Abstract
A remarkable example of maladaptive plasticity is the development of epilepsy after a brain insult or injury to a normal animal or human. A structure that is considered central to the development of this type of epilepsy is the dentate gyrus (DG), because it is normally a relatively inhibited structure and its quiescence is thought to reduce hippocampal seizure activity. This characteristic of the DG is also considered to be important for normal hippocampal-dependent cognitive functions. It has been suggested that the brain insults which cause epilepsy do so because they cause the DG to be more easily activated. One type of brain insult that is commonly used is induction of severe seizures (status epilepticus; SE) by systemic injection of a convulsant drug. Here we describe an alteration in the DG after this type of experimental SE that may contribute to chronic seizures that has not been described before: large folds or gyri that develop in the DG by 1 month after SE. Large gyri appeared to increase network excitability because epileptiform discharges recorded in hippocampal slices after SE were longer in duration when recorded inside gyri relative to locations outside gyri. Large gyri may also increase excitability because immature adult-born neurons accumulated at the base of gyri with time after SE, and previous studies have suggested that abnormalities in adult-born DG neurons promote seizures after SE. In summary, large gyri after SE are a common finding in adult rats, show increased excitability, and are associated with the development of an abnormal spatial distribution of adult-born neurons. Together these alterations may contribute to chronic seizures and associated cognitive comorbidities after SE.
Keywords: Granule cell, Epilepsy, Adult neurogenesis, Pilocarpine, Neuropathology
Introduction
The plasticity of the normal brain of mammals is remarkable and typically associated with beneficial effects on behavior. However, there are examples of plasticity that are ‘maladaptive’ or harmful, such as the plasticity which leads to epilepsy after a brain insult or injury.
One of the most common animal models of this type of epilepsy uses severe seizures (status epilepticus; SE) as the insult to initiate the development of epilepsy (Pitkanen et al. 2006). Experimental SE is typically induced by an injection of the chemoconvulsant pilocarpine or KA (Ben-Ari et al. 1979; Nadler 1981; Turski et al. 1983; Levesque et al. 2016). After SE, diverse changes occur, from structural alterations to changes in gene expression and physiology (Dudek et al. 2002; Leite et al. 2002; DeLorenzo and Sun 2006; Taniura et al. 2006; Lukasiuk and Pitkanen 2007; Debski et al. 2016). Alterations occur in neurons, glia and the vasculature both in and outside the DG (Heinemann et al. 2012; Marchi and Lerner-Natoli 2013; van Vliet et al. 2015; Binder and Hubbard 2016; Vezzani et al. 2016). Together the changes in the DG are thought to contribute to epilepsy, i.e., recurrent spontaneous seizures, in the weeks and months after SE (Dudek and Sutula 2007; Noebels et al. 2012; Goldberg and Coulter 2013; Scharfman 2015).
The DG has been increasingly implicated as a key site in the development of epilepsy after a brain insult. One reason for the focus on the DG is based on the idea that the DG normally acts like an inhibitory filter or gate, making incoming afferent input from the entorhinal cortex unlikely to cause action potentials in the principal cells of the DG, the granule cells. As a result, the effects of entorhinal cortical activity on hippocampal neurons are limited by the DG. Thus, the DG is considered to be a barrier to seizure entry into hippocampus and seizures develop when this barrier weakens (Heinemann et al. 1992; Lothman et al. 1992; Ang et al. 2006; Hsu 2007; Pathak et al. 2007; Pun et al. 2012; Krook-Magnuson et al. 2015). Within the DG, numerous mechanisms have been identified which may contribute to the weakening of the ‘DG inhibitory gate’ that weakens in epilepsy. For example, SE typically leads to neuronal loss in the hilar region, and many changes to the principal cells, the glutamatergic granule cells (GCs; (Mello et al. 1993; El Bahh et al. 1999; Roncon et al. 2015). Another effect of SE is to dramatically change the generation of new GCs in the adult brain, called adult neurogenesis. There is a dramatic increase in proliferation after SE, originally shown by Parent et al. (1997). Many of the newly born GCs develop impairments in structure, location and integration into the preexisting circuitry (Scharfman 2004; Overstreet-Wadiche et al. 2006b; Shapiro et al. 2008; Zhao and Overstreet-Wadiche 2008; Hester and Danzer 2014; Jessberger and Parent 2015). Another structural change is the growth of collaterals or ‘sprouting’ of the axons of GCs (Nadler 2003; Sutula and Dudek 2007; Buckmaster 2012), which often arise from abnormal adult-born neurons (Scharfman et al. 2000; Pierce et al. 2011). Notably, there is evidence that aberrant neurogenesis leads to hyperexcitability and epilepsy (Cho et al. 2015), even without SE (Koyama et al. 2012; Pun et al. 2012; Myers et al. 2013). Importantly, there are studies which suggest that some neurons born after SE, possibly those that mature normally rather than abnormally, may quiet the DG network after SE rather than promote seizures (Jakubs et al. 2006), consistent with the evidence that normal adult-born neurons reduce evoked seizures (Iyengar et al. 2015).
In this context of very active research into the changes in the DG in epilepsy, we report another structural change which to our knowledge has not been described: greatly increased gyrification. Thus, large gyri develop in the GC layer after SE. The large gyri appear to be primarily in one part of the DG: the inferior blade of the most caudal region. The gyri develop after either pilocarpine-induced or KA-induced SE in the adult rat. We first characterized the large gyri with different anatomical markers and quantified them. Then we found that the large gyri appeared to be sites of increased network excitability. To do so we used hippocampal slices to show that epileptiform discharges recorded from the GC layer were prolonged inside gyri relative to adjacent areas of the GC layer outside of the gyri. Finally, we acquired evidence that these large gyri after SE reflect a new type of aberrant neurogenesis. Thus, using an antibody to doublecortin (DCX) to mark immature GCs we found that young adult-born neurons were increasingly clustered at the base of large gyri as animals aged several months after SE. By 7–11 months after SE we found that large gyri are almost exclusively the location of immature neurons in the inferior blade of the caudal DG.
We conclude that gyrification is a striking structural change in the rodent DG after SE in the adult rat and probably has not been reported before because most laboratories study the most anterior part of the DG. In addition, increased gyrification appears to contribute to increased excitability in the DG after SE, and adds to the growing list of abnormalities in adult neurogenesis in experimental epilepsy.
Materials and methods
Animal care and use followed the guidelines set by the National Institute of Health and the New York State Department of Health. All chemicals were purchased from Sigma Chemical Co. unless otherwise stated. Animals were purchased from Taconic Farms Inc. and bred in-house. They were housed in standard rat cages with 1–2 other rats of the same sex, had food (Purina 5001; W.F. Fisher and Son Inc.) and water ad libitum, and were maintained on a 12:12 h light:dark cycle.
Induction of SE
Pilocarpine
Adult male Sprague–Dawley rats (1.5–2-month-old, 180–250 g) were injected with atropine methylbromide (1 mg/kg, s.c.) to decrease peripheral side effects of pilocarpine, and 30 min later with pilocarpine hydrochloride (380 mg/kg, s.c.). The onset of SE was defined as the first stage 4–5 seizure (Racine 1972) that did not end, meaning animals did not resume normal behavior (grooming, feeding, normal gait) after 3 min. Diazepam (5 mg/kg, s.c., Wyeth-Ayerst) was injected 1 h after the onset of SE to decrease the severity of SE. Approximately 5 h after the onset of SE, animals were injected s.c. with 2.5 ml of 5% dextrose in lactated-ringers solution (Henry Schein Inc.). Controls were age-matched and were injected with atropine, saline (0.9% NaCl) and diazepam using the volumes and times that would normally be used for pilocarpine treatment. After SE and for approximately 7 days, food intake was encouraged by placing chow and apples (that were cut open) at the bottom of the cage.
Female rats (2–3-month-old) were also administered pilocarpine. The first eight females that were used did not have SE after 380 mg/kg of pilocarpine and another eight did not have SE when the dose was 400 mg/kg, which we reported before (Scharfman and MacLusky 2014; D’Amour et al. 2015), so KA was used (see below).
KA
KA (12 mg/kg; Milestone Pharmatech USA Inc.) was injected in male and female rats at the same ages as those used for pilocarpine, with methods described elsewhere (Scharfman and MacLusky 2014; D’Amour et al. 2015). All males and females exhibited SE in response to KA (n = 5 and 6, respectively).
KA-induced SE was defined differently from pilocarpine-induced SE because animals appeared to develop SE after KA injection by the time of the first stage 3 seizure but the first stage 4–5 seizure after pilocarpine. In KA-injected rats, normal behavior did not resume after the first stage 3 seizure, and animals had persistent twitching of the body and limbs. In the subsequent hour they had stage 4–5 convulsions intermittently. Therefore, we defined the onset of SE after KA as the first stage 3 seizure that was not followed by a resumption of normal behavior. Diazepam (5 mg/kg s.c.; Wyeth-Ayerst, Collegeville, PA) was injected 1 h after the first stage 3 seizure to reduce the severity of seizures. Animals were administered 5% dextrose in lactated-ringers solution after approximately 5 h, such as the pilocarpine-treated male rats. Post-SE treatment was the same as pilocarpine-treated rats.
Anatomy
Immunohistochemistry
After deep anesthesia with urethane (2.5 g/kg), animals were perfused transcardially with saline for 3 min followed by 4% paraformaldehyde (PAF) in 0.1 M phosphate buffer (PB, pH 7.4) for 3 min, as previously described (Scharfman et al. 2002b). Brains were left in the skull overnight at 4 °C and removed the next day. They were postfixed in 4% PAF in 0.1 M PB (pH 7.4) at 4 °C and sectioned (50 μm) using a vibratome (Vibratome 3000; Ted Pella Inc.).
Immunocytochemistry was performed as previously described (Scharfman et al. 2002b). Sections were processed using free-floating sections that were initially washed twice (5 min each) in 0.1 M Tris buffer (pH 7.6) and treated with 1% H2O2 made in 0.1 M Tris buffer (pH 7.6; 30 s). Sections were then washed in 0.1 M Tris buffer (pH 7.6; 5 min) and then treated with 0.25% Triton X-100 dissolved in 0.1 M Tris buffer (Tris A; 45 min), followed by 5% normal goat serum (for polyclonal antibodies) or 5% horse serum (for monoclonal antibodies), diluted in Tris B (0.1% Triton X-100 dissolved in 0.005% bovine serum albumin; BSA) for 45 min.
Next, sections were washed in Tris A (10 min) and then in Tris B (10 min) and incubated in an antibody to DCX (goat polyclonal, 1:6000, Santa Cruz Biotechnology Inc., diluted in Tris A) overnight at room temperature on a rotating shaker. Details about the antibody are provided in Table 1.
Table 1.
Immunohistochemical information
| Primary antibody | Generation and characterization | Blocking solution | Secondary antibody |
|---|---|---|---|
| Doublecortin Goat polyclonal Purchasing information: #SC-8066 Santa Cruz Biotechnology Inc. Dilution: 1:3000 |
General information: doublecortin is a 40 kDa protein involved in brain development Synthesis of the antibody: from the company website: “raised against a peptide mapping at the C terminus of doublecortin of human origin” Specificity of the antibody: Western blot shows 40 kDa expression using whole cell lysates References: (Brown et al. 2003; Couillard-Despres et al. 2005) |
Normal donkey serum (10%, Vector Laboratories) | Donkey anti-goat (1:400, Jackson ImmunoResearch Inc) |
| BrdU Mouse monoclonal Purchasing information: #11170376001 (Roche) Sigma-Aldrich Dilution: 1:1000 |
General: BrdU is a thymidine analog that is incorporated into DNA during DNA synthesis Synthesis: epitope: BrdU The antibody that was used for this project was originally purchased from Boehringer Mannheim which was purchased by Roche and is now available at Sigma-Aldrich Specificity: the pattern of labeling suggests proliferating cells in the DG are labeled by BrdU, both glia and neurons (Scharfman et al. 2000) |
Normal horse serum (10%, Vector) | Horse anti-mouse (1:400, Vector) |
| NeuN Mouse monoclonal Purchasing information: #MAB377, Millipore Dilution: 1:5000 |
General: NeuN is a nuclear antigen found in neurons. The antibody stains both the nucleus and cytoplasm Synthesis: raised against purified cell nuclei from mouse brain Specificity: References: (Sarnat et al. 1998; Duffy et al. 2011) |
Normal horse serum (10%, Vector) and Avidin D (per manufacturer’s instructions for use of NovaRed) | Horse anti-mouse (1:400, Vector) |
Information about antibodies used for immunohistochemistry
On the following day, sections were treated with Tris A (10 min) followed by Tris B (10 min) and then incubated for 45 min with a biotinylated secondary antibody (1:400, Vector Laboratories). Sections were washed in Tris A (10 min), and finally incubated for 2 h in avidin–biotin horseradish peroxidase complex (ABC) diluted in Tris A (ABC Elite kit, 1:1000, Vector). Sections were washed three times in 0.1 M Tris buffer (5 min each), developed with diaminobenzidine tetrahydrochloride (4.5 mg/20 ml of 0.1 M Tris buffer) with 20 μl of glucose oxidase, 40 μl of ammonium chloride, and 160 μl of D-glucose, and then washed three times (5 min each) in 0.1 M Tris buffer.
For figures, images were captured by a digital camera (Retiga 2000R; Q Imaging) attached to a brightfield microscope (Model BX51; Olympus of America Inc.) and figures were composed in Photoshop (v. 7; Adobe Systems Inc.).
Timm and Cresyl stain
For Timm stain, animals were perfused with 0.9% saline for 3 min followed by 0.37% sodium sulfide in PB (pH 7.2) for 5 min, 0.9% saline for 2 min, and finally 4% PAF for 10 min. Sections (50 μm) were mounted on slides that were pre-treated with gelatin so that sections remained adhered to the slide. They were processed for Timm stain as previously described (Scharfman et al. 2002b). Cresyl violet stain was conducted as previously described (Scharfman et al. 2002b).
Bromodeoxyuridine (BrdU) and NeuN double labeling
Animals were injected with BrdU (50 mg/kg i.p.; Boehringer-Mannheim Inc.) twice per day (approximately 9:00 a.m. and 5:00 p.m.) for six consecutive days. Animals were euthanized at least 4 weeks later, perfusion-fixed, and sectioned as described above (“Immunohistochemistry”). Details of the processing for BrdU are provided elsewhere (Scharfman et al. 2000, 2002a). After the reaction, sections were washed in Tris buffer three times (5 min each) and then processed for NeuN labeling immediately. Processing for NeuN followed previously described procedures for use of NovaRed as a chromagen [Vector; (Scharfman et al. 2000, 2002a)]. Double-labeled cells were defined by a BrdU-labeled nucleus surrounded by a NeuN-labeled cytoplasm as described previously (Scharfman et al. 2000, 2002a).
Analysis
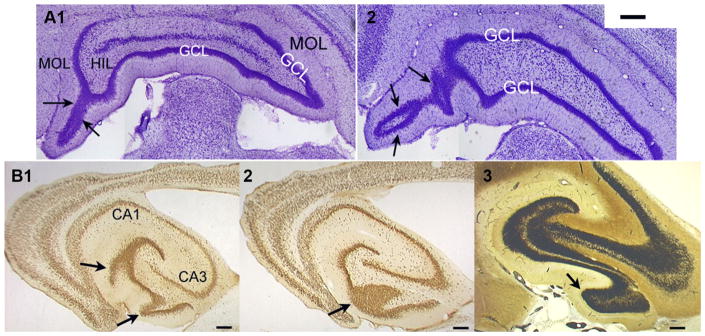
The length of the GC layer was measured after tracing a line corresponding to the center of the GC layer at 10× using Bioquant Lifetools software (Bioquant Inc.) and a brightfield microscope (Axioskop; Carl Zeiss Inc.). In some sections, there were complex invaginations of the GC layer at one of the poles or tips of the DG (i.e., Fig. 2). In these cases, the GC layer was not measured.
Fig. 2.
a Invaginations in the GC layer at the poles of the DG after SE. a1 A Nissl-stained coronal section from a pilocarpine-treated rat that had SE shows invaginations at the dorsal tip (also called the pole) of a section from the posterior DG (arrows). 2 A section from another rat at a more posterior location. In this animal, there was a complex invagination and extension of the GC layer in the dorsal pole. Dorsal is to the left and medial is down. MOL molecular layer. HIL hilus. GCL granule cell layer. Calibration, 200 μm. b Gyri after SE in horizontal sections 1, 2. Two sections from a pilocarpine-treated rat after SE that were cut in the horizontal plane. b1 is more ventral than b2. These sections were stained with an antibody to the neuronal antigen NeuN. Arrows point to areas of the GC layer corresponding to gyri cut at a tangent (1) or along the midline of the gyrus (2). 3 A Timm-stained section from another pilocarpine-treated rat that had SE shows large areas of mossy fiber sprouting in the area corresponding to the gyrus (arrow). Calibration in b1–3, 100 μm
For quantification of labeled cells in the GC layer, the borders of GC layer needed definition. This is difficult because there are cells that are close to the GC layer that some laboratories suggest are normal GCs but dispersed, and others suggest they are part of the molecular layer or hilus and “ectopic.” Here the GC layer was defined as the region where GCs were contiguous. At the border of the GC layer and inner molecular layer or the border of the GC layer and hilus, a cell was defined as outside the GC layer if there was a space between it and the closest cell in the GC layer which was at least the diameter of a GC (10 μm).
Measurements of gyri were made starting at approximately 5.8 mm posterior to Bregma (where the dorsal and ventral DG come together) until approximately 6.2 mm posterior to Bregma (the last section where the CA3 cell layer is detected; (Paxinos and Watson 2007). More posterior parts of the DG were not used because the GC layer was irregular in this location in both controls and epileptic rats, presumably because it is cut at angles to the cell layer where the DG ends.
To measure the number of gyri per hippocampus, we used an approach that took into account the fact that most gyri continued from one 50-μm-thick section to subsequent sections. Therefore, all sections from one hippocampus were examined from 5.8 to 6.2 mm posterior to Bregma to calculate the number of gyri per hippocampus. In addition, sequential sections were used to find the section where a given gyrus was largest, and measurements of depth were made in that section.
Terminology
The terms ‘dorsal’ and ‘ventral’ refer to the surfaces of the brain, not parts of the hippocampus. Therefore, in sections cut in the coronal plane the dorsal part of the section is simply the part closest to the dorsal surface, and does not necessarily mean the dorsal half of the hippocampus. This is important because the dorsal half is often thought of as the part close to the septal pole. Note that the terminology does not apply to the human hippocampus, where the most anterior portion is ventral to the most posterior region.
Electrophysiology
Slice preparation
Animals were anesthetized deeply by placement in a glass jar with isoflurane at the base (Aerrane, Henry Schein Inc.). After loss of consciousness the head was removed with a small animal decapitator and the brain was immediately removed and placed in ice cold buffer (276 mM sucrose, 5 KCl, 2CaCl2, 2 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 10 D-glucose). Slices (400-μm thick) were cut at a 15° angle to the coronal plane using a Vibroslicer (World Precision Instruments). Slices were immediately placed in a plexiglass recording chamber (made in-house) where slices were maintained on a nylon mesh at an interface of ACSF and humidified air. Warm, humidified air was vented into the inner slice chamber from a larger plexiglass outer chamber containing distilled H2O aerated with 95% O2/5% CO2 and maintained at 32 ± 0.5 °C with a feedback temperature controller (Scientific Systems Design Inc). After 30 min, buffer was replaced with one that substituted 125 mM NaCl for sucrose. Recordings began 30 min thereafter. Slices were perfused from below at 1–2 ml/min using a peristaltic pump (Minipuls 2, Gilson Inc.).
Pharmacology
Bicuculline methiodide was dissolved in 0.9% NaCl and stored frozen (−20 °C) as a stock solution (10 mM) until the day of the experiment when it was diluted in ACSF to reach the final concentration (10 μM).
Analysis
Epileptiform burst discharges were bursts of population spikes superimposed on a positive slow potential, corresponding to paroxysmal depolarization shifts in GCs (Rutecki et al. 1985; Hwa et al. 1991; Scharfman 1994a, b; Scharfman et al. 1999; McCloskey and Scharfman 2011) and were evoked by an outer molecular layer stimulus approximately 300 μm away. The stimulating electrode (monopolar, Teflon-coated stainless steel wire, 75 μm wide including the Teflon; A&M Systems Inc.) was placed on the slice surface at the midpoint between the two recording sites (one at the peak of a gyrus and the other at a site adjacent to the gyrus). GC layer recordings were at the GC/hilar border of the cell layer. A 100 μA stimulus was set at duration (typically 30–50 μs) to evoke a burst of population spikes in response to every other stimulus (“threshold”). Stimulus frequency was low so that repeated stimulation would not alter the response (1/60 s). Only one slice was used per animal. Burst duration was measured from the onset of the burst to the point where it returned to the pre-stimulus potential. Burst amplitude was measured from baseline to the peak.
Statistical comparisons
Mean ± standard error of the mean (s.e.m.) is reported in the “Results”. The significance level was p < 0.05. For parametric statistics, a Student’s t test (two-tailed) was used to compare two groups and one-way or two-way ANOVA followed by Tukey–Kramer post hoc tests were used for >2 groups. Interaction of factors are only reported in the Results where they were significant (p < 0.05). For nonparametric statistics, a Mann–Whitney U test was used for two groups and a Kruskal–Wallis ANOVA was used for >2 groups. Non-parametric statistics were used when some values were 0 (i.e., some rats had 0 gyri). Tests were conducted using Graph Pad Prism (GraphPad Software, San Diego, CA).
Results
Subjects
This study is based on more than 70 rats. For pilocarpine-induced SE, there were 47 rats that has pilocarpine and all had SE. There were 12 saline controls. Most rats that had SE were examined >1 month after SE to determine basic characteristics of gyrification. Saline controls were euthanized 8.1 ± 2.0 months after saline (n = 12), an age range that was not statistically different from the pilocarpine-treated rats that were euthanized >1 month after SE (7.6 ± 1.0 months; n = 29; Student’s t test, p = 0.707). To determine the time-dependence of gyrification, additional pilocarpine-treated animals were euthanized 1–3 days (n = 5), 7–8 days (n = 5) or 14–17 days (n = 5) after SE. To determine if the effects of SE in male rats were similar in females, KA was used because it elicits SE reliably in males and females but pilocarpine often fails to elicit SE in females (Scharfman and MacLusky 2014; D’Amour et al. 2015). For these experiments, five male rats and six female rats were injected with KA, had SE, and were euthanized >1 month later. All animals that had SE appeared to develop epilepsy because at least one spontaneous stage 3–5 seizure was detected 4 weeks or longer after SE.
Gyrification in the DG after SE
Using coronal sections, gyri in the GC layer were evident in all rats that had SE but were not detected or small in control rats (Fig. 1; Table 2). Gyri were predominantly in the sections of the DG that were most posterior, and in the inferior blade of these sections (Fig. 1). Large and complex invaginations of the GC layer were also found at the poles of the DG, most easily detected in the coronal plane (Fig. 2a). In horizontal sections, where gyri were cut tangentially, gyri made the GC layer appear to have variations in thickness and location (Fig. 2b).
Fig. 1.
Increased gyrification in the DG after pilocarpine-induced SE. a Nissl-stained coronal sections from one hemisphere of a control rat treated with saline. The most anterior sections are on the top and the most posterior are at the bottom. b Coronal sections in similar locations from a rat that had pilocarpine-induced SE and was perfused 2.5 months later. Arrows point to large gyri in the DG. Calibration in a and b, 250 μm
Table 2.
Gyrification in saline controls and rats that had SE
| Saline All |
With gyri | Pilocarpine | KA | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| 1–3 days | 7–8 days | 14–17 days | >1 month | All | Males | Females | |||
| Mean gyrus length (μm) | |||||||||
| Mean | 89.3 | 178.7 | 173.2 | 194.1 | 280.1 | 348.7 | 343.6 | 322.0 | 361.7 |
| sem | 28.2 | 17.0 | 22.8 | 45.7 | 46.4 | 25.1 | 22.8 | 36.0 | 18.3 |
| n | 12 | 6 | 5 | 5 | 5 | 29 | 11 | 5 | 6 |
| Largest gyrus length (μm) | |||||||||
| Mean | 89.3 | 178.7 | 173.2 | 194.1 | 310.4 | 399.7 | 420.8 | 356.0 | 474.8 |
| sem | 28.2 | 17.0 | 22.8 | 45.7 | 40.6 | 22.0 | 22.2 | 31.4 | 21.4 |
| n | 12 | 6 | 5 | 5 | 5 | 29 | 11 | 5 | 6 |
| Number of gyri | |||||||||
| Mean | 0.5 | 1 | 1 | 1 | 1.6 | 2.1 | 1.9 | 1.6 | 2.2 |
| sem | 0.1 | 0 | 0 | 0 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 |
| n | 12 | 6 | 5 | 5 | 5 | 29 | 11 | 5 | 6 |
Measurements of gyri are shown for saline controls, pilocarpine-treated rats after SE, and KA-treated rats after SE
Figure 3 shows that lamination was maintained using the Timm stain even when gyri were large and numerous. Timm stain of coronal sections was used for this purpose because each sublayer of the DG (e.g., inner, middle and outer molecular layers) is distinct [Fig. 3; (Blaabjerg and Zimmer 2007)]. In epileptic animals, mossy fibers that are normal appear black (i.e., in the hilus); sprouted mossy fibers in the inner molecular layer appear black, the middle molecular layer is clear of Timm stain, and gold stains the outer molecular layer because of a moderate concentration of zinc in the axons of the lateral perforant path [Fig. 3; (Blaabjerg and Zimmer 2007)].
Fig. 3.
Timm stained sections from an animal that had pilocarpine-induced SE show large gyri maintain their lamination. At low power (a) and at higher power (b–d), lamination appears to be normal, i.e., the GC layer and subdivisions of the molecular layer are not distorted in the gyri. IML inner molecular layer, MML middle molecular layer, OML outer molecular layer. Note that b, c are areas of a (indicated by the black bar) that are shown at higher gain. d is an area of c, indicated by the black bar, which is shown at higher power. Calibration, a 250 μm; b, c 100 μm; d 50 μm
Quantification of DG gyri
To determine if gyri were significantly greater in rats that had SE compared to controls, we compared the numbers of gyri and the depth of the gyri. We defined a gyrus as a fold in the DG that was greater than 100 μm because the GC layer is approximately 100 μm wide (measured from the GC layer/hilar border to the GC layer/inner molecular layer border; Fig. 5a). The depth of the gyri were measured from a line drawn between the base or “mouth” of a gyrus to the GC layer/hilar border at the apex or peak of the gyrus (Fig. 4a). The base was defined from a line drawn between the GC layer/hilar border at one edge of the gyrus to the GC layer/hilar border at the other edge of the gyrus (Fig. 4a).
Fig. 5.
Timecourse of increased gyrification after SE. Comparison of mean gyrus length (a), the largest gyrus (b) and the number of gyri (c) at different times after pilocarpine-induced SE showed significant differences between the earliest two time points (1–3 and 7–8 days after SE) and >1 month after SE. One-way ANOVA results for a F(3, 40) 3.68, p = 0.019; for b F(3, 40) 5.93, p = 0.002; for c F(3, 40) 5.65, p = 002). Asterisks indicate significance by Tukey–Kramer post hoc test. Sample sizes are at the base of the bars. There was no significant correlation between the age after SE and mean gyrus length (d, p = 0.320) or the largest gyrus length (e, p = 0.315) and the age after SE. Only animals aged >1 month after SE are shown
Fig. 4.
Quantification of gyri in control and experimental rats. a A schematic illustrates terminology and shows the quantification of gyrus depth. The depth was defined as the distance between the peak and mouth of a gyrus, with the peak defined by the GC layer/hilar border at the apex of the gyrus, and the mouth defined as the area around the base of a gyrus. b1 Mean gyrus length and the largest gyrus length are compared for saline-treated rats with gyri and pilocarpine-treated rats >1 month after SE. Sample sizes are at the base of the bar. A two-way ANOVA showed a significant effect of treatment (F(1, 66) 18.43, p < 0.0001) but not an effect of the test (F(1, 66) 0.65, p = 0.421) and there was no interaction. Bonferroni’s post hoc test showed a significantly greater length in pilocarpine-treated rats (both mean and largest length, p < 0.05). 2 The number of gyri/hippocampus are shown for control and pilocarpine-treated rats that were >1 month after SE. Sample sizes are at the base of the bars. All controls were included. A Mann–Whitney U test showed that there were significantly more gyri in pilocarpine-treated rats (p < 0.0001). c1 KA-treated rats are compared to pilocarpine-treated rats. All animals had gyri. A two-way ANOVA showed no effect of treatment (F(1, 114) 0.24; p = 0.621). 2 Male and female kainic acid-treated rats are compared. All animals were >1 month after SE and had gyri. A two-way ANOVA showed an effect of sex (F(1, 27) 8.95, p = 0.0059) and the type of test (mean gyrus length, largest gyrus length or number of gyri; F(2, 27) 206.60, p < 0.0001) with an interaction (F(2, 27) 3.85, p = 0.034) because the one measurement that was significantly different between males and females was the largest gyrus length; it was significantly larger in females (Bonferroni’s test, p < 0.05)
We first examined pilocarpine-treated rats that were perfused >1 month after saline or pilocarpine. In controls, 6 of 12 controls had 1 gyrus and the other 6 had none. In contrast, all animals had gyri after SE (n = 29). The mean gyrus depth in saline controls was different from pilocarpine-treated rats. This was true when only saline controls with gyri were included (saline controls, n = 6; pilocarpine-treated, n = 29; Fig. 4b1) or when all controls were included, even rats without folds (saline controls, n = 12; pilocarpine-treated, n = 29; Mann–Whitney U test, p < 0.0001).
The largest gyrus was 240 μm deep in saline controls and 780 μm in pilocarpine-treated rats. The means for each group were significantly different when saline controls with gyri were included (saline controls, n = 6; pilocarpine, n = 29; Fig. 4b1) or if all controls were included (saline controls, n = 12; pilocarpine, n = 29; Mann–Whitney U test, p < 0.0001).
When all controls were included, even those without gyri, the mean number of gyri in saline controls (mean 0.50 ± 0.11; range 0–1, n = 12) was different from pilocarpine-treated rats (1.83 ± 0.16, range 1–4, n = 12; Mann–Whitney U test, p < 0.0001; Table 1; Fig. 4b2).
In KA-treated rats examined >1 month after SE, the mean gyrus depth, largest gyrus, and number of gyri were not statistically different from pilocarpine-treated rats >1 month after SE (Table 1; Fig. 4c1, 2). Males that had KA-induced SE were not different from females that had KA-induced SE except for the length of gyri, where the largest gyrus in females was larger than the largest gyrus in males (Fig. 4d1, d2; Table 1).
Time course of gyrification
To determine when gyri develop after SE, we varied the time between SE and perfusion-fixation (Fig. 5a–c). There were few gyri 1–3 days after SE, and they were small (maximum depth, 165 μm, n = 5; Fig. 5a–c). In rats that were evaluated 7–8 days after SE, gyri were also rare and small (maximum depth, 220 μm; Fig. 5a–c). In animals that were examined 14–17 days after SE, gyri were often large (maximum depth, 450 μm, n = 5; Fig. 5a–c). Comparisons of mean gyrus length, largest gyrus length and number of gyri showed that there were significant differences with time after SE, with 1–3 and 7–8 days groups significantly different from rats that were >1 month after SE (one-way ANOVAs and Tukey–Kramer post hoc tests, p < 0.05; Fig. 5a–c). Therefore, gyri increased significantly over the first weeks after SE.
When animals were examined that were perfused >1 month after SE, mean gyrus depth appeared to be independent of time after SE because there was no significant correlation between time after SE and mean gyrus depth (range 1–18 months after SE; n = 29; p = 0.320; Fig. 5d). The correlation between time after SE and mean gyrus length also was not significant (p = 0.315; Fig. 5e).
Physiological function of gyrification after SE
One would predict that gyri might show increased excitability because there are more closely packed GCs than normal, causing nonsynaptic interactions (Jefferys 1995; Dudek and Sutula 2007), such as reduced ability to clear [K+]o (Durand et al. 2010). Elevations in [K+]o cause neurons to depolarize, and they are more likely to reach action potential threshold. Normally there might be no elevation in [K+]o, but one would predict that there would be accumulation of [K+]o in gyri during repetitive action potential firing of GCs and this might cause prolonged GC activity in gyri. To test that hypothesis we caused transient elevation in [K+]o by exposing slices to an antagonist of GABAA receptors (bath-application of 10 μM bicuculline) and then eliciting an epileptiform discharge with an afferent stimulus to the outer molecular layer. The outer molecular layer was chosen because it is the location of perforant path axons that synapse on GC dendrites, a primary source of excitatory input. During epileptiform discharges caused by exposure to bicuculline there were multiple action potentials in GCs, which correspond to population spikes recorded in the GC layer (Fig. 6a).
Fig. 6.
Greater duration of evoked burst discharges inside gyri relative to outside gyri. a1 A schematic illustrates the stimulation site in the outer molecular layer between a gyrus and an area that did not have gyri. There were two recording sites, one inside the peak of the gyrus (1) and the other outside the gyrus (2), which were similar in distance from the stimulating electrode. Recordings were made in the presence of 10 μM bicuculline. 2 The response to a stimulus elicited an epileptiform burst discharge at both recording sites 1 and 2. Arrows mark a similar time after the start of the burst when the burst had ended at site 1 but had not at site 2. 3 A diagram of the measurements of burst amplitude and duration. b Burst amplitude (1) and burst duration (2) are compared for five slices from five different rats. Two-way ANOVA with location (in or outside the gyrus) and type of measurement (amplitude or duration) as factors showed a significant effect of location (F(1, 16) 21.55, p = 0.0003) and type of measurement (F(1, 16) 282.30, p < 0.0001). There also was an interaction of factors (F(1, 16) 22.75, p = 0.0002) because there was a significantly longer burst duration inside gyri relative to outside gyri (post hoc test, p < 0.05). Burst amplitude was not significantly different between sites in and outside gyri (p >0.05)
Notably, bath-application of bicuculline did not elicit spontaneous discharges. In response to stimulation, burst discharges were elicited that were all-or-none, i.e., a burst discharge was produced or there was almost no response to stimulation. These data are consistent with the idea that the epileptiform burst discharges produced in hippocampal slices in response to disinhibition, elevated [K+]o (or other conditions that favor epileptiform activity) are paroxysmal depolarization shifts (PDSs) that are all-or-none sudden, large depolarizations that initiate repetitive firing (Scharfman 1994a, b, 2015; Scharfman et al. 1999; McCloskey and Scharfman 2011).
A stimulation strength and frequency of stimulation was chosen to elicit a burst discharge over 50% of trials. We compared burst discharges at two recording locations in the GC layer that were equidistant from the stimulating electrode: the peak of a gyrus to one side of the stimulating electrode and a point adjacent to that gyrus on the other side of the stimulating electrode (Fig. 6a). Interestingly, when a burst discharge occurred at one recording site, it also occurred at the other recording site, suggesting the ability to evoke a burst discharge was not different.
Amplitude and half-duration of burst discharges were compared (Fig. 6b). Two-way ANOVA with location (in or outside the gyrus) and type of measurement (amplitude or half-duration) as factors showed a significantly greater burst duration in gyri compared to outside gyri, but burst amplitude was not significantly different inside vs. outside gyri (Fig. 6b).
Adult neurogenesis and gyrification after SE
Next we addressed reasons for increased gyrification after SE. We hypothesized that one factor could be adult neurogenesis in the DG, because it increases greatly after SE, as originally shown by Parent et al. (1997) using systemic pilocarpine-induced SE in rats and later using systemic KA-induced SE in rats (Scharfman et al. 2000). We hypothesized that if adult-born GCs accumulated in the DG, the GC layer might fold onto itself to accommodate the large population of GCs. This hypothesis is consistent with the literature about gyrus formation in the developing neocortex where it has been suggested that gyri form as neuronal number increases beyond what can be accommodated by the local constraints on size (Zilles et al. 2013; Sun and Hevner 2014).
To test the idea we first asked if the GC layer increased in length in pilocarpine-treated rats after SE compared to controls. The pilocarpine-treated rats were perfused >1 month after SE. To determine length, animals used to quantify gyri (described above) were chosen. The total length of superior and inferior blades was summed as shown in Fig. 7a, b. A mean was calculated for eight controls and eight pilocarpine-treated rats >1 month after SE. Where there were elongations of the DG poles (Fig. 2a1) measurements were made that included the elongation but when the DG poles exhibited complex invaginations (Fig. 2a2) animals were excluded because tracing the GC layer became difficult to do objectively. The results should be considered in light of this exclusion, which is likely to have underestimated the GC layer length in animals that had SE because invaginations added substantially to the GC layer. Nevertheless, the results showed that the length of the GC layer was larger in pilocarpine-treated rats with SE relative to controls (Student’s t test, p < 0.0001; Fig. 7c).
Fig. 7.
GC layer length increases after SE. a One of the control Nissl-stained sections in Fig. 1 is shown with an arrow pointing to the tracing of the GC layer used to measure its length. b A section from the pilocarpine-treated rat in Fig. 1 is shown with an arrow pointing to the GC layer tracing. c A comparison of GC layer length in control and pilocarpine-treated rats shows that there was a significant increase in the length of the GC layer after pilocarpine-induced SE (Student’s t test, p < 0.0001)
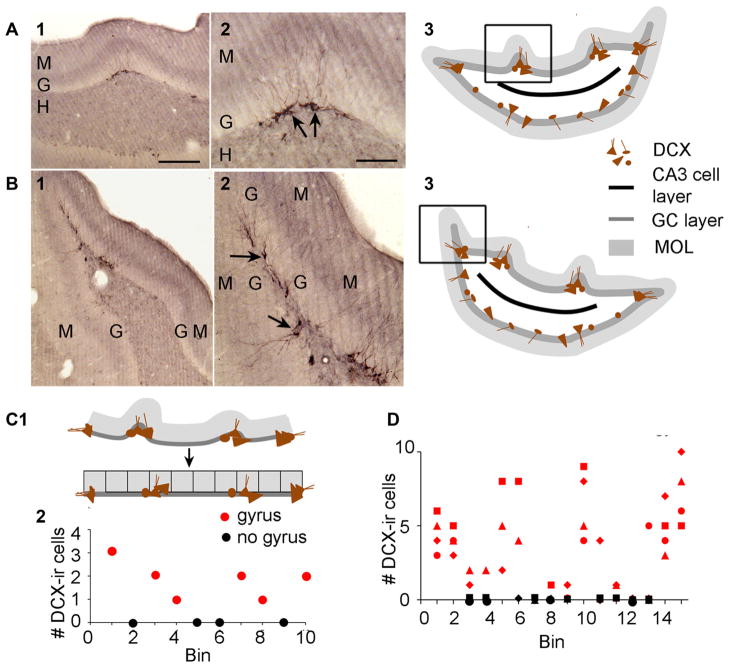
We also stained sections from pilocarpine-treated rats with DCX, a marker of immature neurons (Brown et al. 2003; Couillard-Despres et al. 2005). In animals that were examined 3–11 months after SE (n = 6 pilocarpine-treated rats and 3 KA-treated rats), DCX labeling occurred intermittently in the GC layer of the most posterior part of the DG. As shown in Fig. 8a1, 2, DCX-ir was relatively high in gyri. DCX-ir was also high at the poles of the DG where the GC layer appeared to have extensions (Fig. 8a3, 4). To measure DCX-ir we examined the inferior blade and divided it into bins that were 300 μm in length (Fig. 8c1). Where DCX-labeled cells were detected they were counted and plotted as shown in Fig. 8c2. When four sections (from four different animals) were examined, DCX-ir cells were more prevalent near gyri and at the poles of the DG (Fig. 8d).
Fig. 8.
Gyri in animals after SE contain high numbers of immature GCs. a1, a2 A section from a rat after SE is shown at low power in a1 and higher power in a2. Arrows mark DCX-ir cells in the area of a small gyrus. Calibration, 200 μm (1) or 100 μm (2). 3 Drawing of the orientation of the section in a1, 2 with a box around the areas shown in the micrographs. b1, 2 A section from a different rat than A at one of the DG poles shows DCX-ir is high near the pole. Arrows point to examples of DCX-ir cells. Calibration for b1 is shown in a1. Calibration for b2 is shown in a2. 3 Drawing of the orientation of the section in b1, 2 with a box showing the location of the micrographs. c1 A schematic depicts the preferential location of DCX-ir cells in gyri of the inferior blade of the DG in a section located relatively far from the anterior pole. The methods used to graph DCX-ir cells in d are shown. The inferior blade was stretched out to form a single line. Then it was binned into 300 μm segments. DCX-ir cells in each bin were quantified. 2 A graph of the DCX-ir cell numbers per bin shows that when DCX-ir cells were present, they either were near the poles of the DG or the bin corresponded to the location of a gyrus (denoted by red symbols). When there was no detectable DCX-ir, there was no gyrus (black symbols). d Quantification of DCX-ir cells in bins of the inferior blade in four sections (four rats) show areas of high DCX labeling are where gyri were present. At the start and end of the graph, where the DG poles are located, DCX labeling is also high. When cells are located near a gyrus the symbol is red and when there was no gyrus the symbol is black
In Fig. 9, we used the same approach as in Fig. 8 to examine DCX-ir in animals that were 3–5 months after SE and 7–11 months after SE (Fig. 9a). We found that at the younger ages the DCX-ir was not entirely associated with gyri (Fig. 9b) but at older ages it was remarkably selective for gyri (Fig. 9c).
Fig. 9.
DCX-ir in the DG is altered by time after SE. a A schematic illustrates the groups that were compared, 3–5 months (b) or 7–11 months after SE (c). Immuno = Immunohistochemistry. b1, 2 DCX-ir 4 months after SE. The area in 1 that is outlined by the box is shown at higher gain in 2. There is DCX staining in (arrows in 2) and beside (arrowheads in 2) a small gyrus. 3 A graph shows that animals that were examined 3–5 months after SE (n = 3) had DCX-ir cells in (red) and outside (black) gyri. Data from different rats are denoted by a different symbol. c1, 2 In a rat that was examined 10 months after SE, DCX-ir was only detected in gyri (arrows in 2). 3 A graph of four animals that were examined 7–11 months after SE shows DCX-ir in gyri more selectively than at the earlier ages graphed in b
The preferential localization of DCX-ir cells at the base of gyri suggested that there was preferential localization of adult neurogenesis at the base of gyri, so we conducted BrdU-labeling studies to address this idea. BrdU was injected for six consecutive days at 6 months of age after pilocarpine-induced SE and animals were examined 4 weeks later (n = 5; Fig. 10a). We found that BrdU labeling was extremely rare compared to labeling earlier in life (Fig. 10b), and few cells were double-labeled with NeuN (Fig. 10a–d).
Fig. 10.
BrdU labeling shows decreased adult neurogenesis 6 months after SE. a1 A timeline shows animals were injected with BrdU 6 months after SE (50 mg/kg, i.p., 2 times per day for six consecutive days, n = 5) and examined 1 month later. 2 A timeline shows other animals were injected early after SE (5–11 days after SE; n = 5) and examined 1 month later. b1 A micrograph of a representative section of the DG for a rat that was injected as shown in a1. BrdU-labeled nuclei (black arrow) were rare. NeuN immunoreactivity is orange/brown. Calibration, 10 μm. 2 An example of robust BrdU labeling and double labeling (arrows) in a rat that was injected with BrdU as shown in a2. This image was created by merging three focal planes. c BrdU-labeled cells in the inferior blade of the posterior sections of the DG were quantified for five animals per group, either injected at young (blue) or old (green) ages. The older animals were injected as shown in a1 and the younger group was injected as shown in a2. The total number of double-labeled cells and cells with only BrdU labeling are compared. The total number of BrdU/NeuN-labeled cells was greater in the younger group than the older group (Student’s t test, p < 0.0001; asterisk) but the total number of BrdU-positive cells that lacked NeuN labeling was low in both the younger and older group (p > 0.05). These data suggest a higher proliferation rate and more neurogenesis at young ages. Sample sizes are at the base of the bars. d The percent of cells that were double-labeled is shown for young and old rats. The percentage was defined as the number of double-labeled cells divided by all BrdU-labeled cells. The data in c, d were analyzed by two-way ANOVA with the age as a main factor and the type of comparison a main factor. There was a significant effect of age (F(1, 30) 86.85, p < 0.0001). There also was a significant effect of the type of comparison (F(2, 30) 81.26, p < 0.0001) and an interaction of factors (F(2, 30) 38.78, p < 0.0001). Bonferroni’s post hoc tests showed that there were significantly more double-labeled cells in younger animals than older animals (p < 0.05) in c, d but there was no difference between the numbers of cells that were labeled with BrdU only. The results suggest that the percentage of cells which became neurons was higher in the younger group
These data suggest that DCX-ir cells in large gyri of animals aged 7–11 months after SE were not recently born because the rate of adult neurogenesis is so low that at any time one would not expect a number of DCX-ir cells to arise at one location, such as a gyrus. Instead, DCX-ir in large gyri of older animals might result from accumulation of DCX-ir cells over months. In this situation, DCX-ir may occur for long periods of time because DCX-ir cells stall in their maturation. This hypothesis is supported by studies of rodents after SE where adult-born neurons have delayed maturation (Overstreet-Wadiche et al. 2006a) and studies in the normal rodent where the temporal DG shows a slower maturation than the more anterior portion near the septum (Snyder et al. 2012).
Discussion
Summary
The major finding of this study is that gyri become very large in the inferior GC layer of the most posterior part of the DG after pilocarpine- or KA-induced SE in the adult rat. This increased gyrification was robust, with a single gyrus reaching almost 800 μm in depth and occurring in all rats that were examined >1 month after SE. A second type of GC layer abnormality was found in the poles of the sections which had gyri, which was a complex elongation and twisting or invagination of the GC layer.
Despite the large size of many gyri in the inferior blade, the sublayers of the DG appeared to be maintained, using Timm stain as a marker of individual sublayers. Timm stain also showed that mossy fiber sprouting was similar in and beside gyri. However, Timm stain is only one way to look at DG lamination, and only provides a general view of the circuitry of the DG.
Gyri appeared to increase over the course of the first weeks after SE because in the days after SE the mean gyrus length was similar to controls, but small relative to those found in rats >1 month following SE. However, exactly when the increased gyrification begins is not clear. The animals examined at 1–3 or 7–8 days after SE were not different statistically, but they were significantly different from animals >1 month after SE. These comparisons suggest that gyrification increases after 7–8 days have elapsed following SE but prior to 1 month. There was no correlation of gyrus length with longer times after SE (1–18 months), suggesting that gyri reach a plateau after 1 month.
Robust nature of gyrification
In our studies, gyri were extremely large and found in all animals if they were perfused 1 month or longer after SE. Therefore, it is strange that they have not been noted before. In the published literature, gyri are present, however. For example, large gyri appear in a study of KA-induced SE [Fig. 2; (Bragin et al. 2002)], epilepsy after traumatic brain injury [TBI; Fig. 1e; (Pitkanen et al. 2009)], and epilepsy after perinatal hypoxia [Fig. 11; (Kadam and Dudek 2007)]. Therefore, gyri are likely to be robust but not studied.
These previous reports of animals after SE, TBI or hypoxia suggest that gyri develop because of any epileptogenic insult. However, gyri may develop for other reasons, because there were some gyri in saline controls, although they were small relative to epileptic rats. Interestingly, manipulating lysophosphatidic acid can cause gyri to develop in the cerebral cortex ex vivo in mice (Kingsbury et al. 2003), suggesting that factors which regulate lysophosphatidic acid could influence DG gyri in the absence of an epileptic insult. Interestingly, the mechanism of gyrification was related to reduced neuron death and increased maturation of neural progenitor cells (Kingsbury et al. 2003) see also (Ye et al. 2002; Kingsbury et al. 2004). Therefore, a genetic or environmental change that causes more GCs to accumulate than normal could be sufficient to increase the gyri of the DG. Normally the ratio of accumulation vs. cell death of adult-born neurons may be similar, but genetic, environmental or other factors (e.g., SE) may increase the predisposition for accumulation or decrease cell death—leading to more gyrification.
Mechanisms underlying gyrification
The results showed that most of the increase in gyrification of rats after SE occurs in the first month after SE. This timecourse is consistent with a contribution from neuronal loss, which occurs mainly in the first days after SE and subsequently could trigger a reshaping over several weeks in the part which remains. However, not all animals with large gyri had extensive neuronal loss.
Another possibility is related to adult neurogenesis because there is a very large increase in GC proliferation after SE as first noted by Parent et al. (1997) for pilocarpine-induced SE. Therefore, the increase in gyrification could be caused by increased proliferation of GCs after SE. Although many of the newborn cells died shortly after their birth in the studies of Parent et al. (1997), in our work, the newborn cells persisted for over a year (Scharfman et al. 2000) which may be due to the fact that we used an anticonvulsant to truncate SE much earlier than most laboratories, leading to reduced vulnerability of neural progenitors. The severity of seizures and consequent neuronal damage has been shown to influence adult neurogenesis after SE (Mohapel et al. 2004). Therefore, increased gyrification may be caused by increased adult neurogenesis after SE. Other factors clearly influence gyrification because small gyri were observed in saline controls, as noted above.
Adult-born neurons that were immature were found to cluster at the base of gyri, even in very old animals. Although this observation would suggest that adult neurogenesis continues at these locations long after 1 month following SE, and contributes to increased gyrification >1 month following SE, other data suggest this is not the case. Thus, the rate of adult neurogenesis was extremely low 6 months after SE based on BrdU labeling. In addition, quantification of the gyri showed that they did not significantly increase as animals aged beyond 1 month after SE. Therefore, it appears that there is a slow accumulation of immature neurons at the base of gyri where they stall in their maturation at the stage where DCX is expressed. Importantly, previous studies have shown that adult-born neurons are delayed in their maturation after SE (Overstreet-Wadiche et al. 2006a). In addition, there is a slower rate of maturation in the temporal part of the DG than the part of the DG closest to the septum (Snyder et al. 2012).
The implication of the results are important to consider in the context of many other abnormalities of adult neurogenesis that occur after SE. Immediately after SE, proliferation greatly increases and the newborn cells contribute to a greatly increased number of neurons (Parent et al. 1997). Many of these neurons do not migrate correctly, leading to ectopic granule cells (Scharfman et al. 2000). Other neurons migrate to the GC layer correctly but develop basal dendrites (Spigelman et al. 1998; Ribak et al. 2012). Other GCs hypertrophy or are abnormal in morphology (Murphy et al. 2012; Pun et al. 2012; Hester and Danzer 2014). The current study also suggests that in the most posterior sections of the DG there is an accumulation of immature neurons in gyri and this abnormal spatiotemporal distribution increases as the time after SE increases so that the primary site where immature neurons exists is large gyri, at least for the most posterior parts of the hippocampus.
Relevance to gyrification in neocortex
Cortical gyrification in humans is a normal part of brain development that begins in prenatal life and ends after childhood (Magnotta et al. 1999). Although the DG has gyri in humans, they have not been studied to our knowledge, possibly because of the small size of the DG, making it hard to examine with current imaging techniques. However, cortical gyri have been studied. In disease, abnormal gyrification has been reported, and it is often attributed to errors of cortical development (Armstrong et al. 1995). Abnormal gyrification has been described in schizophrenia (Kulynych et al. 1997; Vogeley et al. 2000; Sallet et al. 2003), dyslexia (Casanova et al. 2004), and autism spectrum disorders (Schmitt et al. 2002; Hardan et al. 2004).
In temporal lobe epilepsy, abnormal cortical gyrification has been discussed but there is not necessarily increased folding; instead, there is often a thinner neocortical width (cortical atrophy), steeper neocortical gyri or changes in curvature of the gyri (Oyegbile et al. 2004; Lin et al. 2007; Ronan et al. 2007). Interestingly, Oyegbile et al. (Oyegbile et al. 2004) showed increased neocortical gyrification was greater in females, although the sample size was small. These data about females are consistent with our studies where the measurement of maximal gyrus length in females was greater than males.
Notably, one study did show that there was increased neocortical complexity in temporal lobe epilepsy, with increased folding (Voets et al. 2011). That study parallels our findings in the DG in an animal model of epilepsy. In this context, it is interesting to consider teleological parallels that have been made between human neocortical and DG gyrification, with stem cells being important to both (Hevner 2016). It is also interesting to note that the sub-ventricular zone gives rise to neurons after brain injury in rodents (Kaplan 1988; Alvarez-Buylla et al. 2000; Gu et al. 2000; Parent 2003). Therefore, in both the epileptic neo-cortex and DG, increased adult neurogenesis after brain insults could lead to increased numbers of neurons, causing increased gyrification in both locations.
Physiological effects of gyrification
We found that epileptiform burst discharges were prolonged in gyri but the amplitude of the burst discharges was similar in and outside gyri. The prolongation of discharges suggests that neurons in gyri were more excitable and mechanisms related to the duration of discharges were particularly affected. One explanation is that increased numbers of GCs in close apposition, as occurs in a large gyrus, would be likely to have reduced astrocytic buffering of [K+]o and the elevation of [K+]o could cause a depolarization of surrounding cells, leading to a more prolonged burst discharge.
There may be a reason why the amplitude of the burst discharges did not appear to differ in and outside gyri. This may be related to an increase in activity of both GABAergic neurons and GCs at the onset of a discharge. Ultimately the activity of GABAergic neurons may lead to depletion of GABA because this has been suggested to occur at the onset of seizures (Zhang et al. 2011, 2012). After that time, after the initial peak in amplitude of the burst discharge, continued depolarization of GCs by elevated [K+]o might cause a greater excitatory influence on GCs than inhibitory mechanisms could control, prolonging the burst discharges.
Whether the increased gyrification contributes to seizures is an interesting question but not clarified by the results. However, it is notable that the ventral hippocampus is thought to be highly excitable (Borck and Jefferys 1999), possibly because of strong recurrent excitatory circuits between the DG and CA3 (Scharfman 2007), and the results presented here suggest another possible mechanism—increased gyrification in the DG. It is also noteworthy that the ventral hippocampus is considered to be a site of origin of seizures after pilocarpine-induced SE, based on studies showing the ventral hippocampus exhibits early neuronal activity at the start of a spontaneous seizure (Toyoda et al. 2013). The corresponding area in humans is considered highly “epileptogenic” (Masukawa et al. 1995; King et al. 1997), but in humans the DG has gyri normally so it remains to be seen if its epileptogenicity is due to an increase in that DG gyrification or other factors. Further studies will be required to prove that the gyrification shown here plays a role in the chronic spontaneous seizures in TLE.
Acknowledgments
NIH NS-037562, NS-081203 and the New York State Department of Health and Office of Mental Health.
References
- Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res. 2000;127:1–11. doi: 10.1016/s0079-6123(00)27002-7. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Lagowska J, Tremblay E, Le Gal La Salle G. A new model of focal status epilepticus: intra-amygdaloid application of kainic acid elicits repetitive secondarily generalized convulsive seizures. Brain Res. 1979;163:176–179. doi: 10.1016/0006-8993(79)90163-x. [DOI] [PubMed] [Google Scholar]
- Binder DK, Hubbard JA. Astrocytes and epilepsy. Academic Press; New York: 2016. [Google Scholar]
- Blaabjerg M, Zimmer J. The dentate mossy fibers: structural organization, development and plasticity. Prog Brain Res. 2007;163:85–107. doi: 10.1016/S0079-6123(07)63005-2. [DOI] [PubMed] [Google Scholar]
- Borck C, Jefferys JG. Seizure-like events in disinhibited ventral slices of adult rat hippocampus. J Neurophysiol. 1999;82:2130–2142. doi: 10.1152/jn.1999.82.5.2130. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Mossy fiber sprouting in the dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies) Bethesda: National Center for Biotechnology Information, US; 2012. [PubMed] [Google Scholar]
- Casanova MF, Araque J, Giedd J, Rumsey JM. Reduced brain size and gyrification in the brains of dyslexic patients. J Child Neurol. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- D’Amour J, Magagna-Poveda A, Moretto J, Friedman D, LaFrancois JJ, Pearce P, Fenton AA, MacLusky NJ, Scharfman HE. Interictal spike frequency varies with ovarian cycle stage in a rat model of epilepsy. Exp Neurol. 2015;269:102–119. doi: 10.1016/j.expneurol.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debski KJ, Pitkanen A, Puhakka N, Bot AM, Khurana I, Harikrishnan KN, Ziemann M, Kaspi A, El-Osta A, Lukasiuk K, Kobow K. Etiology matters—genomic DNA methylation patterns in three rat models of acquired epilepsy. Sci Rep. 2016;6:25668. doi: 10.1038/srep25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sun DA. Basic mechanisms in status epilepticus: role of calcium in neuronal injury and the induction of epileptogenesis. Adv Neurol. 2006;97:187–197. [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res. 2002;135:53–65. doi: 10.1016/S0079-6123(02)35007-6. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arevalo JC, Arancio O, Chao MV, Scharfman HE. A selective role for arms/kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Exp Neurol. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand DM, Park EH, Jensen AL. Potassium diffusive coupling in neural networks. Philos Trans R Soc Lond B Biol Sci. 2010;365:2347–2362. doi: 10.1098/rstb.2010.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bahh B, Lespinet V, Lurton D, Coussemacq M, Le Gal La Salle G, Rougier A. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999;40:1393–1401. doi: 10.1111/j.1528-1157.1999.tb02011.x. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Coulter DA. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci. 2013;14:337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 2004;131:263–268. doi: 10.1016/j.pscychresns.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Heinemann U, Kaufer D, Friedman A. Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60:1251–1257. doi: 10.1002/glia.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy—a possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014;38:105–116. doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF. Evolution of the mammalian dentate gyrus. J Comp Neurol. 2016;524:578–594. doi: 10.1002/cne.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Hwa GG, Avoli M, Oliver A, Villemure JG. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp Brain Res. 1991;83:329–339. doi: 10.1007/BF00231156. [DOI] [PubMed] [Google Scholar]
- Iyengar SS, LaFrancois JJ, Friedman D, Drew LJ, Denny CA, Burghardt NS, Wu MV, Hsieh J, Hen R, Scharfman HE. Suppression of adult neurogenesis increases the acute effects of kainic acid. Exp Neurol. 2015;264:135–149. doi: 10.1016/j.expneurol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Parent JM. Epilepsy and adult neurogenesis. Cold Spring Harb Perspect Biol. 2015;7:a020677. doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SD, Dudek FE. Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy. J Comp Neurol. 2007;505:716–737. doi: 10.1002/cne.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS. Plasticity after brain lesions: contemporary concepts. Arch Phys Med Rehabil. 1988;69:984–991. [PubMed] [Google Scholar]
- King D, Bronen RA, Spencer DD, Spencer SS. Topographic distribution of seizure onset and hippocampal atrophy: relationship between MRI and depth EEG. Electroencephalogr Clin Neurophysiol. 1997;103:692–697. doi: 10.1016/s0013-4694(97)00090-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Ye X, Chun J. Genetics and cell biology of lysophosphatidic acid receptor-mediated signaling during cortical neurogenesis. J Cell Biochem. 2004;92:1004–1012. doi: 10.1002/jcb.20061. [DOI] [PubMed] [Google Scholar]
- Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, Matsuki N, Ikegaya Y. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med. 2012;18:1271–1278. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Bui A, Lew S, Oijala M, Soltesz I. In vivo evaluation of the dentate gate theory in epilepsy. J Physiol. 2015;593:2379–2388. doi: 10.1113/JP270056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulynych JJ, Luevano LF, Jones DW, Weinberger DR. Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry. 1997;41:995–999. doi: 10.1016/S0006-3223(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Levesque M, Avoli M, Bernard C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J Neurosci Methods. 2016;260:45–52. doi: 10.1016/j.jneumeth.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J, Jr, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–2018. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- Lukasiuk K, Pitkanen A. Gene and protein expression in experimental status epilepticus. Epilepsia. 2007;48(Suppl 8):28–32. doi: 10.1111/j.1528-1167.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- Marchi N, Lerner-Natoli M. Cerebrovascular remodeling and epilepsy. Neuroscientist. 2013;19:304–312. doi: 10.1177/1073858412462747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, O’Connor WM, Lynott J, Burdette LJ, Uruno K, McGonigle P, O’Connor MJ. Longitudinal variation in cell density and mossy fiber reorganization in the dentate gyrus from temporal lobe epileptic patients. Brain Res. 1995;678:65–75. doi: 10.1016/0006-8993(95)00167-o. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Scharfman HE. Progressive, potassium-sensitive epileptiform activity in hippocampal area CA3 of pilocarpine-treated rats with recurrent seizures. Epilepsy Res. 2011;97:92–102. doi: 10.1016/j.eplepsyres.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello LE, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis. 2004;15:196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Hofacer RD, Faulkner CN, Loepke AW, Danzer SC. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia. 2012;53:908–921. doi: 10.1111/j.1528-1167.2012.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Bermudez-Hernandez K, Scharfman HE. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS One. 2013;8:e68208. doi: 10.1371/journal.pone.0068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV. Minireview. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. Jasper’s basic mechanisms of the epilepsies. 4. National Center for Biotechnology Information (US); Bethesda: 2012. [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006a;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006b;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile T, Hansen R, Magnotta V, O’Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak HR, Weissinger F, Terunuma M, Carlson GC, Hsu FC, Moss SJ, Coulter DA. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Pierce JP, McCloskey DP, Scharfman HE. Morphometry of hilar ectopic granule cells in the rat. J Comp Neurol. 2011;519:1196–1218. doi: 10.1002/cne.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Schwartzkroin PA, Moshe SL. Models of seizures and epilepsy. Elsevier; New York: 2006. [Google Scholar]
- Pitkanen A, Immonen RJ, Grohn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50(Suppl 2):21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Shapiro LA, Yan XX, Dashtipour K, Nadler JV, Obenaus A, Spigelman I, Buckmaster PS. Seizure-induced formation of basal dendrites on granule cells of the rodent dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s basic mechanisms of the epilepsies. Bethesda: National Center for Biotechnology Information, US; 2012. [PubMed] [Google Scholar]
- Ronan L, Murphy K, Delanty N, Doherty C, Maguire S, Scanlon C, Fitzsimons M. Cerebral cortical gyrification: a preliminary investigation in temporal lobe epilepsy. Epilepsia. 2007;48:211–219. doi: 10.1111/j.1528-1167.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- Roncon P, Soukupova M, Binaschi A, Falcicchia C, Zucchini S, Ferracin M, Langley SR, Petretto E, Johnson MR, Marucci G, Michelucci R, Rubboli G, Simonato M. Microrna profiles in hippocampal granule cells and plasma of rats with pilocarpine-induced epilepsy—comparison with human epileptic samples. Sci Rep. 2015;5:14143. doi: 10.1038/srep14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutecki P, Lebeda FJ, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54:1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- Sallet PC, Elkis H, Alves TM, Oliveira JR, Sassi E, Campi de Castro C, Busatto GF, Gattaz WF. Reduced cortical folding in schizophrenia: an MRI morphometric study. Am J Psychiatry. 2003;160:1606–1613. doi: 10.1176/appi.ajp.160.9.1606. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev. 1998;20:88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience. 1994a;59:245–257. doi: 10.1016/0306-4522(94)90593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Epsps of dentate gyrus granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci. 1994b;14:6041–6057. doi: 10.1523/JNEUROSCI.14-10-06041.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 “backprojection” to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Epilepsy. In: Zigmond MJ, Coyle JT, Rowland L, editors. Neurobiology of brain disorders. Elsevier; New York: 2015. pp. 236–261. [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol Dis. 2014;72(Pt B):180–192. doi: 10.1016/j.nbd.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–5631. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002a;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Smith KL, Jackson MB, Goodman JH. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol. 2002b;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Watts K, Eliez S, Bellugi U, Galaburda AM, Reiss AL. Increased gyrification in Williams syndrome: evidence using 3d MRI methods. Dev Med Child Neurol. 2002;44:292–295. doi: 10.1017/s0012162201002109. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE, Jessberger S. Structural changes for adult-born dentate granule cells after status epilepticus. Epilepsia. 2008;49(Suppl 5):13–18. doi: 10.1111/j.1528-1167.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Ferrante SC, Cameron HA. Late maturation of adult-born neurons in the temporal dentate gyrus. PLoS One. 2012;7:e48757. doi: 10.1371/journal.pone.0048757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman I, Yan XX, Obenaus A, Lee EY, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Sun T, Hevner RF. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Taniura H, Sng JC, Yoneda Y. Histone modifications in status epilepticus induced by kainate. Histol Histopathol. 2006;21:785–791. doi: 10.14670/HH-21.785. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2013;33:11100–11115. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Gorter JA. Blood–brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol. 2015;38:26–34. doi: 10.1016/j.semcdb.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, Loscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets NL, Bernhardt BC, Kim H, Yoon U, Bernasconi N. Increased temporolimbic cortical folding complexity in temporal lobe epilepsy. Neurology. 2011;76:138–144. doi: 10.1212/WNL.0b013e318205d521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P. Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiatry. 2000;157:34–39. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Ye X, Ishii I, Kingsbury MA, Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochem Biophys Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Valiante TA, Carlen PL. Transition to seizure: from “macro”- to “micro”-mysteries. Epilepsy Res. 2011;97:290–299. doi: 10.1016/j.eplepsyres.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Koifman J, Shin DS, Ye H, Florez CM, Zhang L, Valiante TA, Carlen PL. Transition to seizure: ictal discharge is preceded by exhausted presynaptic GABA release in the hippocampal CA3 region. J Neurosci. 2012;32:2499–2512. doi: 10.1523/JNEUROSCI.4247-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CS, Overstreet-Wadiche L. Integration of adult generated neurons during epileptogenesis. Epilepsia. 2008;49(Suppl 5):3–12. doi: 10.1111/j.1528-1167.2008.01632.x. [DOI] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]