Abstract
Individual animals differ consistently in their behaviour, thus impacting a wide variety of ecological outcomes. Recent advances in animal personality research have established the ecological importance of the multidimensional behavioural volume occupied by individuals and by multispecies communities. Here, we examine the degree to which the multidimensional behavioural volume of a group predicts the outcome of both intra- and interspecific interactions. In particular, we test the hypothesis that a population of conspecifics will experience low intraspecific competition when the population occupies a large volume in behavioural space. We further hypothesize that populations of interacting species will exhibit greater interspecific competition when one or both species occupy large volumes in behavioural space. We evaluate these hypotheses by studying groups of katydids (Scudderia nymphs) and froghoppers (Philaenus spumarius), which compete for food and space on their shared host plant, Solidago canadensis. We found that individuals in single-species groups of katydids positioned themselves closer to one another, suggesting reduced competition, when groups occupied a large behavioural volume. When both species were placed together, we found that the survival of froghoppers was greatest when both froghoppers and katydids occupied a small volume in behavioural space, particularly at high froghopper densities. These results suggest that groups that occupy large behavioural volumes can have low intraspecific competition but high interspecific competition. Thus, behavioural hypervolumes appear to have ecological consequences at both the level of the population and the community and may help to predict the intensity of competition both within and across species.
Keywords: animal personality, behavioural hypervolume, insect ecology, species interaction
Consistent individual differences in behaviour, known as animal personality, appear to be ubiquitous throughout the animal kingdom (Gosling, 2001; Kralj-Fišer & Schuett, 2014). Animal personality determines aspects of individuals' ecology such as individual variation in social behaviour (Lichtenstein et al., 2016; Wright, Holbrook, & Pruitt, 2014) space use (Pearish, Hostert, & Bell, 2013; Wilson & McLaughlin, 2007) and diet (Wilson, Coleman, Clark, & Biederman, 1993). The personality literature has long argued that a comprehensive understanding of an individual's behaviour requires a multitrait approach (Sih, Bell, & Johnson, 2004; Sih, Cote, Evans, Fogarty, & Pruitt, 2012). Despite this early emphasis on a multitrait approach and the availability of statistical approaches to evaluate multiple behaviours simultaneously, many studies that relate personality to ecological outcomes continue to examine only one trait at a time (Wolf & Weissing, 2012). Here we examine multiple behaviours simultaneously using a recently developed multivariate measure, the behavioural hypervolume (Pruitt, Bolnick, Sih, DiRienzo, & Pinter-Wollman, 2016; Pruitt et al., 2017).
We define a behavioural hypervolume as the volume of an irregular convex polygon in multidimensional behavioural space occupied by an individual, a group, a population or even a community. Multidimensional behavioural space refers to a space in which each dimension represents standardized performance in a different personality test. In other words, behavioural hypervolumes are a metric of behavioural diversity that incorporates three or more personality traits. Behavioural hypervolumes emphasize behavioural extremes, because observations on the boundaries of these shapes in behavioural space dictate their hypervolume (Pruitt et al., 2016, 2017). Consequently, individuals in convex polygons that occupy more behavioural space at the group or population level tend to be more behaviourally dissimilar to each other, at least, when one considers the most extreme individuals. This dissimilarity may, in turn, map to individuals' ecological niche, including the degree to which they compete with conspecifics and heterospecifics. There is considerable evidence to suggest that this is true: individual variation in personality often predicts how individuals interact with conspecifics (Kurvers et al., 2009; Laskowski & Bell, 2013) and heterospecifics (Chang, Teo, Norma-Rashid, & Li, 2017; DiRienzo, Pruitt, & Hedrick, 2013; Webster, Ward, & Hart, 2009). Thus, behavioural hypervolumes may impact ecological processes.
For instance, recent work has shown that the behavioural hypervolume of a group can influence the outcome of predator–prey interactions (Pruitt et al., 2017), and the behavioural hypervolume of a mixed-species community can determine their stability (Pruitt et al., 2016). Here we examine whether the behavioural hypervolumes of groups of interacting species can predict the outcome of both intra- and interspecific interactions. We evaluate this possibility by examining two insect species that inhabit and feed on the Canada goldenrod, Solidago canadensis. We chose these two species because they are two of the most common insects in old fields, they share a resource and they are found on the same plant parts, especially leaves near the crown, which suggests that they may compete for access to the most preferred regions of their host plant (J. L. L. Lichtenstein & B. McEwen, personal observation). Katydid nymphs from the genus Scudderia and the meadow froghopper, Philaenus spumarius, both feed on the Canada goldenrod (Weaver & King, 1954). Notably, Scudderia nymphs are leaf chewers native to our study site, whereas the froghoppers, P. spumarius, are sap-suckers that are wildly successful invaders across the Nearctic. These froghoppers are in some areas the second most abundant herbivore on goldenrods (Root & Cappuccino, 1992) and have more deleterious effects on goldenrod growth and reproduction than other herbivores (Meyer & Root, 1993; Meyer, 1993). Both species reach high densities on goldenrod leaves over large geographical ranges, thus creating ample opportunities for both species to interact with both conspecifics and each other.
Here, we ask whether behavioural hypervolumes shape how each species interacts with conspecifics (intraspecific interaction) and with other species (interspecific interaction). Specifically, we evaluated two predictions. First, we predicted that single-species groups of katydids with greater behavioural hypervolumes would cluster together in space more than groups with small behavioural hypervolumes. We reasoned that individuals in single-species groups occupying larger hypervolumes differ from each other more in their niches, thus reducing intraspecific competition. This is based on the assertion that personality traits relate to the ecological niche that individuals occupy, such as differences among individuals in diet (Wilson et al., 1993) and space use (Pearish et al., 2013; Wilson & McLaughlin, 2007).
Second, we predicted that greater behavioural hypervolumes in katydids, froghoppers, or both, would decrease the survival of froghoppers due to interspecific competition. There is considerable evidence showing that individuals' personality scores relate to the strategies that animals use when interacting with other species (Belgrad & Griffen, 2016; Chang et al., 2017; DiRienzo et al., 2013; Webster et al., 2009). Therefore, we reasoned that behaviourally diverse groups would contain a greater diversity of these interaction strategies, thus causing them to interact with other species more frequently and intensely. Addressing these hypotheses will allow us to further evaluate the ecological consequences of behavioural hypervolumes and potentially extend the ecological niche concept to behavioural or personality ‘niches’.
METHODS
Collection Site and Study Organisms
We performed our experiments at the Donald S. Wood Field Laboratory (DSW) of the Pymatuning Laboratory of Ecology in June, July, and August 2016. The DSW is located in northwest Pennsylvania (41°34′09.6″N, 80°27′51.4″W). The property is composed of mixed forest and semiannually mowed old field. The old field portion is dominated by the goldenrod species Solidago canadensis, Solidago grandiflora and Solidago rugosa, in order of relative abundance. At our collection site, these species tend to form dense monospecific stands comprising hundreds or thousands of stems with a few lone stems scattered around (J. L. L. Lichtenstein & B. McEwen, personal observation). We collected roughly 1000 froghoppers, P. spumarius (Aphrophoridae), and approximately 400 third- to seventh-instar katydid nymphs from the genus Scudderia (Tettigoniidae) from a 40 × 60 m section of the old field via sweep netting. Philaenus spumarius are an invasive insect native to the Palearctic that has spread over the entire Nearctic and feeds on hundreds of plant species, including S. canadensis (Weaver & King, 1954). At an average length of 6 mm, froghoppers are much smaller than katydid nymphs but they are the longest and fastest-jumping insect species relative to body size yet measured, surpassing even fleas (Burrows, 2003). They reach densities of more than 15 individuals per host plant at our site (J. L. L. Lichtenstein & B. McEwen, personal observation). Katydid nymphs of the two species that occur at our site (Scudderia curvicauda and Scudderia furcata) are indistinguishable as nymphs and preferentially inhabit moist and poorly drained old field areas (Cantrall, 1943). We found both of these insects frequently in S. canadensis stands and observed them feeding on S. canadensis leaves in the laboratory and in situ. After we captured the insects, we kept each individual in a 50 ml tube (12 cm tall, 3 cm diameter) with an S. canadensis leaf. Aside from the insects we used for our personality repeatability trials described below, we did not keep insects in tubes for more than 24 h.
Behavioural Assays
To acquire behavioural hypervolumes, we ran our insects through three behavioural tests: (1) perch height (i.e. habitat use), (2) activity level and (3) boldness. We performed these tests in the same sequence for each individual before returning them to their home containers, with 2 h between tests of different behaviours. To assess the repeatability of these traits, we performed each behavioural assay on each individual once per day for four consecutive days on 13 froghoppers and 12 katydid nymphs. After establishing the repeatability of these behaviours, we concluded that our behavioural assays quantified temporally consistent personality traits in these species. This allowed us to run individuals in each behavioural assay only once for the mesocosm and space use studies (described below).
Perch height
To quantify an individual's perch height, we measured the height at which an individual perched in a novel 50 ml storage tube containing a single leaf of their host plant. We made sure that each tube had a leaf of a similar size (6–7 cm length). This species spends much of their time on goldenrod leaves, particularly near the crown of the plant. Therefore, we measured the specific location on leaves where they might spend most of their time. The trial was initiated by setting an individual at the bottom of a clean tube and then gently placing a leaf so that it extended up the side of the tube, with the stem facing downward. We then permitted the insects 30 min to climb their leaves and to select a site on which to settle and initiate feeding. Most insects ascended to the top of the container within 10–15 min and then settled at a lower point. After 30 min, we measured their height above the ground. For the repeatability trials, we changed each insect's leaf and container between consecutive trials. Height was measured to the 1 mm using digital callipers.
Activity level
Activity level was assessed by placing an individual insect at the bottom of a clean 50 ml tube. The 50 ml tube was placed at a 15° angle to the ground to instigate the insects' tendency to climb up the surface of the container. We then measured the time (in seconds) that each individual spent active (walking, climbing) over the next 5 min. Activity was quantified as the number of seconds during which an individual moved in the 5 min observation, with more active individuals moving for longer durations.
Boldness
To measure the boldness of individual insects, we placed each individual in a clean 700 ml container and allowed it to settle for 15 min. We then gently prodded the insects repeatedly on the head from the front with a toothpick until they jumped or until we reached 50 prods. We quantified boldness as the number of prods required to elicit a flight response. A value of 50 was given if we could not elicit a jump response after 50 prods. This happened for 10.4% of froghoppers and 42.5% of katydids. The rationale here is that bold individuals remain longer on their host plant despite being disturbed by an unknown competitor or predator, whereas shy individuals flee more quickly when disturbed.
Space Use Experiment
We focused on the effects of behavioural hypervolumes on space use in katydids because of the difficulty of visually tracking froghopper groups. To test our prediction that groups of katydids that occupy greater behavioural hypervolumes tend to cluster spatially (potentially indicative of lower competition), we placed groups of five katydids whose behaviour had previously been tested in all three behavioural assays, in 60 × 60 cm steel and mesh cages (1450DSV: BioQuip Products Inc., Compton, CA, U.S.A.) with five nonfocal froghoppers. The presence of froghoppers mimics the conditions that katydids experience in the wild and the context in which these species interact during our staged survival experiments. The cages were kept indoors and contained only a single potted S. canadensis stem. We evaluated the effects of behavioural hypervolumes on space use in a mixed-species situation to mimic the conditions of our survival studies (described below) and because these are the conditions that we most commonly observe these insects in situ. Around 1700 hours on the day before we recorded space use, we placed the insects in mesocosms. The next day, we recorded the spatial position of all individuals in the cage over the course of 8 h. We drew axes along the outside of the cages running between 0 cm and 60 cm, to create a three-dimensional grid at the resolution of 1 cm3 that allowed us to note the position of each individual every hour between 0900 and 1600 hours on the day after establishing the mesocosm. We computed the average distance between each of the katydids using the pdist function in MatLab (Mathworks, Natick, MA, U.S.A.) to obtain an average interindividual distance for each time point.
Survival Experiment
To test the effects of behavioural hypervolumes on species interaction outcomes, we observed froghopper survival in mesocosms (1450DSV: BioQuip Products Inc.) containing katydids, under two conditions: high and low froghopper density. This design allowed us to evaluate possible density-dependent effects of behavioural hypervolumes. Low froghopper density mesocosms contained 10 froghoppers and 10 katydids (N = 16) and high froghopper density mesocosms contained 20 froghoppers and 10 katydids (N = 16). No katydids were used in both the space use and survival experiments. Three weeks prior to initiating our mesocosm trials we transplanted a single goldenrod (S. canadensis) stem into patches of manicured lawn immediately adjacent to our old field site. The day before we deployed the mesocosms, we cut all the plants within 1 m of the stem to 6 cm in height to help ensure that all mesocosms had similar structural environments. The single goldenrod in each mesocosm was 45–55 cm tall at the start of the survival study. We placed one mesocosm of each froghopper density treatment on the ground every other day during the months of June and July and during the first week of August. This procedure resulted in 20 mesocosms being deployed at any given time. Mesocosm enclosures were deployed with detached bottom panels to allow placing them on the ground in the field around living stems. We then lined the bottom edges of the inside and outside of each mesocosm with commercial topsoil to prevent animals from escaping.
Once the mesocosms were prepared, we assembled treatment groups haphazardly using pools of insects that we tested behaviourally the day before, blind to their performance on behavioural tests. Ten days later, we harvested the insects by hand until we could no longer find insects and recorded the number of individuals that had disappeared. We assumed that insects that had gone missing during this time had perished, as opposed to having escaped. This assumption is anecdotally supported by the number of froghopper carcasses recovered at the bottom of each mesocosm. The harvesting process required approximately 1 h per mesocosm to ensure all were collected, although most insects were recovered in the first 15 min.
Statistical Methods
We first calculated the repeatability of each behavioural trait for each species. We did this using the rptR package (Nakagawa & Schielzeth, 2013) in R (version 3.3.1). This package fits GLMMs with ‘individual ID’ designated as a random factor, trial number as a fixed effect and individuals' performance on the test as the response variable. The proportion of variation explained by individual ID was used as our estimate of repeatability. Next, the package establishes confidence intervals for the data using a bootstrapping procedure, and the estimate was deemed to be significant if its 95% CI did not overlap with zero. Our bootstraps used 1000 iterations. We fitted both species' boldness with a Poisson distribution, because this distribution best fit our data for both species, as determined by examining Q–Q plots comparing our data with other distributions. Froghopper perch height and activity level did not conform to a normal distribution, even after log transformation, but they did fit a Poisson distribution, which was used as the link function in these analysis as well. Katydid perch height conformed to a normal distribution after log transformation and fitted this model well. Histograms of each of these personality traits can be found in Supplementary Fig. S1.
To calculate behavioural hypervolumes, we first scaled each of the three behavioural measures using a Z transformation, relative to all conspecifics. Then, we plotted each individual insect as a point in a three-dimensional behavioural space in which each dimension represented performance in one of our behavioural tests. The hypervolume was then calculated as the volume of the smallest possible convex polyhedron defined by a group of individuals of the same species. We calculated the behavioural hypervolume for each species in every space use and survival assessment replicate. We computed all hypervolume calculations using the convhulln function in MatLab. Examples of these volumes can be found in Fig. 1. We also compared the average hypervolume of the froghopper groups in the high- versus low-density treatments to test whether our density treatments confounded density with behavioural hypervolume, because larger groups may occupy larger behavioural hypervolumes by chance alone.
Figure 1.
Example behavioural hypervolume (BHV) pairs from four mesocosms in the ‘survival’ experiment. These behavioural hypervolumes represent the behavioural three-dimensional space that the individuals in each species occupied. Each dimension in this space is defined by performance on one of the three behavioural tests. Pairs (a) and (b) are examples where both species had relatively low hypervolumes, and pairs (c) and (d) are examples where both species had relatively high hypervolumes.
Because we tracked the position of insects hourly over the course of 8 h in the space use experiment, we first examined whether interindividual distances changed over time. Using the rptR package in R, we found that katydid groups were consistent in their interindividual distance across hourly observations in their space use trials (Supplementary Table S1).We therefore proceeded to use the average interindividual distance (IID) across all time points in subsequent analysis. Specifically, we used a normally distributed GLM with katydid average IID as a response variable and katydid behavioural hypervolume as a predictor variable. The model's residuals did not deviate significantly from a normal distribution (Shapiro–Wilk test: W= 0.943, P = 0.619).
To test the effects of behavioural hypervolumes on froghopper survival, we used a normally distributed GLM with the proportion of froghoppers that survived as our response variable and froghopper density treatment, froghopper hypervolumes, katydid hypervolume, and all possible interaction terms as predictor variables. These interaction terms allowed us to examine whether the hypervolumes of either or both species predicted froghopper survival. This model's residuals also did not deviate significantly from a normal distribution (Shapiro–Wilk test: W= 0.965, P = 0.348), once again suggesting that it was a good fit for the model. We ran an identical model for the katydids, the results of which can be found in Supplementary Table S2.We opted to exclude these results from the main text because of the extremely high survival of katydids.
We further evaluated the predictive power of behavioural hypervolumes against univariate metrics of personality (the average personality scores of each species) using the Akaike weight criterion. This model selection procedure selects for models with low AICc and high Akaike weights (Akaike, 1987; Burnham & Anderson, 2002). We performed this procedure separately for models predicting froghopper and katydid survival. We used JMP version 13.0 (SAS Institute, Cary, NC, U.S.A.) for these analyses, with the exception of our repeatability and behavioural hypervolume calculations.
RESULTS
Both insects ranged in perch height from 0.1 cm to 12 cm. Froghoppers perched at an average (±SE) height of 8.07 ± 0.10 cm, and katydids perched at 7.94 ± 0.15 cm. Froghoppers and katydids both spent 0–300 s moving, and froghoppers moved for an average of 50.14 ± 1.82 s and katydids moved for an average of 25.19 ± 1.88 s. Froghoppers moved after an average of 12.55 ± 0.57 pokes, and katydids moved after an average of 30.44 ± 1.16 pokes.
Hypervolumes
We obtained 32 froghopper behavioural hypervolumes and 32 katydid behavioural hypervolumes. Examples of both are shown in Fig. 1. The behavioural hypervolumes of froghoppers did not differ significantly between the high and low froghopper densities (t test: t32 = 1.77, P = 0.09), thus population density and behavioural hypervolume were not conflated in these experiments.
Repeatability Estimates
We found that both katydids and froghoppers were significantly repeatable in their perch height, activity level and boldness (Supplementary Table S1). As we mentioned above, we found that katydid groups were consistent in their interindividual distance across hourly observations in their space use trials (Supplementary Table S1).
Tendency to Associate
Katydid groups' average interindividual distances were negatively correlated with their behavioural hypervolumes (GLM: R2 = 0.660, likelihood ratio test: χ21 =9.709, P = 0.002; Fig. 2), meaning that katydid groups composed of behaviourally unlike individuals tended to remain closer together.
Figure 2.
The relationship between katydid (Scudderia nymphs) behavioural hypervolume and katydid interindividual distance. Line represents a best-fit linear regression.
Survival
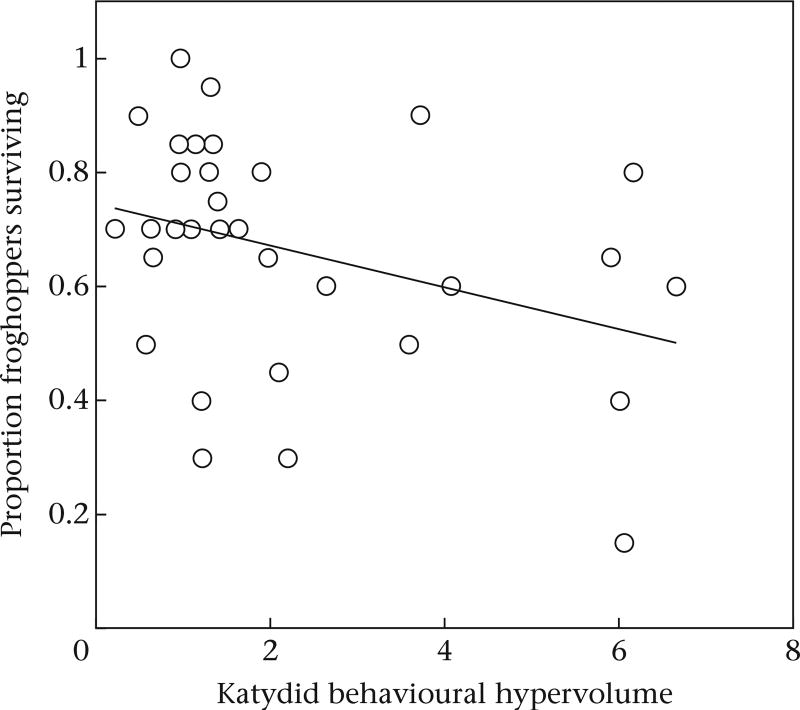
Froghopper survival was best explained by models using behavioural hypervolumes, which exhibited an Akaike weight (AICc = 0.16, AICc weight = 0.85) over nine times greater than the next best model (AICc = 4.6, AICc weight = 0.09) and others using the simple average of any one behaviour (Supplementary Table S3). Froghopper survival was related to the behavioural hypervolumes of both froghoppers and katydids, and was influenced by froghopper density. Our whole model predicting froghopper survival was statistically significant (Table 1), predicting over 35% of variation in froghopper survival. Katydid behavioural hypervolume was negatively correlated with froghopper survival (Table 1, Fig. 3), and we detected a significant katydid hypervolume*froghopper hypervolume interaction term (Table 1) and a significant katydid hypervolume*froghopper hypervolume*froghopper density interaction term (Table 1). Specifically, in high froghopper density mesocosms, more froghoppers survived when both the katydid and froghopper hypervolumes were small (Fig. 4). Examples of large hypervolumes and small hypervolumes like those that disfavoured or favoured froghopper survival (respectively) can be found in Fig.1.
Table 1.
Outputs of normally distributed GLMs predicting the survival of froghoppers
| Predictor variable | R2 | df | L-R χ2 | P |
|---|---|---|---|---|
| Whole model | 0.355 | 25 | 14.45 | 0.0437 |
| Density treatment | 1 | 0.77 | 0.3810 | |
| Froghopper behavioural hypervolume | 1 | 0.05 | 0.8200 | |
| Katydid behavioural hypervolume | 1 | 7.28 | 0.0070 | |
| Froghopper hypervolume*katydid hypervolume | 1 | 4.58 | 0.0324 | |
| Density treatment*froghopper hypervolume | 1 | 0.80 | 0.3714 | |
| Density treatment*katydid hypervolume | 1 | 2.75 | 0.0971 | |
| Density treatment*froghopper hypervolume*katydid hypervolume | 1 | 6.50 | 0.0108 |
L-R: likelihood ratio. The predictor variable column begins with the whole model output, followed by the effect tests of the predictor variables that comprise the whole model. Significant P values are in bold.
Figure 3.
The relationship between katydid (Scudderia nymphs) behavioural hypervolume and the proportion of froghoppers (Philaenus spumarius) that survived. Line represents a best-fit linear regression.
Figure 4.
The survival of froghoppers (Philaenus spumarius) as predicted by the interaction between froghopper behavioural hypervolume and katydid (Scudderia nymphs) behavioural hypervolume at (a) low and (b) high froghopper densities. Dark blue points represent high survival (100–80%), blue points represent mid-high survival (79–66%), light blue points represent mid-low survival (65–51%) and the lightest blue points represent low survival (50–15%).
DISCUSSION
Much prior work has related animal personality to various aspects of individuals' ecology (Johnson et al., 2015; Sih et al., 2012; Wolf & Weissing, 2012), such as their body condition (Johnson, Miles, Trubl, & Hagenmaier, 2014; Wright, Keiser, & Pruitt, 2016) and social niche (Laskowski & Pruitt, 2014), by examining only one personality trait at a time (Smith & Blumstein, 2008; Sweeney et al., 2013). Here we used a multitrait approach to examine whether the behavioural hypervolumes occupied by groups of individuals shaped both intraspecific and interspecific interactions. Consistent with our hypotheses, we found that the behavioural hypervolumes of katydid groups were negatively correlated with their average interindividual distance in staged mesocosms (Fig. 2), conveying that behaviourally diverse groups tolerate each other more. The hypervolumes of these insects had no effect on katydid survival, because few katydids perished. This suggests that the time frame of this experiment might have been too short to track the survival of these sturdy insects. Moreover, katydid hypervolumes were negatively correlated with the survival of an invasive froghopper (P. spumarius; Fig. 3). Froghopper survival was greatest when both froghopper groups and katydid groups exhibited small hypervolumes (Fig. 4), but these interactive effects appeared only under high froghopper density conditions. This suggests that both high density and large behavioural hypervolume each increase interaction intensity subtly on their own, but far more strongly together. Thus, groups with higher densities and large behavioural hypervolumes have more intense interspecific interactions. Had we considered only single behaviours, much variation in froghopper mortality would have gone unexplained (Supplementary Table S3). However, these behavioural measures might be associated with age or size, so population structure may play a further role in explaining our findings. Nevertheless, they hint that behavioural hypervolumes can be helpful for predicting the outcome of both intra- and interspecific interactions.
Considerable evidence suggests that animal personality can determine how individuals interact with conspecifics (Bergmüller & Taborsky, 2010; Kurvers et al., 2009; Pinter-Wollman, Keiser, Wollman, & Pruitt, 2016). Specifically, theoretical and empirical work on social heterosis has found that behavioural diversity improves the performance of social groups (Jandt et al., 2014; Modlmeier & Foitzik, 2011; Modlmeier, Liebmann, & Foitzik, 2012; Nonacs & Kapheim, 2007, 2008; Pruitt & Riechert, 2011). Our findings add to these lines of evidence by showing that katydid groups occupying greater hypervolumes in behavioural space (i.e. those composed of more behaviourally dissimilar individuals) tolerate each other's presence more and potentially even aggregate. It is possible that behaviourally dissimilar katydids only aggregate in the presence of froghoppers. However, katydids live in the presence of froghoppers across most of their geographical range, and thus, to consider the space use of katydids in the absence of froghoppers would be somewhat artificial and at odds with the conditions used for our survival experiments. Drawing evidence from studies linking animal personality with variation in individuals' ecological niches (Boyer, Réale, Marmet, Pisanu, & Chapuis, 2010; Wilson & McLaughlin, 2007; Wilson et al., 1993), we reason that katydids in groups with high behavioural hypervolumes may associate more closely with one another because of low competition. This is consistent with studies of social heterosis, showing that more behaviourally diverse social groups (Burns & Dyer, 2008; Dyer, Croft, Morrell, & Krause, 2009; Modlmeier & Foitzik, 2011; Modlmeier et al., 2012; Pruitt & Riechert, 2011), nonsocial groups (Pruitt et al., 2017), or even whole communities (Pruitt et al., 2016) compete with each other less and therefore exhibit enhanced collective success. Our study provides some evidence that behavioural hypervolumes can shape the outcome of intraspecific competition in territorial species, like katydids, and may have consequences for their population biology under natural conditions.
A growing number of studies has shown that animal personality can predict the outcome of interspecific interactions (Keiser, Slyder, Carson, & Pruitt, 2015; Lichtenstein, Pruitt, & Modlmeier, 2015; Royauté & Pruitt, 2015; Sweeney et al., 2013; Toscano & Griffen, 2014). We found that katydid behavioural hypervolume was negatively correlated with froghopper survival rates (Fig. 3), and that froghopper survival was highest when both species had low behavioural hypervolumes (Fig. 4). This is consistent with our hypothesis that larger behavioural hypervolumes could increase the intensity of the interaction between two competing species. We propose that this increased intensity of interactions might have accelerated the rate at which froghoppers starved to death. Furthermore, our finding that the effects of both species' behavioural hypervolumes on froghopper survival were more pronounced at higher froghopper densities suggests that these effects are density dependent. High densities of competing species typically increase the intensity of their interaction (Hairston, Smith, & Slobodkin, 1960). Because behavioural hypervolumes predict the outcome of species interactions (Fig. 3), behavioural hypervolumes should be more important when the interactions are more intense (Fig. 4). This result does not help us to determine whether intraspecific competition is stronger than interspecific competition (or vice versa). Rather, it suggests that increased intraspecific competition intensifies the signature of interspecific competition. Taken together, these findings illustrate the potential for behavioural hypervolumes to predict the outcome of species interactions, even when single behaviours do not.
Conclusions
We found that behavioural hypervolumes can help predict the outcomes of katydid interactions with conspecifics and heterospecific invaders. Furthermore, models using behavioural hypervolumes outperformed those using univariate personality traits by a factor of nine (Supplementary Table S3). Multiple behavioural traits predict more variation in mortality than single behavioural traits. This adds to the growing body of evidence that animal personality can predict the outcome of species interactions (DiRienzo et al., 2013; Keiser & Pruitt, 2013; Pruitt & Ferrari, 2011; Webster et al., 2009). However, the present study builds on these prior studies by (1) adopting an extrema-based multivariate approach, which has been useful in general ecology, and (2) considering how the behavioural hypervolumes of two interacting species can jointly help to predict the outcome of their interaction (Fig. 4). This expands upon prior studies that used behavioural hypervolumes of only a single species or that lumped multiple species into one volume as a predictive metric. After all, species interactions by definition transpire between populations, thus, we reason that the predictability of most interactions will be enhanced by considering the traits of both (or all) species involved in the interaction module. Behavioural hypervolumes and related trait-based ecology methods therefore provide us with yet another interesting set of tools for predicting system-level dynamics. We suspect that behavioural hypervolumes may shape the outcome of numerous other ecological processes, and we hope our results will serve to inspire others to evaluate these relationships.
Supplementary Material
Acknowledgments
Funding for this research was provided by the University of Pittsburgh, the Pape foundation, the McKinley foundation, the University of California (Santa Barbara), National Science Foundation grant awards to J.N.P. (1352705 and 1455895) and N.P.W. (1456010), and a National Institutes of Health grant awarded to J.N.P. and N.P.W. (R01GM115509). Also, we thank the Pymatuning Laboratory of Ecology for hosting our research, particularly Dr Cori Richards-Zawacki and Chris Davis.
Footnotes
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.anbehav.2017.08.010.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- Belgrad BA, Griffen BD. Predator–prey interactions mediated by prey personality and predator hunting mode. Proceedings of the Royal Society B: Biological Sciences. 2016;283:20152963. doi: 10.1098/rspb.2016.0408. http://dx.doi.org/10.1098/rspb.2015.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmüller R, Taborsky M. Animal personality due to social niche specialisation. Trends in Ecology & Evolution. 2010;25(9):504–511. doi: 10.1016/j.tree.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Boyer N, Réale D, Marmet J, Pisanu B, Chapuis JL. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. Journal of Animal Ecology. 2010;79(3):538–547. doi: 10.1111/j.1365-2656.2010.01659.x. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. 2. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- Burns JG, Dyer AG. Diversity of speed–accuracy strategies benefits social insects. Current Biology. 2008;18(20):R953–R954. doi: 10.1016/j.cub.2008.08.028. [DOI] [PubMed] [Google Scholar]
- Burrows M. Biomechanics: Froghopper insects leap to new heights. Nature. 2003;424(6948):509–509. doi: 10.1038/424509a. [DOI] [PubMed] [Google Scholar]
- Cantrall IJ. The ecology of the Orthoptera and Dermaptera of the George Reserve, Michigan. Ann Arbor, MI: University of Michigan Press; 1943. [Google Scholar]
- Chang C-C, Teo HY, Norma-Rashid Y, Li D. Predator personality and prey behavioural predictability jointly determine foraging performance. Scientific Reports. 2017;7:40734. doi: 10.1038/srep40734. http://dx.doi.org/10.1038/srep40734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo N, Pruitt JN, Hedrick AV. The combined behavioural tendencies of predator and prey mediate the outcome of their interaction. Animal Behaviour. 2013;86:317–322. [Google Scholar]
- Dyer JR, Croft DP, Morrell LJ, Krause J. Shoal composition determines foraging success in the guppy. Behavioral Ecology. 2009;20(1):165–171. [Google Scholar]
- Gosling SD. From mice to men: What can we learn about personality from animal research? Psychological Bulletin. 2001;127(1):45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. American Naturalist. 1960;94(879):421–425. [Google Scholar]
- Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, et al. Behavioural syndromes and social insects: Personality at multiple levels. Biological Reviews. 2014;89(1):48–67. doi: 10.1111/brv.12042. [DOI] [PubMed] [Google Scholar]
- Johnson JC, Halpin R, Stevens D, Vannan A, Lam J, Bratsch K. Individual variation in ballooning dispersal by black widow spiderlings: The effects of family and social rearing. Current Zoology. 2015;61(3):520–528. [Google Scholar]
- Johnson JC, Miles LS, Trubl PJ, Hagenmaier A. Maternal effects on egg investment and offspring performance in black widow spiders. Animal Behaviour. 2014;91:67–73. [Google Scholar]
- Keiser CN, Pruitt JN. Spider aggressiveness determines the bidirectional consequences of host–inquiline interactions. Behavioral Ecology. 2013;25(1):142–151. http://dx.doi.org/10.1093/beheco/art096. [Google Scholar]
- Keiser CN, Slyder JB, Carson WP, Pruitt JN. Individual differences in predators but not producers mediate the magnitude of a trophic cascade. Arthropod–Plant Interactions. 2015;9(3):225–232. [Google Scholar]
- Kralj-Fišer S, Schuett W. Studying personality variation in invertebrates: Why bother? Animal Behaviour. 2014;91:41–52. [Google Scholar]
- Kurvers RH, Eijkelenkamp B, van Oers K, van Lith B, van Wieren SE, Ydenberg RC, et al. Personality differences explain leadership in barnacle geese. Animal Behaviour. 2009;78:447–453. [Google Scholar]
- Laskowski KL, Bell AM. Competition avoidance drives individual differences in response to a changing food resource in sticklebacks. Ecology Letters. 2013;16(6):746–753. doi: 10.1111/ele.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski KL, Pruitt JN. Evidence of social niche construction: Persistent and repeated social interactions generate stronger personalities in a social spider. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1783):20133166. doi: 10.1098/rspb.2013.3166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lichtenstein JL, Pruitt JN, Modlmeier AP. Intraspecific variation in collective behaviors drives interspecific contests in acorn ants. Behavioral Ecology. 2015;27(2):553–559. http://dx.doi.org/10.1093/beheco/arv188. [Google Scholar]
- Lichtenstein JL, Wright CM, Luscuskie LP, Montgomery GA, Pinter-Wollman N, Pruitt JN. Participation in cooperative prey capture and the benefits gained from it are associated with individual personality. Current Zoology. 2016 doi: 10.1093/cz/zow097. http://dx.doi.org/10.1093/cz/zow097. Advance online Publication. [DOI] [PMC free article] [PubMed]
- Meyer GA. A comparison of the impacts of leaf-and sap-feeding insects on growth and allocation of goldenrod. Ecology. 1993;74(4):1101–1116. [Google Scholar]
- Meyer GA, Root RB. Effects of herbivorous insects and soil fertility on reproduction of goldenrod. Ecology. 1993;74(4):1117–1128. [Google Scholar]
- Modlmeier AP, Foitzik S. Productivity increases with variation in aggression among group members in Temnothorax ants. Behavioral Ecology. 2011;22(5):1026–1032. http://dx.doi.org/10.1093/beheco/arr086. [Google Scholar]
- Modlmeier AP, Liebmann JE, Foitzik S. Diverse societies are more productive: A lesson from ants. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1736):2142–2150. doi: 10.1098/rspb.2011.2376. http://dx.doi.org/10.1098/rspb.2011.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4(2):133–142. [Google Scholar]
- Nonacs P, Kapheim K. Social heterosis and the maintenance of genetic diversity. Journal of Evolutionary Biology. 2007;20(6):2253–2265. doi: 10.1111/j.1420-9101.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Nonacs P, Kapheim K. Social heterosis and the maintenance of genetic diversity at the genome level. Journal of Evolutionary Biology. 2008;21(2):631–635. doi: 10.1111/j.1420-9101.2007.01418.x. [DOI] [PubMed] [Google Scholar]
- Pearish S, Hostert L, Bell AM. Behavioral type–environment correlations in the field: A study of three-spined stickleback. Behavioral Ecology and Sociobiology. 2013;67(5):765–774. doi: 10.1007/s00265-013-1500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, Keiser CN, Wollman R, Pruitt JN. The effect of keystone individuals on collective outcomes can be mediated through interactions or behavioral persistence. American Naturalist. 2016;188(2):240–252. doi: 10.1086/687235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, Bolnick DI, Sih A, DiRienzo N, Pinter-Wollman N. Behavioural hypervolumes of spider communities predict community performance and disbandment. Proceedings of the Royal Society B: Biological Sciences. 2016;283(1844):20161409. doi: 10.1098/rspb.2016.1409. http://dx.doi.org/10.1098/rspb.2016.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, Ferrari MC. Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology. 2011;92(10):1902–1908. doi: 10.1890/11-0701.1. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Howell KA, Gladney SJ, Yang Y, Lichtenstein JL, Spicer ME, et al. Behavioral hypervolumes of predator groups and predator–predator interactions shape prey survival rates and selection on prey behavior. American Naturalist. 2017;189(3):254–266. doi: 10.1086/690292. http://dx.doi.org/10.1086/690292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, Riechert SE. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proceedings of the Royal Society B: Biological Sciences. 2011;278(1709):1209–1215. doi: 10.1098/rspb.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root RB, Cappuccino N. Patterns in population change and the organization of the insect community associated with goldenrod. Ecological Monographs. 1992;62(3):393–420. [Google Scholar]
- Royauté R, Pruitt JN. Varying predator personalities generates contrasting prey communities in an agroecosystem. Ecology. 2015;96(11):2902–2911. doi: 10.1890/14-2424.1. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: An ecological and evolutionary overview. Trends in Ecology & Evolution. 2004;19(7):372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A, Cote J, Evans M, Fogarty S, Pruitt J. Ecological implications of behavioural syndromes. Ecology Letters. 2012;15(3):278–289. doi: 10.1111/j.1461-0248.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: A meta-analysis. Behavioral Ecology. 2008;19(2):448–455. [Google Scholar]
- Sweeney K, Cusack B, Armagost F, O'Brien T, Keiser CN, Pruitt JN. Predator and prey activity levels jointly influence the outcome of long-term foraging bouts. Behavioral Ecology. 2013;24(5):1205–1210. doi: 10.1093/beheco/art052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano BJ, Griffen BD. Trait-mediated functional responses: Predator behavioural type mediates prey consumption. Journal of Animal Ecology. 2014;83(6):1469–1477. doi: 10.1111/1365-2656.12236. [DOI] [PubMed] [Google Scholar]
- Weaver CR, King D. Meadow spittlebug, Philaenus leucophthalmus (L.) Wooster, OH: Ohio Agricultural Experiment Station; 1954. [Google Scholar]
- Webster M, Ward A, Hart P. Individual boldness affects interspecific interactions in sticklebacks. Behavioral Ecology and Sociobiology. 2009;63(4):511–520. [Google Scholar]
- Wilson DS, Coleman K, Clark AB, Biederman L. Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus): An ecological study of a psychological trait. Journal of Comparative Psychology. 1993;107(3):250–260. [Google Scholar]
- Wilson AD, McLaughlin RL. Behavioural syndromes in brook charr, Salvelinus fontinalis: Prey-search in the field corresponds with space use in novel laboratory situations. Animal Behaviour. 2007;74:689–698. [Google Scholar]
- Wolf M, Weissing FJ. Animal personalities: Consequences for ecology and evolution. Trends in Ecology & Evolution. 2012;27(8):452–461. doi: 10.1016/j.tree.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Wright CM, Holbrook CT, Pruitt JN. Animal personality aligns task specialization and task proficiency in a spider society. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9533–9537. doi: 10.1073/pnas.1400850111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wright CM, Keiser CN, Pruitt JN. Colony personality composition alters colony-level plasticity and magnitude of defensive behaviour in a social spider. Animal Behaviour. 2016;115:175–183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.