Abstract
The human polyomavirus JC (JCV) infects glial cells and is the etiologic agent of the CNS demyelinating disease progressive multifocal leukoencephalopathy. JCV can infect granule cell neurons of the cerebellum, causing JCV granule cell neuronopathy and cortical pyramidal neurons in JCV encephalopathy. Whether JCV also infects neurons in other areas of the CNS is unclear. We determined the prevalence and pattern of JCV infection of the hippocampus in archival samples from 28 patients with known JCV infection of the CNS and 66 control subjects. Among 28 patients, 11 (39.3%) had JCV infection of hippocampus structures demonstrated by immunohistochemistry. Those included gray matter (dentate gyrus and cornu ammonis, subiculum) in 11/11 and afferent or efferent white matter tracts (perforant path, alveus, fimbria) in 10/11. In the hippocampus, JCV infected granule cell and pyramidal neurons, astrocytes, and oligodendrocytes. Although glial cells expressed either JCV regulatory T Antigen or JCV VP1 capsid protein, infected neurons expressed JCV T Antigen only, suggesting an abortive/restrictive infection. None of the 66 control subjects had evidence of hippocampal JCV protein expression by immunohistochemistry or JCV DNA by in situ hybridization. These results greatly expand our understanding of JCV pathogenesis in the CNS.
Keywords: Epilepsy, Epilepsy surgery biopsies, Hippocampus, JC virus, JCV encephalopathy (JCVE), JCV granule cell neuronopathy (JCV GCN), Progressive multifocal leukoencephalopathy (PML)
INTRODUCTION
The polyomavirus JC (JCV) is the etiologic agent of progressive multifocal leukoencephalopathy (PML). PML is the result of productive and lytic infection of oligodendrocytes and astrocytes, leading to multifocal areas of demyelination in the CNS. PML occurs in immunosuppressed patients with HIV/AIDS, hematological malignancies, organ transplant recipients, and in individuals with autoimmune diseases treated with immunomodulatory medications ( 1 ). In addition, JCV variants can also cause productive infection of cerebellar granule cell neurons, causing JC virus granule cell neuronopathy (JCV GCN) ( 2–9 ) and JC virus encephalopathy (JCVE) ( 10–14 ). JCV can also infect meningeal and choroid plexus epithelial cells, causing meningitis and hydrocephalus (JCVM) ( 15 ). Although PML, JCV GCN, JCVE, and JCVM can occur in isolation, infection of cerebellar granule cell or cortical pyramidal neurons have been reported in cases of PML ( 11 , 16 , 17 ).
Furthermore, PML patients often present with seizures and cognitive dysfunction ( 18 , 19 ). The hippocampus is a key component of the limbic system, which is involved in memory formation and, when damaged, epileptogenesis ( 20 ). Whether JCV infection can involve hippocampal structures and be associated with mesial temporal sclerosis and seizures to our knowledge has not been investigated. We sought to determine whether JCV can infect glial and neuronal cells in the hippocampus.
MATERIALS AND METHODS
Patient Samples
We selected a total of 31 blocks containing hippocampus from 28 patients with known CNS JCV infection for this study. These materials were collected from the Department of Pathology of the Beth Israel Deaconess Medical Center (Boston, Massachusetts) and from the National Neuro AIDS Tissue Consortium ( 21 ). JCV CNS infections included 1 case of JCV meningitis (JCVM) and 27 cases of PML with or without JCV GCN. Of those 28 cases, 22 were HIV-seropositive and 6 were HIV-seronegative (including 3 patients with chronic lymphocytic leukemia, 1 patient had a brain tumor outside of the hippocampus, 1 had a tumor outside of the CNS, and 1 had no other known condition other than PML. We also selected 35 blocks from other parts of the CNS from the same patients that were used as internal positive controls (1–2/patient). In addition, 72 blocks from 66 patients without JCV-related CNS infection were used as controls. Of those 66 control subjects, 24 were epilepsy surgery patients, 24 were from the Honolulu Asia Aging Study cohort ( 22 ) with hippocampal sclerosis found on postmortem examination but no clinical or histological signs of Alzheimer disease, 9 were HIV-seropositive patients and 9 were HIV-seronegative patients.
In Situ Hybridization, Immunohistochemistry, and Immunofluorescence Staining
In situ hybridization (ISH) and single and double immunohistochemistry (IHC) and immunofluorescence (IF) staining assays were performed as previously described ( 10 , 11 , 23 , 24 ). ISH was performed with full length JCV DNA probe (JC Virus BIO-PROBE®, ENZ-40847, Enzo Diagnostics, Farmingdale, NY).
In multiple IHC and IF stainings, primary antibodies from different species (mouse monoclonal and rabbit, chicken, guinea pig, and sheep polyclonal) were used with appropriately matched (species and isotype) highly cross-adsorbed secondary antibodies ( Table 1 ). It was therefore necessary to use different antibodies (raised in different species) for each cell phenotype ( Table 1 ). The secondary antibodies were conjugated either to alkaline phosphatase, horseradish peroxidase, glucose oxidase, or to Alexa Fluor 350, 488, 568, and 647 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Single and double IHC analyses were performed with the Mouse IgG and/or Rabbit IgG Vectastain Elite ABC Kits (Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions. Other kits and chromogens were also used ( 23 , 24 ). To limit steric interference, triple IHC were done with the ImmPRESS™ polymerized reporter enzyme staining system (Vector Laboratories), according to the manufacturer’s instruction. Because our triple and quadruple IHC included primary and secondary antibodies from more than 4 different species, we had to substitute the ImmPRESS species-specific buffers with general antibody diluents (S0809 or S3022, from Dako, Carpinteria, CA) to avoid nonspecific deposits. Negative controls included omission of the primary antibodies (IHC and IF) or probe (ISH) and the use of isotype-matched controls (IHC and IF) as well as sections known to be free of JCV-infected cells. Positive controls were sections of patients known to have JCV-infected cells, including patients for which the presence of JC viral particles had been demonstrated by electron microscopy ( 6 , 10 ).
TABLE 1.
Antibodies
| Antigen; Product Name | Host | Isot. | Target | Source |
|---|---|---|---|---|
| PAB597 | m | NA | JCV VP1 | |
| SV40 T Ag (v-300); sc-20800 | r | IgG | JCV T Ag | Santa Cruz Biotechnology, Santa Cruz, California |
| MAP-2; HM-2; M4403 | m | IgG1 | Neurons | Sigma, St-Louis, Missouri |
| MAP-2; ab5622 | r | IgG | Neurons | Chemicon, Temecula, California |
| MAP-2; LS-B290 | ch | IgY | Neurons | Lifespan Bioscience, Seattle, Washington. |
| MAP-2; ab5392 | ch | IgY | Neurons | Abcam, Cambridge, Massachusetts |
| NeuN (FOX3); ab104225 | r | IgG | Neurons | Abcam |
| NeuN (FOX3); ABN90P | gp | IgG | Neurons | EMD Millipore, Billerica, Massachusetts |
| GFAP; M0761; 6F2 | m | IgG1 | Astrocytes | Dako, Carpinteria, California |
| GFAP; Z0334 | r | IgG | Astrocytes | Dako |
| GFAP; ab4674 | ch | IgY | Astrocytes | Abcam |
| GFAP; ab90601 | sh | IgG | Astrocytes | Abcam |
| GFAP; 173004 | gp | IgG | Astrocytes | Synaptic Systems, Göttingen, Germany |
| CNPase; C5922; 11-5B | m | IgG1 | Myelin/Oligo | Sigma |
| CNPase; ab18527 | r | IgG | Myelin/Oligo | Abcam |
| CNPase; ab50739 | ch | IgY | Myelin/Oligo | Abcam |
| CNP; HPA023266 | r | IgG | Myelin/Oligo | Sigma, |
ch, chicken; CNP and CNPase, CNP, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; GFAP, glial fibrillary acidic protein; gp, guinea pig; m, mouse; Isot., isotype; MAP-2, microtubule-associated protein 2; oligo, oligodendrocytes; r, rabbit; SV40 T Ag, Simian virus 40 T antigen; sh, sheep.
Extent of Demyelination
The extent of demyelination was estimated in all blocks with either a Luxol fast blue staining counterstained with Cresyl violet or an IHC with one of the myelin/oligodendrocytes markers listed in Table 1 .
Phenotypes of JCV-Infected Cells
When JCV-infected cells were present their phenotypes were determined by double IHC staining including a JCV marker (T Ag or VP1) and a phenotype marker for either neurons, oligodendrocytes, or astrocytes ( Table 1 ).
RESULTS
We first performed single IHC staining for JCV T Ag, an early/regulatory protein, and for JCV VP1 capsid protein, which is present in mature viral particles. JCV T Ag was expressed in 11/28 (39.3%) hippocampi from patients with known CNS JCV infection, including in gray and white matter structures; these 11 patients also had VP1 expression but it was mainly in white matter tracts ( Fig. 1 ) All cases had detectable JCV expression in their previously known extrahippocampal site of infection (internal positive controls). ISH showed the presence of JCV DNA in only 8/11 of those cases, and did not reveal any additional case in which the IHC staining had been negative. Of the 11 patients with JCV-infection of the hippocampus, 7 were HIV-positive and 4 were HIV-negative. None of 66 control subjects had any evidence of JCV protein expression by IHC or JCV DNA by ISH in their hippocampi ( Table 2 ).
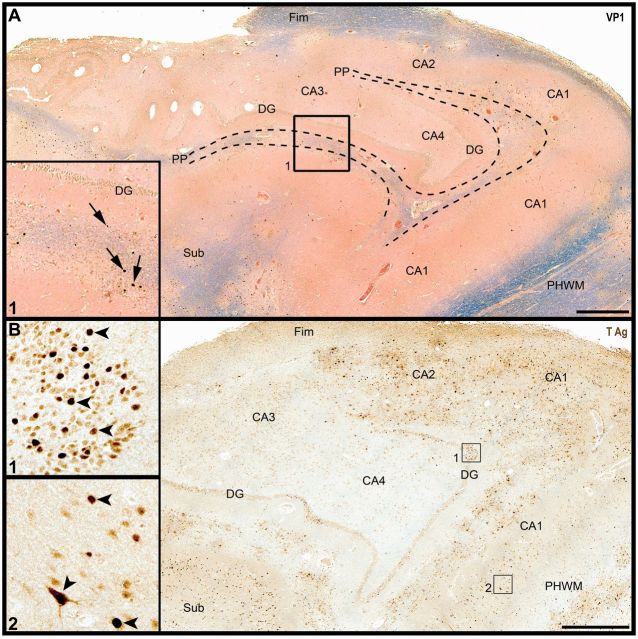
FIGURE 1.
Diffuse JCV infection of hippocampal subregions. (A) Luxol fast blue and hematoxylin and eosin (LFB&H&E) and JCV VP1 staining (arrows) of the hippocampus from a patient with HIV/PML reveals multiple areas of demyelination in the perforant path (PP), fimbria (Fimb), subiculum, and peri-hippocampal white matter (PHWM), with cells expressing JCV VP1 capsid protein (inset 1, arrows). DG: dentate gyrus; CA 1-4: cornu ammonis areas 1-4, sub: subiculum. (B) JCV T Ag immunohistochemistry of the same block shows heavy infection of white matter structures, as well as cells in the DG (inset 1, arrowheads) and in CA areas (inset 2, arrowheads). Scale bars: 1 mm.
TABLE 2.
JCV Infection in Hippocampi of Patients With Known JCV CNS Infection and Controls
| Staining | Patients With JCV CNS Infection (n Positive/n Tested [%]) | Control Subjects |
|---|---|---|
| JCV IHC | 11/28 (39.3) | 0/66 |
| JCV ISH | 8/28 (28.6) | 0/66 |
| JCV infection in hippocampal GM (DG, CA, SUB) | 11/28 (39.3) | 0/66 |
| JCV infection in hippocampal WM | 10/28 (35.7) | 0/66 |
GM, gray matter; DG, dentate gyrus; CA, cornu ammonis; SUB, subiculum; WM, white matter; IHC, immunohistochemistry; ISH, in situ hybridization.
To determine which areas were affected by JCV, we mapped hippocampal structures in the 11 patients with detectable JCV protein expression. Cornu ammonis (CA) areas were affected in 11/11 and dentate gyrus in 9/11; white matter areas showed demyelination in 10/11 ( Table 2 ). While 7/11 had infected cells in each dentate gyrus, CA and white matter, 1/11 had gray matter (CA) involvement only. Demyelination occurred in proximity to areas of neuronal infection in some cases whereas in others, white matter damage was not seen in proximity to involved neurons. We observed subpial demyelination in 5/11 infected cases, suggesting that infection could spread from CSF spaces. Representative examples of JCV involvement of various hippocampal areas are shown in Figure 2 .
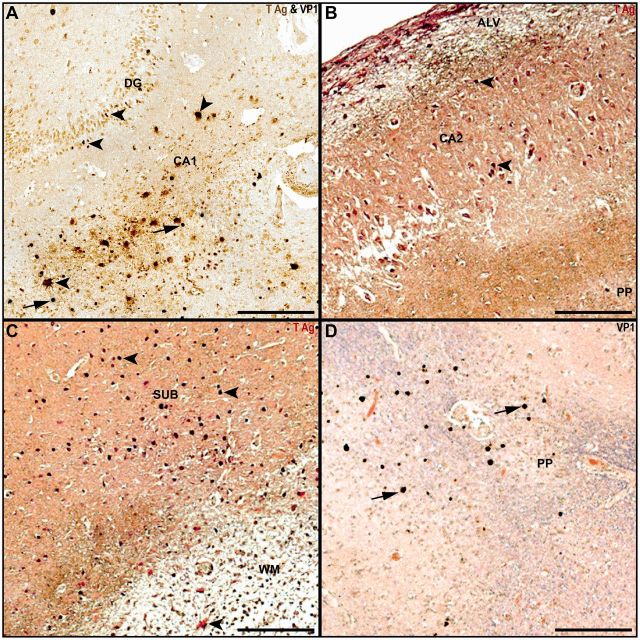
FIGURE 2.
Hippocampal gray and white matter JCV infection in patients with PML. (A) Granule cell neurons in the dentate gyrus and cells with astrocyte phenotype in CA1 express JCV T Ag (brown, arrowheads), whereas other glial cells in CA1 express JCV VP1 (blue-black, arrows). (B) Subpial demyelination (CNPase, brown) in alveus (ALV) and perforant path (PP) flank cells with phenotype of pyramidal neurons in CA2 expressing JCV Tag (red, arrowheads). (C) Multiple cells in the subiculum (SUB) express JCV T Ag (red, arrowheads) adjacent to an area of demyelinated white matter (CNPase, brown) containing an infected astrocyte (arrowhead). (D) Multiple glial cells in areas of demyelination in the perforant path (PP) express JCV VP1 (brown-black, filled arrows, with Luxol fast blue and hematoxylin and eosin counterstaining). Scale bars: 250 µm.
To verify the phenotype of JCV infected cells in hippocampi, we performed double IHC or IFA staining for JCV proteins and cellular markers. Patients with JCV-positive hippocampi had neurons expressing JCV T Ag only, and not JCV VP1. Conversely, astrocytes and oligodendrocytes of those patients were found to express either JCV T Ag or VP1. Examples of JCV-infected hippocampal granule cell and pyramidal neurons, as well as astrocytes and oligodendrocytes are shown in Figure 3 . ISH confirmed JCV infection of granule cell and pyramidal neurons in the hippocampus ( Fig. 4 ).
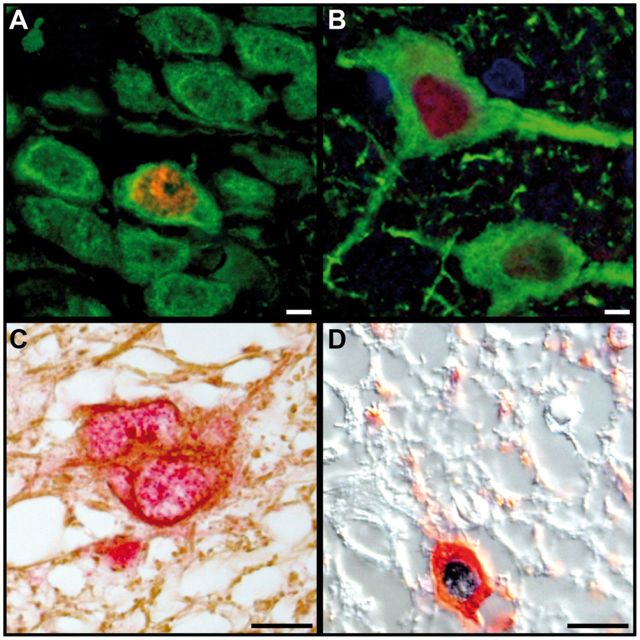
FIGURE 3.
JCV infects granule cell and pyramidal neurons, astrocytes, and oligodendrocytes in the hippocampus of patients with HIV/PML. (A) Double IFA for JCV T Ag (red) and MAP-2 (green) shows a JCV-infected granule cell neuron in the dentate gyrus among uninfected neurons. (B) Double IFA for JCV T Ag (red) and MAP-2 green shows a JCV-infected pyramidal neuron in the cornu ammonis next to an uninfected neuron. (C) Double immunohistochemistry (IHC) for JCV T Ag (red) and GFAP (brown) shows a JCV-infected astrocyte in the hippocampal white matter. (D) Double IHC DIC (differential interference contrast) for JCV VP1 (blue) and CNPase (red) in the hippocampal white matter shows a JCV-infected oligodendrocyte. Scale bars: 10 µm.
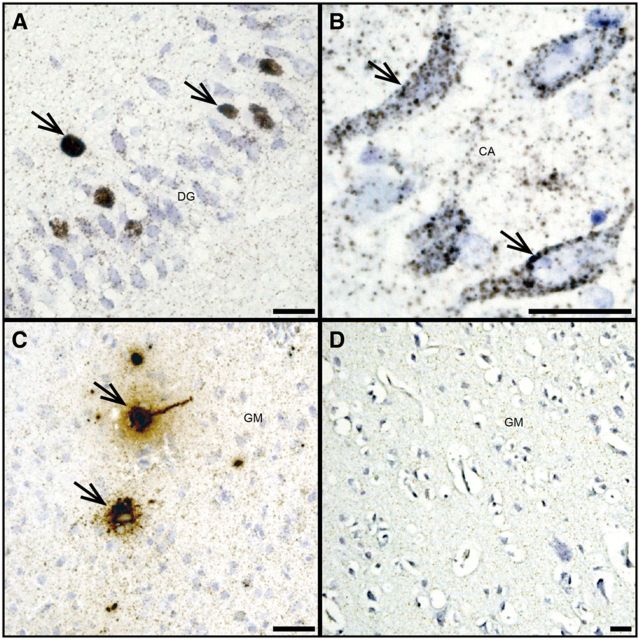
FIGURE 4.
JCV DNA is detected in hippocampal granule cell and pyramidal neurons. (A, B) JCV DNA (brown, arrows) is abundant in some granule cell neurons of the dentate gyrus of a patient with HIV/PML (A) and in some pyramidal neurons of the cornu ammonis of the same patient (brown, arrows) (B) . (C) High level of JCV DNA (brown, arrows) can be seen in pyramidal neuron of the hemispheric gray matter of a JCVE patient known to have almost exclusively JCV-infected neurons, demonstrated by presence of T Ag, VP1 by immunohistochemistry and viral particles by electron microscopy in a previous report ( 10 ), used as a positive control. (D) Absence of JCV DNA in the brain of an HIV-infected patient without PML used as a negative control. Scale bars: 25 µm.
DISCUSSION
The present study sheds a new light on JCV pathogenesis and considerably expands on previous demonstrations that JCV infects glial cells as well as neurons in the CNS ( 11 , 16 ). Whereas JCV can infect granule cell neurons in the cerebellum and pyramidal neurons in the hemispheric cortex independently from glial cells in CNS white matter, our current data show that JCV infection can involve granule cell and pyramidal neurons as well as astrocytes and oligodendrocytes in gray and white matter areas of the hippocampus. Nearly 40% of patients with known CNS JCV infection had affected hippocampi, indicating that this is a frequent occurrence in both HIV-positive and negative individuals. This is significant because patients with PML often suffer from cognitive dysfunction, memory deficits, and seizures ( 19 ), all of which may be caused by hippocampal lesions.
As previously observed in cerebellar granule cell neurons ( 16 ) and cortical pyramidal neurons ( 11 ), hippocampal neurons expressed JCV T Ag and not JCV VP1. This finding suggests that post-mitotic hippocampal neurons can sustain a restrictive/abortive JCV infection, rather than a full replication cycle with production of mature viral particles. We observed the same phenomenon in hippocampal neurons infected with the JCV-related simian virus 40 (SV40) in monkeys immunosuppressed with simian immunodeficiency virus ( 25 ).
Our study has limitations. The hippocampus is a complex structure with numerous connections between gray and white matter structures. Due to the available material, our study was not designed to resolve possible transmission of JCV between glial cells and neurons. Because we tested archival samples from various sources, including brain bank repositories, the clinical data were incomplete and did not allow us to correlate JCV hippocampal infection with cognitive dysfunction, spatial disorientation, seizures, or presence of abnormal signal on MRI in the mesial temporal lobes.
Of note, we did not detect JCV protein expression or JCV DNA in hippocampi of 24 elderly patients with mesial temporal sclerosis ( 26 ) or in 24 adult temporal lobe epilepsy surgery patients. These results indicate that JCV is not associated with those conditions. Our study expands the range of neurons that can be infected by JCV. We have previously found that granule cell neurons in patients with JCV GCN have mutations in the C terminal of the VP1 protein ( 3 , 4 ) and that JCVE is associated with a large deletion in the agnoprotein ( 12 , 14 ). Whether hippocampal neurons, which are functionally and anatomically different than cerebellar or cortical neurons, bear the same or other mutations will require further study. However, these will prove challenging because of the intricate proximity of gray and white matter structures in the hippocampus.
ACKNOWLEDGMENT
We would like to thank the National Neuro AIDS Tissue Consortium for providing some of the samples of this study ( 21 ).
This study was supported by NIH grants NS047029 and NS074995 to I.J.K. and NS08916, U01-AG046871, U01-AG019349, U01-AG017155, an Alzheimer’s Association Zenith fellowship award, MH100868, HD079249, The Nancy Lurie Marks Family Foundation, and Autism Speaks/National Alliance for Autism Research to M.P.A. The funding agencies had no input in the investigations or analysis of the results.
Conflict of interest: Dr Koralnik receives royalties for chapters in the online textbook UpToDate and is a recipient of a research grant from Biogen. He serves on scientific advisory boards for Medimmune and Johnson & Johnson. Ms. Batson, Drs Anderson, White, and Wüthrich declare that they have no competing interests.
REFERENCES
- 1. Gheuens S, Wuthrich C, Koralnik IJ. Progressive multifocal leukoencephalopathy: Why gray and white matter . Ann Rev Pathol 2013. ; 8 : 189 – 215 [DOI] [PubMed] [Google Scholar]
- 2. Dang L, Dang X, Koralnik IJ , et al. . JC polyomavirus granule cell neuronopathy in a patient treated with rituximab . JAMA Neurol 2014. ; 71 : 487 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dang X, Koralnik IJ. A granule cell neuron-associated JC virus variant has a unique deletion in the VP1 gene . J Gen Virol 2006. ; 87 : 2533 – 7 [DOI] [PubMed] [Google Scholar]
- 4. Dang X, Vidal JE, Oliveira AC , et al. . JC virus granule cell neuronopathy is associated with VP1 C terminus mutants . J Gen Virol 2012. ; 93 : 175 – 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koralnik IJ, Wuthrich C, Dang X , et al. . JC virus granule cell neuronopathy: A novel clinical syndrome distinct from progressive multifocal leukoencephalopathy . Ann Neurol 2005. ; 57 : 576 – 80 [DOI] [PubMed] [Google Scholar]
- 6. Du Pasquier RA, Corey S, Margolin DH , et al. . Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual . Neurology 2003. ; 61 : 775 – 82 [DOI] [PubMed] [Google Scholar]
- 7. Schippling S, Kempf C, Buchele F , et al. . JC virus granule cell neuronopathy and GCN-IRIS under natalizumab treatment . Ann Neurol 2013. ; 74 : 622 – 6 [DOI] [PubMed] [Google Scholar]
- 8. Agnihotri SP, Dang X, Carter JL , et al. . JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene . Neurology 2014. ; 83 : 727 – 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dang X, Koralnik IJ. Gone over to the dark side: Natalizumab-associated JC virus infection of neurons in cerebellar gray matter . Ann Neurol 2013. ; 74 : 503 – 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wuthrich C, Dang X, Westmoreland S , et al. . Fulminant JC virus encephalopathy with productive infection of cortical pyramidal neurons . Ann Neurol 2009. ; 65 : 742 – 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy . J Neuropathol Exp Neurol 2012. ; 71 : 54 – 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dang X, Wuthrich C, Gordon J , et al. . JC virus encephalopathy is associated with a novel agnoprotein-deletion JCV variant . PLoS One 2012. ; 7 : e35793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellis LC, Koralnik IJ. JC virus nucleotides 376-396 are critical for VP1 capsid protein expression . J Neurovirol 2015. ; 21 : 671 – 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis LC, Norton E, Dang X , et al. . Agnogene deletion in a novel pathogenic JC virus isolate impairs VP1 expression and virion production . PloS One 2013. ; 8 : e80840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agnihotri SP, Wuthrich C, Dang X , et al. . A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient . Ann Neurol 2014. ; 76 : 140 – 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wuthrich C, Cheng YM, Joseph JT , et al. . Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy . J Neuropathol Exp Neurol 2009. ; 68 : 15 – 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miskin DP, Koralnik IJ. Novel syndromes associated with JC virus infection of neurons and meningeal cells: No longer a gray area . Curr Opin Neurol 2015. ; 28 : 288 – 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lima MA, Drislane FW, Koralnik IJ. Seizures and their outcome in progressive multifocal leukoencephalopathy . Neurology 2006. ; 66 : 262 – 4 [DOI] [PubMed] [Google Scholar]
- 19. Miskin DP, Herman ST, Ngo LH , et al. . Predictors and characteristics of seizures in survivors of progressive multifocal leukoencephalopathy . J Neurovirol epub ahead of print (Dec 16, 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartsch T, Wulff P. The hippocampus in aging and disease: From plasticity to vulnerability . Neuroscience 2015. ; 309 : 1 – 16 [DOI] [PubMed] [Google Scholar]
- 21. Morgello S, Gelman BB, Kozlowski PB , et al. . The National NeuroAIDS Tissue Consortium: A new paradigm in brain banking with an emphasis on infectious disease . Neuropathol Appl Neurobiol 2001. ; 27 : 326 – 35 [DOI] [PubMed] [Google Scholar]
- 22. Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: Epidemiologic and neuropathologic research on cognitive impairment . Curr Alzheimer Res 2012. ; 9 : 664 – 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wuthrich C, Kesari S, Kim WK , et al. . Characterization of lymphocytic infiltrates in progressive multifocal leukoencephalopathy: Co-localization of CD8(+) T cells with JCV-infected glial cells . J Neurovirol 2006. ; 12 : 116 – 28 [DOI] [PubMed] [Google Scholar]
- 24. Wuthrich C, Popescu BF, Gheuens S , et al. . Natalizumab-associated progressive multifocal leukoencephalopathy in a patient with multiple sclerosis: A postmortem study . J Neuropathol Exp Neurol 2013. ; 72 : 1043 – 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaliyaperumal S, Wuthrich C, Westmoreland SV , et al. . Simian virus 40 infection in the spinal cord of simian immunodeficiency virus-immunosuppressed rhesus macaques . J Neuropathol Exp Neurol 2015. ; 74 : 1071 – 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walker MC. Hippocampal sclerosis: Causes and prevention . Semin Neurol 2015. ; 35 : 193 – 200 [DOI] [PubMed] [Google Scholar]