Abstract
Measurement of oocyte and embryo biomechanical properties has recently emerged as an exciting new approach to obtain a quantitative, objective estimate of developmental potential. However, many traditional methods for probing cell mechanical properties are time consuming, labor intensive and require expensive equipment. Microfluidic technology is currently making its way into many aspects of assisted reproductive technologies (ART), and is particularly well suited to measure embryo biomechanics due to the potential for robust, automated single-cell analysis at a low cost. This review will highlight microfluidic approaches to measure oocyte and embryo mechanics along with their ability to predict developmental potential and find practical application in the clinic. Although these new devices must be extensively validated before they can be integrated into the existing clinical workflow, they could eventually be used to constantly monitor oocyte and embryo developmental progress and enable more optimal decision making in ART.
Keywords: embryology, microfluidics, biomechanics, oocyte maturation, fertilization, embryo development
Introduction
In recent years, numerous studies on cell biomechanics have proven that cells can sense and actively respond to the physical properties of their environment. Extracellular forces may influence a cell's developmental fate, and a cell's biomechanical properties may serve as biomarkers of disease or indicators of its internal molecular state (Engler et al., 2006; Kumar and Weaver, 2009; Guck and Chilvers, 2013). Physicians have long been able to diagnose tumors by using palpation to sense differences in tissue stiffness (Huang and Ingber, 2005). More recently, it has been shown that extracellular matrix stiffness is greatly increased in cancerous tissue, and cancer cells have different viscoelastic properties compared with normal cells (Suresh et al., 2005; Zaman et al., 2006; Cross et al., 2007; Li et al., 2008; Lekka et al., 2012; Rebelo et al., 2013). Although cancer is the best studied example of mechanics reflecting disease state, there are also examples of mechanical biomarkers being used to identify malaria, sickle cell anemia and Type 2 diabetes (Suresh et al., 2005; Suresh, 2006; Jin et al., 2010). Along with improvements in biomechanical measurement techniques, these discoveries have recently inspired a renewed interest in understanding the interactions between an embryo's physical environment, its mechanical properties and its developmental potential.
Some of the most common techniques for measuring cell biomechanical properties include micropipette aspiration, atomic force microscopy and optical tweezers. While these techniques have been extensively used in scientific studies, they have not been widely translated into clinical applications because they require expensive equipment, are low throughput and require significant labor and training to use. In recent years, improvements in microfluidic technology have made it possible to automate many biomechanical measurement techniques, thereby reducing cost, drastically increasing throughput and reducing inter-user variability in measurement. This review will cover the growing body of literature focused on microfluidic methods of measuring oocyte and embryo mechanical properties, and using these properties as biomarkers of developmental potential. It will also explore how these methods can find practical application in the IVF clinic, through integration with automated, microfluidic platforms and in combination with existing viability assessment technologies.
Advantages of using mechanical biomarkers in assisted reproductive technologies
There has been a great deal of effort directed toward developing objective, quantitative and accurate markers of oocyte and embryo developmental potential. The advent of extended culture to the blastocyst stage greatly improved identification of viable embryos simply through attrition, and has helped to reduce the incidence of multiple gestation pregnancy and contributed to higher rates of elective single-embryo transfer in recent years (Gardner et al., 1998, 2004; Forman et al., 2013). Although extended time in the artificial environment of the IVF laboratory can affect certain aspects of embryo health and development, currently the benefits of improved selection ability vastly outweigh the drawbacks of time in culture (Dumoulin et al., 2010; Nelissen et al., 2012, 2013; El Hajj and Haaf, 2013). Techniques such as preimplantation genetic screening (PGS) for aneuploidy detection have been a valuable addition to the IVF toolbox as they can identify chromosomal abnormalities with very high accuracy. However, PGS may not be indicated for all patient populations as it is highly invasive and expensive, can fail to detect mosaicism and cannot identify embryos, which are euploid but fail to implant for other reasons (Gardner et al., 2015). Time-lapse imaging of cell-cycle parameters has recently been developed as a non-invasive approach to embryo viability prediction, and is showing promising results in initial clinical trials (Conaghan et al., 2013; Kaser and Racowsky, 2014; Vermilyea et al., 2014). Some effort has also been directed toward correlating embryo metabolic activity and protein content to developmental potential, but most potential biomarkers have been found to be difficult to measure reliably or poorly predictive of viability (Gardner et al., 2015).
More recently, measurement of embryo mechanical properties has emerged as a potential new biomarker to predict blastocyst development and pregnancy (Murayama et al., 2008a; Yanez et al., 2016). Although this approach is still relatively new and will require extensive clinical validation, it could have significant benefits over currently available techniques for embryo selection. In particular, mechanics can be measured as early as the oocyte or zygote stage, and could be used to conduct embryo transfer immediately after fertilization, minimizing time in the stressful culture environment while improving rates of singleton pregnancy. Mechanical biomarkers could also fill the void in available predictors of oocyte quality, allowing physicians to make better choices about how many oocytes to collect for cryopreservation and to evaluate the effectiveness of hormonal stimulation protocols. Rather than attempting to fertilize all oocytes at once, physicians could elect to fertilize a subgroup of high quality oocytes, potentially avoiding the creation of supernumerary embryos and the waste of clinic time and resources on oocytes that will likely not be transferred.
Importance of biomechanics in reproductive biology
There are many physical stimuli and mechanical changes that happen during oocyte maturation, ovulation, fertilization and subsequent embryo development and that can influence developmental outcomes. An overly stiff or dense culture environment has been shown to impair follicle growth, alter levels of endocrine hormones and reduce oocyte quality (West et al., 2007). Increased cortex thickness and stiffness is also seen in women with polycystic ovarian syndrome (PCOS), and has been suggested to contribute to the impaired follicle development and oocyte quality associated with this condition (Hughesdon, 1982; West et al., 2007). Recently, fertilized embryos also experience significant mechanical forces as peristaltic motion pushes them through the fallopian tubes and directs them to an appropriate implantation site in the uterus (Eytan et al., 2001).
While oocytes and embryos are subject to significant mechanical inputs from their environment over the course of development, they also exhibit large variation and changes in their own mechanical properties. The most well known of these changes is the increase in stiffness experienced by the zona pellucida (ZP) during fertilization which is called ‘zona hardening’. Although the function of zona hardening is still unclear, there is some evidence it could contribute to prevent polyspermy in some species (Downs et al., 1986; Coy et al., 2008). At first, the degree of hardening was measured by quantifying the zona's resistance to dissolution by biochemical agents, which is destructive to the oocyte or embryo within and is therefore not clinically practical (De Felici et al., 1985). A variety of methods have since been developed to measure ZP physical properties in a non-invasive or minimally invasive fashion, which will be discussed in detail in this review. It is important to note that resistance to dissolution is not always coupled with mechanical stiffness, so care must be taken when comparing zona hardness results obtained via biochemical or physical methods (Drobnis et al., 1988).
Biochemical studies on ZP hardness have demonstrated that the ZP becomes softer over the course of meiotic maturation from the germinal vesicle (GV) and to the metaphase II (MII) stage (Murayama et al., 2013). Spontaneous zona hardening has also been observed in response to post-ovulatory aging in vivo and in some in vitro culture conditions, and is correlated with significantly reduced rates of fertilization (Longo, 1981; de Felici and Siracusa, 1982; Gianfortoni and Gulyas, 1985; Fukuda et al., 1992; Kim et al., 2004; Miao et al., 2009; Vajta et al., 2010; Lord and John Aitken, 2013). When human oocytes fail to fertilize during IVF due to their excessive stiffness, ICSI may be attempted to directly induce fertilization (Schiewe et al., 1995). Although this so-called ‘rescue ICSI’ is capable of inducing fertilization, especially if it is performed as early as possible, the resulting embryos are often of poor quality and have increased rates of early pregnancy loss and abnormalities in offspring (Wilcox et al., 1998; Yuzpe et al., 2000; Kuczyński et al., 2002; Chen and Kattera, 2003; Nagy et al., 2006). Interestingly, one study found that ICSI produces slightly stiffer embryos compared with IVF, although it is unclear whether this could affect subsequent development (Manna et al., 2001). It appears that zona hardening in oocytes may be correlated with maturation and post-ovulatory aging, and an optimally soft ZP may therefore serve as a biomarker of high fertilization and developmental potential.
The cause of zona hardening in response to post-ovulatory oocyte aging remains unclear. In normal fertilization, cortical granules arranged around the perimeter of the oocyte release their contents into the perivitelline space (PVS), which then induce zona hardening (De Felici et al., 1985). Prolonged in vitro culture and post-ovulatory aging have been demonstrated to induce partial, premature cortical granule exocytosis in mouse, and may be a contributing factor to zona hardening in the absence of fertilization (Miao et al., 2009; Lord and John Aitken, 2013). The surface of the ZP in aged oocytes displays a rough, cobblestone-like appearance, which is similar to that of fertilized embryos and immature oocytes (Miao et al., 2009). It is thought that substances released by cortical granules may induce an increase in crosslinking between ZP proteins, which can be measured as an increase in stiffness (Cohen, 1991).
The mechanical characteristics of the oolemma and cytoplasm have also been correlated with oocyte maturation, post-ovulatory aging and developmental outcomes. Aged oocytes exhibit decreased oolemma fluidity, which may inhibit sperm fusion and decrease the likelihood of successful fertilization (Takahashi et al., 2003). In these oocytes, the microvilli present in the oolemma extend abnormally far into the PVS and may have other structural alterations (Miao et al., 2009). Qualitative observations of ICSI in oocytes showed that the response of the membrane to needle puncture is predictive of embryo morphology and survival in culture (Palermo et al., 1996; Ebner et al., 2003). Recently, the appearance, size and persistence of the ICSI injection funnel was also correlated with oocyte maturity, suggesting that cytoplasmic viscosity increases over the course of maturation (Krause et al., 2016).
Because mechanical changes in oocytes can adversely affect fertilization and further development, significant effort has been directed toward preserving a healthy mechanical phenotype in culture. Special formulations of in vitro culture media containing serum, fetuin or other compounds can reduce the degree of zona hardening and improve fertilization rates in mouse and other species (de Felici and Siracusa, 1982; Downs et al., 1986; Ducibella et al., 1990; Schroeder, 1990; Eppig et al., 1992; Vandevoort et al., 2007). Other culture media characteristics such as redox potential, osmolality and pH may also affect zona hardening, but their effect on oocyte and embryo biomechanics is still unknown. Cooling of oocytes during in vitro culture may promote release of cortical granules and therefore induce premature zona hardening, so the temperature of the culture environment must be precisely controlled to avoid compromising viability (Johnson et al., 1988). This finding has been an important consideration in the development of cryopreservation protocols, to avoid causing excessive zona hardening after thawing. There is some evidence that heat shock may accelerate cytoplasmic maturation and lower rates of blastocyst formation, but the effect on zona hardening is still unclear (Payton, 2004; Edwards et al., 2005).
There is significant evidence in the literature that oocyte and embryo mechanical properties change dynamically over the course of development, and may need to be tightly regulated in order to ensure successful embryogenesis. Their mechanical phenotype may provide useful insight into events at the molecular level which are indicative of maturation, aging and external stressors, and which may be predictive of fertilization, development in culture and pregnancy. Traditional biochemical methods of measuring ZP mechanics by dissolution and imaging oolemma microstructure via electron microscopy are destructive and therefore not clinically practical. Assessment of cytoplasmic mechanics may be possible non-invasively, but is currently difficult and not clinically practical without automated analysis techniques. Recent advances in microfluidic technology and automation can address both of these problems by measuring oocyte and embryo mechanics in an automated and non-invasive manner.
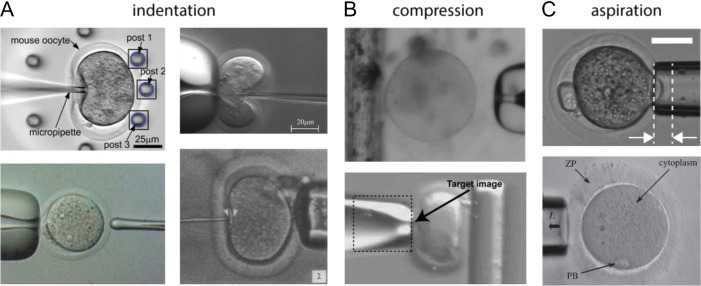
The technologies described in this review use a variety of mechanical models and technological approaches to describe oocyte and embryo mechanical phenotype. Like other cells, oocytes and embryos are often described as viscoelastic materials, which can exhibit both liquid-like and solid-like mechanical properties. Several methods have been developed to measure these properties in oocytes and embryos, both at the whole-embryo level and in sub-components such as the zona or cytoplasm only. Figure 1 contains images of the main categories of microfluidic methods of probing oocyte and embryo mechanical properties, which include indentation, compression or aspiration. The following sections will survey these methods and highlight studies that have discovered mechanical biomarkers of developmental potential.
Figure 1.
Examples of microfluidic devices used to probe oocyte and embryo mechanical properties. The approaches to measure mechanics can be separated into three main categories. (A) Approaches involving indentation. The top left image is from Liu et al. (2012) and shows an oocyte being pushed against a group of flexible posts. The top right image is from Sun et al. (2003) and shows an oocyte deformed by a force-sensing microneedle. The bottom right image is from Green (1987) and shows an oocyte compressed by a quartz-fiber ‘poker’. The bottom left image is from Murayama et al. (2004) and shows the oocyte being probed by a MTS from the left side. (B) Approaches involving compression. The top image is from Abadie et al. (2014) and shows an oocyte about to be compressed between a micropipette and the edge of a floating platform. The bottom image is from Wacogne et al. (2008) and shows an oocyte being compressed between a micropipette and a flexible post (side view). The ‘target image’ text refers to the algorithm used to track the pipette displacement. (C) Approaches involving aspiration. The top image is from Yanez et al. (2016) and shows an embryo partially aspirated into a micropipette. The region between the arrows is the aspiration depth into the micropipette. The bottom image is from Khalilian et al. (2010b) and shows a portion of the ZP being aspirated into a micropipette.
Oocyte maturation mechanics
The literature covered in this section uses a variety of approaches to non-invasively probe oocyte mechanics. Although not all of these are implemented directly on a microfluidic chip, they typically involve glass capillaries, micro-needles or other structures, which lend themselves to an on-chip implementation. Some of the earliest studies on oocyte mechanics were performed several decades ago and measured oocyte stiffness over the course of meiotic maturation in starfish and other animal species (Hiramoto, 1976; Nakamura and Hiramoto, 1978; Nemoto et al., 1980). These studies used parallel plate compression and negative pressure application via micropipette aspiration to measure parameters such as cell surface stiffness, membrane surface tension and intracellular pressure. They found that oocytes experience a general decline in stiffness over the course of maturation, with spikes at the time of polar body extrusion.
After a long gap in the literature, Murayama et al. revisited the topic of understanding how oocyte mechanical properties change during maturation. Using advancements in microelectromechanical (MEMS) technology, they devised a microtactile sensor (MTS) consisting of a cylindrical ceramic probe only 20 μm across (Fig. 1A, lower left). This probe is composed of piezoelectric transducers for driving its vibration and for detecting its resonance frequency (Murayama and Omata, 2004; Murayama et al., 2004). Based on the slope of the change in the probe's resonance frequency upon indentation of the ZP, they were able to calculate that the Young's modulus of the ZP declines from 22.8 kPa in GV mouse oocytes to 8.26 kPa in MII mouse oocytes (Murayama et al., 2006). Because they only measured at two time points, they did not observe the spikes during polar body extrusion that had been found in early studies on starfish oocytes. However, their results confirmed the general trend of softening observed during oocyte maturation.
Other MEMS devices have been developed to measure oocyte stiffness, including a micro-beam that acts as a force sensor during oocyte compression (Fig. 1B, bottom) (Wacogne et al., 2008). Based on knowledge of the oocyte and beam deformation, along with the beam's stiffness, the oocyte's stiffness can be calculated. This study proposed that a ‘flattening parameter’, which describes the ellipsoidal deformation of the oocytes, may be a better parameter to distinguish between them compared with stiffness alone. Another type of oocyte characterization device used a force sensor composed of magnetic springs (Fig. 1B, top) (Abadie et al., 2014). A micropipette is used to compress an oocyte against the edge of a floating platform, and the compression lengths of the attached magnetic springs are measured and used to calculate the force applied to the oocyte. Although no Young's modulus value was reported, the authors did report oocyte stiffness values of 3.1 and 11.2 mN/m for MII oocytes and values of 7.2 and 9.3 for GV/MI oocytes. This sample size was very small, but suggests that there could be significant variation in oocyte mechanical properties within the MII stage. The authors also provided force-compression curves, which could be used to better understand the nonlinear and plastic behavior of oocytes.
One group developed a microfluidic cell holding device fabricated from poly(dimethylsiloxane) (PDMS) to observe deformation of individual oocytes during micropipette indentation (Fig. 1A, top left) (Liu et al., 2010, 2012). On the device, each oocyte is contained in its own microwell and surrounded by tall, flexible posts. A microneedle applies an indentation force to the oocyte, compressing it and pressing it against the posts that also deform. Based on the displacement of the posts and knowledge about their mechanical properties, the mechanical properties of the oocyte can be calculated. The authors reported a Young's modulus of 3.1 kPa from young mouse oocytes, and 1.6 kPa from aged mouse oocytes, indicating that mechanics are linked with pre-ovulatory aging. While aged mouse oocytes were significantly softer, they were also more viscous, had a lower density of ZP glycoproteins and had reduced amounts of F-actin in the subcortical region of the cytoplasm. This study was the first to report differences between the mechanical properties of oocytes of (presumed) high and low developmental potential, and suggests that mechanics may add predictive value to morphological evaluations of oocyte quality.
Because the ZP surrounds the oocyte and is most easily accessible to external measurement probes, most of the literature on oocyte mechanics has focused on calculating the viscoelastic properties of the ZP only. It is likely that the oocyte cytoplasm, oolemma and PVS may also undergo mechanical changes over the course of development and maturation, and may provide a more accurate prediction of developmental potential if measured directly. One study elected to remove the ZP and measure oocyte cortical mechanics directly with micropipette aspiration (Larson et al., 2010). Over the course of meiotic maturation, it was found that the oocyte cortex mirrors the mechanical changes in the ZP; its tension drops by a factor of ~6 and then increases by a factor of ~1.6 upon fertilization. Cortical tension is ~2.5-fold higher near the meiotic spindle of MII oocytes compared with the end opposite the spindle, indicating that the MII egg is mechanically polarized. A follow-up study using this method focused on cortical changes in response to post-ovulatory aging (Mackenzie et al., 2016). Although oocytes experience an increase in ZP hardness in response to post-ovulatory aging, their cortical tension is lowered by nearly 50% due to aberrant cytoskeletal dynamics. This finding strengthens the evidence that post-ovulatory aging affects oocyte mechanical phenotype, and thus mechanics may serve as a biomarker of oocyte developmental competence.
The literature covered in this section has mostly focused on quantifying zona hardness and cortical mechanics over the course of meiotic maturation and in response to post-ovulatory aging. There has been a general agreement that oocytes soften as they progress from the GV to the MI and MII stages, which matches previous findings of a decrease in the zona's resistance to dissolution during maturation. A summary of these findings is presented in Fig. 2. Only two studies have linked oocyte mechanics with markers of developmental potential: Liu et al. (2010) have linked zona mechanics with the degree of pre-ovulatory aging and Mackenzie et al. (2016) have demonstrated that cortical mechanics correlate with post-ovulatory aging. Follow-up studies will be required to further characterize how changes at the molecular level are reflected in oocyte mechanics, and to find which mechanical properties in human oocytes are predictive of clinical outcomes.
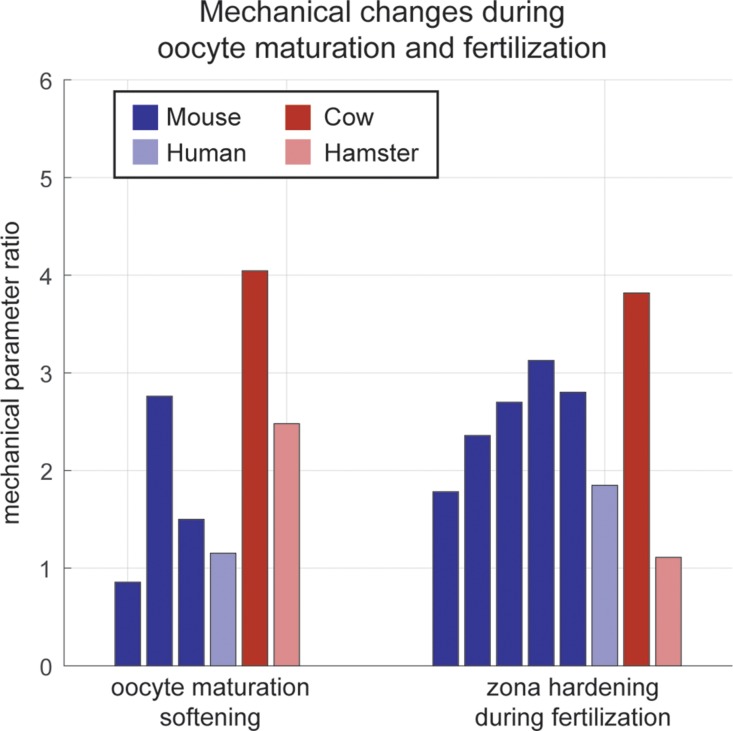
Figure 2.
Comparison of measured values of oocyte and embryo mechanical properties using different measurement devices and in different species. Y-axis represents ratio of measured mechanical parameters (for example, Young's modulus or stiffness) between immature and mature oocytes, or between mature oocytes and fertilized embryos. Data are from the following studies: (Drobnis et al., 1988; Murayama et al., 2006; Papi et al., 2010; Abadie et al., 2014; Yanez et al., 2016) for oocyte softening and (Drobnis et al., 1988; Sun et al., 2003; Murayama et al., 2006; Papi et al., 2010; Khalilian et al., 2010b; Yanez et al., 2016) for zona hardening, from left to right. Some studies measured mechanics for multiple species. Results show a fairly consistent degree of zona softening during oocyte maturation and zona hardening during fertilization.
There is also a need for more comprehensive models of oocyte mechanical behavior, which will enable the measurement of components beyond the ZP alone. The oocyte is a mechanically heterogeneous structure composed of a cytoplasm (which has mechanically distinct sub-compartments in relation to the meiotic spindle location) and a PVS, which are surrounded by the ZP. Before the GV stage, the PVS does not exist and the oolemma is tightly coupled to the ZP (Dandekar and Talbot, 1992; Talbot and Dandekar, 2003). As the oocyte gains the ability to regulate its size on its own, it uncouples its membrane from the ZP and initiates the formation of the PVS, a process that has been linked to increased meiotic competence if performed before the end of the GV stage (Inoue et al., 2007; Tartia et al., 2009). The complex mechanical events involved in this process highlight the importance of modeling the oocyte as a heterogeneous structure, where each component has a unique set of liquid-like and solid-like properties, and which exhibits varying degrees of mechanical coupling to other components.
Fertilization and zygote mechanics
The first successful attempt to measure zona hardness via physical stimulus used a quartz fiber to poke a hamster egg zona with increasing amounts of force, and concluded that the zona acts as a purely elastic shell in which sperm cannot penetrate solely by mechanical force (Fig. 1A, bottom right) (Green, 1987). The following year, zona hardening was characterized as a mechanical phenomenon rather than solely a biochemical one for the first time, by measuring deformation in response to a capillary suction mechanism similar to micropipette aspiration (Drobnis et al., 1988). This study showed that hamster fertilized embryos are only slightly stiffer than hamster eggs, while mouse fertilized embryos are nearly 1.8 times stiffer than mouse eggs. These discrepancies led the authors to conclude that mechanical zona hardening may contribute to the zona block to polyspermy, although the degree to which this occurs may be species dependent.
One group attached an ICSI needle to a micro-robot with an MEMS-based force sensor to measure mouse oocyte and embryo resistance to injection (Fig. 1A, top right) (Sun et al., 2003). They measured the force-deformation curve measured during an injection and fit the data to a biomembrane point-load mechanical model. They found that the force required to puncture a fertilized embryo ZP was 13 μN, almost twice the 7.5 µN required to puncture oocyte ZP. Similarly, they found that the Young's modulus of the ZP increases from 17.9 kPa in oocytes to 42.2 kPa in embryos, which represents a 2.4-fold hardening. The MTS developed by Murayama et al. (2004) was also used to measure ZP Young's modulus during fertilization and at fixed intervals afterward. They found that mouse oocyte ZP has a Young's modulus of 8.26 kPa, which increases to 22.3 kPa after fertilization, representing a 2.4-fold increase. The zona gradually softens again after fertilization, and becomes softer than the mature egg by the 8-cell stage.
Recently, Khalilian et al. (2010b) used micropipette aspiration to apply varying pressures to human and mouse egg zona before and after fertilization, and calculated the amount of zona hardening based on different mechanical models (Fig. 1C, bottom) (Khalilian et al., 2010a,b). Models used for micropipette aspiration experiments were first developed for cells such as chondrocytes and neutrophils, and so they do not take the multi-layered structure of the embryo into account (Hochmuth, 2000). These models also assume the embryo is an isotropic, homogeneous elastic medium (half-space) and do not take into account variations in stiffness between the ZP, cytoplasm and PVS or the degree of coupling between them. This may bias estimation of its mechanical properties such as Young's modulus. The studies by Khalilian et al. (2010a,b) used a layered model, which treats the cell as a half-space and the ZP as a layer with mechanical properties distinct from the cytoplasm, and a shell model similar to the layered model but with the assumption of zero stiffness for the cytoplasm. They found that traditional half-space models underestimate the Young's modulus of the ZP, and an accurate estimate of the Young's modulus ZP is 11.8 kPa for mouse oocytes, 36.9 kPa for mouse embryos, 7.34–7.47 for human oocytes (depending on the model used) and 13.18–14.19 for human embryos. Thus, they estimated that during fertilization the zona hardens by a factor of 1.8 in humans and 3.1 in mouse.
Another study by Yanez et al. (2016) used micropipette aspiration to demonstrate that mechanical properties of embryos measured hours after fertilization are predictive of blastocyst formation in humans and live birth in mouse (Fig. 1C, top) (Yanez et al., 2016). The embryo response to a step negative pressure was fit to a bulk mechanical model and it was found that embryos that were too soft or too stiff exhibited reduced developmental potential. Embryos with low developmental potential as predicted by mechanics showed significantly different patterns of gene expression than embryos with high developmental potential, particularly in genes associated with DNA repair and processes important for oocyte maturation. The authors speculated that embryos with mechanics indicative of low developmental potential may have had trouble completing zona hardening during fertilization, or may have not softened sufficiently during oocyte maturation. Although this study did not directly report the degree of zona hardening, it can be calculated from data in the paper to be ~2.8.
Unlike the study by Khalilian et al. (2010b) that estimated the Young's modulus of the mouse embryo ZP to be 36.9 kPa, the study by Yanez et al. (2016) used a bulk mechanical model of the zygote-stage embryo and reported a Young's modulus of 0.5 kPa for the mouse embryo as a whole (rather than the ZP alone). This value is more similar to that of cells, which lack a ZP but exhibit solid-like behavior such as endothelial cells and chondrocytes (Sato et al., 1990; Jones et al., 1999). The discrepancy between the bulk embryo Young's modulus and the ZP Young's modulus may be due to the mechanical model used and the heterogeneous structure of the embryo, which consists of a relatively soft cell surrounded by a stiff ZP. Studies that apply a small deformation to the outside the embryo will measure the high Young's modulus of the ZP, whereas studies that apply a high deformation will tend to measure the Young's modulus of the embryo as a whole. In the model used by Yanez et al. (2016), it is unclear which specific structures within the embryo are contributing to the biomechanical properties measured. Further studies will be required to determine the correlation between the mechanical properties of the embryo's cytoplasm, PVS and ZP and the bulk mechanics of the embryo as a whole.
The literature reviewed in this section shows a strong agreement that the ZP experiences an ~2-fold hardening during fertilization, which is in concordance with previous biochemical studies on ZP dissolution time. A summary of the literature on zona hardening is presented in Fig. 2. It is possible that the degree of zona hardening may serve as a biomarker of viability, as embryos with impaired developmental competence may undergo incomplete fertilization and a suboptimal degree of zona hardening. Alternatively, embryos that are the product of excessive post-ovulatory aging or insufficient maturation may have been overly stiff before fertilization and may therefore have altered mechanical properties post-fertilization as well.
Although two studies so far have demonstrated that embryo mechanical properties are predictive of developmental potential, further investigation is needed to better understand this relationship. Particular caution must be taken when choosing a time point to measure embryo mechanics, and a developmental endpoint to designate an embryo as ‘viable’. In studies on in vitro egg activation, cytochalasin B has been used to produce diploid parthenogenetic mouse embryos that are capable of blastocyst formation and implantation but arrest before the ninth day of gestation (Balakier and Tarkowski, 1976). Because cytochalasin B can severely alter the cortical cytoskeleton, mechanical parameters measured at this stage, which are predictive of blastocyst formation may not ultimately predict live birth at all. There is also some data that parthenogenetic and normally fertilized mouse embryos may undergo measurably different degrees of zona hardening (Mintz and Gearhart, 1973; Gulyas and Yuan, 1985; Murayama et al., 2008b). Using blastocyst formation as a viability endpoint for both types of embryos would yield an artificially large range of ZP mechanics, which are predictive of normal development. Therefore, embryos in these studies should be followed to pregnancy and live birth whenever possible.
Nanoscale modeling of zona mechanical properties
Rather than measuring the bulk mechanical properties of the embryo and its ZP, some studies chose to focus on the nanoscale mechanical properties of the ZP. The first studies on the ZP's nanoscale structure were performed with electron microscopy and revealed the presence of cylindrical fibril bundles arranged in concentric layers (Jackowski and Dumont, 1979; Flechon et al., 2004; Pelletier et al., 2004). These bundles are composed of sulfated glycoproteins, which are present in different ratios (or not at all) depending on species (Bleil and Wassarman, 1980; Green, 1997). Before fertilization, the surface of the mature oocyte ZP appears rough and porous; after fertilization, it becomes smooth and remains that way through preimplantation development (Jackowski and Dumont, 1979; Vanroose et al., 2000).
These observations motivated some recent studies on ZP nanoscale mechanical properties using atomic force spectroscopy and atomic force microscopy (AFM). It was discovered that the bovine mature oocyte ZP behaves like an elastic material when low forces are applied, and has a Young's modulus of 22 kPa (Papi et al., 2009). When a force greater than 2.1 nN is applied to deform the ZP by >550 nm, the non-covalent interactions between ZP protein chains are forced to dissociate, after which they recoil and form new non-covalent interactions closer to the stretched position. This phenomenon where the ZP does not return to its original shape after applied stress is called plastic deformation.
Follow-up work on immature, mature and fertilized bovine oocytes revealed that immature oocyte ZP exhibits a purely elastic behavior and has a Young's modulus of 89 kPa, while mature oocyte ZP has a Young's modulus of 22 kPa, which means bovine oocytes undergo a 4× softening over the course of maturation (Papi et al., 2010). Fertilized oocyte ZP has the same Young's modulus as immature oocyte ZP, but exhibits plastic deformation at high enough forces and therefore is not as resilient to indentation as immature oocyte ZP. This initial work led the authors to hypothesize that incomplete ZP stiffening through the depth of the ZP may contribute to high rates of polyspermy seen in cows. However, further investigation showed that fertilized oocyte ZP filaments are not much more resistant to plastic deformation compared with unfertilized oocyte ZP, and bovine sperm flagella can generate enough energy to potentially displace filaments in both fertilized and unfertilized oocyte ZP (Papi et al., 2012). Therefore, zona hardening during fertilization may not be a significant factor in preventing polyspermy in bovine oocytes.
Another study on ZP mechanics by Boccaccio et al. (2012) combined AFM nanoindentation measurements with nonlinear finite element simulations to better characterize the nonlinear behavior and heterogeneous structure of the ZP. They found that ZP biomechanical heterogeneity increases significantly after fertilization, and confirmed that zona hardening during fertilization is due to an increase in inter-filament crosslinking. Unlike the previous work by Papi et al. (2010, 2012), Boccaccio et al. (2012) found that the gradient of the Young's modulus value in fertilized oocyte ZP is 40 times steeper than in mature oocyte ZP. This gradient may be caused by release of cortical granules, which will increase zona crosslinking in the immediate vicinity of the plasma membrane but leave the outer layer relatively soft.
Imaging approaches to measure cytoplasm mechanics
There has also been a considerable amount of work done on mechanically characterizing the oocyte and embryo cytoplasm. Most types of studies on cytoplasm mechanics use microrheology to locally probe viscoelastic properties, which involves video tracking of either (i) micrometer-sized tracer particles injected into the cytoplasm or (ii) structures and features already present in the cytoplasm. The majority of the work in this field has been done in non-mammalian oocytes such as Drosophila, C. elegans and Xenopus, but may still have high applicability to human oocytes.
One study in Drosophila oocytes tracked the motions of injected fluorescent beads with sampling frequencies into the kHz range and calculated the viscoelasticity of the cytoplasm in different locations (Wessel et al., 2015). They found that the part of the cytoplasm between the nuclear layer and yolk was homogeneous and highly viscous, while the part in the nuclear layer was both highly elastic and highly viscous. This increase in viscous and elastic moduli was largely due to an increased microtubule density, with little contribution from actin filaments. In contrast, cytoplasmic extracts from Xenopus eggs were found to form a soft viscoelastic solid with elastic modulus around 2–10 Pa, where both actin filaments and microtubules contribute to mechanical strength (Valentine et al., 2005).
Studies on living starfish oocytes used multiple particle tracking and laser particle tracking to study mechanical properties of their cytoplasm over varying time scales (Pesce et al., 2009, 2011). They found that the mechanical properties of the cytoplasm vary based on time scale; the semiflexible behavior of the actin network on very short time scales (<1 ms), the stiff behavior similar to a soft-glass material on intermediate time scales (1 ms–1 s) and viscous behavior on long time scales (>1 s). As oocytes mature, they experience an increase in cytoplasmic viscosity, which the authors concluded may be caused by a reorganization of the actin network. Although the findings of these studies do not match exactly, it nonetheless indicates that cytoplasmic mechanical properties are reflective of some combination of the distribution of microtubules and actin filaments.
The oocyte cytoskeleton performs a wide variety of functions vital for establishing developmental competence, including nutrient distribution, spindle localization and morphogen gradient establishment (Hecht et al., 2009; Verchot-Lubicz and Goldstein, 2010; Yi et al., 2011). Therefore, measurement of local cytoplasmic viscoelasticity and imaging of cytoplasmic streaming may provide insight into oocyte quality and maturation. One group measured rhythmic oscillatory changes in mouse oocytes after fertilization and found that they were correlated with blastocyst formation and live birth (Ajduk et al., 2011). Using particle imaging velocimetry, they showed that sperm entry triggers rhythmic cytoplasmic movements that depend on cytoskeleton integrity and that may indicate embryo quality. Embryos with a low speed of cytoplasmic streaming or with very frequent speed peaks rarely developed to the blastocyst stage.
Mechanical stimuli can improve development
It is well known that oocytes experience a wide array of mechanical inputs during in vivo maturation, fertilization and further development. Traditional static in vitro culture systems keep embryos on stiff, inert plastic surfaces that do not accurately mimic the dynamic fluid flows in the fallopian tubes, or the forces exerted by the uterus and oviduct (Fauci and Dillon, 2006). Consequently, many microfluidic devices have been developed with the intent of better reproducing the developing embryo's natural environment to improve development rates. There already exist thorough reviews on devices that feature smaller volumes of media and high embryo density to increase the concentration of secreted growth factors and better replicate the in vivo biochemical environment (Krisher and Wheeler, 2010; Swain and Smith, 2011). Other devices have focused on replicating the physical environment inside the fallopian tubes and have attempted to optimize fluid flow and physical contact with embryos. It is thought that gentle mechanical stimulation of embryos may activate trophic signaling pathways and promote development, although the mechanism remains unclear and excess shear forces may be harmful (Xie et al., 2007, 2008; Swain et al., 2013). In light of this, it is important that designers of microfluidic culture environments be mindful to apply finely tuned mechanical inputs to embryos.
So-called dynamic culture platforms that apply mechanical stimulation to embryos have existed in some form for many years, starting with a simple setup that placed mouse oocytes on a laboratory shaker during fertilization (Hoppe and Pitts, 1973). Since then, mechanical micro-vibration was applied to embryos for a few seconds at a time at frequencies between 20 and 44 Hz and was shown to cause an increase in pregnancy rates in humans, blastocyst formation rates in mice and maturation in pig oocytes (Isachenko et al., 2010, 2011; Mizobe et al., 2010; Hur et al., 2013). Another group developed a tilting embryo culture system that aimed to mimic the shear stress, compression and friction force, which the embryo experiences in the fallopian tubes (Matsuura et al., 2010; Hara et al., 2013). This system subjected embryos to a maximum of 20 degrees of tilt at 1 degree/s so that embryos would move along the bottom of the dish at around 1 mm/min. Human and mouse embryos subjected to this mechanical stimulation exhibited enhanced blastocyst development, cell number and morphology.
Although they showed promising results, the devices described thus far for mechanical stimulation are not true microfluidic systems, but instead conventional culture dishes placed on a moving platform inside an incubator. A few microfluidic devices have been developed to mechanically stimulate embryos, including a dynamic micro-funnel culture system to expose embryos to a gentle pulsatile flow of media (Heo et al., 2010). Using this system, it was possible to achieve increased development rates in mouse embryos compared with those in a static culture environment. Another microfluidic culture system employed a partially constricted channel to mimic peristaltic muscle contractions (Kim et al., 2009). The authors found that bovine embryos placed in a constricted channel on this device showed higher rates of development to the 8-cell stage compared with those cultured in a straight channel. Although some microchannel widths improved embryo development compared with a control group, it was evident that microchannel geometry greatly affects the amount of mechanical force applied to embryos and must be precisely controlled to avoid causing damage.
Bae et al. (2011) designed a membrane-based microfluidic culture system, which enables the application of an in vivo-like compressive force to embryos. The study measured the effect of various pressure amplitudes and stimulus durations on embryo morphology, and found that overly large values of these variables could significantly impair embryo development. This study highlights the importance of appropriately tuning mechanical stimuli to replicate physiological conditions because excessive applied stress can cause harm compared with simply leaving embryos in static culture.
Potential for high-throughput and automated measurement devices
Advances in robotics and automation are quickly finding applications in life sciences and medical devices, and are already showing promise in their applications to assisted reproductive technologies (ART). Specifically, microfluidic devices have been developed for sperm selection, which are reviewed by Smith et al. (2015). Although not yet validated in randomized clinical trials, many of these devices have shown higher effectiveness in isolating motile sperm compared with traditional methods. Devices have been made to tackle aspects of oocyte manipulation, including cumulus cell stripping and zona removal, and there have also been promising advances in robotic devices to automate ICSI (Zeringue et al., 2001, 2005). One particularly creative device integrated both the holding pipette and injection needle used in ICSI with a micro-gripper tool (Garcés-Schröder et al., 2015). This platform is also equipped with a force sensor to measure oocyte mechanical properties during the injection process, thus integrating two separate procedures. Although still early in its development, this micro-gripper illustrates a future direction of automation in IVF, where one device can combine a standard procedure such as ICSI or embryo handling with a measurement to monitor the embryo's quality and health.
As of yet, there has been no fully automated microfluidic device that can measure oocyte and embryo mechanical properties and predict developmental potential. Most of the existing methods reviewed above employ small volumes of liquid, glass capillaries and/or microneedles and have not yet been automated or implemented in an on-chip form. There have, however, been many advances in automated single-cell mechanical analysis, which could potentially present a roadmap forward for adapting existing techniques of oocyte and embryo mechanical measurement. Some particularly interesting approaches are illustrated in Fig. 3 and will be briefly reviewed here. One common design is to flow cells one by one through microchannels or constrictions and observe how they squeeze through these obstacles (Fig. 3B) (Guo et al., 2012; Zheng et al., 2013; Luo et al., 2014). High-speed image processing is typically employed to measure parameters such as channel transit time, cell elongation and recovery time. Using impedance measurements rather than live imaging can enable throughput to rise above 100 cells/s, although this is far beyond what would be necessary for applications in ART.
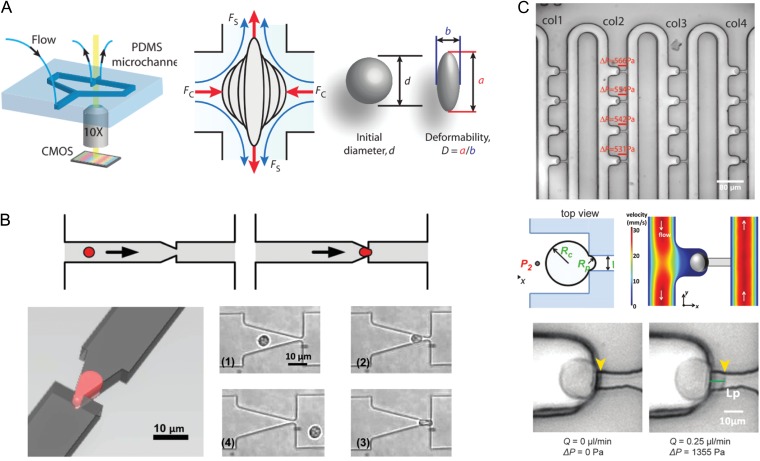
Figure 3.
Devices for automated and high-throughput microfluidic mechanical characterization, which could be adapted to measure embryos and oocytes. (A) Hydrodynamic cell stretcher to measure deformability parameter (Tse et al., 2013). The left side of the image shows the microfluidic chip, which contains a channel that contains cells to be tested and a perpendicular channel to stretch them under continuous flow. The middle part of the image shows a schematic of a single cell being stretched, where it is subjected to a compressive (FC) and a shear (FS) force. The right part of the image shows how a ‘deformability’ parameter is calculated. (B) Micropipette aspiration translated to a microfluidic chip (Guo et al., 2012). The top panels show a schematic of a single cell being deformed to simulate micropipette aspiration, and the left panel shows the aspiration process from another angle. The panels numbered (1) through (4) show micrographs of a single neutrophil as it is deformed through a funnel constriction. (C) Device that can autonomously perform micropipette aspiration on many cells simultaneously (Lee and Liu, 2015). The top panel shows loading of single cells into columns with individual trapping structures. The middle left panel shows a schematic of a single cell undergoing aspiration, where RC is the radius of the cell and RP is the radius of the aspiration micropipette. The middle right panel shows a numerical simulation of the flow velocity around the cell. The bottom panels show a demonstration of micropipette aspiration using the device.
A device has also been developed to replicate an existing technique, micropipette aspiration, on a microfluidic chip to measure multiple cells in parallel (Fig. 3C) (Lee and Liu, 2015). Traditional micropipette aspiration can be very time consuming, requiring ~1 min per cell, a skilled operator and bulky equipment. The microfluidic pipette array developed by Lee and Liu (2015) can autonomously trap multiple cells, perform an aspiration measurement and observe their deformation. This type of device could be used to bring micropipette aspiration into clinical use, and efficiently measure biomarkers of viability while requiring minimal training and effort from clinical staff.
Other automated approaches to measure cell mechanics include microfluidic hydrodynamic stretching and optical stretching. By taking advantage of inertial focusing, Gossett et al. (2012) were able to deliver individual cells through a microchannel into a region with an extensional flow, capture an image of the cell's deformation and calculate a deformability parameter. This technique, termed deformability cytometry, was applied to accurately identify malignancy in pleural effusion samples from patients (Fig. 3A) (Tse et al., 2013). Optical stretching has also been implemented in a microfluidic device, and was shown to have high sensitivity in distinguishing normal, cancerous and metastatic cells. Unlike deformation through microchannels or micropipette aspiration, hydrodynamic and optical stretching do not require any contact with cells and could be used to apply deformation to an entire oocyte or embryo.
When designing these types of devices, channel geometry and surface properties must be finely tuned in order to maximize sensitivity, while avoiding problems such as channel clogging or adhesion to cell surfaces. Embryos and oocytes may also present a unique challenge in constriction-based or stretching approaches due to the presence of the zona and polar body (and possibly multiple cells if measured past the zygote stage). Since oocyte mechanical properties may differ depending on the measurement location, automated devices that measure their mechanics will have to control or account for orientation (Larson et al., 2010). The problem of autonomously controlling oocyte orientation with a micropipette has been solved using computer vision to track polar body position, so this can likely be translated onto a more high-throughput device as well (Leung et al., 2012). Still, models used to calculate mechanical parameters will have to carefully account for the heterogeneity of the oocyte's structures and mechanical properties.
In addition to automating embryo handling and measurement in the IVF clinic, microfluidic devices can also be used as a scientific tool to more efficiently conduct mechanistic studies of embryo development. Ozil et al. (2005, 2006, 1990) developed a microfluidic chamber to hold oocytes in a fixed position while precisely controlling the pattern of Ca2+ oscillations after fertilization with high temporal resolution. Both premature termination and excessive stimulation of Ca2+ oscillations compromised oocyte ability to develop to term, although blastocyst formation rates appeared unaffected. A follow-up study by Banrezes et al. (2011) adapted this device to modify oocyte mitochondrial activity at the PN stage by changing the redox potential of the media. They found that oocytes are fairly robust to transient energetic depression at this stage, but at the cost of post-natal growth.
These studies offer a preview into the types of experiments that will be enabled by microfluidic devices, where multiple types of culture conditions can be tested in parallel with precise temporal and spatial control. It is well known that various aspects of ART can negatively impact embryo development, pregnancy and even the resulting offspring (Hiura et al., 2012; Pandey et al., 2012). Microfluidic chambers, which can physically manipulate embryos, rapidly exchange media, modify media characteristics in real time and even vary their mechanical characteristics, will enable scientists to better understand how ART affects embryos. The impact of physical stimuli and culture environment on embryos could even be monitored throughout preimplantation development by measuring their biomechanical properties or other characteristics at regular intervals.
It is important to mention, however, that the ability to gather so much information then raises the problem of how to synthesize it into a clinically relevant and actionable form. As high-throughput sequencing data have dramatically multiplied the amount of information generated from genomics studies, researchers have had to develop new analysis techniques to separate signal from noise in these data sets and carefully design experiments to be able to draw meaningful conclusions. Similarly, high-throughput and automated experiments on embryo development will generate a great deal of information as both the number of experimental variables (culture time, media formulation, physical stimuli, etc.) and the available readouts (mechanical properties, imaging, metabolomics, gene expression, epigenetics, developmental stage) will multiply. Moving forward a great deal of care must be taken in experimental design and data analysis in order to draw meaningful conclusions that can positively influence the clinical practice of IVF.
Conclusions
Microfluidic devices are already showing promise in revolutionizing the entire process of fertilization, embryo culture and embryo selection for transfer. Although autonomous microfluidic devices have been developed for processes such as sperm selection and oocyte denuding, in the future the entire process of ART could be performed in a ‘womb-on-a-chip’, fully automated from the beginning of oocyte culture up to embryo selection for transfer. These types of devices could streamline the IVF process, reduce variability between laboratory personnel and reduce the need for clinical staff to perform repetitive, tedious work. Insights from biomechanical studies could be used to optimize oocyte and embryo culture by providing mechanical inputs, which best replicate those experienced in vivo. In addition to improving the clinical practice of IVF, microfluidic devices could also become important tools in scientific studies on embryo development. By applying temporally and spatially controlled mechanical and biochemical inputs to an oocyte or embryo during maturation and development, researchers will be able to test many different variables in parallel and observe their effect on developmental outcomes.
Regular measurement of oocyte and embryo biomechanical properties could be performed on an automated microfluidic device to monitor progression through development, and to estimate developmental potential. These regular mechanical measurements could be integrated with other monitoring methods such as metabolic profiling and time-lapse imaging to provide a type of comprehensive, real-time embryo health monitoring. At the time of transfer, a combination of these parameters could be used to make an optimal choice regarding which and how many embryos to transfer to maximize pregnancy rates while minimizing the incidence of multiple gestation pregnancies. Now that effective cryopreservation techniques for oocytes are finding wide application in fertility clinics, mechanical measurements could also provide information about oocyte quality and enable physicians to better estimate how many oocytes to cryopreserve. An accurate mechanical measurement of oocyte quality could also help to evaluate the effectiveness of hormonal stimulation protocols and also streamline the process of IVF by only spending clinic time and resources fertilizing high quality oocytes.
There are still some technological challenges that must be overcome before microfluidic devices can be practically used in the clinic to measure oocyte and embryo biomechanical properties. These devices must be designed to integrate with the existing clinical workflow without requiring too much extra time from embryologists or supporting equipment. Devices that measure oocyte and embryo mechanics must be extensively tested for robustness and safety, and must be able to convert mechanical parameter values into useful clinical information. Future studies on oocyte and embryo biomechanics will also have to pay attention to culture media formulation and environmental conditions as a potential confounding variable. Because an embryo's culture environment can have a significant effect on its ZP stiffness and other biomechanical properties, it may have to be included as a variable in a predictive model of viability based on biomechanical properties. If microfluidic devices and mechanical measurement techniques can overcome these hurdles and become practical in regular clinical use, then they have the potential to improve clinic efficiency and success rates, and even drive down cost to bring IVF to a wider population.
Acknowledgements
This work was supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was also supported by Coulter and Hellman awards from Stanford University.
Authors’ roles
L.Z.Y. wrote and edited the manuscript and D.B.C. edited the manuscript.
Conflict of interest
The authors have no conflict of interest to report.
References
- Abadie J, Roux C, Piat E, Filiatre C, Amiot C. Experimental measurement of human oocyte mechanical properties on a micro and nanoforce sensing platform based on magnetic springs. Sens Actuators B Chem 2014;190:429–438. [Google Scholar]
- Ajduk A, Ilozue T, Windsor S, Yu Y, Seres KB, Bomphrey RJ, Tom BD, Swann K, Thomas A, Graham C et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun 2011;2:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae CY, Kim MS, Park JK. Mechanical stimulation of bovine embryos in a microfluidic culture platform. Biochip J 2011;5:106–113. [Google Scholar]
- Balakier H, Tarkowski AK. Diploid parthenogenetic mouse embryos produced by heat-shock and cytochalasin B. J Embryol Exp Morphol 1976;35:25–39. [PubMed] [Google Scholar]
- Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS One 2011;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil JD, Wassarman PM. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida mouse oocytes and isolated zonae pellucida. Dev Biol 1980;76:185–202. [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Frassanito MC, Lamberti L, Brunelli R, Maulucci G, Monaci M, Papi M, Pappalettere C, Parasassi T, Sylla L et al. Nanoscale characterization of the biomechanical hardening of bovine zona pellucida. J R Soc Interface 2012;9:2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Kattera S. Rescue ICSI of oocytes that failed to extrude the second polar body 6 h post-insemination in conventional IVF. Hum Reprod 2003;18:2118–2121. [DOI] [PubMed] [Google Scholar]
- Cohen J. Assisted hatching of human embryos. J Vitr Fertil Embryo Transf 1991;8:179–190. [DOI] [PubMed] [Google Scholar]
- Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, Baker VL, Adamson GD, Abusief ME, Gvakharia M et al. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril 2013;100:412–419. [DOI] [PubMed] [Google Scholar]
- Coy P, Grullon L, Canovas S, Romar R, Matas C, Aviles M. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 2008;135:19–27. [DOI] [PubMed] [Google Scholar]
- Cross SE, Jin Y-S, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol 2007;2:780–783. [DOI] [PubMed] [Google Scholar]
- Dandekar P, Talbot P. Perivitelline space of mammalian oocytes: extracellular matrix of unfertilized oocytes and formation of a cortical granule envelope following fertilization. Mol Reprod Dev 1992;31:135–143. [DOI] [PubMed] [Google Scholar]
- Downs SM, Schroeder AC, Eppig JJ. Serum maintains the fertilizability of mouse oocytes matured in vitro by preventing hardening of the zona pellucida. Gamete Res 1986;15:115–122. [Google Scholar]
- Drobnis EZ, Andrew JB, Katz DF. Biophysical properties of the zona pellucida measured by capillary suction: is zona hardening a mechanical phenomenon. J Exp Zool 1988;245:206–219. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Kurasawa S, Rangarajan S, Kopf GS, Schultz RM. Precocious of cortical granules during mouse oocyte meiotic maturation in correlation with an egg induced modification of the zona pellucida. Dev Biol 1990;137:46–55. [DOI] [PubMed] [Google Scholar]
- Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, Schreurs IL, Dunselman GA, Kester AD, Geraedts JP et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod 2010;25:605–612. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Sommergruber M, Puchner M, Wiesinger R, Tews G. Developmental competence of oocytes showing increased cytoplasmic viscosity. Hum Reprod 2003;18:1294–1298. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Saxton AM, Lawrence JL, Payton RR, Dunlap JR. Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J Dairy Sci 2005;88:4326–4333. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, O'Brien MJ. Comparison of embryonic developmental competence of mouse oocytes grown with and without serum. Mol Reprod Dev 1992;32:33–40. [DOI] [PubMed] [Google Scholar]
- Eytan O, Jaffa AJ, Elad D. Peristaltic flow in a tapered channel: application to embryo transport within the uterine cavity. Med Eng Phys 2001;23:473–482. [DOI] [PubMed] [Google Scholar]
- Fauci LJ, Dillon R. Biofluidmechanics of reproduction. Annu Rev Fluid Mech 2006;38:371–394. [Google Scholar]
- De Felici M, Siracusa G. Spontaneous hardening of the zona pellucida of mouse oocytes during in vitro culture. Gamete Res 1982;6:107–113. [Google Scholar]
- De Felici M, Salustri A, Siracusa G. “Spontaneous” hardening of the zona pellucida of mouse oocytes during in vitro culture. II. The effect of follicular fluid and glycosaminoglycans. Gamete Res 1985;12:227–235. [Google Scholar]
- Flechon JE, Kopecny V, Pivko J, Pavlok A, Motlik J. Texture of the zona pellucida of the mature pig oocyte. The mammalian egg envelope revisited. Reprod Nutr Dev 2004;44:207–218. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff NR, Scott RT. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril 2013;100:100–107. e1. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Roudebush WE, Thatcher SS. Influences of in vitro oocyte aging on microfertilization in the mouse with reference to zona hardening. J Assist Reprod Genet 1992;9:378–383. [DOI] [PubMed] [Google Scholar]
- Garcés-Schröder M, Leester-Schädel M, Schulz M, Böl M, Dietzel A. Micro-Gripper: a new concept for a monolithic single-cell manipulation device. Sens Actuators A Phys 2015;236:130–139. [Google Scholar]
- Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update 2015;21:727–747. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod 1998;13:3434–3440. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril 2004;81:551–555. [DOI] [PubMed] [Google Scholar]
- Gianfortoni JG, Gulyas BJ. The effects of short-term incubation (Aging) of mouse oocytes on in vitro fertilization, zona solubility, and embryonic development. Gamete Res 1985;11:59–68. [Google Scholar]
- Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci 2012;109:7630–7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DP. Three-dimensional structure of the zona pellucida. Rev Reprod 1997;2:147–156. [DOI] [PubMed] [Google Scholar]
- Green DPL. Mammalian sperm cannot penetrate the zona-pellucida solely by force. Exp Cell Res 1987;169:31–38. [DOI] [PubMed] [Google Scholar]
- Guck J, Chilvers ER. Mechanics meets medicine. Sci Transl Med 2013;5:212–241. [DOI] [PubMed] [Google Scholar]
- Gulyas BJ, Yuan LC. Cortical reaction and zona hardening in mouse oocytes following exposure to ethanol. J Exp Zool 1985;233:269–276. [DOI] [PubMed] [Google Scholar]
- Guo Q, Park S, Ma H. Microfluidic micropipette aspiration for measuring the deformability of single cells. Lab Chip 2012;12:2687–2695. [DOI] [PubMed] [Google Scholar]
- El Hajj N, Haaf T. Epigenetic disturbances in in vitro cultured gametes and embryos: Implications for human assisted reproduction. Fertil Steril 2013;99:632–641. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Hara T, Matsuura K, Kodama T, Sato K, Kikkawa Y, Muneto T, Tanaka J, Naruse K. A tilting embryo culture system increases the number of high-grade human blastocysts with high implantation competence. Reprod Biomed Online 2013;26:260–268. [DOI] [PubMed] [Google Scholar]
- Hecht I, Rappel W-J, Levine H. Determining the scale of the Bicoid morphogen gradient. Proc Natl Acad Sci USA 2009;106:1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod 2010;25:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y. Mechanical properties of starfish oocytes. Dev growth Differ 1976;18:205–209. [DOI] [PubMed] [Google Scholar]
- Hiura H, Okae H, Miyauchi N, Sato F, Sato A, Pette Van De M, John RM, Kagami M, Nakai K, Soejima H et al. Characterization of DNA methylation errors in patients with imprinting disorders conceived by assisted reproduction technologies. Hum Reprod 2012;27:2541–2548. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech 2000;33:15–22. [DOI] [PubMed] [Google Scholar]
- Hoppe PC, Pitts S. Fertilization in vitro and development of mouse ova. Biol Reprod 1973;8:420–426. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell 2005;8:175–176. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv 1982;37:59–77. [DOI] [PubMed] [Google Scholar]
- Hur YS, Park JH, Ryu EK, Park SJ, Lee JH, Lee SH, Yoon J, Yoon SH, Hur CY, Lee WD et al. Effect of micro-vibration culture system on embryo development. J Assist Reprod Genet 2013;30:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Akiyama T, Nagata M, Aoki F. The perivitelline space-forming capacity of mouse oocytes is associated with meiotic competence. J Reprod Dev 2007;53:1043–1052. [DOI] [PubMed] [Google Scholar]
- Isachenko E, Maettner R, Isachenko V, Roth S, Kreienberg R, Sterzik K. Mechanical agitation during the in vitro culture of human pre-implantation embryos drastically increases the pregnancy rate. Clin Lab 2010;56:569–576. [PubMed] [Google Scholar]
- Isachenko V, Maettner R, Sterzik K, Strehler E, Kreinberg R, Hancke K, Roth S, Isachenko E. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod Biomed Online 2011;22:536–544. [DOI] [PubMed] [Google Scholar]
- Jackowski S, Dumont JN. Surface alterations of the mouse zona pellucida and ovum following in vivo fertilization: correlation with the cell cycle. Biol Reprod 1979;20:150–161. [DOI] [PubMed] [Google Scholar]
- Jin H, Xing X, Zhao H, Chen Y, Huang X, Ma S, Ye H, Cai J. Detection of erythrocytes influenced by aging and type 2 diabetes using atomic force microscope. Biochem Biophys Res Commun 2010;391:1698–1702. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Pickering SJ, George MA. The influence of cooling on the properties of the zona pellucida of the mouse oocyte. Hum Reprod 1988;3:383–387. [DOI] [PubMed] [Google Scholar]
- Jones WR, Ping Ting-Beall H, Lee GM, Kelley SS, Hochmuth RM, Guilak F. Alterations in the young's modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J Biomech 1999;32:119–127. [DOI] [PubMed] [Google Scholar]
- Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update 2014;20:617–631. [DOI] [PubMed] [Google Scholar]
- Khalilian M, Navidbakhsh M, Valojerdi MR, Chizari M, Yazdi PE, Rezazadeh Valojerdi M, Eftekhari Yazdi P. Alteration in the mechanical properties of human ovum zona pellucida following fertilization: experimental and analytical studies. Exp Mech 2010. a;51:175–182. [Google Scholar]
- Khalilian M, Navidbakhsh M, Valojerdi MR, Chizari M, Yazdi PE, Yazdi E, Yazdi PE, Yazdi E. Estimating Young's modulus of zona pellucida by micropipette aspiration in combination with theoretical models of ovum’. J R Soc Interface 2010. b;7:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Ko D-S, Lee H-TH-CH-J, Lee H-TH-CH-J, Park W-I, Kim SS, Park J-K, Yang B-C, Park S-B, Chang W-K et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet 2004;21:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Bae CY, Wee G, Han YM, Park JK. A microfluidic in vitro cultivation system for mechanical stimulation of bovine embryos. Electrophoresis 2009;30:3276–3282. [DOI] [PubMed] [Google Scholar]
- Krause I, Pohler U, Grosse S, Shebl O, Petek E, Chandra A, Ebner T. Characterization of the injection funnel during intracytoplasmic sperm injection reflects cytoplasmic maturity of the oocyte. Fertil Steril 2016;106:1–6. [DOI] [PubMed] [Google Scholar]
- Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev 2010;22:32–39. [DOI] [PubMed] [Google Scholar]
- Kuczyński W, Dhont M, Grygoruk C, Pietrewicz P, Redzko S, Szamatowicz M. Rescue ICSI of unfertilized oocytes after IVF. Hum Reprod 2002;17:2423–2427. [DOI] [PubMed] [Google Scholar]
- Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 2009;28:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SM, Lee HJ, Hung P, Matthews LM, Robinson DN, Evans JP. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol Biol Cell 2010;21:3182–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Liu AP. A microfluidic pipette array for mechanophenotyping of cancer cells and mechanical gating of mechanosensitive channels. Lab Chip 2015;15:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J, Jaczewska J, Lekki J, Stachura Z. Cancer cell recognition—mechanical phenotype. Micron 2012;43:1259–1266. [DOI] [PubMed] [Google Scholar]
- Leung C, Lu Z, Zhang XP, Sun Y. Three-dimensional rotation of mouse embryos. IEEE Trans Biomed Eng 2012;59:1049–1056. [DOI] [PubMed] [Google Scholar]
- Li QS, Lee GYH, Ong CN, Lim CT. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun 2008;374:609–613. [DOI] [PubMed] [Google Scholar]
- Liu X, Fernandes R, Jurisicova A, Casper RF, Sun Y. In situ mechanical characterization of mouse oocytes using a cell holding device. Lab Chip 2010;10:2154–2161. [DOI] [PubMed] [Google Scholar]
- Liu X, Shi J, Zong Z, Wan K-T, Sun Y. Elastic and viscoelastic characterization of mouse oocytes using micropipette indentation. Ann Biomed Eng 2012;40:2122–2130. [DOI] [PubMed] [Google Scholar]
- Longo FJ. Changes in the zonae pellucidae and plasmalemmae of aging mouse eggs. Biol Reprod 1981;25:399–411. [DOI] [PubMed] [Google Scholar]
- Lord T, John Aitken R. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013;146. [DOI] [PubMed] [Google Scholar]
- Luo YN, Chen DY, Zhao Y, Wei C, Zhao XT, Yue WT, Long R, Wang JB, Chen J. A constriction channel based microfluidic system enabling continuous characterization of cellular instantaneous Young's modulus. Sens Actuators B Chem 2014;202:1183–1189. [Google Scholar]
- Mackenzie ACL, Kyle DD, McGinnis LA, Lee HJ, Aldana N, Robinson DN, Evans JP. Cortical mechanics and myosin-II abnormalities associated with post-ovulatory aging: implications for functional defects in aged eggs. Mol Hum Reprod 2016;22:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna C, Rienzi L, Greco E, Sbracia M, Rahman A, Poverini R, Siracusa G, De Felici M. Zona pellucida solubility and cortical granule complements in human oocytes following assisted reproductive techniques. Zygote 2001;9:201–210. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Hayashi N, Kuroda Y, Takiue C, Hirata R, Takenami M, Aoi Y, Yoshioka N, Habara T, Mukaida T et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod Biomed Online 2010;20:358–364. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 2009;15:573–585. [DOI] [PubMed] [Google Scholar]
- Mintz B, Gearhart JD. Subnormal zona pellucida change in parthenogenetic mouse embryos. Dev Biol 1973;31:178–184. [DOI] [PubMed] [Google Scholar]
- Mizobe Y, Yoshida M, Miyoshi K. Enhancement of cytoplasmic maturation of in vitro-matured pig oocytes by mechanical vibration. J Reprod Dev 2010;56:285–290. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Constantinou CE, Omata S. Micro-mechanical sensing platform for the characterization of the elastic properties of the ovum via uniaxial measurement. J Biomech 2004;37:67–72. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Mizuno J, Kamakura H, Fueta Y, Nakamura H, Akaishi K, Anzai K, Watanabe A, Inui H, Omata S. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Hum Cell 2006;19:119–125. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Omata S. Fabrication of micro tactile sensor for the measurement of micro-scale local elasticity. Sens Actuators A Phys 2004;109:202–207. [Google Scholar]
- Murayama Y, Yoshida K, Takahashi H, Mizuno J, Akaishi K, Inui H. Softening of the mouse zona pellucida during oocyte maturation. Annu Int Conf IEEE Eng Med Biol Soc 2013:6834–6837. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Yoshida M, Mizuno J, Nakamura H, Watanabe Y, Akaishi K, Inui H, Constantinou CE, Omata S. Elasticity measurement of zona pellucida using a micro tactile sensor to evaluate embryo quality. J Mamm Ova Res 2008. a;25:8–16. [Google Scholar]
- Murayama Y, Yoshida M, Mizuno J, Nakamura H, Watanabe Y, Akaishi K, Inui H, Constantinou CE, Omata S. Elasticity measurement of zona pellucida using a micro tactile sensor to evaluate embryo quality. Bio One 2008. b;25:8–16. [Google Scholar]
- Nagy ZP, Rienzi LF, Ubaldi FM, Greco E, Massey JB, Kort HI. Effect of reduced oocyte aging on the outcome of rescue intracytoplasmic sperm injection. Fertil Steril 2006;85:901–906. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Hiramoto Y. Mechanical properties of the cell surface in starfish eggs. Dev Growth Differ 1978;20:317–327. [DOI] [PubMed] [Google Scholar]
- Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, Land JA, Evers JL, Dumoulin JC. Further evidence that culture media affect perinatal outcome: Findings after transfer of fresh and cryopreserved embryos. Hum Reprod 2012;27:1966–1976. [DOI] [PubMed] [Google Scholar]
- Nelissen ECM, Van Montfoort AP, Smits LJM, Menheere PPCA, Evers JLH, Coonen E, Derhaag JG, Peeters LL, Coumans AB, Dumoulin JCM. IVF culture medium affects human intrauterine growth as early as the second trimester of pregnancy. Hum Reprod 2013;28:2067–2074. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Yoneda M, Uemura I. Marked decrease in the rigidity of starfish oocytes induced by 1-Methyladenine. Dev Growth Differ 1980;22:315–325. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 2006;300:534–544. [DOI] [PubMed] [Google Scholar]
- Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca 2+]cyt signal in the mouse. Dev Biol 2005;282:39–54. [DOI] [PubMed] [Google Scholar]
- Ozil JP. The parthenogenetic development of rabbit oocytes after repetitive pulsatile electrical stimulation. Development 1990;109:117–127. [DOI] [PubMed] [Google Scholar]
- Palermo GD, Alikani M, Bertoli M, Colombero LT, Moy F, Cohen J, Rosenwaks Z. Oolemma characteristics in relation to survival and fertilization patterns of oocytes treated by intracytoplasmic sperm injection. Hum Reprod 1996;11:172–176. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Papi M, Brunelli R, Familiari G, Frassanito MC, Lamberti L, Maulucci G, Monaci M, Pappalettere C, Parasassi T, Relucenti M et al. Whole-depth change in bovine zona pellucida biomechanics after fertilization: how relevant in hindering polyspermy. PLoS One 2012;7:e45696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi M, Brunelli R, Sylla L, Parasassi T, Monaci M, Maulucci G, Missori M, Arcovito G, Ursini F, Spirito De M et al. Mechanical properties of zona pellucida hardening. Eur Biophys J 2010;39:987–992. [DOI] [PubMed] [Google Scholar]
- Papi M, Sylla L, Parasassi T, Brunelli R, Monaci M, Maulucci G, Missori M, Arcovito G, Ursini F, Spirito De M. Evidence of elastic to plastic transition in the zona pellucida of oocytes using atomic force spectroscopy. Appl Phys Lett 2009;94:17–19. [Google Scholar]
- Payton RR. Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol Reprod 2004;71:1303–1308. [DOI] [PubMed] [Google Scholar]
- Pelletier C, Keefe DL, Trimarchi JR. Noninvasive polarized light microscopy quantitatively distinguishes the multilaminar structure of the zona pellucida of living human eggs and embryos. Fertil Steril 2004;81:850–856. [DOI] [PubMed] [Google Scholar]
- Pesce G, Selvaggi L, Caporali A, De Luca AC, Puppo A, Rusciano G, Sasso A. Mechanical changes of living oocytes at maturation investigated by multiple particle tracking. Appl Phys Lett 2009;95:093702. [Google Scholar]
- Pesce G, Selvaggi L, Rusciano G, Sasso A. High- and low-frequency mechanical properties of living starfish oocytes. J Biophotonics 2011;4:324–334. [DOI] [PubMed] [Google Scholar]
- Rebelo LM, Sousa JS, de, Mendes Filho J, Radmacher M. Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology 2013;24:055102. [DOI] [PubMed] [Google Scholar]
- Sato M, Theret DP, Wheeler LT, Ohshima N, Nerem RM. Application of the micropipette technique to the measurement of cultured porcine aortic endothelial cell viscoelastic properties. J Biomech Eng 1990;112:263–268. [DOI] [PubMed] [Google Scholar]
- Schiewe MC, Araujo E, Asch RH, Balmaceda JP. Enzymatic characterization of zona pellucida hardening in human eggs and embryos. J Assist Reprod Genet 1995;12:2–7. [DOI] [PubMed] [Google Scholar]
- Schroeder AC. Fetuin inhibits zona pellucida hardening and conversion of ZP2 to ZP2f during spontaneous mouse oocyte maturation in vitro in the absence of serum. Biol Reprod 1990;43:891–897. [DOI] [PubMed] [Google Scholar]
- Smith GD, Monteiro A, Keller L. Microfluidics for sperm selection. Non-Invasive Sperm Sel Vitr Fertil Nov Concepts Methods 2015:51–58. [Google Scholar]
- Sun Y, Wan K-T, Roberts KP, Bischof JC, Nelson BJ. Mechanical property characterization of mouse zona pellucida. IEEE Trans Nanobioscience 2003;2:279–286. [DOI] [PubMed] [Google Scholar]
- Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater 2005;1:15–30. [DOI] [PubMed] [Google Scholar]
- Suresh S. Mechanical response of human red blood cells in health and disease: Some structure-property-function relationships. J Mater Res 2006;21:1871–1877. [Google Scholar]
- Swain JE, Lai D, Takayama S, Smith GD. Thinking big by thinking small: application of microfluidic technology to improve ART. Lab Chip 2013;13:1213–1224. [DOI] [PubMed] [Google Scholar]
- Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update 2011;17:541–557. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev 2003;66:143–152. [DOI] [PubMed] [Google Scholar]
- Talbot P, Dandekar P. Perivitelline space: does it play a role in blocking polyspermy in mammals. Microsc Res Tech 2003;61:349–357. [DOI] [PubMed] [Google Scholar]
- Tartia AP, Rudraraju N, Richards T, Hammer M-A, Talbot P, Baltz JM. Cell volume regulation is initiated in mouse oocytes after ovulation. Development 2009;136:2247–2254. [DOI] [PubMed] [Google Scholar]
- Tse HTK, Gossett DR, Moon YS, Masaeli M, Sohsman M, Ying Y, Mislick K, Adams RP, Rao J, Di Carlo D. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med 2013;5:212ra163. [DOI] [PubMed] [Google Scholar]
- Vajta G, Rienzi L, Bavister BD. Zona-free embryo culture: is it a viable option to improve pregnancy rates. Reprod Biomed Online 2010;21:17–25. [DOI] [PubMed] [Google Scholar]
- Valentine MT, Perlman ZE, Mitchison TJ, Weitz DA. Mechanical properties of Xenopus egg cytoplasmic extracts. Biophys J 2005;88:680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoort CA, Hung PH, Schramm RD. Prevention of zona hardening in non-human primate oocytes cultured in protein-free medium. J Med Primatol 2007;36:10–16. [DOI] [PubMed] [Google Scholar]
- Vanroose G, Nauwynck H, Soom AV, Ysebaert MT, Charlier G, Oostveldt PV, De Kruif A. Structural aspects of the zona pellucida of in vitro-produced bovine embryos: a scanning electron and confocal laser scanning microscopic study. Biol Reprod 2000;62:463–469. [DOI] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Goldstein RE. Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma 2010;240:99–107. [DOI] [PubMed] [Google Scholar]
- Vermilyea MD, Tan L, Anthony JT, Conaghan J, Ivani K, Gvakharia M, Boostanfar R, Baker VL, Suraj V, Chen AA et al. Computer-automated time-lapse analysis results correlate with embryo implantation and clinical pregnancy: a blinded, multi-centre study. Reprod Biomed Online 2014;29:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacogne B, Pieralli C, Roux C, Gharbi T. Measuring the mechanical behaviour of human oocytes with a very simple SU-8 micro-tool. Biomed Microdevices 2008;10:411–419. [DOI] [PubMed] [Google Scholar]
- Wessel AD, Gumalla M, Grosshans J, Schmidt CF. The mechanical properties of early drosophila embryos measured by high-speed video microrheology. Biophys J 2015;108:1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007;28:4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod 1998;13:394–397. [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE, Rappolee DA. Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev 2008;75:689–697. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev 2007;74:1287–1294. [DOI] [PubMed] [Google Scholar]
- Yanez LZ, Han J, Behr BB, Pera RAR, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 2011;13:1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzpe AA, Liu Z, Fluker MR. Rescue intracytoplasmic sperm injection (ICSI) – Salvaging in vitro fertilization (IVF) cycles after total or near-total fertilization failure. Fertil Steril 2000;73:1115–1119. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, Siemeski A, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 2006;103:10889–10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeringue HC, Beebe DJ, Wheeler MB. Removal of cumulus from mammalian zygotes using microfluidic techniques. Biomed Microdevices 2001;3:219–224. [Google Scholar]
- Zeringue HC, Wheeler MB, Beebe DJ. A microfluidic method for removal of the zona pellucida from mammalian embryos. Lab Chip 2005;5:108–110. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Nguyen J, Wei Y, Sun Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013;13:2464–2483. [DOI] [PubMed] [Google Scholar]