Summary
Epstein-Barr virus (EBV) is a causative agent of infectious mononucleosis and is associated with 200,000 new cases of cancer and 140,000 deaths annually. Subunit vaccines against this pathogen have focused on the gp350 glycoprotein and remain unsuccessful. We isolated human antibodies recognizing the EBV fusion machinery (gH/gL and gB) from rare memory B cells. One anti-gH/gL antibody, AMMO1, potently neutralized infection of B cells and epithelial cells; the two major cell types targeted by EBV. We determined a cryo-electron microscopy reconstruction of the gH/gL-gp42-AMMO1 complex and demonstrated that AMMO1 bound to a discontinuous epitope formed by both gH and gL at the Domain-I/Domain-II interface. Integrating structural, biochemical and infectivity data, we propose that AMMO1 inhibits fusion of the viral and cellular membranes. This work identifies a crucial epitope that may aid in the design of next-generation subunit vaccines against this major public health burden.
eTOC Blurb
Epstein-Barr virus is a cancer-associated pathogen for which there is no vaccine. Snijder et al isolate a monoclonal antibody that neutralizes infection of the major cell types infected by EBV. Structural analysis of the antibody-gH/gL glycoprotein complex reveals a key site of EBV vulnerability that may pave the way for a next-generation EBV vaccine.

Introduction
Epstein-Barr virus (EBV) infects the majority of adults worldwide. Although most primary infections are asymptomatic, EBV is a causative agent of infectious mononucleosis in children and young adults, and is associated with numerous hematopoietic and epithelial cell cancers (Cohen et al., 2011; Young and Rickinson, 2004). EBV also causes lymphoproliferative disorders in immunocompromised patients such as those with HIV/AIDS or in patients undergoing immune suppression for organ transplantation (Taylor et al., 2015). Thus, a vaccine that prevents EBV infection would be of major public health benefit (Cohen et al., 2011). EBV targets B cells and epithelial cells during primary infection. Host cell entry is a complex process mediated by several viral glycoproteins that define tropism and mediate membrane fusion. Three virally encoded surface glycoproteins, gH, gL and gB, share a conserved function among herpesviruses and are required for EBV infection (Connolly et al., 2011).
gB is a type III transmembrane fusion protein that promotes merger of the viral and host membranes (Backovic et al., 2009). gB activity is dependent upon the heterodimeric gH/gL complex which acts as an adaptor that triggers gB-mediated fusion upon binding a cell-surface receptor on target cells (Mohl et al., 2016; Stampfer and Heldwein, 2013).
gH/gL assumes an elongated structure comprised of four distinct domains designated D-I to D-IV. D-I is formed by gL and the N-terminus of gH, whereas the rest of gH comprises D-II through D-IV (Matsuura et al., 2010). D-I and D-II are separated by a prominent groove connected by a linker helix (Matsuura et al., 2010). Mutations that affect membrane fusion have been identified throughout gH/gL, but most map to D-I and the DI/D-II interface, including the linker helix and the groove between D-I and D-II (Chen et al., 2013a; Mohl et al., 2014; Omerovic et al., 2005; Plate et al., 2011; Sathiyamoorthy et al., 2016; Wu et al., 2005), indicating that this region of gH/gL is important for gB activation.
αvβ5, αvβ6, or αvβ8 integrins (Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009), and the ephrin receptor A2 (EphA2) (Chen et al., 2018; Zhang et al., 2018) have been identified as epithelial cell surface receptors that interact directly with gH/gL to trigger gB-mediated fusion. An exposed Lys-Gly-Asp (KGD) motif on D-II has been proposed to mediate gH/gL binding to integrins (Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009), while the binding site of EphA2 on gH/gL is unknown.
B cell infection requires an additional viral glycoprotein, gp42, which forms a 1:1 complex with gH/gL (Kirschner et al., 2006). The N-terminus of gp42 mediates high-affinity interactions with gH/gL and the C-terminus binds to the B chain of human leukocyte antigen (HLA) class II which leads to triggering of gB-mediated fusion through the gH/gL-gp42 complex (Haan et al., 2000; Sathiyamoorthy et al., 2014; Spriggs et al., 1996). Although gp42 is necessary for B cell infection, it inhibits epithelial cell infection (Kirschner et al., 2007; Kirschner et al., 2006; Wang et al., 1998). Virions produced in B cells contain lower levels of gp42 than virions produced in epithelial cells. Thus, virions that shed from one cell type preferentially infect the other (Borza and Hutt-Fletcher, 2002).
gp350 is the most abundant EBV surface glycoprotein (Edson and Thorley-Lawson, 1981). It promotes viral attachment to target cells through a high affinity interaction with CD21 (Tanner et al., 1987) or CD35 (Ogembo et al., 2013) without mediating fusion. Anti-gp350 antibodies can inhibit B cell infection in vitro, but some enhance infection of CD21-negative epithelial cells (Turk et al., 2006).
Sera from EBV-infected individuals can neutralize both B cell and epithelial cell infection in vitro (Miller et al., 1972; Moss and Pope, 1972; Sashihara et al., 2009; Tugizov et al., 2003). However, the antigens and epitope specificities targeted by the corresponding neutralizing antibodies are not known. To date, only a handful of anti-EBV neutralizing monoclonal antibodies (MAbs) have been characterized, all of murine origin. The 72A1 MAb binds to the CD21/CD35 binding site on gp350 (Ogembo et al., 2013; Tanner et al., 1987) and potently neutralizes B cell infection (Hoffman et al., 1980; Sashihara et al., 2009), but has no effect on infection of CD21-negative epithelial cells (Molesworth et al., 2000; Tugizov et al., 2003). The C1 MAb binds to an unknown epitope on gp350 and inhibits B cell infection, but promotes epithelial cell infection (Thorley-Lawson and Geilinger, 1980; Turk et al., 2006). F-2-1 targets gp42 and inhibits B cell but not epithelial cell infection (Chesnokova and Hutt-Fletcher, 2011; Li et al., 1995; Molesworth et al., 2000; Strnad et al., 1982). CL55 is the only EBV gB-specific MAb and it is non-neutralizing (Chesnokova and Hutt-Fletcher, 2011; Wu et al., 2005).
E1D1, CL59, and CL40 bind gH/gL and neutralize epithelial cell infection but fail to efficiently block B cell infection (Balachandran et al., 1987; Chesnokova and Hutt-Fletcher, 2011; Li et al., 1995; Molesworth et al., 2000). Mutagenesis and negative-stain electron microscopy (EM) studies have mapped the CL59 epitope to gH/gL D-IV (Sathiyamoorthy et al., 2017; Wu et al., 2005). High-resolution structures demonstrate that E1D1 binds exclusively to gL (Sathiyamoorthy et al., 2016), and that CL40 binds to an epitope at the D-II/D-III interface of gH (Sathiyamoorthy et al., 2017), but the mechanisms by which they neutralize EBV infection have not been elucidated.
The isolation and structural characterization of neutralizing MAbs elicited during natural human infections with human immunodeficiency virus (HIV), influenza virus, respiratory syncytial virus, human cytomegalovirus (CMV) and dengue virus have defined critical epitopes on these pathogens and advanced vaccine design (Rappuoli et al., 2016). To better characterize the humoral immune response to EBV, we sought to isolate antigen-specific memory B cells from EBV-infected individuals. We obtained several anti-gB antibodies one of which neutralized epithelial cell infection. We also isolated one anti-gH/gL antibody, called AMMO1 that potently neutralized both epithelial and B cell infection in vitro. Using a combination of cryo-electron microscopy (cyroEM) and X-ray crystallography we determined the structure of the gH/gL-gp42-AMMO1 complex. This analysis demonstrated that AMMO1 binds to a discontinuous epitope on gH/gL at the interface between D-I and D-II, which is implicated in triggering gB-mediated fusion.
The isolation and characterization of AMMO1 described herein may help pave the way for the design of a gH/gL-based subunit vaccine. The dual-tropic inhibition of EBV entry by AMMO1 indicates that this MAb could also have therapeutic potential in blocking EBV infection, reactivation, and amplification in immunocompromised individuals and transplant recipients.
RESULTS
Anti-EBV human monoclonal antibodies isolated from infected individuals
We sought to isolate memory B cells specific for gp42, gp350, gH/gL and gB using recombinant ectodomain glycoproteins as bait. All glycoproteins were recognized by known anti-EBV MAbs and serum antibodies from 15 out of 16 donors indicating that they were properly folded and exhibited native antigenicity (Fig. S1).
We used phycoerythrin (PE) conjugated EBV glycoproteins to identify antigen-specific class-switched B cells. The majority stained positive with gp350 or gp42 (Fig. 1A) due to their ability to bind CD21 (Tanner et al., 1987) and CD35 (Ogembo et al., 2013) or MHC class II (Haan et al., 2000; Spriggs et al., 1996), respectively. Attempts to completely block non-specific gp350 and gp42 binding to these receptors with commercially available antibodies were unsuccessful and we did not pursue further efforts to sort these B cells.
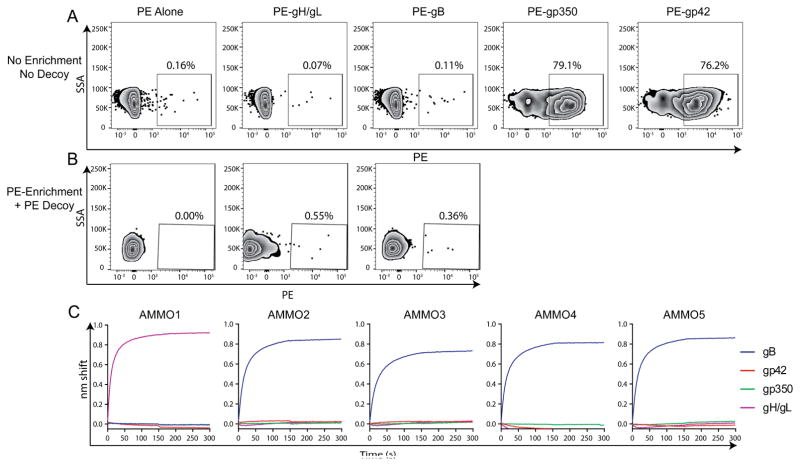
Figure 1. Isolation of anti-EBV antibodies from antigen specific B cells.
(A) Class switched B cells were stained with SA-PE, or SA-PE conjugated to gH/gL, gB, gp350, or gp42 as indicated. (B) Peripheral blood mononuclear cells were stained with a decoy protein conjugated to SA-PE-DL650 and SA-PE alone, or SA-PE conjugated to gH/gL or gB as indicated. A positive magnetic enrichment using anti-PE microbeads was performed and then cells were stained as in A. PE-DL-650−/PE+ class switched B cells are shown. Numbers indicate the % of PE+ class switched B cells in each panel in A and B. (C) Binding of the AMMO1, AMMO2, AMMO3, AMMO4, and AMMO5, antibodies cloned from B cells sorted using the approach in B to gB, gp42, gp350 and gH/gL by BLI as indicated. SA-PE: streptavidin-phycoerythrin SSA: side-scatter area in A and B. See also Figures S2 and S3.
B cell staining with gH/gL or gB was comparable to that with the unconjugated PE control (Fig. 1A). This observation indicated that gH/gL- or gB-specific B cells are quite rare, and led us to use complimentary approaches to isolate them. We employed a magnetic bead-based enrichment coupled with fluorescent decoy protein (Taylor et al., 2012), which reduced background staining and allowed us to more confidently identify gH/gL- and gB-specific B cells (Fig. 1A). Sorting was carried out starting with a total of 8×108 cryopreserved peripheral blood mononuclear cells from three separate donors (Table S1). B cells were individually sorted into irradiated feeder cell cultures in the presence of recombinant cytokines. The supernatants were subsequently screened for antigen reactivity by ELISA. Despite the implementation of an enrichment step, most sorted cells were false positives and we identified only two gH/gL- and seven gB-positive wells (Table S1). From these, we successfully recovered paired variable heavy (VH) and variable light (VL) chain transcripts for one gH/gL antibody (AMMO1) and four gB-specific antibodies (AMMO2-AMMO5). All were derived from distinct heavy and light chain genes, and displayed VH mutation frequencies ranging from 8–10% and VK/VL frequencies ranging from ~5–8% at the nucleotide level (Fig. S3). We cloned the VH/VL sequences into recombinant expression vectors and verified by biolayer interferometry (BLI) that AMMO1 specifically bound to gH/gL, and that AMMO2-AMMO5 specifically bound to gB (Fig. 1C). These results demonstrate that we have isolated human MAbs that target the EBV fusion machinery.
AMMO1 inhibited EBV infection of both epithelial and B cells
We assessed the ability of AMMO1-AMMO5 to neutralize EBV infection of epithelial cells (Fig. 2A) and B cells (Fig. 2B). For comparison, we also included the murine anti-gH/gL MAbs CL40, CL59 and E1D1, which block epithelial cell infection but poorly inhibit B-cell infection (Chesnokova and Hutt-Fletcher, 2011; Wu et al., 2005). AMMO1, CL40 and CL59 had comparable potency in an epithelial cell infection assay, whereas E1D1 displayed incomplete neutralization at the highest concentration tested (Fig. 2A and Table S2). Among the anti-gB MAbs AMMO5 neutralized EBV infection of epithelial cells, while AMMO2-AMMO4 were non-neutralizing (Fig. 2A and Table S2).
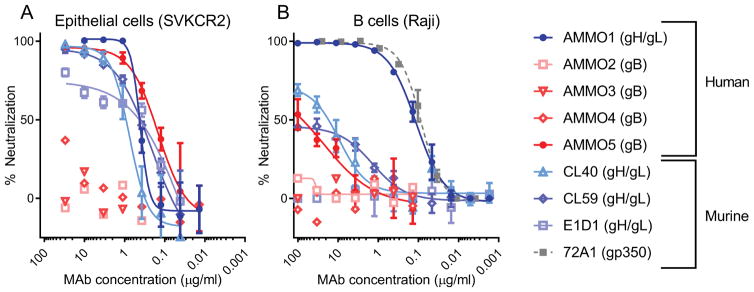
Figure 2. Isolated MAb neutralizes EBV infection in epithelial cells and B cells.
Serial dilutions of the indicated antibodies were evaluated for their ability to neutralize (A) AKTA-GFP EBV infection of epithelial (SVKCR2) cells or (B) B95.8/F EBV infection of B (Raji) cells. Anti-gH/gL antibodies are shown in shades of blue, anti-gB antibodies are shown in shades of red, and the anti-gp350 antibody (72A1) is shown in gray in B. The human or murine origins of the antibodies are indicated in the legend. Data points are mean ± SD of duplicate wells. Representative curves from 2 to 6 replicates are shown.
AMMO1 was the only anti-gH/gL MAb that completely neutralized B cell infection; it displayed comparable potency to the anti-gp350 MAb 72A1 (Fig. 2B and Table S2). Despite not being completely neutralizing, CL40 was able to reduce B cell infection to 50%, however it was ~100 times less potent than AMMO1 (Fig. 2B and Table S2). CL59 did not reproducibly achieve 50% neutralization, and E1D1 was completely ineffective at neutralizing B cell infection (Fig. 2B). These results demonstrate that AMMO5 can prevent EBV infection of epithelial but not B cells, while AMMO1 can prevent infection of both cell types.
AMMO1 recognized a unique discontinuous epitope on gH/gL
To understand observed differences in the neutralization potency of AMMO1 and other anti-gH/gL MAbs, we determined their binding affinities for gH/gL. AMMO1 bound 1–2 orders of magnitude more tightly to gH/gL than other MAbs tested (Table S3). Although membrane fusion with Raji B cells can occur independently of endocytosis (Miller and Hutt-Fletcher, 1992), we measured binding at pH 5.0 to assess whether differences in the neutralization potency of B cell infection might result from exposure to the acidic endosomal compartment which could affect antibody binding. Only E1D1 binding was affected by pH (Table S3). Therefore, the higher potency of AMMO1 could not be attributed to dissociation of the other MAbs from gH/gL due to exposure to the low endosomal pH upon virion internalization.
Based on these results, we surmised that epitope differences may explain why AMMO1 is the only gH/gL MAb that completely neutralized infection of both B cells and epithelial cells. To delineate the AMMO1 epitope, we analyzed the gH/gL-gp42-AMMO1 complex by cryoEM (Fig. 3A–B, Fig. S4, and Table S4). We determined a reconstruction of the complex at 4.8Å resolution, which resolved secondary structural elements and several amino acid side chains as well as densities corresponding to N-linked glycans on gH, gL and gp42. The local resolution varied between ~4Å in the region of the map corresponding to the gH core and 4.5–6.0Å for gL, gp42 and AMMO1 (Fig. S4). We also determined a 1.6Å crystal structure of the unliganded AMMO1 antigen binding fragment (Fab, Table S5) and generated a model of the gH/gL-gp42-AMMO1 complex using Rosetta (DiMaio et al., 2015) and available EBV glycoprotein structures (Matsuura et al., 2010; Sathiyamoorthy et al., 2016) (Fig. 3C–D and Table S4). The architecture of gH/gL-gp42 in the complex is in good agreement with the gH/gL-gp42-E1D1 crystal structure (Cα root mean square deviation of 1.1Å over 915 aligned residues of gH/gL-gp42) (Sathiyamoorthy et al., 2016).
Figure 3. AMMO1 targets a unique discontinuous epitope on gH/gL.
(A) CryoEM reconstruction of gH/gL-gp42-AMMO1 complex at 4.8 Å resolution. (B) 90° rotation from A: AMMO1 heavy chain is shown in dark purple, AMMO1 light chain in light purple, gL in light blue, gH D-I in dark blue, gH D-II in wheat, gH D-III in green, gH D-IV in yellow and gp42 in cherry. (C–D) Ribbon diagram of the gH/gL/gp42-AMMO1 atomic model rendered with the same colors as panels A and B. (E) Zoomed-in view of the AMMO1 epitope with regions of interest labeled. (F) AMMO1 footprint on the gH/gL-gp42 complex. Only the AMMO1 CDR loops are shown interacting with gH/gL-gp42 rendered in surface representation. Residues that have been identified as being important for AMMO1 binding (K73 and Y76) are shown in dark red. (G) The gH N60 glycan is shown in stick representation in the corresponding region of cryoEM density to highlight the putative contacts made with AMMO1. The star shows the position of the gH KGD motif. See Figures S4 and S5.
AMMO1 bound to a discontinuous epitope spanning gH/gL D-I and D-II, which comprised residues of both gH and gL, burying a surface area of 1160Å2 (Fig. 3E–F). CDRL2, CDRH1 and CDRH3 contacted the 2α-4 helix (Fig. 3E–F). CDRH3 also bound the short helix between the 2β-7 and 2β-8 strands, and the loop between 2α-1 and 2β-1. CDRL1 bound to the gH/gL interface, contacting the gH 2α-1 linker helix between D-I/D-II, and the gL C-terminal Lα-3 helix (Fig. 3E–F).
We introduced several mutations in gH and gL at residues which were predicted to contact AMMO1, and assessed binding of the MAb to cell surface expressed gH/gL (Fig. S5). When mutated to alanine, two residues, K73 and Y76 in the 2α-1 linker helix led to a reduction in AMMO1 binding (Fig. S5D and Fig. 3F). Substitution of K73 with a bulkier tryptophan residue virtually abrogated AMMO1 binding (Fig. S5D), as predicted by the atomic model.
The N-linked glycan on gH-N60 is oriented such that it could putatively contact the AMMO1 CDRL2 and framework region (Fig. 3G). We profiled N-linked glycosylation in gH, gL and gp42 by mass-spectrometry, which verified the presence of various glycans at gH-N60 (Fig. S4H and Data S1). A gH-T62A substitution which disrupts the N60 glycosylation sequon, altered AMMO1 binding kinetics, which indicated that the glycan could interact with the antibody (Table S3). The first residue of the AMMO1 heavy chain, a pyroglutamic acid, appeared to directly interact with the gp42 C-domain (CTD). Substitution of this residue by an asparagine did not substantially affect the neutralization potency of AMMO1 (Fig. S6) or its affinity for gH/gL (Table S3).
Our cryoEM structure indicated that the AMMO1 epitope was distinct from that of E1D1 and CL59, which bind exclusively to gL and to D-IV, respectively (Sathiyamoorthy et al., 2016; Sathiyamoorthy et al., 2017). Comparison of the gH/gL-gp42-AMMO1 cryoEM structure with the gH/gL-gp42-N-terminal domain-CL40 crystal structure (Sathiyamoorthy et al., 2017), showed not only that the epitopes of the two antibodies overlap at the 2α-4 helix, but also that their Fab fragments would clash upon binding to gH/gL (Fig. 4A–C). To confirm this observation and assess whether AMMO1 affects the binding of other anti-gH/gL MAbs, we performed reciprocal competitive binding experiments using BLI. In agreement with the structural data, we observed reduced binding of immobilized AMMO1 to gH/gL when an excess of soluble CL40 was present (Fig. 4D, green trace) and complete inhibition of CL40 binding to gH/gL in the presence of an excess of AMMO1 (Fig. 4E red trace). We detected an increase in the nm shift for the other combinations of gH/gL-MAbs consistent with the formation of higher-molecular weight complexes (Fig. 4F and G). Collectively these results demonstrate that AMMO1 binds to a unique epitope on gH/gL that is distinct from that of CL59 and E1D1 but partially overlaps with the CL40 epitope.
Figure 4. AMMO1 and CL40 share partially overlapping epitopes.
Ribbon diagrams of the gH/gL/gp42-AMMO1 (A) and gH/gL/gp42-CL40 complexes (B, PDB 5W0K). gp42 is omitted from A and B for clarity. (C) gH/gL residues experiencing a change in accessible surface area upon AMMO1 or CL40 binding are colored purple, or light pink, respectively. Areas that experience a change in accessible surface area upon binding of either AMMO1 or CL40 are shown in dark pink. The binding of AMMO1 (D), CL40 (E), CL59 (F), or E1D1 (G) to gH/gL alone, or gH/gL pre-complexed with the indicated antibodies were measured by BLI. BLI traces from one experimental replicate are shown in D–G.
A clash between the gp42 N173 glycan and AMMO1 can displace the gp42 CTD
CL40 was reported to displace the gp42 CTD upon binding to the gH/gL-gp42 complex as a consequence of the overlap of their interaction sites on gH/gL (Sathiyamoorthy et al., 2017). Although the footprints of AMMO1 and gp42 are distinct, gH residue N240 experienced a change of accessible surface area upon binding to either of these two proteins (Fig. 5A). Since AMMO1 and gp42 are located on opposite sides of this residue, their interactions with N240 do not appear mutually exclusive (Fig. 5B). Using a distinct subset of particles identified by three-dimensional classification of the cryoEM dataset, we obtained a second reconstruction of the gH/gL-gp42-AMMO1 complex at 10 Å resolution in which the gp42 C-domain density is displaced relative to gH/gL (Fig. 5C–D and S4G).
Figure 5. An AMMO1-gp42 N173 glycan clash displaces the gp42 CTD.
(A) gH/gL residues that experience a change in accessible surface area upon AMMO1 binding are colored light purple, those that experience a change in accessible surface area upon gp42 binding are colored cherry. Asn 240 (colored in magenta) experiences a change in accessible surface area upon binding to AMMO1 and to gp42. (B) Zoomed-in view of the region around gH Asn 240. Ribbons are rendered with the same color scheme as in Figure 3 and amino acid side chains are colored grey (carbon), blue (nitrogen) and red (oxygen). (C) CryoEM reconstructions of the gH/gL/gp42-AMMO1 complex show that most particle images harbor the gp42 C-domain bound on top of gH D-II. (D) A fraction of particle images are characterized by a displacement of the gp42 C-domain. The map is shown at high contour (isosurface) and at low contour (mesh) level to demonstrate that the gp42 CTD is much less well ordered than the rest of the complex. (E) The 4.8 Å resolution reconstruction shown in C reveals that the gp42 N173 glycan points toward the AMMO1 framework region. The glycan is rendered in stick representation with the corresponding region of cryoEM density (blue mesh). (F) AMMO1 binding to gH/gL pre-incubated with the indicated concentrations of gp42 (left) or gp42-T175A (right) was measured by BLI. Representative curves from two independent replicates are shown in F. See also Figures S4 and S7.
Two-dimensional classification of negatively stained gH/gL-gp42-AMMO1 complexes (Fig. S7B–C) also showed that a fraction of AMMO1-bound complexes had a displaced gp42 CTD (Fig. S7C). In contrast, gp42 was displaced from all gH/gL-gp42-CL40 complexes under the same conditions (Fig. S7D).
In the 4.8Å cryoEM map, the gp42-N173 glycan points toward the framework region of the AMMO1 light chain indicating that it might interfere with AMMO1 binding (Fig. 5E). Using mass spectrometry, we verified the presence of various glycans at this position (Fig. S4H, and Data S2). In line with these observations, binding experiments revealed partial competition between AMMO1 and gp42 for binding to gH/gL (Fig. 5F, left panel). This competition was abrogated by the introduction of a T175A mutation, which disrupts the N173 glycosylation sequon (Fig. 5F, right panel). These data revealed that AMMO1 can sterically displace the gp42 CTD from gH/gL through the gp42 N173 glycan.
AMMO1 blocks membrane fusion
Although AMMO1 could alter the conformation of gp42 in the gH/gL-gp42 complex, binding to HLA class II was unaffected by the presence of the AMMO1 Fab (Fig. 6A). We also assessed whether AMMO1 binding to gH/gL-gp42 could affect the ability of the complex to bind to B cells. As expected, gH/gL bound to Raji cells in the presence of gp42 (Fig. 6B). An excess of AMMO1, but not CL40 or the control MAb AMMO4, reduced but did not completely inhibit gH/gL-gp42 binding to B cells (Fig. 6B).
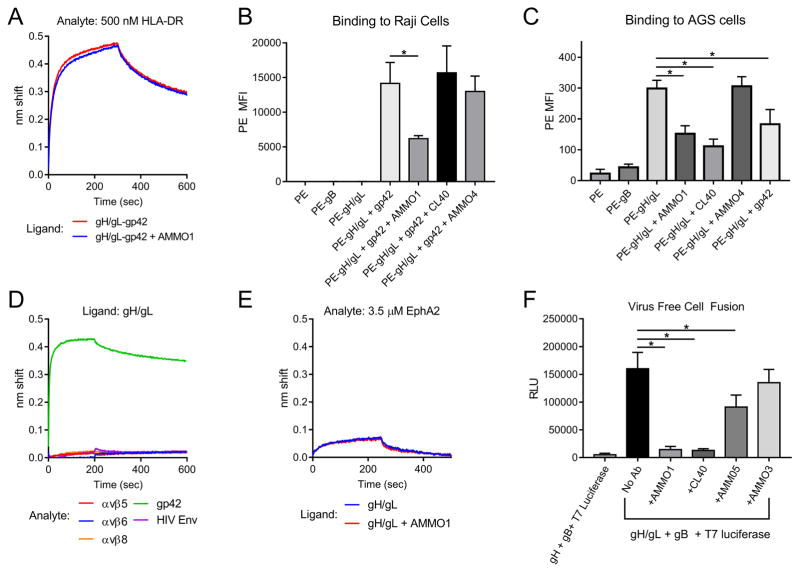
Figure 6. AMMO1 interferes with cell fusion.
(A) Binding of gH/gL-gp42, or gH/gL-gp42-AMMO1 complexes to HLA-DR were measured using BLI. (B) B cell surface staining with SA-PE alone, SA-PE conjugated to biotinylated gH/gL, biotinylated gB, or biotinylated gH/gL bound to gp42 +/− an excess of the indicated MAbs. (C) Epithelial cell surface staining with SA-PE alone, or SA-PE conjugated to biotinylated gH/gL +/− an excess of the indicated MAbs. Data are represented as mean ± SD, and * indicates a statistical difference in the mean fluorescence intensity of PE in B and C. (D) BLI was used to measure gH/gL binding to a 1 μM solution of αvβ5, αvβ6, αvβ8, gp42, or an HIV-1 Envelope protein. (E) BLI was used to measure gH/gL or gH/gL-AMMO1 binding to a 3.5 μM solution of EphA2 by BLI. (F) CHO-K1 cells were transfected with expression plasmids encoding the indicated proteins, and then overlaid on HEK293 cells stably expressing T7 polymerase, +/− the indicated MAbs. * indicates a statistical difference in the mean RLU (n=5 wells). Data from one experimental replicate is shown in A–F.
During epithelial cell infection, gH/gL binds directly to receptors on the surface of epithelial cells that presumably trigger it and lead to the subsequent activation of gB (Borza et al., 2004; Chen et al., 2012). We observed weak binding of gH/gL to the surface of the AGS epithelial cell line (Fig. 6C). Pre-incubation with AMMO1, CL40, or gp42 reduced, but did not abrogate gH/gL binding to AGS cells (Fig. 6C), whereas the anti-gB MAb AMMO4 had no effect.
gH/gL is thought to interact directly with αvβ5, αvβ6, or αvβ8 integrins present at the surface of epithelial cells through the KGD motif on gH D-II (Chen et al., 2012; Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009). Our results suggested that AMMO1 binding could restrict integrin access to the KGD motif due to the proximity of its epitope (Fig. 3G). We were unable to detect binding of gH/gL to αvβ5, αvβ6 or αvβ8 integrins (Fig. 6D). These observations are in line with a recent report (Sathiyamoorthy et al., 2017), but contrast with previous studies (Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009).
A direct interaction between gH/gL and EphA2 has recently been shown to be important for EBV viral entry (Chen et al., 2018; Zhang et al., 2018). We observed a very weak binding signal between EphA2 and gH/gL that was unaltered by pre-incubation of AMMO1 (Fig. 6E) suggesting that AMMO1 does not prevent a gH/gL-EphA2 interaction.
The observation that AMMO1 potently neutralized EBV infection of both B cells and epithelial cells pointed to a common mechanism of viral inhibition. Since AMMO1 fails to completely block binding of gH/gL to epithelial cells or gH/gL-gp42 to B cells, we hypothesized that AMMO1 is likely interfering with gB activation and membrane fusion. Using a virus-free cell fusion assay (McShane and Longnecker, 2005), we observed that the anti-gH/gL MAbs AMMO1 and CL40, and the anti-gB MAb AMMO5 significantly reduced cell fusion, whereas the non-neutralizing MAb AMMO3 had no effect (Fig. 6E).
Collectively these results indicated that AMMO1 can restrict gH/gL or gH/gL-gp42 access to cell surface receptors and that it is capable of blocking membrane fusion.
DISCUSSION
Orally transmitted EBV establishes infection in permissive cells in the oral mucosa such as epithelial cells or infiltrating B cells (Rickinson et al., 2014; Taylor et al., 2015). EBV has tropism for both cell types and it is not clear which one is preferentially infected during primary infection (Tangye et al., 2017). The virus could first infect epithelial cells and lytic viral replication would subsequently seed B cell infections. Alternatively, the virus may initially infect infiltrating B cells, which would lead to subsequent targeting of epithelial cells. Neither scenario is mutually exclusive; thus a combination of epithelial and B cell neutralizing antibodies may be required to most effectively block incoming virus at the oral mucosa (Herrman et al., 2015).
gp350 MAbs can inhibit infection of B cells but not CD21 negative epithelial cells. Conversely, anti-gH/gL MAbs are effective at preventing epithelial cell infection but fail to prevent B cell infection. These observations suggest that gp350 or gH/gL antibodies alone might not be sufficient to prevent EBV infection, and that an effective EBV subunit vaccine might need to elicit both types of antibodies to prevent an initial infection event.
Previous EBV subunit vaccine studies in humans focused on gp350. Recombinant gp350 elicits antibodies that can neutralize B cell infection in vitro (Moutschen et al., 2007; Sokal et al., 2007). Although a phase II clinical trial of a gp350 subunit vaccine showed clinical benefit by reducing the incidence of infectious mononucleosis, it failed to protect from EBV infection (Sokal et al., 2007). From these results emerged the idea that the efficacy of EBV subunit vaccines could be improved if they were formulated with additional glycoproteins (Cohen et al., 2013). The potent in vitro neutralizing activity of AMMO1 suggests that a gH/gL-based vaccine capable of eliciting AMMO1-like antibodies could be as effective as gp350 at blocking EBV entry into B cells, while also preventing epithelial cell infection. Moreover a gH/gL vaccine would avoid the potential enhancement of epithelial cell infection reported for anti-gp350 antibodies (Turk et al., 2006). In support of this notion, rabbit immunization with gH/gL elicited higher antibody titers blocking B cell infection than gp350 (Cui et al., 2016).
AMMO1 may also have utility as a therapeutic agent. Passive administration of the 72A1 MAb demonstrated potential to prevent EBV infection during the early post-transplant period in high-risk EBV-seronegative transplant recipients. However, administration of 72A1 led to the production of anti-drug antibodies, which were attributed to its murine origin (Haque et al., 2006). Since AMMO1 can neutralize infection of the two main cell types targeted by EBV, and is human-derived, it may present a more effective and safer alternative than 72A1.
The unique ability of AMMO1 among anti-gH/gL MAbs to neutralize both epithelial and B cell infection points to a common mechanism of neutralization, most likely by interfering with gB triggering. Mutagenesis studies identified that the D-I/D-II linker region is key for membrane fusion (Omerovic et al., 2005). Several of these critical residues are buried by AMMO1. Thus, AMMO1 could inhibit fusion activation by preventing direct interaction between gB and the linker-helix on gH/gL (Fig. 7A). Residues within the D-I/D-II groove were shown to mediate membrane fusion as well (Chen et al., 2013a; Matsuura et al., 2010; Plate et al., 2009), suggesting that conformational changes within the D-I/D-II groove might be required for triggering of, or interaction with gB (Chen et al., 2013a; Chesnokova and Hutt-Fletcher, 2011). Since AMMO1 binds to elements of D-I (including gL), D-II, and the linker helix, it could inhibit gB activation by acting as a molecular clamp preventing movements within and across D-I and D-II (Fig. 7B).
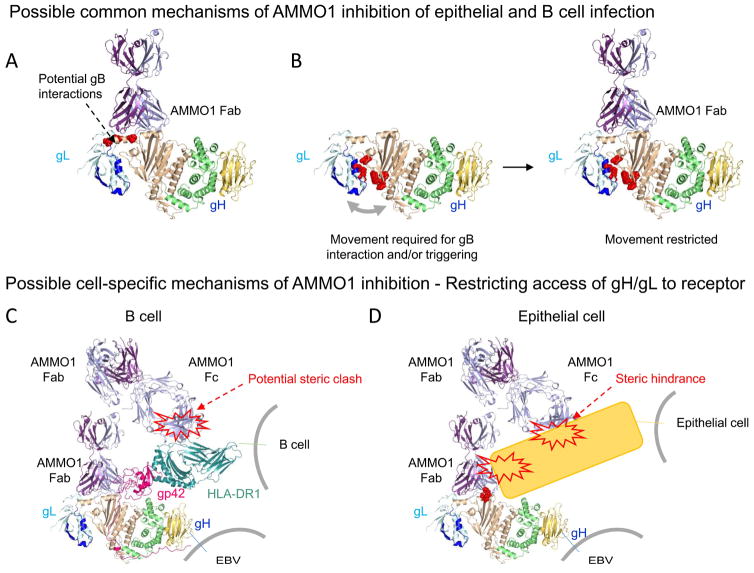
Figure 7. Possible mechanisms of AMMO1-mediated neutralization.
(A) Direct inhibition of gB binding. Residues in the linker helix which have been previously shown to affect cell fusion (L65, L69 and L74) when mutated are shown in red. AMMO1 binding to gH/gL could block a putative gB interaction site. (B) Molecular clamp preventing gB triggering. Residues within the D-I/D-II groove (L55, L207, R152, H154, T174, K94) that have been shown to affect membrane fusion when mutated are shown in red. AMMO1 could restrict movements across the D-I/D-II groove that are required for gB interaction and/or triggering. (C) Restriction of B cell receptor interactions. Although AMMO1 binds away from the HLA-II binding site on the gH/gL-gp42 complex, it could restrain access to membrane anchored receptors through the second FAb arm or the Fc region of the antibody. HLA-DR1 is shown in blue-green and positioned to bind its predicted binding site of gp42 (PDB ID 1KG0). (D) Restriction of epithelial cell receptor interactions. AMMO1 could inhibit binding to one or more epithelial cell receptors by directly restricting access to the interacting site or by indirect steric hindrance mediated through the second FAb arm or the Fc region of the antibody. The KGD motif which has been implicated in gH/gL binding to integrins is shown in red.
The architecture of CMVgH/gL resembles that of EBVgH/gL (Chandramouli and Malito, 2017). It has been proposed that binding of the CMVgH/gL/UL128/UL130/UL131 pentamer to a cell surface receptor induces a D-I rearrangement around the linker helix, which leads to a widening of the D-I/D-II groove and gB triggering (Chandramouli and Malito, 2017). Thus, the linker helix may be a target for antibodies that neutralize CMV infection as well. The MAb 13H11, which neutralizes CMV infection of both epithelial cells and fibroblasts, binds in proximity of the CMVgH/gL linker helix, although higher resolution data will be needed to compare the 13H11 and AMMO1 binding modes (Ciferri et al., 2015; Macagno et al., 2010).
The proposed fusion inhibition activity of AMMO1 is further supported by studies of neutralizing MAbs against gH/gL from other herpesviruses. MAbs that inhibit fusion of varicella zoster virus bind to epitopes bridging the first two domains of gH/gL (Xing et al., 2015). Escape mutations from the LP11 antibody, which blocks the herpes simplex virus gH/gL-gB interaction, also map to a similar epitope region on gH/gL (Chowdary et al., 2010).
Previous studies suggest that displacement of the gp42 CTD by CL40 prohibits a conformation of the gH/gL-gp42-HLA class II complex necessary for triggering gB-mediated fusion during B cell infection (Sathiyamoorthy et al., 2017). The observation that CL40 more readily displaces gp42 than AMMO1 but fails to potently neutralize B-cell infection, indicated that this is not the primary mechanism of B-cell neutralization by AMMO1. Future studies that shed light on the elusive nature of the gH/gL-gB interaction will be necessary to determine whether, and how AMMO1 disrupts gB function.
Our data also indicated that AMMO1 could contribute to neutralization by restricting access of gH/gL or gH/gL-gp42 to cell surface receptors during epithelial and B cell infection, respectively (Fig. 7C–D). αVβ5, αVβ6, and αVβ8 integrins (Chesnokova and Hutt-Fletcher, 2011; Chesnokova et al., 2009), and EphA2 (Chen et al., 2018; Zhang et al., 2018) have been implicated as cell surface EBV receptors during epithelial cell infection. AMMO1 did not affect EphA2 binding to gH/gL, therefore it is unlikely that AMMO1 inhibits EBV infection of epithelial cells by blocking this interaction.
Although we could not detect an interaction between gH/gL and soluble αVβ5, αVβ6 or αVβ8 integrins by BLI, the reduced binding of gH/gL to epithelial cells in the presence of AMMO1 indicates that the MAb may restrict binding to cell-surface anchored integrins, to membrane anchored EphA2, or to an alternative receptor (Fig. 7D).
In this study, we also isolated AMMO5, an anti-gB MAb which could inhibit entry into epithelial cells. The inability of AMMO5 to antagonize B cell infection points to putative differences in gB triggering and/or fusion during EBV entry into the two cell types. This notion is supported by mutagenesis studies of gH/gL, which revealed distinct effects on fusion with B cells and with epithelial cells (Chen et al., 2013a; Mohl et al., 2014; Sathiyamoorthy et al., 2016; Wu et al., 2005).
Most of the anti-gB MAbs isolated in this study were non-neutralizing. We emphasize that the gB protein used to sort B cells is most likely in the post-fusion conformation (Backovic et al., 2009) and might fail to bait out neutralizing antibodies recognizing the pre-fusion state, as was reported for the RSV fusion protein (Magro et al., 2012; McLellan et al., 2013).
In summary, we report the isolation of anti-EBV neutralizing antibodies arising from natural infection in humans and identify the anti-gH/gL antibody AMMO1 that potently neutralizes both epithelial and B-cell infection. The near-atomic resolution cryoEM structure of the gH/gL-gp42-AMMO1 complex presented here defines a key site of EBV vulnerability and may help pave the way for the design of next-generation subunit vaccines. The moderate level of somatic mutation in AMMO1 is within the range that could be elicited with current human vaccine regimens implemented for influenza or HIV (Easterhoff and Moody, 2017; Joyce et al., 2016; Moody et al., 2011; Wrammert et al., 2008). Since AMMO1 is of human origin, it could also be used to treat and/or prevent EBV-related complications such as lymphoproliferative diseases in organ transplant recipients and immunocompromised individuals.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Andrew T. McGuire (amcguire@fredhutch.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Subjects
Peripheral blood mononuclear cells (PBMC) and serum were collected from seven HIV-uninfected, and eight HIV-infected adults recruited at the Seattle HIV Vaccine Trials Unit (Seattle, Washington, USA) as part of the study “Establishing Immunologic Assays for Determining HIV-1 Prevention and Control”, also referred to as Seattle Assay Control or SAC. All participants signed informed consent, and the following institutional human subjects review committee approved the protocol prior to study initiation: Fred Hutchinson Cancer Research Center IRB (Seattle, Washington, USA). PBMC samples from donors were blindly selected at random with no considerations were made for age, or sex.
Cell lines
All cell lines were incubated at 37°C in the presence of 5% CO2 and were not tested for mycoplasma contamination. 293T (human female) and NIH-3T3 (murine male) cells were grown in DMEM containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (cDMEM). CHO K-1 (hamster female) and AGS (human female) cells were maintained in Ham’s F-12 + 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (cF-12). Raji cells (human male), as well as the 72A1 (murine, sex unknown), CL55(murine, sex unknown), (Wu et al., 2005), F-2-1 (murine sex unknown), (Strnad et al., 1982), CL40 (murine, sex unknown), CL59 (murine, sex unknown), (Molesworth et al., 2000), and E1D1 (Balachandran et al., 1987) hybridomas, were maintained in RMPI + 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (cRPMI). 293–2089 cells (human female) (Delecluse et al., 1998) were grown in cRPMI containing 100μg/ml hygromycin. AKATA (human female) B cells harboring EBV in which the thymidine kinase gene has been replaced with a neomycin and GFP cassette virus (AKTA-GFP) (Molesworth et al., 2000), were grown in cRMPI containing 350μg/ml G418. SVKCR2 cells (human male) (Li et al., 1992) were grown in DMEM containing 10% cosmic calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, 10 ng/ml cholera toxin and 400 μg/ml G418. 293-T7 cells (human female) (Omerovic et al., 2005) were maintained in cDMEM containing 100μg/ml Zeocin. 293F (human female) cells were maintained in Freestyle 293 media with gentle shaking.
METHOD DETAILS
Plasmids
cDNA encoding to gH (AA 19–679, Genbank AFY97969.1), gp42 (amino acids 33–223, genbank: AFY97939.1), gp350 (AA 1–470, Genbank: AAD51697.1), and gB AA 23–683, Genbank: AFY97983.1) with the residues WY112–113 mutated to HR and WLIW193–196 mutated to RVEA (Backovic et al JIV 2007) were synthesized with an N-terminal TPA leader peptide (MDAMKRGLCCVLLLCGAVFVSPSAS) and a C-terminal HIS-Avi Tag (GSGSGHHHHHHGLNDIFEAQKIEWHE) and cloned into the EcoRI and NotI sites of the pTT3 plasmid. gL (AA 24–137 Genbank: AFY97944.1), was synthesized with an N-terminal TPA leader peptide (MDAMKRGLCCVLLLCGAVFVSPSAS) without a C terminal tag and cloned into the EcoRI and NotI sites of the pTT3 plasmid. Site directed mutagenesis was used to introduce stop codons into gH and gp42 between the HIS and Avi tags to produce expression plasmids without the Avi-Tag, and to introduce the T62A and T175A mutations into gH and gp42, respectively. The murine CD40L sequence flanked by an XbaI-Kozak sequence on the 5′ end and a Not I site on the 3′ end was synthesized by Genscript and cloned into the XbaI and NotI sites of pCDH-EF-MCS-IRES-RFP vector (Systembio) to create pCDH-muCD40L-RFP.
pCAGGS expression plasmids for gH, gL, gB, and pT7EMCLuc (which carries a luciferase-containing reporter plasmid under the control of the T7 promoter) were kindly provided by Dr. R. Longnecker (Haan et al., 2001; Okuma et al., 1999; Plate et al., 2011). Plasmids for the expression of humanized, recombinant 72A1 were provided by Dr. F. Wang (Herrman et al., 2015). p509, an expression plasmid encoding BZLF1, was provided by Dr. W. Hammerschmidt (Delecluse et al., 1998). p2670, an expression plasmid encoding BALF4, was provided by Dr. H. Delecluse (Neuhierl et al., 2002).
Recombinant Antibody Cloning
Codon optimized cDNAs encoding a murine leader sequence MGWSCIILFLVATATGVHS followed by the human IgG1, IgL or IgK constant regions were synthesized by IDT and cloned into the EcoRI and BamHI sites of pTT3. VH and VL sequences recovered from sorted B cells using RT PCR (see below) were amplified with gene-specific primers or synthesized by IDT and assembled in frame into the appropriate linearized IgG IgK or IgL plasmid using InFusion cloning. The sequences of the recombinant antibody plasmids were verified by Sanger sequencing.
Recombinant Protein Expression
Plasmids encoding EBV proteins, or antibody heavy and light chains were transfected into 293F cells at a density of 106 cells/ml in Freestyle 293 media using the 293Free transfection reagent according to the manufacturer’s instructions. Expression was carried out in Freestyle 293 media for 6 days after which cells and cellular debris were removed by centrifugation at 4,000 × g followed by filtration through a 0.22 μm filter. Clarified cell supernatant containing EBV proteins was passed over Ni-NTA resin pre-equilibrated with Ni-NTA binding buffer (0.3 M NaCl, 20 mM Tris,10mM imidazole, pH 8.0), followed by extensive washing with Ni-NTA binding buffer, and then eluted with 250 mM imidazole, 0.3 M NaCl, 20 mM Tris, pH 8.0 (Ni-NTA elution buffer). Purified proteins were then dialyzed overnight into PBS. AVI-tagged gB, gp350, and gp42 were biotinylated in vitro using the In Vitro Biotin Ligase Kit.
Proteins were further purified by size exclusion chromatography (SEC) using a 10/300 Superdex 200 column (GE Healthcare) equilibrated into HBSE (10 mM HEPES, 150 mM NaCl, 2 mM EDTA pH 7.4), which also served to remove un-ligated biotin and BirA enzyme. Biotinylated proteins were flash frozen and stored at −20°C until use.
Clarified cell supernatant containing recombinant antibodies was passed over Protein A Agarose, followed by extensive washing with PBS, and then eluted with 1 ml of Pierce IgG Elution Buffer, pH 2.0 into 0.1 ml of Tris HCl, pH 8.0. Purified antibodies were then dialyzed overnight into PBS.
Recombinant Protein Biotinylation
AMMO1, E1D1, CL40, CL59, and gH/gL were biotinylated at a theoretical 1.5:1 biotin/protein with the EZ-Link Sulfo-NHS-Biotin Kit. Free biotin was removed by 3 successive rounds of dilution with PBS and concentration with a 30,000 MCOW concentrator (Amicon).
Antigen Binding Fragment preparation
AMMO1 Fab was produced by digesting AMMO1 IgG with Endoproteinase Lys-C overnight at 37°C (10 μg IgG: 1ng Lys C). Fab fragments were separated from Fc fragments with protein A agarose, then further purified with SEC. CL40 Fab was produced by digesting CL40 IgG with activated immobilized ficin at 37°C for 48 hrs. Fab fragments were separated from Fc fragments with protein A agarose, then further purified by SEC.
3T3-CD40L cell line generation
Lentiviruses were produced by co-transfecting the pCDH-muCD40L-RFP, psPAX2 and pMD2.G plasmids at a 4:2:1 ratio (6, 3, and 1.5 μg, respectively) into 293T cells using the GeneJuice Transfection Reagent. 48 hours later the supernatant was collected and passed through a 0.22 μm filter. Supernatant was then transferred to 3T3 cells followed by the addition of 4 μg/ml polybrene and incubated overnight in cDMEM. Cells were then passaged for 7 days. CD40L-expressing cells were identified with a PE-labeled CD154 antibody and sorted by FACS on a Beckman FACS AriaII. Sorted cells were expanded and cultured indefinitely.
Biolayer Interferometry (BLI)
BLI assays were performed on the Octet Red instrument at 30°C with shaking at 1,000 RPM.
Validation of EBV glycoprotein antigenicity using murine hybridomas
Anti-EBV antibodies were captured using Anti-Mouse IgG Fc capture (AMC) biosensors (Fortebio) by immersing sensors directly into hybridoma culture supernatants for 600s. A baseline signal was recorded for 1 min in kinetics buffer (KB: 1X PBS, 0.01% BSA, 0.02% Tween 20, and 0.005% NaN3,) at pH 7.4 or pH 5.0 as indicated. Sensors were then immersed into solutions containing a 0.5μM concentration of each EBV glycoprotein for 250 s to measure association, followed by immersion in KB for 500 s to measure dissociation. All measurements of antibody binding were corrected by subtracting the signal obtained from simultaneous traces performed with the corresponding envelopes in the absence of antibody, using PBS only.
Kinetic analysis
Anti-Human IgG Fc capture (AHC) sensors (for human antibodies), AMC (for murine antibodies), or streptavidin sensors (for biotinylated antibodies or biotinylated gH/gL or gH T62A/gL) were immersed in KB containing 10 μg/ml of purified antibody or biotinylated protein for 200s. After loading, the baseline signal was then recorded for 1 min in KB. The sensors were immersed into wells containing serial dilutions of purified recombinant gH/gL or gH-T62A/gL in KB for 250s (association phase), followed by immersion in KB for an additional 750s (dissociation phase). The background signal from each analyte-containing well was measured using empty reference sensors, and subtracted from the signal obtained with each corresponding ligand-coupled sensor. The background signal of ligand-coupled sensors in KB was subtracted from each sensor at each time-point. Kinetic analyses were performed at least twice with an independently prepared analyte dilution series. Curve fitting was performed using a 1:1 binding model and the ForteBio data analysis software. Mean kon, koff values were determined by averaging all binding curves that matched the theoretical fit with an R2 value of ≥0.99. Binding analyses at pH 5.0 were carried out as above using biotinylated antibodies and Streptavidin sensors.
Antibody competition binding assays
Biotinylated antibodies were diluted to 10 μg/ml and captured onto streptavidin sensors for 120s. The baseline interference was then read for 60s in KB buffer, followed by immersion for 250s (association phase) in a 250 nM solution of gH/gL alone, or gH/gL that had been pre-incubated with 500 nM of non-biotinylated Abs for 20 min in KB. Sensors were then immersed in KB for 750 s (dissociation phase).
gH/gL/gp42-HLA-DR binding assays
Biotinylated gH/gL was immobilized on streptavidin biosensors, and then immersed into KB buffer for 60 seconds followed by into a 1 μM solution of gp42 for 200 seconds. One biosensor was then immersed into a 1 μM solution of the AMMO1 Fab, while the other was immersed in KB for 200 seconds. The biosensors were then immersed into a 500 nM solution of HLA-DR for 300 seconds (association phase) and then into KB for 300 seconds (dissociation phase). One gH/gL-gp42 loaded sensor was immersed in buffer as a reference during the association and dissociation steps and used to subtract the background signal.
gH/gL-integrin binding assays
Biotinylated gH/gL was diluted to 10 μg/ml and captured onto streptavidin sensors for 120s. The baseline interference was then read for 60s in KB buffer containing followed by immersion for 200s (association phase) into KB buffer containing 1 μM of avβ5, avβ6, avβ8, gp42, or an HIV-1 Envelope protein (426c TM4 ΔV1-3(McGuire et al., 2016)). Sensors were then immersed in KB containing for 400s (dissociation phase).
gH/gL EphA2 binding assays
Biotinylated gH/gL was diluted to 30 μg/ml and immobilized on streptavidin biosensors for 120s, and then immersed in KB buffer for 60 seconds. Biosensors were then immersed into either KB buffer or into a 1.5 μM solution of AMMO1 for 60 seconds. The biosensors were then immersed into a 3.5 μM solution of EphA2 for 250 seconds (association phase) and then into KB for 250 seconds (dissociation phase). To control for non-specific binding due to the high analyte concentration, we subtracted the binding signal to biotinylated gp42 immobilized on biosensors assayed under identical conditions.
Serum ELISA
50 ng/well of gp350, gp42 or gB, were adsorbed onto 96 well Immulon 2HB ELISA plates at either 37°C for 1 hour or room temperature overnight in a solution of 0.1 M NaHCO3 pH 9.4–9.6. Plates were then washed 4 times with ELISA washing buffer (1× PBS, 0.02% Tween 20) prior to blocking at 37°C for 1 hour or 4°C overnight with 250 μl per well of PBS containing 10% Non-Fat Milk and 0.02% Tween 20 (blocking buffer). After blocking, plates were washed 4× with ELISA washing buffer. Serum was diluted in blocking buffer and three-fold serial dilutions were performed in duplicate followed by a 1 hour incubation at 37°C. Following 4 additional washes with ELISA washing buffer, a 1:3000 dilution of goat anti-human Ig HRP in blocking buffer was added to each well and incubated at 37°C for 1 hour followed by 4 washes with wash buffer. 50 μl/well of SureBlue Reserve TMB Microwell Peroxidase substrate was added. After 3 min, 50 μl/well of 1N Sulfuric Acid was added and the A450 of each well was read on a Molecular Devices SpectraMax M2. Analysis was performed using the Prism 6 package (Graphpad Software).
ELISAs against gH/gL were performed essentially the same as above except for the following changes. 100 μl/well of a 1 μg/ml solution of His-tagged gH/gL was immobilized on Nickel Coated Plates in 1× PBS and 0.5% Tween 20 overnight at 4°C. The blocking buffer consisted of PBS containing 10% Non-Fat Milk, 0.02% Tween20, and 10 mM imidazole. The background of serum reactivity with the nickel-coated plates of each donor’s sera was subtracted from that of gH/gL at each serum dilution.
Conjugation of antigens to fluorescently labeled streptavidin
Biotinylated EBV glycoproteins were mixed with streptavidin-phycoerythrin at a 4:1 biotin to streptavidin ratio.
B cell sorting
Cryopreserved PBMC were thawed into cRMPI, at a concentration of 4 million PBMC/ml, followed by centrifugation at 300 × g for 5 min. Cells were suspended in ~100μl cRPMI and incubated with an irrelevant, Avi-tagged recombinant decoy protein from Plasmodium yoelii (PY-gamma, a kind gift from Dr. D.N. Sather) conjugated to streptavidin PE labeled with Dylite 650 (SA-PE-DL650) to a final concentration of 10 nM of PE-DL650 for 10 min at 4°C.
Antigen (gB, gH/gL, gp42, or gp350) conjugated to streptavidin-phycoerythrin (SA-PE) was then added to a final concentration of 10nM and incubated for 20 min at 4°C. 25 μl of Anti-PE MicroBeads (Miltenyi Biotech) were added and incubated for an additional 30 min at 4°C. The cell/bead mixture was then passed over a LS MACS separation column (on a MACS separator and washed with 6 ml of FACS buffer (PBS + 1% FBS). The column was removed from the MACS separator and eluted two times with 5 ml of FACS buffer. PE-enriched PBMC were then pelleted by centrifugation at 300 × g for 5 min, and then re-suspended in 100 μl of FACS buffer. Enriched PBMC were then stained with the following antibodies: IgM-FITC at a 1:20 dilution, IgD-PerCP-Cy5.5 at a 1:100 dilution, CD27-PE-Cy7 at a 1:200 dilution, CD20-eF450 at a 1:100 dilution, CD3-BV711 at a 1:100 dilution, CD14-BV711 at a 1:100 dilution, CD16-BV711 at a 1:100 dilution, CD19-BV786 at a 1:100 dilution, and a fixable viability dye eFluor 506 at a 1:200 dilution for 25 min at 4°C. Stained cells were diluted to 5 ml with FACS buffer, pelleted by centrifugation at 300 × g for 5 min and then suspended in 0.5 ml of FACS buffer and subjected to FACS on a BD FACS Aria II.
Live, antigen-positive class-switched B cells (Live/dead−, CD3−, CD14−, CD16−, CD19+ CD20+ IgM−, IgD−, PE-DL650−, PE+) were sorted individually into 96 well plates containing 2.86×104 irradiated 3T3-CD40L cells in 100 μl/well IMDM containing 10% FBS, 1× glutamax, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 U/ml IL2 and 50 ng/ml IL21 (Huang et al., 2013). Cells were cultured at 37°C, 5% CO2. 13 days later the 80 μl of supernatant was transferred into 96 well round-bottom plates. 20 μl of lysis buffer (15 mM Tris pH 8.0, containing RNAse inhibitor) was added to the wells containing cells. The cells were then frozen on dry ice and stored at −80°C.
Sorted Cell Supernatant Screening
Nunc 384 well MaxiSorp plates were coated overnight at room temperature with 50 μl of a 0.5 μg/ml solution of unlabeled goat anti human IgG or gB, in 0.1 M NaHCO3 pH 9.4–9.6. 100 μl/well of a 1 μg/ml solution of His-tagged gH/gL was immobilized 96 well nickel coated plates in 1× PBS and 0.5% Tween 20 overnight at 4°C. ELISA plates were then washed 4× with ELISA wash buffer, and cell supernatant diluted 1:1 with blocking buffer was then added to the appropriate wells (one well per antigen). ELISA was performed as described under “Serum ELISA”, except that all volumes were halved in the 384 well plates. Wells were scored as positive if the A450 was > 2 standard deviations of the A450 recorded with the feeder cell only control supernatants (n=4 wells).
VH/VL Recovery from Sorted Cells
Wells containing sorted cells that were antigen positive by ELISA were thawed and 15 μl of cell lysate was transferred to a thin wall PCR tube and mixed with 10 μl of reverse trasnscription (RT) mix containing 5 μl of first strand buffer, 1.25 μl of 100 mM DTT, 0.06 μl of IGEPAL, 125 ng of random hexamers, 2 μl of 10 mM dNTP mix, 0.5 μl of RNAse inhibitor, and 1 μl of superscript III reverse transcriptase. RT was carried out at 42°C for 10 min, 25°C for 10 min, 50°C for 60 mi n, and 94°C for 5 min, followed by a hold at 4°C.
3 μl of cDNA was used as a template for a two-step nested VH, VK, or VL amplification using the primer set and protocol developed by Doria-Rose and colleagues (Doria-Rose et al., 2015). VH and VL amplicons were Sanger sequenced and then cloned into recombinant expression vectors and expressed as recombinant IgG1 proteins.
Virus Production
To produce B-cell tropic GFP reporter viruses (B95-8/F), 9×106 293–2089 cells were seeded on a 15 cm tissue culture plate in cRPMI containing 100 μg/ml hygromycin. 24 hours later the cells were washed 2× with PBS, media was replaced with cRMPI without hygromycin, and the cells were transfected with 15 μg of each of p509 (Delecluse et al., 1998) and p2670 (Neuhierl et al., 2002) expressing BZLF1 and BALF4, respectively, using GeneJuice transfection reagent. 72 hours later the cell supernatant was collected, cell debris removed by centrifugation at 300 × g for 5 min and then passed through a 0.8 μm filter. To produce epithelial cell tropic virus, B cells harboring AKATA-GFP EBV were suspended at 4×106 cells/ml in RPMI containing 1% FBS. Anti-human IgG was added to a final concentration 100 μg/ml and incubated at 37°C for 4 hours. Cells were then diluted to 2×10 6 cells/ml in RPMI containing 1% FBS and incubated for 72 hours. Cells were pelleted by centrifugation at 300 × g for 10 min and then the supernatant was passed through a 0.8 μm filter. Bacitracin was added to a final concentration of 100 μg/ml. Virions were concentrated 25× by centrifugation at 25000 × g for 2 hours and re-suspended in RPMI containing 100μg/ml bacitracin. Virus was stored at 4°C for up to 2 weeks.
B cell Neutralization Assay
B cell neutralization assays were carried out in Raji cells essentially as described (Sashihara et al., 2009). Monoclonal antibodies were serially diluted in duplicate wells of 96 well round-bottom plates containing 25 μl of cRPMI in duplicate. 12.5 μl of B95-8/F virus (diluted to achieve an infection frequency of 1–5% at the final dilution) was added and incubated at 37°C for 1 hour. 12.5 μl of cRMPI containing 4×106 Raji cells/ml was added to each well and incubated for another hour at 37°C. The cells were then pelleted, washed once with cRPMI, and re-suspended in cRMPI. Antibody concentration is reported relative to the final infection volume (50 μl). After 3 days at 37°C, cells were fixed in 2% paraformaldehyde. The percentage of GFP+ Raji cells as determined on a BD LSRII cytometer.
To account for any false positive cells due to auto-fluorescence in the GFP channel, the %GFP+ cells in negative control wells (no virus, n=5) was subtracted from each well. % neutralization in each well was defined as: [%GFP+ cells in the positive control wells containing virus alone (n=5 wells) – %GFP+ cells in the antibody containing well]/%GFP+ cells in the positive control wells × 100.
The % neutralization for each well was plotted as a function of the log10 of the MAb concentration. The neutralization curve was fit using the log(inhibitor) vs response-variable slope (four parameters) analysis in Graphpad Prism 6 software.
Epithelial Cell Neutralization Assay
1.5×104 SVKCR2 cells per well were seeded into a 96 well tissue culture plate. The following day antibodies were serially diluted in duplicate wells containing 20 μl of media in a 96 well round bottom plate followed by the addition of 20 μl of 25× concentrated epithelial cell-tropic virus and incubated for 15 min. Media was aspirated from the SVKCR2 cells and replaced by the antibody-virus mixture followed by a 3 hour incubation at 37°C. The antibody-virus mixture was then aspirated and replaced with media. 48 hours later the cells were trypsinized and the percentage of GFP positive cells were determined on an BD LSRII cytometer. Percent neutralization was determined as in the B cell neutralization assay.
Cell Surface Binding Assays
Streptavidin-PE conjugated to 0.5 μg of gH/gL biotin (gH/gL-PE), or to 0.5 μg of gB (gB-PE) was diluted in 10μl of PBS to individual wells of a 96 well plate. An equimolar amount of gp42 was added to select wells containing gH/gL-PE. 7 μg of monoclonal antibodies, AMMO1, AMMO4, or CL40 were added to select wells containing gH/gL ± gp42 and incubated for 1.5 hours at room temperature. Meanwhile, adherent AGS cells were trypsinized, washed with cF-12 and then allowed to recover at 37°C 5% CO 2 for 30 min. The cells were gently agitated and then returned to 37°C 5% CO2 for an additional 30 min. Recovered AGS, and Raji cells were pelleted by centrifugation at 300 × g for 3 min and then resuspended at a density of 1 ×106 cells/ml in ice-cold 0.5% bovine serum albumin (BSA) in PBS. 100 μl of AGS or Raji cells were added to wells containing SA-PE, SA-gB, SA-PE gH/gL ± gp42 and ± antibodies in quadruplicate, and incubated on ice for 1h. Cells were pelleted by centrifugation at 300 × g for 3 min, washed with 200 μl of ice cold 0.5% BSA in PBS, pelleted again and resuspended in 10% phosphate buffered formalin. The amount of PE staining was determined on a BD LSRII cytometer.
Virus Free Fusion Assay
CHO-K1 cells were seeded onto six-well plates at a density of 3×105 cells/well. 24 hours later, the cells were transfected with 0.5 μg each of pCAGGS-gH, pCAGGS-gL, pCAGGS-gB (Haan et al., 2001) and 0.8 μg of pT7EMCLuc, which carries a luciferase-containing reporter plasmid under the control of the T7 promoter (Okuma et al., 1999), using GeneJuice Transfection Reagent.
Meanwhile, 293-T7 cells were seeded into a 96 well plate at a density of 1×104 cells per well in a volume of 100 μl/well of cF-12 without Zeocin selection.
8 hours later, the transfected CHO cells were trypsinized, washed once with cF-12, and re-suspended at a density of 1×105 cells/ml in F-12 media. 100 μl/well of CHO-K1 suspension was added to the plate containing 293-T7 cells. Immediately after the addition of CHO-K1 cells, 2 μg of AMMO1, AMMO3, AMMO5, or CL40 were added to 6 wells in parallel. 24 hours later, the media was aspirated and the cells were lysed in 100 μl of Steady-Glo luciferase reagent. 75 μl of cell lysate was transferred to a white bottom assay plate and luciferase activity was read on a Fluroskan Ascent FL fluorimeter.
Crystallization of the AMMO1 FAb and data collection
Crystals of AMMO1 Fab were obtained using a NT8 dispensing robot and screening was done with Rigaku Wizard Precipitant Synergy block#2, Molecular Dimensions Proplex screen HT-96, Hampton Research Crystal Screen HT by the vapor diffusion method. Crystals used for diffraction data were grown in 16.75% PEG 400, 13.4% PEG 3350, 0.1M MgCl2, 0.1M Tris pH 8.5. Crystals were cryo-protected in solutions containing 30% molar excess of their original reagents and 20% Glycerol. Crystal diffracted to 1.6 Å (See Table S4). Data was collected at ALS 5.1 and 5.2 and processed using HKL2000 (Otwinowski and Minor, 1997).
Structure solution and refinement
The structure of AMMO1 Fab was solved by molecular replacement using Phaser in CCP4 (Collaborative Computational Project, 1994) and PDB ID 4FQQ_L (light chain) and 4JPK_H (heavy chain) as search model. COOT(Emsley et al., 2010) and PHENIX (Adams et al., 2010) were used for model building and refinement of the structure, which included TLS parameters. A cross validation (Rfree) test set consisting of 5% of the data was used throughout the refinement process. The refinement statistics are summarized in Table S3. Structural figures were made with Pymol (DeLano, 2002), or USCF Chimera(Pettersen et al., 2004).
Negative stain electron microscopy
Stock solution of gH/gL, gp42, AMMO1 and CL40 were diluted to an estimated concentration of 25 nM of each component in 50 mM HEPES pH 7.5, 150 mM NaCl. We used carbon-coated Ted Pella G400 copper grids, glow discharged immediately before use. A volume of 3.5 μL of sample was deposited on the grid for 20–30 s before excess solution was blotted away using Whatman No. 1 filter paper. This was immediately followed by two rounds of staining in 3.5 μL of 2% (w/v) of uranyl formate. Data was collected on an FEI Tecnai Spirit transmission electron microscope equipped with a US4000 CCD camera. Images were acquired at a nominal magnification of 67,000× at a defocus range between -1 μm and -4 μm. CTF parameters were estimated using GCTF(Zhang, 2016). Particles were picked using DoG Picker(Voss et al., 2009). Particle images were extracted using a box size of 192 pixels binned by a factor of 2 to an effective pixel size of 3.2 Å/pixel and analyzed using RELION 2.0 (Kimanius et al., 2016).
Cryo Electron Microscopy Sample Preparation and Data Collection
Stock solutions of gH/gL, gp42 and AMMO1 were diluted to an estimated concentration of 3 μM of each component in 50 mM HEPES pH 7.5, 150 mM NaCl and 0.01% (v/v) NP40. We used Protochips C-flat 1.2/1.3–4C-T carbon-coated copper grids, glow discharged immediately before use. Particles showed a preferred orientation in ice in the absence of NP40, limiting the overall resolution of the reconstructions. Addition of NP40 resulted in more diverse particle orientations, but resulted in significantly fewer particles in ice. To overcome this issue, a multiple blotting strategy was employed, as previously described (Snijder et al., 2017). After two rounds of sample application and blotting on the lab bench using Whatman No. 1 filter paper, a third volume of sample was applied to the grids, which were then mounted in an FEI Mark I Vitrobot for a final round of blotting and plunge-freezing in liquid ethane, using a 9 s blotting time with -3 mm offset at room temperature and 80–90% relative humidity.
Data were collected using the Leginon software (Suloway et al., 2005) on an FEI Titan Krios electron microscope, equipped with a Gatan Quantum GIF energy filter, operated in zero-loss mode with a slit-width of 20 eV, and a Gatan K2 Summit direct electron detector. The dose rate was adjusted to 8 counts/pixel/s, and each movie was acquired in counting mode fractionated in 75 frames of 200 ms. 2300 micrographs were collected in a single session with a defocus range comprised between 2.0 μm and 4.0 μm.
CryoEM data processing
Movie frames were aligned with MotionCor2 (Zheng et al., 2017), with the use of dose weighting. CTF parameters were estimated from the aligned micrographs without applied dose weighting, using GCTF (Zhang, 2016). Particles were picked from aligned dose-weighted micrographs using DoG Picker (Voss et al., 2009). Particle images were extracted using a box size of 224 pixels binned by a factor of 2 to an effective pixel size of 2.72 Å/pixel and analyzed with RELION 2.0 (Kimanius et al., 2016). After 2 rounds of reference-free 2D classification, 137,000 particles were selected for 3D classification in 5 classes, starting with an initial model was generated from 2D class averages using the e2initialmodel.py function in EMAN2 (Tang et al., 2007). One predominant class of 104,000 particles was selected for further classification and refinement using the re-extracted particles with original pixel size of 1.36 Å. In addition, one minor class of 15,000 particles with the displaced gp42 CTD was refined using the 2× binned images to a resolution of 10.5 Å. The major class of gH/gL-gp42-AMMO1 was further classified in 3 classes with a finer angular sampling (HEALPix order 3 with oversampling) and local searches. One dominant class of 72,000 particles was selected to generate the final map, at 4.8 Å resolution, using a solvent mask and the solvent fcs flag in RELION 2.0. A B-factor of −400 Å2 was applied to sharpen the map. Reported resolutions are based on the gold-standard FSC=0.143 criterion (Rosenthal and Henderson, 2003; Scheres and Chen, 2012) and Fourier shell correlation curves were corrected for the effects of soft masking by high-resolution noise substitution (Chen et al., 2013b).
Model building
UCSF Chimera (Goddard et al., 2007) and Coot (Brown et al., 2015) were used to fit the crystal structures of gH/gL/gp42 (PDB 5T1D) and of AMMO1 into the cryoEM map. The quality of the reconstruction is highest for the density corresponding to the gH core, for which several amino acid side chains are resolved. The quality of the reconstruction is lower for the regions corresponding to gL, gp42 and AMMO1. Refinement of the model was carried out using Rosetta density-guided iterative refinement (DiMaio et al., 2015) and Rosetta Relax (DiMaio et al., 2009). Deviations from the input structures were allowed only if supported by density or to resolve stereochemical issues. Glycans were initially docked into the density and their geometry was then refined using Rosetta, optimizing the fit-to-density as well as the energetics of protein/glycan contacts. The model of the gH/gL/gp/42/AMMO1 complex showing the displacement of gp42 was obtained by rigid-body docking the gp42 C-terminal domain into the corresponding density. The quality of the final model was validated using Molprobity (Chen et al., 2010) and Privateer (Agirre et al., 2015). Structure analysis was assisted by the PISA server (Krissinel and Henrick, 2007).
gH/gL mutant binding analysis
Targeted mutations were introduced into pCAGGS-gH or pCAGGS-gL using the Quickchange XL site-directed mutagenesis kit All mutations were confirmed by Sanger sequencing. Wildtype and mutant gH or gL plasmids were co-transfected into 4ml of 293F cells at a density of 106 cells/ml in Freestyle 293 media using the 293Free transfection reagent. 24h later, cells expressing wildtype gH/gL, each gH/gL mutant, or mock transfected cells were pelleted by centrifugation at 300 × g for 3 min and then re-suspended in 200 μl of PBS containing 0.5% BSA and 2 μg of the CL59 MAb and incubated on ice for 1h. Meanwhile 3-fold serial dilutions of AMMO1 labeled with Dylite 650 were prepared in 50 μl of PBS containing 0.5% BSA in 96 well plates. 20 μl of PBS containing 0.5% BSA and 0.5 μl of PE-anti-mouse IgG was added to each well. Cells expressing gH/gL variants, as well as mock-transfected cells were washed with 3ml of PBS containing 0.5% BSA and resuspended in 650 μl of PBS containing 0.5% BSA. 30 μl of cell suspension was added to wells containing serially diluted AMMO1 and anti-mouse PE in duplicate, followed by a 1h incubation on ice. Each well was washed twice with 200 μl of PBS containing 0.5% BSA and then resuspended in 100 μl of 10% phosphate buffered formalin. gH/gL positive cells were determined by PE (CL59) staining, using mock-transfected cells as a reference. The level of AMMO1 binding to gH/gL was determined by measuring the mean fluorescence intensity (MFI) of DL650 staining of PE+ cells. To account for differences in gH/gL expression the MFI of PE staining for each gH/gL variant (16 wells total) was averaged and used to normalize the DL650 staining of each well. The normalized MFI of DL650 was plotted against the concentration of AMMO1 for each well and fit to a sigmoidal dose response curve using GraphPad Prism software.
Mass spectrometry to identify glycopeptides
Approximately 250 pmol of gH/gL and gp42 were separately prepared for mass spectrometry analysis of glycopeptides. Stock solutions were denatured, reduced and alkylated by dilution to 5 μM in 50 μL of buffer containing 100 mM Tris pH 8.5, 10 mM TCEP, 40 mM iodoacetamide and 2% (w/v) sodium deoxycholate. Samples were first heated to 95°C for 10 min and then incubated for an additional 20–30 min at room temperature in the dark. The samples were split in two for digestion with trypsin and chymotrypsin in parallel, by diluting 20 μL of sample for each protease in a total volume of 100 μL 50 mM ammonium bicarbonate pH 8.5. Protease was added to the samples in a ratio of 1:75 by weight and left to incubate at 37°C overnight. After digestion, 2 μL of formic acid was added to the samples to precipitate the sodium deoxycholate from solution. After centrifugation for 20 min at maximum speed in a bench top centrifuge, 80 μL of the supernatant was collected. For each sample 8 μL was injected on a Thermo Scientific Orbitrap Fusion Tribrid mass spectrometer. A 35-cm analytical column and a 3-cm trap column filled with ReproSil-Pur C18AQ 5 μm beads (Dr. Maisch) were used. Nanospray LC-MS/MS was used to separate peptides over a 110-min gradient from 5% to 30% acetonitrile with 0.1% formic acid. A positive spray voltage of 2,100 V was used with an ion-transfer-tube temperature of 350°C. An electron-transfer/higher-energy collision dissociation ion-fragmentation scheme (Frese et al., 2013) was used with calibrated charge-dependent ETD parameters and supplemental higher-energy collision dissociation energy of 0.15. A resolution setting of 120,000 with an AGC target of 2 × 105 was used for MS1, and a resolution setting of 30,000 with an AGC target of 1 × 105 was used for MS2. Data was searched with Protein Metrics Byonic software (Bern et al., 2012), using a small custom database of recombinant protein sequences including gH, gL, gp42, other viral glycoproteins and the proteases used to prepare the glycopeptides. Reverse decoy sequences were also included in the search. Specificity of the search was set to C-terminal cleavage at R/K (trypsin) or F/W/Y/M/L (chymotrypsin), allowing up to two missed cleavages, with EThcD fragmentation (b/y- and c/z-type ions). We used a precursor mass and product mass tolerance of 12 ppm and 24 ppm respectively. Carbamidomethylation of cysteines was set as fixed modification, methionine oxidation as variable modification, and all four software-provided N-linked glycan databases were used to identify glycopeptides. All glycopeptide hits were manually inspected and only those with quality peptide sequence information are reported here.
QUANTIFICATION AND STATISTICAL ANALYSIS
Unless otherwise noted, a two-tailed, unpaired t-test was used to assess statistical significance. Statistical calculations were performed in Graphpad Prism. The number of replicates and a description of the statistical method are provided in the applicable figure legends. Data were considered statistically significant at * p<0.05.
DATA AND SOFTWARE AVAILABILITY
Sequences for the VH/VL regions of the antibodies reported here can be found at genbank under the accession numbers: KY631779-KY631788. Glycopeptide LC-MS/MS raw data and Byonic search results have been deposited in proteomics identification (PRIDE) database under PXD006403. The glycopeptides corresponding to gH, gL and gp42 can be found in Data S1 and S2. The cryoEM maps have been deposited in the Electron Microscopy Data Bank with accession code EMD-7344 (4.8Å) and EMD-7345 (10 Å). The atomic models of AMMO1 (PDB ID: 6BLA) and gH/gL-gp42-AMMO1 (PDB ID: 6C5V) have been deposited in the Protein Data Bank.
Supplementary Material
Data S1. gH and gL glycopeptides identified by mass spectrometry, related to Figure 3.
Data S2. gp42 glycopeptides identified by mass spectrometry, related to Figure 5.
Highlights.
Isolated neutralizing antibodies arising from natural EBV infection in humans.
Anti-gH/gL antibody AMMO1 neutralizes EBV infection of B cells and epithelial cells.
CryoEM structure of gH/gL-gp42-AMMO1 complex defines a site of EBV vulnerability.
Acknowledgments
We are particularly grateful to Leonidas Stamatatos for use of laboratory space, equipment and for helpful discussions. This research was supported by a Vaccine and Infectious Disease Division Faculty Initiative Grant through the Fred Hutchinson Cancer Research Center (ATM) and the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number R01GM120553 (DV), the Seattle Structural and Genomics Center for Infectious Disease from the National Institute of Allergy and Infectious Diseases under contract number HHSN272201700059C (D.V.), a Pew Biomedical Scholars Award (D.V.), the Netherlands Organization for Scientific Research (NWO, Rubicon 019.2015.2.310.006; JS) and the European Molecular Biology Organisation (EMBO, ALTF 933-2015; JS). The authors acknowledge the use of instruments at the Electron Imaging Center for NanoMachines supported by NIH (1U24GM116792, 1S10RR23057 and 1S10OD018111), NSF (DBI-1338135) and CNSI at UCLA. We thank the James B. Pendleton Charitable Trust for its generous support of Formulatrix robotic instruments. We thank Dr. Anna Wald for serum samples for the EBV seronegative donor (P); Drs. J. Agirre and Y. Yang for helpful discussion on analysis and representation of N-linked glycosylation by MS; Stephen P. Voght for careful technical editing of the manuscript, and Ashley R. Clayton and Abigail Wall for assistance with figure preparation. X-ray diffraction data was collected at the Berkeley Center for Structural Biology beamlines 5.0.1 and 5.0.2, which are supported in part by the National Institutes of Health, National Institute of General Medical Sciences. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. This work was also partly supported by the University of Washington’s Proteomics Resource (UWPR95794).
Footnotes
Author Contributions:
M.S.O., J.S., C.W., A.B.S., D.V., A.T.M, and M.D.G. performed experiments, M.J.M. provided clinical samples, M.P., D.V. and A.T.M designed experiments, analyzed data, and all authors contributed to the writing of the manuscript.
Declaration of Interests
A.T.M discloses that he has filed provisional patents, 62/504,447 and 62/560,061 related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirre J, Iglesias-Fernandez J, Rovira C, Davies GJ, Wilson KS, Cowtan KD. Privateer: software for the conformational validation of carbohydrate structures. Nature structural & molecular biology. 2015;22:833–834. doi: 10.1038/nsmb.3115. [DOI] [PubMed] [Google Scholar]
- Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2880–2885. doi: 10.1073/pnas.0810530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N, Oba DE, Hutt-Fletcher LM. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. Journal of virology. 1987;61:1125–1135. doi: 10.1128/jvi.61.4.1125-1135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern M, Kil YJ, Becker C. Byonic: advanced peptide and protein identification software. Current protocols in bioinformatics. 2012;Chapter 13(Unit13):20. doi: 10.1002/0471250953.bi1320s40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nature medicine. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- Borza CM, Morgan AJ, Turk SM, Hutt-Fletcher LM. Use of gHgL for attachment of Epstein-Barr virus to epithelial cells compromises infection. Journal of virology. 2004;78:5007–5014. doi: 10.1128/JVI.78.10.5007-5014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Long F, Nicholls RA, Toots J, Emsley P, Murshudov G. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta crystallographica Section D, Biological crystallography. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli S, Malito E. Structural basis for potent antibody-mediated neutralization of human cytomegalovirus. 2017;2 doi: 10.1126/sciimmunol.aan1457. [DOI] [PubMed] [Google Scholar]
- Chen J, Jardetzky TS, Longnecker R. The large groove found in the gH/gL structure is an important functional domain for Epstein-Barr virus fusion. Journal of virology. 2013a;87:3620–3627. doi: 10.1128/JVI.03245-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rowe CL, Jardetzky TS, Longnecker R. The KGD motif of Epstein-Barr virus gH/gL is bifunctional, orchestrating infection of B cells and epithelial cells. mBio. 2012;3 doi: 10.1128/mBio.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS, Longnecker R. Ephrin receptor A2 is a functional entry receptor for Epstein–Barr virus. Nature Microbiology. 2018 doi: 10.1038/s41564-017-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, McMullan G, Faruqi AR, Murshudov GN, Short JM, Scheres SH, Henderson R. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy. 2013b;135:24–35. doi: 10.1016/j.ultramic.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta crystallographica Section D, Biological crystallography. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Hutt-Fletcher LM. Fusion of Epstein-Barr virus with epithelial cells can be triggered by alphavbeta5 in addition to alphavbeta6 and alphavbeta8, and integrin binding triggers a conformational change in glycoproteins gHgL. Journal of virology. 2011;85:13214–13223. doi: 10.1128/JVI.05580-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nature structural & molecular biology. 2010;17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Chandramouli S, Leitner A, Donnarumma D, Cianfrocco MA, Gerrein R, Friedrich K, Aggarwal Y, Palladino G, Aebersold R, et al. Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies. PLoS pathogens. 2015;11:e1005230. doi: 10.1371/journal.ppat.1005230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: an important vaccine target for cancer prevention. Science translational medicine. 2011;3:107fs107. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine. 2013;31(Suppl 2):B194–196. doi: 10.1016/j.vaccine.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nature reviews Microbiology. 2011;9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Cao Z, Chen Q, Arjunaraja S, Snow AL, Snapper CM. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine. 2016;34:4050–4055. doi: 10.1016/j.vaccine.2016.06.021. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8245–8250. doi: 10.1073/pnas.95.14.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio F, Song Y, Li X, Brunner MJ, Xu C, Conticello V, Egelman E, Marlovits TC, Cheng Y, Baker D. Atomic-accuracy models from 4.5-A cryo-electron microscopy data with density-guided iterative local refinement. Nature methods. 2015;12:361–365. doi: 10.1038/nmeth.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio F, Tyka MD, Baker ML, Chiu W, Baker D. Refinement of protein structures into low-resolution density maps using rosetta. Journal of molecular biology. 2009;392:181–190. doi: 10.1016/j.jmb.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose NA, Bhiman JN, Roark RS, Schramm CA, Gorman J, Chuang GY, Pancera M, Cale EM, Ernandes MJ, Louder MK, et al. New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. Journal of virology. 2015;90:76–91. doi: 10.1128/JVI.01791-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterhoff D, Moody MA. Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial. 2017;13:e1006182. doi: 10.1371/journal.ppat.1006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson CM, Thorley-Lawson DA. Epstein-Barr virus membrane antigens: characterization, distribution, and strain differences. Journal of virology. 1981;39:172–184. doi: 10.1128/jvi.39.1.172-184.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta crystallographica Section D, Biological crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese CK, Zhou H, Taus T, Altelaar AF, Mechtler K, Heck AJ, Mohammed S. Unambiguous phosphosite localization using electron-transfer/higher-energy collision dissociation (EThcD) Journal of proteome research. 2013;12:1520–1525. doi: 10.1021/pr301130k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. Journal of structural biology. 2007;157:281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Haan KM, Kwok WW, Longnecker R, Speck P. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. Journal of virology. 2000;74:2451–2454. doi: 10.1128/jvi.74.5.2451-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan KM, Lee SK, Longnecker R. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology. 2001;290:106–114. doi: 10.1006/viro.2001.1141. [DOI] [PubMed] [Google Scholar]
- Haque T, Johannessen I, Dombagoda D, Sengupta C, Burns DM, Bird P, Hale G, Mieli-Vergani G, Crawford DH. A mouse monoclonal antibody against Epstein-Barr virus envelope glycoprotein 350 prevents infection both in vitro and in vivo. The Journal of infectious diseases. 2006;194:584–587. doi: 10.1086/505912. [DOI] [PubMed] [Google Scholar]
- Herrman M, Muhe J, Quink C, Wang F. Epstein-Barr Virus gp350 Can Functionally Replace the Rhesus Lymphocryptovirus Major Membrane Glycoprotein and Does Not Restrict Infection of Rhesus Macaques. Journal of virology. 2015;90:1222–1230. doi: 10.1128/JVI.02531-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GJ, Lazarowitz SG, Hayward SD. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Doria-Rose NA, Longo NS, Laub L, Lin CL, Turk E, Kang BH, Migueles SA, Bailer RT, Mascola JR, et al. Isolation of human monoclonal antibodies from peripheral blood B cells. Nature protocols. 2013;8:1907–1915. doi: 10.1038/nprot.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MG, Wheatley AK, Thomas PV, Chuang GY, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, et al. Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell. 2016;166:609–623. doi: 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimanius D, Forsberg BO, Scheres SH, Lindahl E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife. 2016;5 doi: 10.7554/eLife.18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AN, Lowrey AS, Longnecker R, Jardetzky TS. Binding-site interactions between Epstein-Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion. Journal of virology. 2007;81:9216–9229. doi: 10.1128/JVI.00575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AN, Omerovic J, Popov B, Longnecker R, Jardetzky TS. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. Journal of virology. 2006;80:9444–9454. doi: 10.1128/JVI.00572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. Journal of molecular biology. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Li Q, Turk SM, Hutt-Fletcher LM. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. Journal of virology. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QX, Young LS, Niedobitek G, Dawson CW, Birkenbach M, Wang F, Rickinson AB. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. Journal of virology. 2010;84:1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Kirschner AN, Longnecker R, Jardetzky TS. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22641–22646. doi: 10.1073/pnas.1011806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Gray MD, Dosenovic P, Gitlin AD, Freund NT, Petersen J, Correnti C, Johnsen W, Kegel R, Stuart AB, et al. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nature communications. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane MP, Longnecker R. Analysis of fusion using a virus-free cell fusion assay. Methods Mol Biol. 2005;292:187–196. doi: 10.1385/1-59259-848-x:187. [DOI] [PubMed] [Google Scholar]
- Miller G, Niederman JC, Stitt DA. Infectious mononucleosis: appearance of neutralizing antibody to Epstein-Barr virus measured by inhibition of formation of lymphoblastoid cell lines. The Journal of infectious diseases. 1972;125:403–406. doi: 10.1093/infdis/125.4.403. [DOI] [PubMed] [Google Scholar]
- Miller N, Hutt-Fletcher LM. Epstein-Barr virus enters B cells and epithelial cells by different routes. Journal of virology. 1992;66:3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohl BS, Chen J, Sathiyamoorthy K, Jardetzky TS, Longnecker R. Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus. Molecules and cells. 2016;39:286–291. doi: 10.14348/molcells.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohl BS, Sathiyamoorthy K, Jardetzky TS, Longnecker R. The conserved disulfide bond within domain II of Epstein-Barr virus gH has divergent roles in membrane fusion with epithelial cells and B cells. Journal of virology. 2014;88:13570–13579. doi: 10.1128/JVI.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molesworth SJ, Lake CM, Borza CM, Turk SM, Hutt-Fletcher LM. Epstein-Barr virus gH is essential for penetration of B cells but also plays a role in attachment of virus to epithelial cells. Journal of virology. 2000;74:6324–6332. doi: 10.1128/jvi.74.14.6324-6332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PloS one. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DJ, Pope JH. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. The Journal of general virology. 1972;17:233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Moutschen M, Leonard P, Sokal EM, Smets F, Haumont M, Mazzu P, Bollen A, Denamur F, Peeters P, Dubin G, et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine. 2007;25:4697–4705. doi: 10.1016/j.vaccine.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Neuhierl B, Feederle R, Hammerschmidt W, Delecluse HJ. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15036–15041. doi: 10.1073/pnas.232381299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogembo JG, Kannan L, Ghiran I, Nicholson-Weller A, Finberg RW, Tsokos GC, Fingeroth JD. Human complement receptor type 1/CD35 is an Epstein-Barr Virus receptor. Cell reports. 2013;3:371–385. doi: 10.1016/j.celrep.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology. 1999;254:235–244. doi: 10.1006/viro.1998.9530. [DOI] [PubMed] [Google Scholar]
- Omerovic J, Lev L, Longnecker R. The amino terminus of Epstein-Barr virus glycoprotein gH is important for fusion with epithelial and B cells. Journal of virology. 2005;79:12408–12415. doi: 10.1128/JVI.79.19.12408-12415.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods in enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Plate AE, Reimer JJ, Jardetzky TS, Longnecker R. Mapping regions of Epstein-Barr virus (EBV) glycoprotein B (gB) important for fusion function with gH/gL. Virology. 2011;413:26–38. doi: 10.1016/j.virol.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate AE, Smajlovic J, Jardetzky TS, Longnecker R. Functional analysis of glycoprotein L (gL) from rhesus lymphocryptovirus in Epstein-Barr virus-mediated cell fusion indicates a direct role of gL in gB-induced membrane fusion. Journal of virology. 2009;83:7678–7689. doi: 10.1128/JVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R, Bottomley MJ, D’Oro U, Finco O, De Gregorio E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. The Journal of experimental medicine. 2016;213:469–481. doi: 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson AB, Long HM, Palendira U, Munz C, Hislop AD. Cellular immune controls over Epstein-Barr virus infection: new lessons from the clinic and the laboratory. Trends in immunology. 2014;35:159–169. doi: 10.1016/j.it.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. Journal of molecular biology. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Sashihara J, Burbelo PD, Savoldo B, Pierson TC, Cohen JI. Human antibody titers to Epstein-Barr Virus (EBV) gp350 correlate with neutralization of infectivity better than antibody titers to EBV gp42 using a rapid flow cytometry-based EBV neutralization assay. Virology. 2009;391:249–256. doi: 10.1016/j.virol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Hu YX, Mohl BS, Chen J, Longnecker R, Jardetzky TS. Structural basis for Epstein-Barr virus host cell tropism mediated by gp42 and gHgL entry glycoproteins. Nature communications. 2016;7:13557. doi: 10.1038/ncomms13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Jiang J, Hu YX, Rowe CL, Mohl BS, Chen J, Jiang W, Mellins ED, Longnecker R, Zhou ZH, et al. Assembly and architecture of the EBV B cell entry triggering complex. PLoS pathogens. 2014;10:e1004309. doi: 10.1371/journal.ppat.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathiyamoorthy K, Jiang J, Mohl BS, Chen J, Zhou ZH, Longnecker R, Jardetzky TS. Inhibition of EBV-mediated membrane fusion by anti-gHgL antibodies. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E8703–e8710. doi: 10.1073/pnas.1704661114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres SH, Chen S. Prevention of overfitting in cryo-EM structure determination. Nature methods. 2012;9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder J, Borst AJ, Dosey A, Walls AC, Burrell A, Reddy VS, Kollman JM, Veesler D. Vitrification after multiple rounds of sample application and blotting improves particle density on cryo-electron microscopy grids. Journal of structural biology. 2017 doi: 10.1016/j.jsb.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. The Journal of infectious diseases. 2007;196:1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Armitage RJ, Comeau MR, Strockbine L, Farrah T, Macduff B, Ulrich D, Alderson MR, Mullberg J, Cohen JI. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. Journal of virology. 1996;70:5557–5563. doi: 10.1128/jvi.70.8.5557-5563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer SD, Heldwein EE. Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry. Current opinion in virology. 2013;3:13–19. doi: 10.1016/j.coviro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad BC, Schuster T, Klein R, Hopkins RF, 3rd, Witmer T, Neubauer RH, Rabin H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. Journal of virology. 1982;41:258–264. doi: 10.1128/jvi.41.1.258-264.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. Journal of structural biology. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. EMAN2: an extensible image processing suite for electron microscopy. Journal of structural biology. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Palendira U, Edwards ES. Human immunity against EBV-lessons from the clinic. The Journal of experimental medicine. 2017;214:269–283. doi: 10.1084/jem.20161846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J, Weis J, Fearon D, Whang Y, Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987;50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annual review of immunology. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Martinez RJ, Titcombe PJ, Barsness LO, Thomas SR, Zhang N, Katzman SD, Jenkins MK, Mueller DL. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. The Journal of experimental medicine. 2012;209:2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Geilinger K. Monoclonal antibodies against the major glycoprotein (gp350/220) of Epstein-Barr virus neutralize infectivity. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:5307–5311. doi: 10.1073/pnas.77.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Berline JW, Palefsky JM. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nature medicine. 2003;9:307–314. doi: 10.1038/nm830. [DOI] [PubMed] [Google Scholar]
- Turk SM, Jiang R, Chesnokova LS, Hutt-Fletcher LM. Antibodies to gp350/220 enhance the ability of Epstein-Barr virus to infect epithelial cells. Journal of virology. 2006;80:9628–9633. doi: 10.1128/JVI.00622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss NR, Yoshioka CK, Radermacher M, Potter CS, Carragher B. DoG Picker and TiltPicker: software tools to facilitate particle selection in single particle electron microscopy. Journal of structural biology. 2009;166:205–213. doi: 10.1016/j.jsb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kenyon WJ, Li Q, Mullberg J, Hutt-Fletcher LM. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. Journal of virology. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Borza CM, Hutt-Fletcher LM. Mutations of Epstein-Barr virus gH that are differentially able to support fusion with B cells or epithelial cells. Journal of virology. 2005;79:10923–10930. doi: 10.1128/JVI.79.17.10923-10930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Oliver SL, Nguyen T, Ciferri C, Nandi A, Hickman J, Giovani C, Yang E, Palladino G, Grose C, et al. A site of varicella-zoster virus vulnerability identified by structural studies of neutralizing antibodies bound to the glycoprotein complex gHgL. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6056–6061. doi: 10.1073/pnas.1501176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nature reviews Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Y, Wang H-B, Zhang A, Chen M-L, Fang Z-X, Dong X-D, Li S-B, Du Y, Xiong D, et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein–Barr virus entry. Nature Microbiology. 2018 doi: 10.1038/s41564-017-0080-8. [DOI] [PubMed] [Google Scholar]
- Zhang K. Gctf: Real-time CTF determination and correction. Journal of structural biology. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, Agard DA. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nature methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. gH and gL glycopeptides identified by mass spectrometry, related to Figure 3.
Data S2. gp42 glycopeptides identified by mass spectrometry, related to Figure 5.