregon State University-PNNL) by the National Institute of Environmental Health Sciences and the Laboratory Directed Research and Development program at the Pacific Northwest National Laboratory (PNNL) and is a contribution of the Global Forensic Chemical Exposure Assessment for the Environmental Exposome project. PNNL is a multi-program national laboratory operated by Battelle for the DOE under Contract DE-AC05-76RLO 1830. We would also like to thank the external peer reviewers for their comments and suggestions that have enabled us to enhance the content of this manuscript.
Footnotes
Disclaimer
The U.S. Environmental Protection Agency has provided administrative review and has approved this paper for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. Environmental Protection Agency.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. Adverse Outcome Pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 2.Groh KJ, Carvalho RN, Chipman JK, Denslow ND, Halder M, Murphy CA, Roelofs D, Rolaki A, Schirmerand KH, Watanabe K. Development and application of the Adverse Outcome Pathway framework for understandingand predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere. 2015;120:764–777. doi: 10.1016/j.chemosphere.2014.09.068. [DOI] [PubMed] [Google Scholar]
- 3.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. Adverse Outcome Pathway (AOP) development I: Strategies and principles. Toxicol Sci. 2014;142(2):312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. Adverse Outcome Pathway Development II: Best practices. Toxicol Sci. 2014;142(2):321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council (NRC) Using 21st Century Scienceto Improve Risk-Related Evaluations. National Academies Press; Washington, DC, US: 2017. [PubMed] [Google Scholar]
- 6.National Research Council (NRC) Exposure Science in the21st Century: A Vision and a Strategy. National Academies Press; Washington, DC, US: 2012. [PubMed] [Google Scholar]
- 7.O’Connell SG, Kincl LD, Anderson KA. Silicone wristbands as personal passive samplers. Environ Sci Technol. 2014;48(6):3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motorykin O, Schrlau J, Jia Y, Harper B, Harris S, Harding A, Stone D, Kile M, Sudakin D, MasseySimonich SL. Determination of parent and hydroxy PAHs in personal PM2.5 and urine samples collected during NativeAmerican fish smoking activities. Sci Total Environ. 2015;505:694–703. doi: 10.1016/j.scitotenv.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone A, Delistraty D. Sources of toxicity and exposure information for identifying chemicals of high concern to children. Environ Impact Assess Rev. 2010;30(6):380–387. [Google Scholar]
- 10.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten J-W, Santos LBdS, Bourne PE, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obrst L, Cassidy P. Geological Society of America Special Papers. Vol. 482. Geological Society of America; 2011. The need for ontologies: Bridging the barriers of terminology and data structure; pp. 99–123. [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services; 2009. p. 530. [Google Scholar]
- 13.Ciffroy P, Alfonso B, Altenpohl A, Banjac Z, Bierkens J, Brochot C, Critto A, De Wilde T, Fait G, Fierens T, et al. Modelling the exposure to chemicals for risk assessment: a comprehensive library of multimedia and PBPK modelsfor integration, prediction, uncertainty and sensitivity analysis – the MERLIN-Expo tool. Sci Total Environ. 2016;568:770–784. doi: 10.1016/j.scitotenv.2016.03.191. [DOI] [PubMed] [Google Scholar]
- 14.Dudzina T, Delmaar CJE, Biesterbos JWH, Bakker MI, Bokkers BGH, Scheepers PTJ, van Engelen JGM, Hungerbuehler K, von Goetz N. The probabilistic aggregate consumer exposure model(PACEM): Validation and comparison to a lower-tierassessment for the cyclic siloxane D5. Environ Int. 2015;79:8–16. doi: 10.1016/j.envint.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Fenner K, Scheringer M, Macleod M, Matthies M, McKone T, Stroebe M, Beyer A, Bonnell M, Le Gall AC, Klasmeier J, et al. Comparing estimates of persistence and long-range transport potential among multimediamodels. Environ Sci Technol. 2005;39(7):1932–1942. doi: 10.1021/es048917b. [DOI] [PubMed] [Google Scholar]
- 16.Liebig M, Moltmann JF, Knacker T. Evaluation of measured and predicted environmental concentrations ofselected human pharmaceuticals and personal care products. Environ Sci Pollut Res Int. 2006;13(2):110–119. doi: 10.1065/espr2005.08.276. [DOI] [PubMed] [Google Scholar]
- 17.Oldring PKT, O’Mahony C, Dixon J, Vints M, Mehegan J, Dequatre C, Castle L. Development ofa new modelling tool (FACET) to assess exposure to chemical migrants from food packaging. Food Addit Contam, Part A. 2014;31(3):444–465. doi: 10.1080/19440049.2013.862348. [DOI] [PubMed] [Google Scholar]
- 18.Hardy B, Apic G, Carthew P, Clark D, Cook D, Dix I, Escher S, Hastings J, Heard DJ, Jeliazkova N, et al. Toxicology ontology perspectives. ALTEX. 2012;29(2):139–156. doi: 10.14573/altex.2012.2.139. [DOI] [PubMed] [Google Scholar]
- 19.Smith B, Ashburner M, Rosse C, Bard J, Bug W, Ceusters W, Goldberg LJ, Eilbeck K, Ireland A, Mungall CJ, et al. The OBO Foundry: Coordinated evolution of ontologies to support biomedical data integration. Nat Biotechnol. 2007;25(11):1251–1255. doi: 10.1038/nbt1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaman S, Sarntivijai S, Abernethy DR. Use of biomedical ontologies for integration of biological knowledge for learning and prediction of adverse drug reactions. Gene Regul Syst Biol. 2017;11:1177625017696075. doi: 10.1177/1177625017696075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattingly CJ, McKone TE, Callahan MA, Blake JA, Hubal EAC. Providing the missing link: The exposure science ontology ExO. Environ Sci Technol. 2012;46(6):3046–3053. doi: 10.1021/es2033857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teeguarden JG, Tan Y-M, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, et al. Completing the link between exposure science and toxicology for improved environmental health decision making: The Aggregate Exposure Pathway framework. Environ Sci Technol. 2016;50(9):4579–4586. doi: 10.1021/acs.est.5b05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Society for Testing and Materials (ASTM) Standard Guide for Developing Conceptual Site Models for Contaminated Sites. 2014. pp. E1689–95. [Google Scholar]
- 24.Allen TEH, Goodman JM, Gutsell S, Russell PJ. De ning molecular initiating events in the Adverse Outcome Pathway framework for risk assessment. Chem Res Toxicol. 2014;27(12):2100–2112. doi: 10.1021/tx500345j. [DOI] [PubMed] [Google Scholar]
- 25.Gordon A. Aggregate Exposure Pathway Workshop; May 9–10, 2016; Durham, NC, Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment; 2017. p. 55. EPA/600/R-17/357. [Google Scholar]
- 26.Webster E, Mackay D, Wania F. Evaluating environmental persistence. Environ Toxicol Chem. 1998;17(11):2148–2158. [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry (ATSDR) [accessed Apr 3, 2017];Chapter 6: Exposure evaluation, Evaluating exposure pathways|PHA guidance manual. https://www.atsdr.cdc.gov/hac/phamanual/ch6.html#6.2.
- 28.Cohen Hubal EA, Richard A, Aylward L, Edwards S, Gallagher J, Goldsmith M-R, Isukapalli S, TorneroVelez R, Weber E, Kavlock R. Advancing exposure characterization for chemical evaluation and risk assessment. J Toxicol Environ Health, Part B. 2010;13(2–4):299–313. doi: 10.1080/10937404.2010.483947. [DOI] [PubMed] [Google Scholar]
- 29.Mendez W, Dederick E, Cohen J. Drinking water contribution to aggregate perchlorate intake of reproductive-age women in the United States estimated by dietary intake simulation and analysis of urinary excretion data. J Exposure Sci Environ Epidemiol. 2010;20(3):288–297. doi: 10.1038/jes.2009.50. [DOI] [PubMed] [Google Scholar]
- 30.Baek JH, Gu MB, Sang B-I, Kwack SJ, Kim KB, Lee BM. Risk reduction of adverse effects due to di-(2-ethylhexyl) phthalate (DEHP) by utilizing microbial degradation. J Toxicol Environ Health, Part A. 2009;72(21–22):1388–1394. doi: 10.1080/15287390903212733. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Cornwell JC, Stoecker DK, Owens MS. Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences. 2012;9(7):2697–2710. [Google Scholar]
- 32.Ma W, Nie C, Gao X, Qu D, Lun X, Chen B. Sorption characteristics and factors affecting the adsorption behavior of bisphenol A and 17b-estradiol/ethinyl estradiol in river and farmland-based artificial groundwater recharge with reclaimed water. Desalin Water Treat. 2016;57(17):8015–8025. [Google Scholar]
- 33.MacLeod M, Scheringer M, McKone TE, Hungerbuhler K. The State of multimedia mass-balance modeling in environmental science and decision-making. Environ Sci Technol. 2010;44(22):8360–8364. doi: 10.1021/es103297w. [DOI] [PubMed] [Google Scholar]
- 34.Bessonneau V, Ings J, McMaster M, Smith R, Bragg L, Servos M, Pawliszyn J. In vivo microsampling to capture the elusive exposome. Sci Rep. 2017;7:44038. doi: 10.1038/srep44038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM, Wambaugh JF, Isaacs KK, Judson R, Williams AJ, et al. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environ Int. 2016;88:269–280. doi: 10.1016/j.envint.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Ma P, Zhang Q, Lewis J, Lacey M, Furukawa Y, O’Reilly SE, Meaux S, McLachlan J, Zhang S. Endocrine disrupting chemicals in New Orleans surface waters and Mississippi Sound sediments. J Environ Monit. 2012;14(5):1353–1364. doi: 10.1039/c2em30095h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKone TE, Feng L. Building a human health risk assessment ontology (RsO): A proposed framework. Risk Anal. 2015;35(11):2087–2101. doi: 10.1111/risa.12414. [DOI] [PubMed] [Google Scholar]
- 38.National Research Council (NRC) Science and Decisions: Advancing Risk Assessment. Committee on Improving Risk Analysis Approaches Used by the U.S. EPA, National Academies Press (US); Washington, DC: 2009. [Google Scholar]
- 39.United States Environmental Protection Agency (USEPA) Interim Procedures and Guidelines for Health Risk and Economic Impact Assessments of Suspected Carcinogens, EPA/R-109. Washington, DC, USA: 1976. [Google Scholar]
- 40.United States Environmental Protection Agency (USEPA) Framework for Ecological Risk Assessment, EPA/630/R-92/001. Risk Assessment Forum; Washington, DC, USA: 1992. [Google Scholar]
- 41.United States Environmental Protection Agency (USEPA) Guidelines for Ecological Risk Assessment, EPA/630/R-95/002F. Risk Assessment Forum; Washington, DC, USA: 1998. [Google Scholar]
- 42.Suter G, Vermeire T, Munnsjr W, Sekizawa J. An integrated framework for health and ecological risk assessment. Toxicol Appl Pharmacol. 2005;207(2):611–616. doi: 10.1016/j.taap.2005.01.051. [DOI] [PubMed] [Google Scholar]