Abstract
Background
Parkinson disease (PD) is neurodegenerative disorder characterized by tremor, rigidity and bradykinesia and pathologically by the deposition of alpha-synuclein within different tissues. We, and others, have reported the detection of cutaneous alpha-synuclein in individuals with PD.
Objective
The goal of the present study was to detect alpha-synuclein deposition by immunohistochemical staining of skin samples in pathologically confirmed cases of PD.
Methods
Post-mortem skin biopsy samples from 11 individuals with PD, and 5 non-synucleinopathy control subjects were paraffin embedded and stained for total alpha-synuclein and protein gene product 9.5.
Results
Alpha-synuclein deposition was greater in both scalp and abdominal skin biopsy PD samples compared to control samples in pilomotor nerves (P<0.05), sudomotor nerves (P<0.05) and vasomotor nerves (P<0.05). Deposition of alpha-synuclein in scalp and abdominal tissue did not correlate with age, duration of PD, or severity of PD.
Conclusions
There is greater deposition of alpha-synuclein within pilomotor, sudomotor and vasomotor nerve fibers of paraffin embedded samples from autopsy confirmed cases of PD compared to control samples. However, assessment of alpha-synuclein deposition in post-mortem paraffin embedded tissue has many limitations and the utility of this technique in clinical and research studies is uncertain.
Keywords: Parkinson’s disease, Alpha-synuclein, Skin biopsy, Autonomic
INTRODUCTION
Parkinson disease (PD) is neurodegenerative disorder characterized by tremor, rigidity and bradykinesia and pathologically by the deposition of alpha-synuclein within Lewy bodies and Lewy neurites [1]. We, and others, have reported the detection of cutaneous alpha-synuclein in individuals with Parkinson’s disease [2–6]. We have utilized an antibody that was polyclonal and recognized multiple binding sites from amino acids 111-131, thereby detecting both native and phosphorylated alpha-synuclein. This technique does not distinguish PD from control subjects by the presence or absence of the antibody immunostaining, but detects the difference based on the quantity of alpha-synuclein present within autonomic nerve fibers.[2, 7] To date, this technique has only been implemented in individuals with a possible or probable clinical diagnosis of Parkinson’s disease, without neuropathological confirmation after autopsy [2–6]. Prior studies have investigated the presence of phosphorylated alpha-synuclein in post-mortem sample of individuals with PD, with varying methodologies and results.[8, 9] We report the first systematic study to validate our method for immunohistochemical detection of cutaneous alpha-synuclein in paraffin-embedded sections from pathologically confirmed cases of Parkinson’s disease. If successful, this would allow more widespread use of this technique with standardized automated platforms for immunostaining of alpha-synuclein using paraffin embedded tissue sections.
MATERIALS AND METHODS
Subjects
Eleven individuals with Parkinson’s disease, and 5 non-synucleinopathy disease control subjects participated in the Banner Sun Health Research Institute’s Brain and Body Donation Program [10]. All subjects had annual standardized examinations (Unified Parkinson’s Disease Rating Scale – UPDRS) and complete autopsy staging of Parkinson’s disease, as previously described [10]. Skin samples were taken from the scalp and the abdomen at the time of autopsy and were paraffin embedded [10]. Selection of skin samples sites was made as part of the Banner Sun Health Donation Program, and was not designed based on this study. Samples were chosen at random from the Banner Sun Health Research Institute Donation program. Data provided on disease duration, severity and test scores are taken from the last annual observation prior to death.
Immunohistochemistry
Tissue blocks were fixed in 10% formalin, washed in alcohol followed by dehydration and paraffin embedding at Banner Sun Health Research Institute. These paraffin embedded skin biopsy tissue blocks were cut into 5 μm sections and placed on slides that were sent to Beth Israel Deaconess Medical Center for immunohistochemical processing. Sections were immersed in xylene, washed in alcohol, followed by stepwise rehydration. Slides were microwaved to enhance antigen exposure. The primary antibody used to detect alpha-synuclein was polyclonal and recognized multiple binding sites from amino acids 111-131 (Chemicon) [2, 11]. The tissue sections were incubated with anti-alpha synuclein at 1:10,000 dilution overnight at 4°C, followed by incubation with biotinylated secondary antibody for 2 hours at room temperature. Sections were washed and incubated in HRP-labled streptavidin at a diluation of 1:5000 for 30 minutes at room temperature. Following washout, the sections were incubated with Cy3 labeled tyramide for 10 minutes. The tissue sections were then incubated with rabbit anti-PGP 9.5 (Chemicon) overnight at 4°C, followed by incubation with Cy3 conjugated donkey anti-rabbit for 1 hour at room temperature. Sections were mounted on glass slides and cover-slipped in PBS glycerol (90%). Complete details of our immunohistochemical methods have been previously reported [2, 12].
Confocal imaging
All tissue sections were examined under a fluorescent microscope (Zeiss-Axioplan2) and autonomic substructures were imaged by confocal microscopy (Zeiss LSM5 Pascal Exciter; Carl Zeiss, Thornwood, NY). A single optical section was acquired of all dermal structures (Carl Zeiss, Lens Plan-Apochromat ×20/0.8).
Alpha-synuclein
Alpha-synuclein deposition
The deposition of alpha-synuclein within PGP 9.5 positive nerve fibers was confirmed by merged confocal images. Alpha-synuclein deposition was measured with a semi-quantitative 0-4 scale. Alpha synuclein was considered present only if co-localized on a merged confocal image within PGP 9.5 positive nerve fibers as seen in figure 1. Alpha-synuclein was quantified as the number of alpha-synuclein containing fibers divided by the number of 40X high power fields that contained dermal structures (i.e., the average number of positive fibers per high power field rounded to the first decimal. The following scale was used: 0= no alpha-synuclein present in any tissue section, 1= alpha-synuclein rarely present (≤1 fiber per field), 2= alpha-synuclein present in 1.1-2.0 fibers per field, 3= alpha-synuclein present in 2.1-3.0 fibers per field and 4= present in >3 fibers per field. Five control subjects and 11 Parkinson’s subjects had biopsy samples obtained from the scalp and abdomen. Each biopsy specimen was paraffin embedded, and 6-7 sections from each biopsy were studied.
Figure 1.
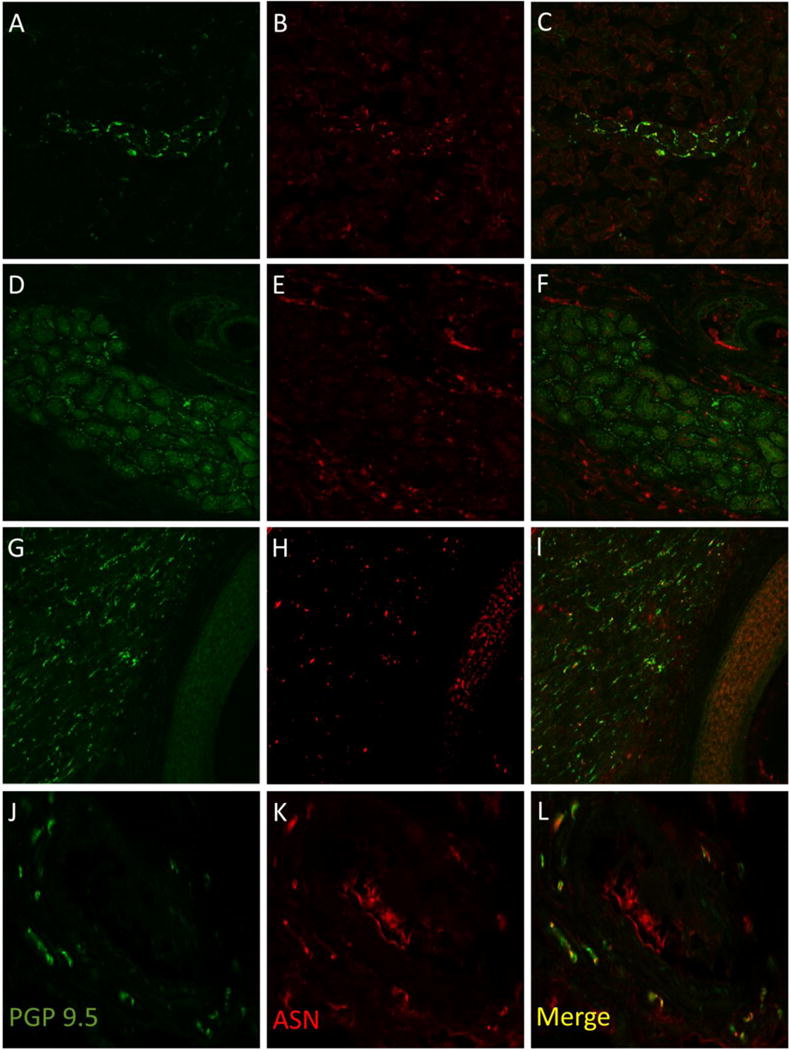
Examples of dermal structures immunostained with PGP 9.5 (A, D, G, J) and alpha synuclein (B, E, H, K) with merged images shown on the right (C, F, I, L). In A-C, a sweat gland is shown from a subject with Parkinson disease with overlapping immunostaining between PGP 9.5 and alpha-synuclein seen as gold color (C). In D-F, a sweat gland from a control biopsy demonstrates little overlapping immunostaining (F). In G-I, a pilomotor muscle from a Parkinson biopsy is shown with overlap between PGP9.5 and alpha-synuclein seen in gold (I). In J-L, a blood vessel from a Parkinson biopsy is shown with overlap between PGP9.5 and alpha-synuclein as gold (L).
Alternative methods of quantifying alpha-synuclein were considered and tested, but due to thin tissue sections (5 micrometer) and therefore relatively small total volumes of tissue for analysis our prior quantitative methods were unable to be used.[2, 7] The establishment of a semi-quantitative method was the only viable option for use.
Statistical analysis
Statistical data analysis was performed using SPSS v17.0 (IBM, Chicago, IL). Unpaired t-test and Fisher’s exact test were used to compare differences between PD and controls. Pearson correlations are reported for relationships between variables. P values of <0.05 were considered significant. Raw data are presented in tabular form.
RESULTS
Subject information
Parkinson subjects
Tissue samples from eleven subjects with Parkinson disease were analyzed. Subjects had a mean age of 79±6 years, a mean duration of disease of 17±4 years, a mini-mental status examination of 24±5, and a United Parkinson Disease Rating Score (UPDRS) score of 37±18. All individuals with Parkinson’s disease had autopsy confirmation of Lewy Body Stage 3 or 4.[10] Individual data are shown in Table 1.
Table 1.
| Disease | Age | MMSE | UPDRS - Total | Disease Duration | Unified Lewy Body Stage | Pilomotor αSN | Sudomotor αSN | Vasomotor αSN |
|---|---|---|---|---|---|---|---|---|
| Control | 95 | 28 | 8 | 0 | Stage 0 | 1 | 1 | 0 |
| Control | 87 | 26 | 1 | 0 | Stage 0 | 0 | 0 | 1 |
| Control | 81 | 29 | 5 | 0 | Stage 0 | 2 | 0 | 1 |
| Control | 68 | 29 | 3 | 0 | Stage 0 | 0 | 1 | 0 |
| Control | 75 | 29 | 2 | 0 | Stage 0 | 0 | 1 | 2 |
| PD | 87 | 13 | 57 | 21 | Stage 4- Neocortical | 0 | 2 | 1 |
| PD | 75 | 27 | 32 | 20 | Stage 3- Brainstem/Limbic | 2 | 1 | 3 |
| PD | 81 | 19 | 74 | 14 | Stage 3- Brainstem/Limbic | 3 | 0 | 2 |
| PD | 79 | 29 | 38 | 14 | Stage 3- Brainstem/Limbic | 2 | 1 | 3 |
| PD | 69 | 24 | 21 | 10 | Stage 3- Brainstem/Limbic | 1 | 2 | 2 |
| PD | 91 | 26 | 17 | 12 | Stage 3- Brainstem/Limbic | 2 | 2 | 1 |
| PD | 75 | 26 | 27 | 21 | Stage 4- Neocortical | 3 | 0 | 3 |
| PD | 74 | 27 | 51 | 17 | Stage 3- Brainstem/Limbic | 3 | 2 | 2 |
| PD | 78 | 24 | 51 | 19 | Stage 3- Brainstem/Limbic | 2 | 3 | 3 |
| PD | 75 | N/A | 17 | 20 | Stage 4- Neocortical | 0 | 2 | 3 |
| PD | 84 | N/A | 28 | 20 | Stage 3- Brainstem/Limbic | 2 | 2 | 1 |
MMSE: mini mental status examination, UPDRS: Unified Parkinson’s Disease Rating Scale, Unified Lewy Body Stage: Scale of 0 (none) to 4 (neocortical involvement). Pilomotor ASN: scoring of alpha synuclein deposition within pilomotor nerves (0-4 scale). Sudomotor ASN: Sudomotor alpha synuclein deposition within sudomotor nerves (0-4 scale).
Control subjects
Tissue samples from 5 control subjects were used in this study. Subjects were free of parkinsonism or dementia on standardized clinical assessments by cognitive and movement specialized neurologists, had a mean age of 81±9 years and a variety of underlying non-synucleinopathy diseases, with individual data shown in Table 1.
Skin biopsy results
Dermal structures (pilomotor muscles, sweat glands and blood vessels) were seen in 55% of tissue sections with 20% of tissue sections containing 2 or more dermal structures. All subjects had at least 3 analyzable tissue sections. Pilomotor, sudomotor and vasomotor nerve fibers were detected using PGP 9.5 within all dermal pilomotor muscles, sweat glands and blood vessels.
Of the control samples, 60 tissue sections (30 scalp and 30 abdomen) were analyzed and 35 tissue sections contained dermal structures that included: 28 pilomotor muscles, 23 sweat glands and 16 blood vessels. In the Parkinson’s samples, 140 tissue sections (70 scalp and 70 abdomen) were analyzed and 75 sections contained dermal structures that included: 59 pilomotor muscles, 42 sweat glands and 32 blood vessels. Tissue sections from PD and control subjects with dermal structures with PGP9.5 positive fibers all contained alpha-synuclein positive regions that co-localized with the PGP9.5 immunostained fibers.
Alpha-synuclein deposition in abdominal tissue, measured using the above semi-quantitative scale, was greater in PD samples compared to control samples in pilomotor nerves (1.8±1.1 PD vs. 0.67±0.82 Control, P<0.05), sudomotor nerves (1.54±0.93 PD vs. 0.50±0.55 Control, P<0.05) and vasomotor nerves (2.00±1.41 PD vs. 0.67±1.03 Control, P<0.05). The alpha-synuclein deposition in scalp tissue was greater in PD samples compared to control samples in the pilomotor nerves (1.70±0.91 PD vs. 0.59±0.88 Control, P<0.05), in sudomotor nerves (1.63±1.21 PD vs. 0.57±0.71 Control, P<0.05) and in vasomotor nerves (2.13±1.28 PD vs. 1.01±0.73 Control, P<0.05). There was no correlation between the deposition of alpha-synuclein and the age, duration of Parkinson’s disease, or severity of Parkinson’s disease in either the scalp or abdominal tissue.
DISCUSSION
We report our initial experience in detection of cutaneous alpha-synuclein in post-mortem skin obtained from autopsy confirmed cases of Parkinson’s disease. Unlike our prior studies that included individuals with a clinically established diagnosis [3, 13], the present study included subjects that had both had a clinical diagnosis of PD as well as autopsy confirmation of disease stage. We note greater deposition of alpha-synuclein within pilomotor, sudomotor and vasomotor nerve fibers of tissue samples from subjects with Parkinson’s disease compared to control subjects using an antibody targeting multiple alpha-synuclein binding domains from amino acids 111-131. We did not detect any difference in nerve density or alpha-synuclein deposition between the scalp and abdominal tissue, although this study was not powered to investigate that question.
Although we are able to detect alpha-synuclein from post-mortem cutaneous tissue of patients with pathologically confirmed Parkinson’s disease, there are several key limitations to the assessment of alpha-synuclein in paraffin-embedded tissue. 1) In contrast to 50μm frozen-sections acquired from a standard in-vivo skin biopsy, paraffin embedded autopsy tissue sections are 5μm thick and therefore provide only a fraction of the volume of frozen tissue sections. This is particularly important because alpha-synuclein is predominantly located within the autonomic nerves of dermal structures (such as blood vessels, sweat glands and pilomotor muscles) that are scattered randomly throughout the skin; a smaller sampling volume decreases the odds of adequate specimen acquisition. 2) Paraffin embedded samples are fixed in 10% formalin for 24-72 hours (which tends to over-fix and reduce peripheral nerve detection) while cutaneous skin biopsies are typically fixed in a 2% paraformaldehyde solution for 18-24 hours for optimized detection of peripheral nerve fibers [14]. As a consequence, the paraffin embedded tissue sections have a lower nerve density counts due to over-fixation, which reduces the visual detection of PGP9.5 immunostained fibers, without which, co-localization cannot be performed and a reliable alpha-synuclein ratio cannot be calculated [2]. The alpha-synuclein ratio normalizes for the presence of a peripheral neuropathy, a common feature of Parkinson’s disease and lower nerve density counts distort the calculation of the ratio [15]. 3) Substantially greater numbers of paraffin embedded tissue sections need to be processed in order to provide equivalent sampling to thick frozen tissue sections that are acquired during a standard in-vivo skin biopsy. 4) In the thinner paraffin embedded tissue sections the nerve fibers also appear as ‘dots’ rather than traceable nerve fibers thus reducing the ability to definitively co-localize alpha-synuclein within immunostained nerve fibers. Co-localization is critical to maintain the sensitivity and the specificity of the technique because true alpha-synuclein deposits may be missed and artifacts may be considered positive in the absence of co-localization with PGP9.5. 5) In addition, the thin paraffin embedded tissue sections are unable to detect morphologic abnormalities to nerve fibers that might suggest pathologic changes, such as increased nerve fiber tortuosity, changes in fiber thickness or nerve fiber aggregation.[5, 16, 17] 6) Finally, the thin tissue sections prevent accurate assessment of autonomic nerve fiber density using standard methodology.[18, 19]
The choice of a non-selective antibody for alpha-synuclein, or use of an antibody specific for phosphorylated alpha-synuclein is also an important consideration. Phosphorylated alpha-synuclein is the pathologic form of alpha-synuclein, and considered a specific marker for a synucleinopathy.[5, 6] However, in prior studies phosphorylated alpha-synuclein tends to be present in very small deposits that can be confused with artifact if not co-localized to cutaneous nerves immunostained with a pan-axonal marker [3, 5]. In contrast, antibodies against total alpha-synuclein allow for easy identification of nerve fibers, but are present within controls samples (although in smaller amounts) [2]. The choice of antibodies therefore makes a large difference in study design and outcomes. This requires further investigation.
In the present study, and in contrast to our prior work [2], we did not detect a relationship between alpha-synuclein deposition and Parkinson’s disease duration or severity. However, our current study was a small pilot study, and was not powered to detect such a relationship. Furthermore, the mean duration of Parkinson’s was 17 years (and none of the samples from individuals with PD of less than 10 years), therefore it is not possible to draw firm conclusions between alpha-synuclein deposition and disease duration. Despite these challenges, we report that cutaneous alpha-synuclein can be detected in post-mortem tissue from patients with Parkinson’s disease. However this study, and work by others,[5, 6] suggests that the results of trials that use paraffin embedded tissue sections with low volumes of tissue for analysis or study of alpha-synuclein without co-localization with PGP 9.5 should be interpreted with caution. It is essential that co-localization of alpha-synuclein with PGP9.5 be incorporated into the methodology, without which artifacts can easily be misinterpreted. The present data suggest that paraffin embedded tissue sections may have limited value as a diagnostic biomarker but are not likely to demonstrate value as a prognostic biomarker or as a biomarker for assessing therapeutic efficacy.
There are many advantages to the use of paraffin embedded tissue sections for the analysis of peripheral alpha-synuclein deposition. The capacity for more widespread access to the methodology and the ability to apply platform-based automation are of potential interest for clinical and research purposes, but, despite these advantages, the technical challenges that are inherent to studying paraffin embedded tissue suggest that the utility of this approach in the study of cutaneous alpha-synuclein deposition may be limited.
Acknowledgments
FUNDING SOURCES
This work was supported by the Michael J. Fox Foundation (RF) and NIH grant U54NS065736 (RF). Skin samples supplied by the Banner Sun Health Research Institute’s Brain and Body Donation Program. The Banner Sun Health Research Institute Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Funding Sources for Study: Supported by the Michael J. Fox Foundation (R.F.) and NIH U54NS065736 (R.F.).
Footnotes
Financial Disclosure/Conflict of Interest: None relevant to this research. Full disclosures provided at the end of the manuscript.
FINANCIAL DISCLOSURES:
C. Gibbons received personal compensation for serving on the scientific advisory boards of Pfizer Inc. and Lundbeck Inc. N. Wang reports no disclosures relevant to the manuscript. R. Freeman received personal compensation for serving on scientific advisory boards of Astellas, Biogen, Dong, Glenmark, Hydra, Johnson and Johnson, Lundbeck, Pfizer, and Spinifex; and has received personal compensation for his editorial activities (Editor) with Autonomic Neuroscience: Basic and Clinical.
References
- 1.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del TK, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang N, Gibbons CH, Lafo J, Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81(18):1604–10. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, Capellari S, Avoni P, Baruzzi A, Liguori R. Skin nerve alpha-synuclein deposits: A biomarker for idiopathic Parkinson disease. Neurology. 2014;82(15):1362–9. doi: 10.1212/WNL.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 4.Zange L, Noack C, Hahn K, Stenzel W, Lipp A. Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain. 2015;138(Pt 8):2310–21. doi: 10.1093/brain/awv138. [DOI] [PubMed] [Google Scholar]
- 5.Doppler K, Ebert S, Uceyler N, Trenkwalder C, Ebentheuer J, Volkmann J, Sommer C. Cutaneous neuropathy in Parkinson’s disease: a window into brain pathology. Acta Neuropathol. 2014;128(1):99–109. doi: 10.1007/s00401-014-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doppler K, Weis J, Karl K, Ebert S, Ebentheuer J, Trenkwalder C, Klebe S, Volkmann J, Sommer C. Distinctive Distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Movement disorders: official journal of the Movement Disorder Society. 2015 doi: 10.1002/mds.26293. [DOI] [PubMed] [Google Scholar]
- 7.Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology. 2016;87(5):505–12. doi: 10.1212/WNL.0000000000002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Murayama S. Lewy body pathology involves cutaneous nerves. Journal of neuropathology and experimental neurology. 2008;67(10):945–53. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- 9.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology: official journal of the Japanese Society of Neuropathology. 2015;35(4):354–89. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Gibbons CH, Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2011;59(4):382–90. doi: 10.1369/0022155410396931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haga R, Sugimoto K, Nishijima H, Miki Y, Suzuki C, Wakabayashi K, Baba M, Yagihashi S, Tomiyama M. Clinical Utility of Skin Biopsy in Differentiating between Parkinson’s Disease and Multiple System Atrophy. Parkinson’s disease 2015. 2015:167038. doi: 10.1155/2015/167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbons CH, Wang N, Freeman R. Capsaicin induces degeneration of cutaneous autonomic nerve fibers. Annals of neurology. 2010;68(6):888–98. doi: 10.1002/ana.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kass-Iliyya L, Javed S, Gosal D, Kobylecki C, Marshall A, Petropoulos IN, Ponirakis G, Tavakoli M, Ferdousi M, Chaudhuri KR, Jeziorska M, Malik RA, Silverdale MA. Small fiber neuropathy in Parkinson’s disease: A clinical, pathological and corneal confocal microscopy study. Parkinsonism & related disorders. 2015;21(12):1454–60. doi: 10.1016/j.parkreldis.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauria G, Morbin M, Lombardi R, Borgna M, Mazzoleni G, Sghirlanzoni A, Pareyson D. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61(5):631–636. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66(2):256–8. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- 18.Nolano M, Provitera V, Caporaso G, Stancanelli A, Vitale DF, Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology. 2010;75(12):1089–97. doi: 10.1212/WNL.0b013e3181f39cf4. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 2009;72(17):1479–86. doi: 10.1212/WNL.0b013e3181a2e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]


