Abstract
STUDY QUESTION
Is resveratrol able to prevent the lipopolysaccharide (LPS)-induced preterm labor in 15-day pregnant BALB/c mice?
SUMMARY ANSWER
Resveratrol prevented the LPS-induced onset of preterm labor in 64% of the cases and showed anti-inflammatory and tocolytic effects by downregulating COX-2 and iNOS expression and NOS activity, and by changing the uterine prostaglandin and endocannabinoid profiling.
WHAT IS KNOWN ALREADY
Genital tract infections by Gram-negative bacteria are a common complication in human pregnancy and have been shown to increase risk of preterm delivery. Bacterial LPS elicits a strong maternal inflammatory response that results in preterm delivery and fetal death in a murine model endotoxin-induced preterm labor.
STUDY DESIGN, SIZE, DURATION
An in vivo animal study was conducted. On Day 15 of pregnancy, mice received at 8:00 h a dose of vehicle (40% ethanol in saline solution) or resveratrol (3 mg/kg in vehicle) via oral gavage followed by two doses of LPS or vehicle administered intraperitoneally (i.p.), the first one at 10:00 h (0.17 mg/kg in 0.1 ml of sterile saline solution) and the second at 13:00 h (0.5 mg/kg in 0.1 ml of sterile saline solution). The mice were closely observed for any signs of morbidity (piloerection, decreased movement, and diarrhea), vaginal bleeding or preterm delivery. The beginning of preterm delivery was defined by early delivery of the first pup. Normal term labor occurs on Day 19 of gestation.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Time of labor, pregnancy outcome and morphological features were evaluated after LPS and/or resveratrol administration. Uterine stripes were collected 5 h after the last LPS injection and prostaglandin and endocannabinoid profiling was analyzed by mass spectrometry. Nitric oxide synthase (NOS) activity was measured by radioconversion assay. Cyclooxygenase-2 (Cox-2) and 15-hydroxyprostaglandin dehydrogenase (15-Pgdh) mRNA levels were analyzed by RT-PCR whilst the protein expression of inducible nitric oxide synthase (iNOS), COX-1 and COX-2 were studied by western blot.
MAIN RESULTS AND THE ROLE OF CHANCE
In vivo treatment of 15-day pregnant BALB/c mice with resveratrol prevented the LPS-induced preterm birth in 64% of the cases, whereas only 15% of mice with LPS alone escaped preterm birth. Treatment with resveratrol resulted in a reduced NOS activity (P < 0.05) in the uterus of LPS-treated mice. Similarly, resveratrol reduced the expression of LPS-induced pro-inflammatory agents such as iNOS (P < 0.05), COX-2 (P < 0.05), prostaglandin E2 (PGE2) (P < 0.05) and anandamide (AEA) (P < 0.05). Moreover, resveratrol administration resulted in changes in the uterine endocannabinoid profiling altered by LPS.
LARGE SCALE DATA
N/A.
LIMITATIONS, REASONS FOR CAUTION
Since our experimental design involves the use of mice, the extrapolation of the results presented here to humans is limited.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings provide evidence for the tocolytic effects of resveratrol.
STUDY FUNDING AND COMPETING INTEREST(S)
Dr Ana María Franchi was funded by Agencia Nacional para la Promoción Científica y Tecnológica (PICT 2013/0097) and by Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2012/0061). Dr Heather B. Bradshaw was funded by NIH (DA006668). The authors have no competing interests.
Keywords: resveratrol, endocannabinoid system, preterm labor, prostaglandin, lipidomics
Introduction
Premature birth, before 37 completed weeks of gestation, is the leading cause of mortality and morbidity in neonates and infants. Around 1 million children die annually due to complications of preterm birth (Menon, 2012), and despite the advances in prenatal care, the rate of prematurity has not decreased over the past decade (Goldenberg et al., 2008). Even though preterm birth is considered a complex syndrome, ~30% of all premature deliveries are associated with an underlying infection (Romero et al., 2006; Park et al., 2009). In order to study the pathological process involved in the prematurity elicited by infection, we developed a murine model of preterm labor by exposing 15-day pregnant mice to bacterial endotoxin (Cella et al., 2010; Domínguez Rubio et al., 2014; Bariani et al., 2015). Lipopolysaccharide (LPS) stimulates a maternal inflammatory response with cytokine and chemokine production and leukocyte infiltration of uterine, placental and fetal tissues, with the subsequent release of prostaglandins, endocannabinoids, nitrogen and oxygen reactive species and metalloproteinases (Romero et al., 2006). Prostaglandins, nitric oxide (NO) and TNFα have been associated with triggering preterm labor in both animal models (Cella et al., 2010; Burdet et al., 2013; Domínguez Rubio et al., 2014) and humans (Törnblom et al., 2005).
The endocannabinoid system (eCS) is composed of the cannabinoid receptors CB1 and CB2, their endogenous ligands of lipid nature (named endocannabinoids; eCBs), and enzymes regulating synthesis and degradation of these lipids. Since the uterus contains large amounts of the eCB anandamide (N-arachidonoyl ethanolamine; AEA) (Schmid et al., 1997), and blood levels of AEA increase during normal labor (Habayeb et al., 2004; Nallendran et al., 2010), it has been suggested that the eCS plays an important role in reproductive events (Habayeb et al., 2004; Bariani et al., 2015). Preterm labor is a complex syndrome with a difficult clinical management. Even though β2-adrenergic receptor agonists are considered the standard tocolytic treatment worldwide (Simhan and Caritis, 2007), they are not devoid of potentially harmful side effects (Croen et al., 2011; Gidaya et al., 2016). Therefore, the search for newer and safer tocolytic agents is needed. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a naturally occurring polyphenol (Frémont, 2000) that has been shown to exert beneficial effects by targeting some of the pathophysiological mechanisms triggered during preterm labor (Furuya et al., 2015; Deng et al., 2016) and other complications of pregnancy (Bourque et al., 2012; Poudel et al., 2013). It has been shown that resveratrol activates sirtuin-1 (SIRT-1), a NAD+-dependent protein deacetylase (Lagouge et al., 2006), which is widely expressed in reproductive tissues (Lappas et al., 2011). Activation of SIRT-1 has been shown to exert anti-inflammatory effects by downregulating pro-inflammatory cytokines (Shen et al., 2009; Lappas et al., 2011), nitric oxide (NO) production and inducible NO synthase (iNOS) expression (Lee et al., 2009), modulating NFκB transcriptional activity (Bagul et al., 2015) and maintaining fetal vascular homeostasis by inhibiting accelerated senescence of cord blood endothelial progenitor cells (Vassallo et al., 2014).
The aim of the present study was to investigate the protective effects of resveratrol on the pregnancy outcome in our model of systemic LPS-induced preterm labor. We also focused on the effects of resveratrol on pro-inflammatory parameters as well as prostaglandin and endocannabinoid production in the uterus of LPS-treated mice.
Materials and Methods
Reagents
LPS from Escherichia coli 05:B55 and resveratrol were purchased from Sigma Chemical Co. (St Louis, MI, USA). The anti-COX-1, anti-COX-2, anti-iNOS and anti-α-Tubulin antibodies were purchased from Abcam (Cambridge, UK). Secondary horse radish peroxidase (HRP) conjugated antibodies were purchased from Jackson Immunosearch (Baltimor Pike, USA). Western blotting reagents and nitrocellulose membranes (Trans-Blot, 0.45 μm) were from Bio-Rad Inc. (Hercules, CA, USA) and molecular weight marker was purchased by GE Healthcare Bio-Science Corp. (Piscataway, NJ, USA). Trizol reagent, RNAse-free DNAse I, Moloney Murine Leukemia virus reverse transcriptase (M-MLVRT) and random primers were purchased from Invitrogen (Life Technologies, Argentina). GoTaq DNA Polymerase was purchased from Promega (Biodynamics, Argentina). All other chemicals were analytical grade.
Animals
Female BALB/c mice from our own colony were housed in a standard animal room with food and water ad libitum, in controlled conditions of humidity, temperature (21 ± 2°C) and luminosity (200 lux), under a 12 h light/dark lighting schedule (lights on at 7:00 h). For mating, a single male mouse was mated with two nulliparous females of the same strain. Time of pregnancy was determined by visual inspection of the vaginal plug, which was defined as Day 0 of pregnancy. The experimental procedures reported here were approved by the Animal Care Committee of the Center for Pharmacological and Botanical Studies, National Research Council, and by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL) from the School of Medicine, University of Buenos Aires (No. 1163/2016), and were carried out in accordance.
Treatments
As shown in Supplementary Fig. S1, mice were randomly allocated into four experimental groups (Control, Resveratrol, LPS, or LPS + Resveratrol). On Day 15 of pregnancy, mice received at 8:00 h a dose of vehicle (40% ethanol in saline solution) or resveratrol (3 mg/kg in vehicle) via oral gavage followed by two doses of LPS or vehicle administered intraperitoneally (i.p.), the first one at 10:00 h (0.17 mg/kg in 0.1 ml of sterile saline solution) and the second at 13:00 h (0.5 mg/kg in 0.1 ml of sterile saline solution). The mice were closely observed for any signs of morbidity (piloerection, decreased movement and diarrhea), vaginal bleeding or preterm delivery. The beginning of preterm delivery was defined by early delivery of the first pup. Normal term labor occurs on Day 19 of gestation.
A second set of mice were euthanized by cervical dislocation on Day 15 of pregnancy, 5 h after the second injection of vehicle or LPS. Fetuses were immediately removed, and the underlying uterine tissue was collected and stored at −80°C until further use.
RT-PCR
Uterine tissues were homogenized in a glass homogenizer on ice in 1 ml of TRIzol reagent and total RNA was isolated according to the manufacturer's recommendations (Invitrogen). Following extraction, 8 μg of RNA were treated with RNAse-free DNAse I to digest contaminating genomic DNA. Total mRNA was synthesized to cDNA using MMLV-RT, random primers and RNAse inhibitors. PCR was performed with 4 μg of cDNA and specific primers designed using the Primer3 Software package and checked for self-complementarity with OligoCalc Software package. The sequences of primers used in this experiment were as follows: Cyclooxygenase-2 (Cox-2) forward, 5′-TCCTCCTGGAACATGGACTC-3′; Cox-2 reverse, 5′-CCCCAAAGATAGCAT CTGGA-3′; NAD+-dependent 15-hydroxyprostaglandin dehydrogenase (15-Pgdh) forward, 5′-CACCTCCGTTTTGCTTACTCA-3′; 15-Pgdh reverse, 5′-GTTCGTCCAGTGTGATGTGG-3′; β-actin forward: 5′-TGTTACCAACTG GGACGACA–3′; β-actin reverse: 5′-TCTCAGCTGTGGTGGTGAAG–3′. PCR cycle parameters were as follows: for Cox-2 and β-actin, 30 cycles of 94°C 40 s, 57°C 30 s, 72°C 1 min; for 15-Pgdh, 30 cycles of 94°C 40 s, 59°C 30 s, 72°C 1 min.
PCR products were loaded into a 2% agarose gel and bands were visualized on a transilluminator after ethidium bromide staining. Images were taken using a digital camera Olympus C-5060 and analyzed using the Image J software package (open source, NIH). The relative mRNA level was normalized to β-actin and results were expressed as relative optical density (Cox-2/β-actin; 15-Pgdh/β-actin).
Determination of NOS activity
The underlying uterine tissue from each implantation site were collected and immediately frozen at –80°C until NOS activity determination. A modification of the method of Bredt and Snyder (Bredt and Snyder, 1989) was used. This method measures the conversion of [14C]arginine to [14C]citrulline, as citrulline remains in the sample, whereas the equimolar amounts of NO produced are rapidly destroyed. Briefly, uterine tissue was homogenized and incubated at 37°C in a buffer containing 20 mmol/l Hepes, 10 μM [14C]arginine (0.3 μCi), 25 mM valine, 1 mM DTT, 0.45 mM CaCl2 and 1 mM NADPH. Valine, which inhibits the conversion of l-arginine to l-citrulline by arginases, was included in the reaction mixture to increase assay specificity. After 15 min of incubation, samples are centrifuged for 10 min at 3000 g and applied to a 1 ml DOWEX AG50WX8 column (Na + form) and [14C]citrulline was eluted in 3 ml water. The radioactivity was measured by liquid scintillation counting. Enzyme activity is reported as fmol [14C]citrulline per mg of wet tissue per 15 min.
Western blot analysis
A group of mice was euthanized on Day 15 of pregnancy 5 h after the second injection of vehicle or LPS. The uteri were immediately removed and homogenized (Ultra Turrax, T25 basic, IKA Labortechnik, Staufen, Germany) in lysis buffer (10 mM Hepes, 5 mM MgCl2, 142.5 mM KCl, 0.1% SDS, 1% Nonidet-40, 5 mM EDTA, 0.5% sodium deoxycholate in PBS) with a freshly added protease inhibitor cocktail (10 μg/ml leupeptin, 2 μg/ml aprotinin, 100 μg/ml soybean-trypsin inhibitor, 1 mmol/l EDTA, 1 mg/ml benzamidine, 10 μg/ml DTT and 1 mg/ml caproic acid). Tissues were sonicated (Ultrasonic Cell Disrupter, Microson, Heat systems, Inc. New York, NY, USA) for 30 s, centrifuged at 1500 g for 10 min and protein concentration was determined. Samples containing 30 μg of protein were loaded in each lane. Samples were separated by electrophoresis in 7.5–12% SDS–PAGE gel and transferred to a 0.45 μm nitrocellulose membrane. Membranes were blocked with 5% w/v dried non-fat milk and then incubated with the primary antibodies. Membranes were washed with PBS-T (10 mM Tris, 100 mM NaCl and 0.1% Tween 20, pH 7.5) followed by 1 h incubation with HRP-conjugated secondary antibody (1:5000) and developed using the ECL system. Images of immunoreactive bands were acquired using the ImageQuant blot documentation instrument (GE Healthcare Life Sciences, Pittsburgh, PA, USA) and analyzed using the Image J Software Package (developed at the National Institutes of Health). Relative protein levels were normalized to α-Tubulin and results were expressed as relative optical density (iNOS/α-Tubulin, COX-1/α-Tubulin, COX-2/α-Tubulin).
Mass spectrometric analysis of lipids in fractions
For lipid analysis, uterine explants were obtained from each BALB/c pregnant mice, flash frozen and stored at −80°C until used for extraction. For extraction preparation, explants were weighed and placed in 2 ml of HPLC-grade methanol. [2H8]-NAGly (500 pmol) was added to each sample as an internal measure of extraction efficiency. Samples were centrifuged and the supernatant was decanted and diluted with HPLC-grade water to make a 75% aqueous solution. Lipids were extracted as previously described (Bradshaw et al., 2006; Wolfson et al., 2015). Briefly, 500 mg C8 Bond Elut solid phase extraction columns (Agilent) were conditioned with 5 ml HPLC-grade methanol, followed by 2.5 ml HPLC water. The 75% aqueous solutions containing the fractions were loaded onto separate columns, which were then washed with 2.5 ml water. Four sequential elutions (1.5 ml each of 60, 75, 85 and 100% methanol) were collected for mass spectrometric analysis. As described previously (Bradshaw et al., 2006), sample analysis of lipids was carried out as follows. A 20 μL aliquot of each of the eluates was loaded using a Shimadzu SCL10Avp (Wilmington, DE, USA) autosampler onto a reversed phase Zorbax 2.1 × 50 mm2 C18 column maintained at 40°C. HPLC gradient formation at a flow rate of 200 ml/min was achieved by a system comprised of a Shimadzu controller and two Shimadzu LC10ADvp pumps. Lipid levels in the samples were analyzed in multiple reaction monitoring (MRM) mode on an API3000 (Applied Biosystems/MDS SCIEX, Foster City, CA, USA) triple quadrupole mass spectrometer with electrospray ionization. Methods for lipid analysis were created and optimized by flow injection of lipid standards. All calculations for quantitation experiments were based on calibration curves using synthetic standards as previously described (Bradshaw et al., 2006).
Data analysis and statistical procedures
Data were analyzed by means of one-way ANOVA procedures in a completely randomized design. Comparisons were made with the Tukey's post hoc test. Results were considered significant at P < 0.05. The assumptions of normality and homogeneity of variance were studied analytically by Shapiro–Wilks test and the Levene test, respectively. All statistical analyses were performed using the statistical program InfoStat (FCA, Universidad de Córdoba, Córdoba, Argentina).
Results
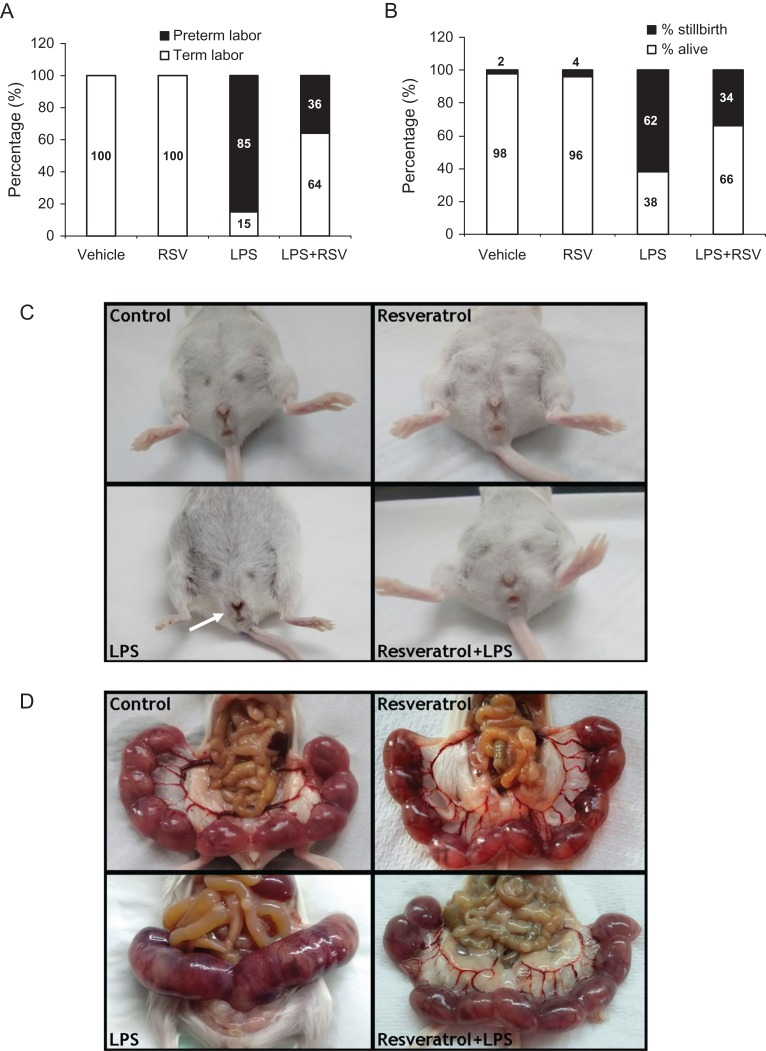
To study the effects of an oral administration of resveratrol on LPS-induced preterm labor, preterm delivery was induced in 15-day pregnant BALB/c mice with two consecutive i.p. injections of the endotoxin. Exposure to LPS-induced preterm delivery in 85% of mice, whilst co-treatment with resveratrol, which did not modify pregnancy length per se, prevented the endotoxin-induced preterm delivery in 64% of the cases (Fig. 1A). Next, we assessed the stillbirth and pup mortality rate in control and treated mice. LPS-treated mice presented a 62% rate of stillbirth whereas the treatment with resveratrol reduced pup mortality to 34% (Fig. 1B). Macroscopic examination of the vagina showed antepartum hemorrhage in LPS-treated mice, which was absent in mice treated with vehicle, resveratrol alone or LPS+resveratrol (Fig. 1C). Additionally, 5 h after the second injection of the endotoxin, the uterus from LPS-treated mice showed signs of edema, inflammation, loss of intersite definition and fetal death when compared to mice treated with vehicle, resveratrol alone or LPS + resveratrol (Fig. 1D).
Figure 1.
The treatment with LPS-induced preterm labor in 85% of cases, whereas resveratrol prevented LPS-induced preterm delivery in 64% of cases. Control animals or animals treated with resveratrol alone delivered at term in 100% of cases (n = 11) (A) Percentage of stillbirth and pup mortality in control or treated mice. LPS-treated mice presented a stillbirth rate of 62% whereas the treatment with resveratrol reduced pup mortality to 34% (B) Representative photographs of vaginal bleeding in LPS-exposed BALB/c pregnant mice (white arrow). Vaginal bleeding was absent in mice treated with resveratrol (C) Representative photographs of uterus morphology at 5 h after the second injection of vehicle or LPS. Note the less defined intersites in uteri from mice treated with LPS. Resveratrol administration prevented the deleterious effects of LPS on uterus morphology (D).
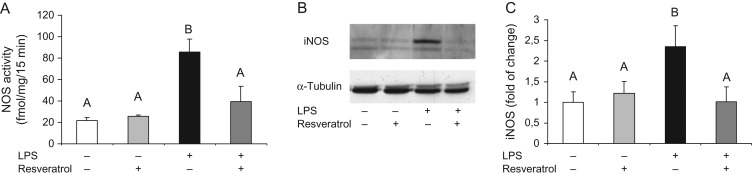
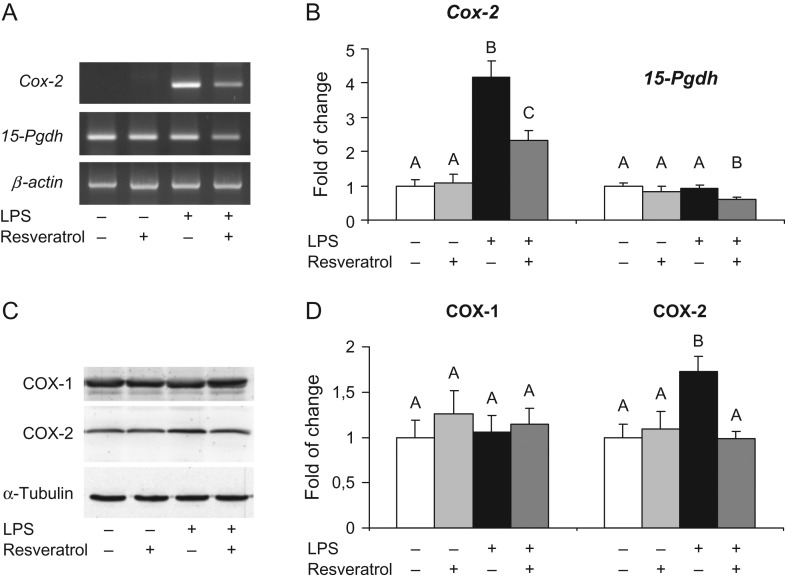
In order to understand the molecular mechanisms involved in the protective effects of resveratrol on preventing LPS-induced preterm labor, NOS activity was assessed 5 h after the last injection of LPS. As shown in Fig. 2A, the treatment with resveratrol prevented the LPS-induced increase in uterine activity of NOS. Moreover, resveratrol also prevented the LPS-induced increase of uterine iNOS protein expression (Fig. 2B). Similarly, resveratrol reduced the uterine overexpression of cyclooxygenase-2 (COX-2) mRNA (Fig. 3A) and protein (Fig. 3C) induced by LPS. Interestingly, the 15-hydroxyprostaglandin dehydrogenase (15-Pgdh) mRNA levels and COX-1 protein expression remained unchanged by LPS and/or resveratrol administration.
Figure 2.
Five hours after the second injection of vehicle or LPS, NOS activity (A) and iNOS protein levels (B and C) were significantly higher in the uterus from LPS-treated mice in the absence of resveratrol, which significantly decreased the effect of the endotoxin on these parameters. Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 6). A representative Western blot of iNOS levels is shown in panel B.
Figure 3.
Cox-2 and 15-Pdgh mRNA levels were analyzed in uterine strips collected from treated pregnant BALB/c mice. LPS treatment induced and increased expression of Cox-2 mRNA with no changes in 15-Pgdh mRNA levels. Administration of resveratrol prevented the LPS-induced effects on uterine Cox-2 mRNA levels (A). Densitometry analysis of Cox-2 and 15-Pgdh mRNA levels. Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 6) (B). COX-1 and COX-2 protein levels were analyzed in uterine strips collected from treated pregnant BALB/c mice. LPS treatment induced and increased protein expression of COX-2 with no changes in COX-1 protein levels. Administration of resveratrol prevented the LPS-induced effects on uterine COX-2 protein levels (C). Densitometry analysis of COX-1 and COX-2 western blots (D). Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 6).
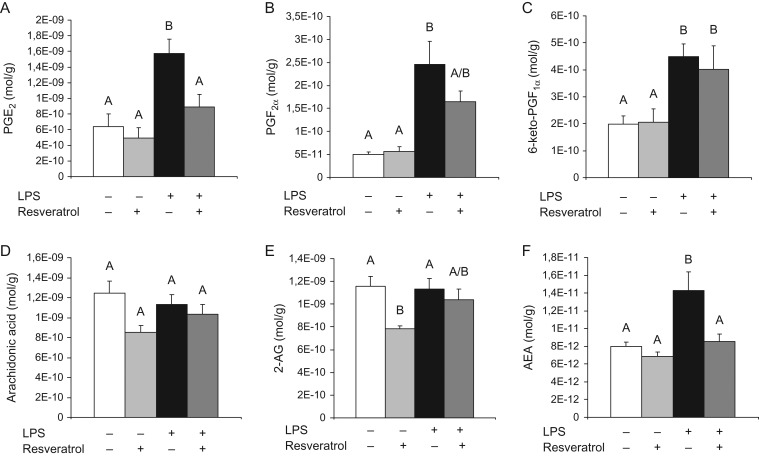
Since we found that resveratrol was able to modulate the LPS-induced expression of COX-2 and since this enzyme has a central role in the production of eicosanoids, we decided to analyze the uterine content of the main prostaglandins, PGE2 and PGF2α as well as 6-keto-PGF1α, the stable hydrolyzed product of the unstable prostacyclin (also known as prostaglandin I2). We found that the treatment with resveratrol prevented the increase in uterine PGE2 (Fig. 4A) whereas the effect on PGF2α (Fig. 4B) was only partial and it had no effects on the uterine content of 6-keto-PGF1α (Fig. 4C). Since arachidonic acid the main substrate of COX-2, we decided to study the effects of LPS and/or resveratrol treatment on this polyunsaturated fatty acid. Interestingly, neither LPS nor resveratrol treatment modified the uterine content of arachidonic acid (Fig. 4D). Next, we analyzed whether the treatment with LPS and/or resveratrol altered the uterine content of endocannabinoids and endocannabinoid-like lipids. LPS administration did not modify the uterine content of 2-arachidonoylglycerol (2-AG) but the treatment with resveratrol reduced alone the content of this endocannabinoid (Fig. 4E). In the case of anandamide (AEA), the administration of LPS induced an increase in the uterine content of this endocannabinoid, whereas the treatment with resveratrol completely prevented this effect (Fig. 4F). Furthermore, LPS administration to 15-day pregnant BALB/c mice modified the uterine content of several endocannabinoid-related lipids belonging to the family of N-acyl ethanolamines (NAEs); N-acyl glycines (NAGlys); N-acyl serines (NASers) and 2-acyl-sn-glycerols (Table I). Additionally, LPS administration induced an increase in the uterine content of linoleic acid (Table SI; Supplementary Table S1). The treatment with resveratrol was able to normalize the uterine content of several of these lipids. Both Table I and Supplementary Table S1 show whether the effects of LPS and/or resveratrol were significant or only showed a tendency. Taken collectively, our results show that resveratrol prevented LPS-induced qualitative and quantitative changes in the endocannabinoid and related lipid profiling in the uterus from 15-day pregnant mice.
Figure 4.
LPS treatment to BALB/c pregnant mice increased the uterine levels of the prostaglandins PGE2 (A), PGF2α (B) and 6-keto-PGF1α (C). Treatment with resveratrol completely prevented the LPS-induced increased production of PGE2, partially prevented the increased production of PGF2α and had no effect on LPS-induced 6-keto-PGF1α levels. Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 8–14). LPS treatment to BALB/c pregnant mice had no effects on the uterine levels of arachidonic acid (D) or 2-AG (E). Treatment with resveratrol decreased the uterine basal levels of 2-AG but had no effects on the basal levels of arachidonic acid. Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 8–14). LPS treatment to BALB/c pregnant mice increased the uterine levels of AEA (F). Treatment with resveratrol completely prevented the LPS-induced increased production of AEA. Data are shown as mean ± S.E.M. Statistics: A ≠ B, P < 0.05 (n = 8–14).
Table I.
LPS administration to 15-day pregnant BALB/c mice induced changes in uterine tissue levels of several N-acyl ethanolamines (NAEs), such as N-oleoyl ethanolamide (OEA) and N-linoleoyl ethanolamine (LEA).
| Uterus | Change with Resveratrol (relative to vehicle) | Change with LPS (relative to vehicle) | Change with Resveratrol + LPS (relative to vehicle) | Change with LPS (relative to resveratrol) | Change with Resveratrol + LPS (relative to resveratrol) | Change with Resveratrol + LPS (relative to LPS) |
|---|---|---|---|---|---|---|
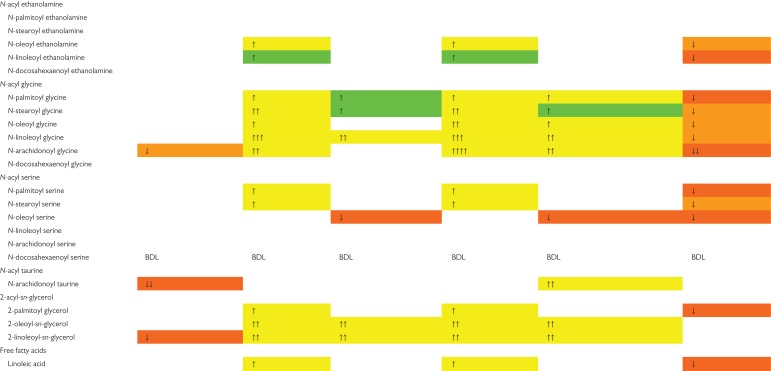 | ||||||

The administration of resveratrol two hours previous to the first LPS injection, was able to modulate the effects of the endotoxin on OEA and LEA. Regarding N-acyl glycines (NAGlys): LPS administration increased the uterine tissue levels of several NAGlys, such as N-plamitoyl glycine (PGly), N-oleoyl glycine (OGly), N-stearoyl glycine (SGly), N-linoleoyl glycine (LGly) and N-arachidonoyl glycine (AGly). The levels of N-docosahexaenoyl glycine (DGly) remained unchanged. Treatment with resveratrol completely restored the tissue levels of PGly and AGly and showed a tendency to restore the uterine levels of SGly, OGly and LGly. In the case of the N-acyl serines (NASers), LPS administration to 15-day pregnant BALB/c mice induced increased uterine levels of N-palmitoyl serine (PSer) and N-stearoyl serine (SSer), with no changes in N-oleoyl serine (OSer), N-linoleoyl serine (LSer) and N-arachidonoyl serine (ASer). Treatment with resveratrol completely restored the uterine levels of PSer and showed a tendency to restore the levels of SSer. In the case of OSer, the administration of both LPS and resveratrol decreased the levels of this NASer below basal levels. Similarly, the administration of resveratrol reduced the uterine basal levels of N-arachidonoyl taurine, with LPS having no effects on its levels. Regarding 2-acyl-sn-glycerols, LPS administration to 15-day pregnant BALB/c mice induced an increase in the uterine levels of 2-palmitoyl glycerol (2-PG), 2-oleyl-sn-glycerol (2-OG) and 2-linoleyl-sn-glicerol (2-LG). Treatment with resveratrol only was able to restore 2-PG to uterine basal level. Similarly, treatment with LPS increased the uterine tissue levels of linoleic acid and resveratrol was able to fully prevent the effects of the endotoxin (n = 8–14).
Discussion
Here we show that resveratrol prevents the systemic LPS-induced preterm labor in an experimental model that mimics the main features of infection-induced preterm delivery in humans (Domínguez Rubio et al., 2014; Bariani et al., 2015). LPS administration to 15-day pregnant BALB/c mice induced premature parturition in 85% of cases, along with antepartum hemorrhage, fetal toxicity and offspring mortality, whereas the treatment with resveratrol prevented these endotoxin-elicited effects in 64% of the cases. Our observations are in agreement with previous reports showing that resveratrol exerted tocolytic effects by suppressing peritoneal macrophage-induced inflammation (Furuya et al., 2015) and preventing spontaneous preterm birth in a genetic model of accelerated decidual senescence (Deng et al., 2016).
The beneficial effects of resveratrol in our inflammation-elicited preterm delivery appear to be mediated by a plethora of pathways including reducing uterine NOS activity and uterine iNOS protein expression. Excessive NO production leads to the formation of other reactive nitrogen species (RNS) which are associated with tissue damage and extensive oxidative and nitrative stress (Roberts et al., 2009). Likewise, Duan et al. (2016) showed that resveratrol downregulated LPS-induced iNOS expression on adrenal glands while Zhang et al. (2014) reported a similar effect in murine lungs. Moreover, Liu et al. (2013) demonstrated that resveratrol protected from retinal damage by downregulating iNOS as well as other pro-inflammatory markers. Interestingly, it has been shown that resveratrol increases both the endothelial and inducible NOS in rat myocardium (Das et al., 2005; Fukuda et al., 2006), suggesting that the effects of resveratrol on NOS isoenzymes are tissue specific.
Our results show that LPS-induced maternal inflammation is associated with an increased uterine COX-2 expression. COX-2 plays a central role in the spontaneous initiation of labor and cervical ripening by producing large amounts of PGE2 and PGF2α. Therefore, the untimely increased expression of COX-2 is associated with the endotoxin-induced pathological initiation of preterm labor. We found that the administration of resveratrol resulted in a downregulation of both uterine COX-2 mRNA and protein expression. Accordingly, Zong et al. (2012) showed that resveratrol inhibited LPS-induced COX-2 expression in murine macrophage RAW 264.7 cell line, whereas Furuya et al. (2015) found that resveratrol administration downregulated COX-2 expression in peritoneal macrophages from LPS-treated pregnant mice. Furthermore, these authors showed that resveratrol prevented the LPS-elicited increased production of TNF-α and IL-1β levels by peritoneal macrophages (Furuya et al., 2015). This resveratrol-induced suppression of pro-inflammatory cytokines could also explain the lower expression of prostaglandins, since it has been shown that both TNF-α and IL-1β induce PGE2 and PGF2α in human choriodeciual tissues (Mitchell et al., 2016). We found that treatment with resveratrol resulted not only in a lower expression of COX-2 but also in a lower uterine content of PGE2 and had a limited effect on uterine content of PGF2α, which could explain the tocolytic effect of the polyphenol. These results are in agreement with those reported by Deng et al. (2016).
Interestingly, it has been recently shown that resveratrol promoted uterine quiescence in non-pregnant rats by blocking Ca2+ influx from both receptor-operated Ca2+ channels and voltage-operated Ca2+ channels (Hsia et al., 2011). In addition, Novaković et al. (2015) reported that resveratrol inhibited uterine contractility of human term pregnant myometrium by modulating K+ channels. Furthermore, Zhu et al. (2015) showed that resveratrol reduced uterine contractility and COX-2 expression in a murine model of adenomyosis.
Cumulative evidence points toward the participation of the eCS in both term and preterm parturition. Thus, it has been shown that levels of AEA increases with the induction of labor in humans (Habayeb et al., 2004; Nallendran et al., 2010). Conflicting reports regarding the uterotonic or utero-relaxant effects of AEA have been reported. Dennedy et al. (2004) showed that AEA exerted a relaxant effect on explants of human pregnant myometrium, whereas Mitchell et al. (2008) showed that activation of CB1 receptor on explants of human term amnion and choriodecidua led to an enhanced production of PGE2, a prostaglandin with a central role in membrane rupture. We have previously reported that CB1 mediated the LPS-elicited increase production of PGF2α in a model of inflammation-induced preterm labor (Bariani et al., 2015), suggesting a participation of the eCS in the loss of uterine quiescence induced by the endotoxin (Mackler et al., 2003; Ross et al., 2004; Adams Waldorf et al., 2008; Hutchinson et al., 2014). Interestingly, Faah−/− mice showed similar gestational lengths when compared to wild type (WT) mice (Sun et al., 2016). However, when Faah−/− mice were challenged with LPS, they became more susceptible to preterm labor than their WT counterparts (Sun et al., 2016), suggesting that a sustained endocannabinoid signaling results in a deleterious effect for pregnancy outcome. Accordingly, we found here that LPS altered the uterine content of AEA and related endocannabinoid-like lipids. This observation is in agreement with a previous report from our lab, where we showed that LPS treatment upregulated the uterine expression of NAPE-PLD (Bariani et al., 2015), suggesting an increased production of endocannabinoids. Moreover, it has been shown that LPS-elicited cytokines affect endocannabinoid production by targeting protein expression and enzymatic activity of FAAH. Thus, Maccarrone et al. (2001) have shown that the anti-inflammatory cytokines IL-4 and IL-10 enhanced FAAH activity, whereas the pro-inflammatory cytokines IL-12 and IFN-γ reduced FAAH activity and protein expression in peripheral lymphocytes. A lower activity of FAAH suggests increased levels of endocannabinoids. Conversely, the treatment with resveratrol restored AEA and other endocannabinoid-like lipids back to control levels. The mechanisms by which resveratrol modulates the uterine content of endocannabinoids and similar lipids are not fully understood. The LPS-elicited increased uterine production of AEA was associated with an increased percentage of preterm labor and the tocolytic effects of resveratrol coincide with a lower uterine content of this endocannabinoid. Overall, these results suggest that the eCS mediates the harmful effects of LPS. Conversely, the precise role of endocannabinoid-like lipids belonging to the general class of NAEs, NAGlys, NASer and 2-acyl-sn-glycerols on reproductive events remains to be explored. Increased levels of N-oleoyl ethanolamine (OEA) have been detected in peripheral blood of women with ectopic pregnancy (Gebeh et al., 2013) and in women undergoing termination of pregnancy with the progesterone antagonist RU486 (Karasu et al., 2014), suggesting a role for this NAE during pregnancy. It has been reported that neither NAGlys nor NASers show activity at CB1 or CB2 receptors (reviewed by Connor et al., 2010). NASers have been shown to modulate calcium-activated potassium channels (Godlewski et al., 2009) and of N-type calcium channels (Guo et al., 2008). Interestingly, it has been reported that NAGlys can be metabolized by COX-2 (Prusakiewicz et al., 2002) and FAAH (Grazia Cascio et al., 2004; Bradshaw et al., 2009), although with a lower catalytic efficiency, suggesting that these compounds may act as endogenous inhibitors of these enzymes. More recently, McHugh et al. (2012) have shown that N-arachidonoyl glycine binds to GPR18 and induces migration of the human endometrial cell line HEC-1B. Nevertheless, there is a lack of data on the roles of lipids such as N-plamitoyl glycine, N-oleoyl glycine, N-stearoyl glycine, N-linoleoyl glycine, N-palmitoyl serine, N-stearoyl serine, 2-palmitoyl glycerol, 2-oleyl-sn-glycerol and 2-linoleyl-sn-glicerol during reproductive events. Similarly, the role of polyunsaturated fatty acids during pregnancy still is poorly understood. Linoleic acid belongs to the omega-6 family of polyunsaturated fatty acids. Although the exact role of this lipid during pregnancy physiology remains elusive, it has been proposed that omega-6 fatty acids can be precursors to endocannabinoids, lipoxins, and omega-6 eicosanoids. Moreover, a higher ratio of omega-6 to omega-3 fatty acids results in an increased production of PGE2 and PGF2 α (reviewed by Coletta et al., 2010). Accordingly, Cheng et al. (2005) found that dietary supplementation of linoleic acid to pregnant ewes resulted in an increased basal production of PGE2 and PGF2α by the endometrium. Our results showed a higher uterine content of linoleic acid in LPS-treated pregnant mice, with resveratrol fully preventing this increase. Whether this LPS-driven increment in uterine content of linoleic acid is directly associated with the higher uterine content of N-linoleoyl ethanolamine, N-linoleoyl glycine, PGE2 or PGF2α, it remains to be determined.
We are aware of the limitations of our model to study the pathophysiological events triggered by the endotoxin during pregnancy and delivery. Despite these limitations, mainly due to differences in the anatomy and physiology of pregnancy and delivery between mice and humans (with mice having a bicornuate uterus and a larger litter), we believe that mice are still useful to provide information about the mechanisms involved in both term and preterm labor and delivery. Here we provide evidence that the uterine content of lipids is altered in LPS-induced preterm delivery and that resveratrol is able to prevent this effect. Further research is needed in order to fully understand their role during the pathophysiology of pregnancy.
Collectively, our results suggest that resveratrol prevents LPS-induced preterm labor by suppressing the LPS-enhanced expression of pro-inflammatory mediators (iNOS and COX-2) and the production of prostaglandins and endocannabinoids. Since resveratrol is capable of crossing the placenta and accessing the fetal circulation, more research is warranted to establish whether this polyphenol has embryo protective effects in addition to its tocolytic properties.
Supplementary Material
Acknowledgments
We thank Ramona Morales and Maximiliano Cella for their excellent technical support. We would also like to thank the animal care technicians Marcela Márquez and Daniel González, for their excellent care of the animals used in this study.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Authors’ roles
M.V.B. and F.C. performed the experiments, the statistical analyses and prepared the figures. A.P.D.R., A.A. and A.S. collaborated with animal preparation, tissue sampling, and data collection. E.L. and H.B.H. performed the mass spectrometry analysis. AMF designed the experiments. AMF and HBH financially supported the study. FC wrote the article text. All authors reviewed the article.
Funding
Grants Proyectos de Investigación Científica y Tecnológica (PICT) 2013/0097 from Agencia Nacional para la Promoción Científica y Tecnológica (ANPCyT) and Proyectos de Investigación Plurianuales (PIP) 2012/0061 from Consejo Nacional de Investigaciones Científicas y Técnicas to A.M.F. and NIH (DA006668) to H.B.B.
Conflict of interest
The authors have no conflict of interest to declare.
References
- Adams Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility, cytokines, and prostaglandins in rhesus monkeys. Reprod Sci 2008;15:121–127. doi:10.1177/1933719107310992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagul PK, Deepthi N, Sultana R, Banerjee SK. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J Nutr Biochem 2015;26:1298–1307. doi:10.1016/j.jnutbio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Bariani MV, Domínguez Rubio AP, Cella M, Burdet J, Franchi AM, Aisemberg J. Role of the endocannabinoid system in the mechanisms involved in the LPS-induced preterm labor. Reproduction 2015;150:463–472. doi:10.1530/REP-15-0211. [DOI] [PubMed] [Google Scholar]
- Bourque SL, Dolinsky VW, Dyck JRB, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 2012;33:449–452. doi:10.1016/j.placenta.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Hu SS, Burstein S, Walker JM. Novel endogenous N-acyl glycines identification and characterization. Vitam Horm 2009;81:191–205. doi:10.1016/S0083-6729(09)81008-X. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol 2006;291:R349–R358. doi:10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA 1989;86:9030–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdet J, Sacerdoti F, Cella M, Franchi AM, Ibarra C. Role of TNF-α in the mechanisms responsible for preterm delivery induced by Stx2 in rats. Br J Pharmacol 2013;168:946–953. doi:10.1111/j.1476-5381.2012.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Farina M, Dominguez Rubio A, Di Girolamo G, Ribeiro M, Franchi A. Dual effect of nitric oxide on uterine prostaglandin synthesis in a murine model of preterm labour. Br J Pharmacol 2010;161:844–855. doi:10.1111/j.1476-5381.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Elmes M, Kirkup SE, Chin EC, Abayasekara DRE, Wathes DC. The effect of a diet supplemented with the n-6 polyunsaturated fatty acid linoleic acid on prostaglandin production in early- and late-pregnant ewes. J Endocrinol 2005;184:165–178. doi:10.1677/joe.1.05910. [DOI] [PubMed] [Google Scholar]
- Coletta JM, Bell SJ, Roman AS. Omega-3 fatty acids and pregnancy. Rev Obstet Gynecol 2010;3:163–171. [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Vandenberg RJ. N-Acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets. Br J Pharmacol 2010;160:1857–1871. doi:10.1111/j.1476-5381.2010.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Connors SL, Matevia M, Qian Y, Newschaffer C, Zimmerman AW. Prenatal exposure to β2-adrenergic receptor agonists and risk of autism spectrum disorders. J Neurodev Disord 2011;3:307–315. doi:10.1007/s11689-011-9093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Alagappan VKT, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS–VEGF–KDR–eNOS after resveratrol consumption. Vascul Pharmacol 2005;42:281–289. doi:10.1016/j.vph.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y, Dey SK. p53 Coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest 2016;126:2941–2954. doi:10.1172/JCI87715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennedy MC, Friel AM, Houlihan DD, Broderick VM, Smith T, Morrison JJ. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol 2004;190:2–9. doi:10.1016/j.ajog.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Domínguez Rubio AP, Sordelli MS, Salazar AI, Aisemberg J, Bariani MV, Cella M, Rosenstein RE, Franchi AM. Melatonin prevents experimental preterm labor and increases offspring survival. J Pineal Res 2014;56:154–162. doi:10.1111/jpi.12108. [DOI] [PubMed] [Google Scholar]
- Duan G-L, Wang C-N, Liu Y-J, Yu Q, Tang X-L, Ni X, Zhu X-Y. Resveratrol alleviates endotoxemia-associated adrenal insufficiency by suppressing oxidative/nitrative stress. Endocr J 2016;63:569–580. doi:10.1507/endocrj.EJ15-0610. [DOI] [PubMed] [Google Scholar]
- Frémont L. Biological effects of resveratrol. Life Sci 2000;66:663–673. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kaga S, Zhan L, Bagchi D, Das DK, Bertelli A, Maulik N. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys 2006;44:43–49. doi:10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- Furuya H, Taguchi A, Kawana K, Yamashita A, Inoue E, Yoshida M, Nakamura H, Fujimoto A, Inoue T, Sato M et al. Resveratrol protects against pathological preterm birth by suppression of macrophage-mediated inflammation. Reprod Sci 2015;22:1561–1568. doi:10.1177/1933719115589413. [DOI] [PubMed] [Google Scholar]
- Gebeh AK, Willets JM, Bari M, Hirst RA, Marczylo TH, Taylor AH, Maccarrone M, Konje JC. Elevated anandamide and related N-acylethanolamine levels occur in the peripheral blood of women with ectopic pregnancy and are mirrored by changes in peripheral fatty acid amide hydrolase activity. J Clin Endocrinol Metab 2013;98:1226–1234. doi:10.1210/jc.2012-3390. [DOI] [PubMed] [Google Scholar]
- Gidaya NB, Lee BK, Burstyn I, Michael Y, Newschaffer CJ, Mortensen EL. In utero exposure to β-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics 2016;137:e20151316 doi:10.1542/peds.2015-1316. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, Davis MI, Zhang L, Razdan RK, Milman G et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther 2009;328:351–361. doi:10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. doi:10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazia Cascio M, Minassi A, Ligresti A, Appendino G, Burstein S, Di Marzo V. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun 2004;314:192–196. [DOI] [PubMed] [Google Scholar]
- Guo J, Williams DJ, Ikeda SR. N-arachidonoyl L-serine, a putative endocannabinoid, alters the activation of N-type Ca2+ channels in sympathetic neurons. J Neurophysiol 2008;100:1147–1151. doi:10.1152/jn.01204.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habayeb OMH, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, Konje JC. Plasma levels of the endocannabinoid anandamide in women—a potential role in pregnancy maintenance and labor. J Clin Endocrinol Metab 2004;89:5482–5487. doi:10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- Hsia S-M, Wang K-L, Wang PS. Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca2+ mobilization in rats in vivo and in vitro. Endocrinology 2011;152:2090–2099. doi:10.1210/en.2010-1223. [DOI] [PubMed] [Google Scholar]
- Hutchinson JL, Rajagopal SP, Yuan M, Norman JE. Lipopolysaccharide promotes contraction of uterine myocytes via activation of Rho/ROCK signaling pathways. FASEB J 2014;28:94–105. doi:10.1096/fj.13-237040. [DOI] [PubMed] [Google Scholar]
- Karasu T, Marczylo TH, Marczylo EL, Taylor AH, Oloto E, Konje JC. The effect of mifepristone (RU486) on the endocannabinoid system in human plasma and first-trimester trophoblast of women undergoing termination of pregnancy. J Clin Endocrinol Metab 2014;99:871–880. doi:10.1210/jc.2013-2922. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006;127:1109–1122. doi:10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod 2011;84:167–178. doi:10.1095/biolreprod.110.086983. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Song M-Y, Song E-K, Kim E-K, Moon WS, Han M-K, Park J-W, Kwon K-B, Park B-H. Overexpression of SIRT1 protects pancreatic-cells against cytokine toxicity by suppressing the nuclear factor-B signaling pathway. Diabetes 2009;58:344–351. doi:10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-Q, Wu B-J, Pan WHT, Zhang X-M, Liu J-H, Chen M-M, Chao F-P, Chao H-M. Resveratrol mitigates rat retinal ischemic injury: the roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J Ocul Pharmacol Ther 2013;29:33–40. doi:10.1089/jop.2012.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol 2001;166:7183–7189. [DOI] [PubMed] [Google Scholar]
- Mackler AM, Ducsay TC, Ducsay CA, Yellon SM. Effects of endotoxin and macrophage-related cytokines on the contractile activity of the gravid murine uterus. Biol Reprod 2003;69:1165–1169. doi:10.1095/biolreprod.103.015586. [DOI] [PubMed] [Google Scholar]
- McHugh D, Page J, Dunn E, Bradshaw HB. Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol 2012;165:2414–2424. doi:10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R. Preterm birth: a global burden on maternal and child health. Pathog Glob Health 2012;106:139–140. doi:10.1179/204777312X13462106637729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Rice GE, Vaswani K, Kvaskoff D, Peiris HN. Differential regulation of eicosanoid and endocannabinoid production by inflammatory mediators in human choriodecidua. PLoS One 2016;11:e0148306 doi:10.1371/journal.pone.0148306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Sato TA, Wang A, Keelan JA, Ponnampalam AP, Glass M. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab 2008;294:E352–E356. doi:10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- Nallendran V, Lam P, Marczylo T, Bankart M, Taylor A, Taylor D, Konje J. The plasma levels of the endocannabinoid, anandamide, increase with the induction of labour. BJOG 2010;117:863–869. doi:10.1111/j.1471-0528.2010.02555.x. [DOI] [PubMed] [Google Scholar]
- Novaković R, Radunović N, Marković-Lipkovski J, Ćirović S, Beleslin-Čokić B, Ilić B, Ivković B, Heinle H, Živanović V, Gojković-Bukarica LJ. Effects of the polyphenol resveratrol on contractility of human term pregnant myometrium. Mol Hum Reprod 2015;21:545–551. doi:10.1093/molehr/gav011. [DOI] [PubMed] [Google Scholar]
- Park C-W, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta 2009;30:56–61. doi:10.1016/j.placenta.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel R, Stanley JL, Rueda-Clausen CF, Andersson IJ, Sibley CP, Davidge ST, Baker PN. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One 2013;8:e64401 doi:10.1371/journal.pone.0064401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Kingsley PJ, Kozak KR, Marnett LJ. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem Biophys Res Commun 2002;296:612–617. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EMG, Doorn JA, Slikker W. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci 2009;112:4–16. doi:10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–326. doi:10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Sathishkumar K, Naik AK, Bawankule DU, Sarkar SN, Mishra SK, Prakash VR. Mechanisms of lipopolysaccharide-induced changes in effects of contractile agonists on pregnant rat myometrium. Am J Obstet Gynecol 2004;190:532–540. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Paria BC, Krebsbach RJ, Schmid HH, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proc Natl Acad Sci USA 1997;94:4188–4192. doi:10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol 2009;296:G1047–G1053. doi:10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med 2007;357:477–487. doi:10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- Sun X, Deng W, Li Y, Tang S, Leishman E, Bradshaw HB, Dey SK. Sustained endocannabinoid signaling compromises decidual function and promotes inflammation-induced preterm birth. J Biol Chem 2016;291:8231–8240. doi:10.1074/jbc.M115.707836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnblom S, Maul H, Klimaviciute A, Garfield RE, Byström B, Malmström A, Ekman-Ordeberg G. mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol 2005;3:33 doi:10.1186/1477-7827-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo PF, Simoncini S, Ligi I, Chateau A-L, Bachelier R, Robert S, Morere J, Fernandez S, Guillet B, Marcelli M et al. Accelerated senescence of cord blood endothelial progenitor cells in premature neonates is driven by SIRT1 decreased expression. Blood 2014;123:2116–2126. doi:10.1182/blood-2013-02-484956. [DOI] [PubMed] [Google Scholar]
- Wolfson ML, Correa F, Leishman E, Vercelli C, Cymeryng C, Blanco J, Bradshaw HB, Franchi AM. Lipopolysaccharide-induced murine embryonic resorption involves changes in endocannabinoid profiling and alters progesterone secretion and inflammatory response by a CB1-mediated fashion. Mol Cell Endocrinol 2015;411:214–222. doi:10.1016/j.mce.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chen N, Liu J, Wu J, Zhang J, Zhang Y, Jiang X. Protective effect of resveratrol against acute lung injury induced by lipopolysaccharide via inhibiting the myd88-dependent Toll-like receptor 4 signaling pathway. Mol Med Rep 2014;10:101–106. doi:10.3892/mmr.2014.2226. [DOI] [PubMed] [Google Scholar]
- Zhu B, Chen Y, Zhang H, Liu X, Guo S-W. Resveratrol reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice with induced adenomyosis. Reprod Sci 2015;22:1336–1349. doi:10.1177/1933719115572479. [DOI] [PubMed] [Google Scholar]
- Zong Y, Sun L, Liu B, Deng Y-S, Zhan D, Chen Y-L, He Y, Liu J, Zhang Z-J, Sun J et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One 2012;7:e44107 doi:10.1371/journal.pone.0044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.