Abstract
STUDY HYPOTHESIS
What factors in mouse oocytes are involved in the ageing-related decline in oocyte quality?
STUDY FINDING
The maternal effect gene Mater is involved in ageing-related oocyte quality decline in mice.
WHAT IS KNOWN ALREADY
Premature loss of centromere cohesion is a hallmark of ageing-related oocyte quality decline; the maternal effect gene Mater (maternal antigen that embryos require, also known as Nlrp5) is required for preimplantation embryo development beyond the 2-cell stage, and mRNA expression of Mater decreases with maternal ageing.
STUDY DESIGN, SAMPLES/MATERIALS, METHODS
Mater protein expression level in mature oocytes from 7 young (5–8 weeks old) to 7 old mice (41–68 weeks old) was compared by immunoblotting analysis. Wild-type and Mater-null mice were used to examine whether Mater is necessary for maintaining normal centromere cohesion by means of cytogenetic karyotyping, time-lapse confocal microscopy and immunofluorescence staining.
MAIN RESULTS AND THE ROLE OF CHANCE
Mater protein is decreased in mature oocytes from old versus young mice (P = 0.0022). Depletion of Mater from oocytes leads to a reduction in centromere cohesion, manifested by precocious sister chromatid separation, enlargement of sister centromere distance and misalignment of chromosomes in the metaphase plate during meiosis I and II.
LIMITATIONS, REASONS FOR CAUTION
This study was conducted in mice. Whether or not the results are applicable to human remains further elucidation. In addition, we were unable to confirm if the strain of mice (C57BL/6XSv129) at the age of 41–68 weeks old has the ‘cohesin-loss’ phenotype.
WIDER IMPLICATIONS OF THE FINDINGS
Investigating Mater's functional mechanisms could provide fresh insights into understanding how the ageing-related oocyte quality decline occurs.
LARGE SCALE DATA
N/A.
STUDY FUNDING AND COMPETING INTEREST(S)
This work was supported by the research grant from Chinese NSFC to P.Z. (31071274). We have no conflict of interests to declare.
Keywords: oocyte, maternal ageing, aneuploidy, cohesion, Mater
Introduction
Reproductive competence decreases dramatically with the advance of maternal age in mammals. For example, human females aged over 35 years display a drastic decline in reproductive ability (Hawkes and Smith, 2010). The female reproductive ageing is attributed mainly to the decline of oocyte quality rather than uterine function (Navot et al., 1991; Hunt and Hassold, 2008). Although some cytoplasmic abnormalities are reported, aneuploidy arising during meiosis I is considered as the hallmark of the oocyte quality decline in maternal ageing (Hunt and Hassold, 2008; Nagaoka et al., 2012). Hypotheses to explain the age-related aneuploidy include defects in meiotic recombination, failure of spindle assembly checkpoint (Battaglia et al., 1996; Steuerwald et al., 2001; Hassold et al., 2007; Yun et al., 2014a; Sakakibara et al., 2015), defective kinetochore microtubule establishment (Shomper et al., 2014) and most prevalently, the premature loss of centromere cohesion (Vogt et al., 2008; Chiang et al., 2010; Lister et al., 2010; Duncan et al., 2012; Merriman et al., 2013). Compelling evidence comes from the observation of an age-dependent weakening of centromere cohesion in a natural ageing mouse model (Chiang et al., 2010; Lister et al., 2010) as well as in the Smc1ß−/− mouse model (Hodges et al., 2005). The association between reduced chromosome cohesion and aneuploidy is seen even in oocytes from young mice (1–3 months old) (Merriman et al., 2013). Although premature cohesion loss has now been widely recognized as the hallmark of ageing-dependent oocyte quality decline (Jessberger, 2012), factors in the oocyte that are involved in this quality decline remain largely unknown and this represents a challenging open question for future work.
Mater (maternal antigen that embryos require, official name Nlrp5) belongs to the NACHT, leucine-rich repeat and PYD containing (NALP) gene family and is the first identified maternal effect gene in mammals (Tong et al., 2000). Mater mRNAs are restrictively expressed in growing oocytes and encode cytoplasmic proteins. Although the transcripts are no longer detectable in metaphase II (MII) oocytes and preimplantation embryos, the protein persists until the blastocyst stage (Tong et al., 2004; Ohsugi et al., 2008). Knocking out Mater does not block the initiation and completion of meiosis in oocytes, but the embryos cannot survive beyond the 2-cell stage without maternal Mater protein (Tong et al., 2000). Further studies reported the detection of Mater in mitochondria (Tong et al., 2004), the possible roles of Mater in regulating mitochondrial functions, endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes and embryos (Fernandes et al., 2012; Kim et al., 2014). Mater is evolutionarily conserved across mammalian species. The orthologues are found in human (Tong et al., 2002), rhesus monkey (Zou et al., 2009), bovine (Pennetier et al., 2004) and porcine (Ma et al., 2009; Pisani et al., 2010), and the expression of Mater displays similar restriction and dynamics in these species. Furthermore, a recent study on rhesus monkey reported its essential role in preimplantation embryo development (Zou et al., 2009). MATER mutations in human are also associated with reproductive wastage and multilocus imprinting disorders (Docherty et al., 2015).
Although mouse oocytes without Mater protein exhibit grossly normal competences to accomplish meiosis and to extrude the first polar body, they display aberrant cytoplasmic F-actin organization and spindle migration to cortex during the first meiotic division (Zheng et al., 2013). Furthermore, the mRNA abundance of Mater in oocytes of old mice (42–45 weeks old) decreases by about 3-fold when compared with that of young mice (5–6 weeks old) (Hamatani et al., 2004). These observations suggest that Mater could be associated with age-dependent oocyte quality decline. In this study, we examined in detail the influence of Mater depletion on oocyte quality. Our results show that oocytes from Mater-null females are characterized by severe centromere cohesion weakening as manifested by a high incidence of aneuploidy, precocious sister chromatid separation, enlargement of overall sister centromere distance and misalignment of chromosomes in metaphase I and II. Moreover, Mater protein level in mature oocytes of old mice (41–68 weeks old) decreases by ∼5-fold when compared with that of young mice (5–8 weeks old). Taken together, these data suggest that oocyte-specific gene Mater is associated with reproductive ageing in female mice. Mutation of MATER can cause human reproductive wastage (Docherty et al., 2015). Thus, the results obtained from mice can have implications for human maternal ageing.
Materials and Methods
Mice and immature (germinal vesicle stage, GV) and mature (metaphase II stage, MII) oocyte collection
Seven young (5–8 weeks old) and seven old (41–68 weeks old, near the end of the reproductive lifespan) female mice (C57BL/6XSv129) were used for the collection of mature oocytes for immunoblotting analysis of Mater protein expression. To collect MII oocytes, female mice were injected i.p. with 5 IU pregnant mare serum gonadotrophin (PMSG) followed by 5 IU hCG 48 h later. Twelve hours after hCG injection, the mature MII oocytes were retrieved from oviduct.
Mater+/+ and Mater-null female mice (5–12 weeks old) (Tong et al., 2000) were used for GV and MII oocyte collection. To collect GV oocytes, ovaries were dissected from anaesthetized mice and grown follicles were punctured to release cumulus-oocyte complexes (COCs) into M2 medium. COCs were denuded of cumulus cells and healthy looking GV oocytes were utilized.
In vitro transcription of mRNAs
Plasmid for in vitro transcription of Eb1 (microtubule-associated protein, RP/EB family, member 1, also known as Eb1) fused with green fluorescent protein (Eb1-GFP) was described previously (Zheng et al., 2013). For in vitro mRNA synthesis, expression constructs were linearized with Sca I (NEB, MA, USA) and capped mRNAs were synthesized with T7 polymerase (mMessage mMachine Kit, Life technologies, CA, USA). Isolated mRNAs were dissolved in injection buffer (10 mM Tris–HCl, pH 7.5 and 0.1 mM EDTA) and stored at −80°C.
In vitro maturation of GV oocytes and time-lapse microscopy
GV oocytes were matured in KSOM (K+-modified simplex optimized medium) (Sutton-McDowall et al., 2010) and the time-lapse microscopy was performed as described (Zheng et al., 2013). In brief, in vitro transcribed mRNAs (500 ng/l) were injected into the cytoplasm of GV oocytes maintained in M2 medium containing 50 ng/ml IBMX (Sigma-Aldrich, MO, USA). After mRNA injection, oocytes were cultured in KSOM with 50 ng/ml of IBMX for at least 3 h to allow the translation of injected mRNAs. DNA was labelled with 2 ng/ml bisbenzimide (Hoechst 33342, Sigma-Aldrich) for 15 min and oocytes were then transferred into 100 µl of KSOM covered with mineral oil (Sigma-Aldrich) in an environmental chamber (37°C, 5% CO2) for time-lapse imaging in which projections were acquired at 7 min intervals with a LSM 510 confocal microscope (Carl Zeiss, Germany). All the experiments were repeated two times with >16 oocytes examined in each group.
Immunoblotting analysis and immunofluorescence staining
For immunoblotting, MII oocytes were lysed in RIPA lysis buffer (Beyotime, China) and proteins were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred to polyvinylidene diflouride membrane. After blocking with blocking buffer (Catalog number 11096176001, Roche, Germany), the membrane was incubated with primary antibodies (rabbit anti-Mater antibody, gift from Dr. Jurrien Dean; mouse anti-β-Actin antibody from OriGene Technologies, MD, USA) and secondary antibodies (horse-radish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G and HRP-conjugated goat anti-mouse IgG, Thermo Scientific, MA, USA). Bands were detected with the enhanced chemiluminescence detection system (Beyotime, China) and developed with X-ray films (Kodak, China). Following detection of Mater protein, the membrane was stripped to examine the expression of β-Actin protein. Integrated density values of the bands for Mater and β-Actin were measured with Image J software (National Institutes of Health, USA, http://imagej.nih.gov/ij/index.html). The density value of β-Actin was used as the loading control and the expression of Mater protein was normalized to the loading control.
For immunofluorescence staining, mature oocytes were fixed in 3.7% paraformaldehyde (Sigma-Aldrich) for 15 min at 37°C, rinsed in phosphate-buffered saline (PBS) containing 0.3% polyvinylpyrrolidone (Sigma-Aldrich) followed by permeabilization in 0.25% Triton X-100 (Sigma-Aldrich) for 30 min. Oocytes were then blocked in 1% bovine serum albumin (BSA) for 1 h at room temperature and incubated with the fluorescein isothiocyanate (FITC)-conjugated anti-α tubulin antibody (Sigma-Aldrich) prior to imaging on an LSM 510 confocal microscope. DNA was labelled with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich).
Metaphase chromosome spread, sister centromere distance measurement
In vivo matured MII oocytes or in vitro cultured prometaphase I oocytes were used to prepare metaphase chromosome spreads according to the protocol described by others (Hodges and Hunt, 2002). Chromosome spreads were stained with DAPI and examined under UV light using a Zeiss Axioplan 2 microscope (Zeiss, Germany). To be conservative, only the spreads containing hyperploidy or single sister chromatids were considered as aneuploidy to avoid preparation artefacts that could result in hypoploidy. The centromere could be clearly visualized by bright DAPI staining. The distance between the center of DAPI staining of sister centromeres was measured and considered as the sister centromere distance. Student's T-test in Graphpad Prism 5 software was used to determine statistical significance. P value<0.05 was considered as significant.
Cortical granule staining with lens culinaris agglutinin
Cortical granule (CG) staining was conducted according to a previous report (Liu et al., 2005). Briefly, MII oocytes were separated from the zona pellucida by incubation in acid M2 medium. Oocytes were fixed with 3.7% paraformaldehyde in PBS for 30 min. After washing three times in blocking solution (M2 with 0.3% BSA and 100 mM glycine), oocytes were permeabilized with 0.1% Triton X-100 in M2 for 5 min followed by washing in blocking solution. Oocytes were incubated in M2 containing 100 µg/mL FITC-conjugated lens culinaris agglutinin (FITC-LCA, Sigma-Aldrich) for 30 min. Oocytes were then washed three times in M2 containing 0.3% BSA and 0.01% Triton X-100 and stained with DAPI to visualize DNA. After washing, oocytes were mounted on a slide and observed using a LSM 510 confocal microscope.
Ethical approval
Procedures involving animals and their care were conducted in compliance with the guidelines of the Animal Care and Use Committees of Kunming Institute of Zoology, the Chinese Academy of Sciences.
Results
Mater protein expression in mouse oocytes decreases with maternal ageing
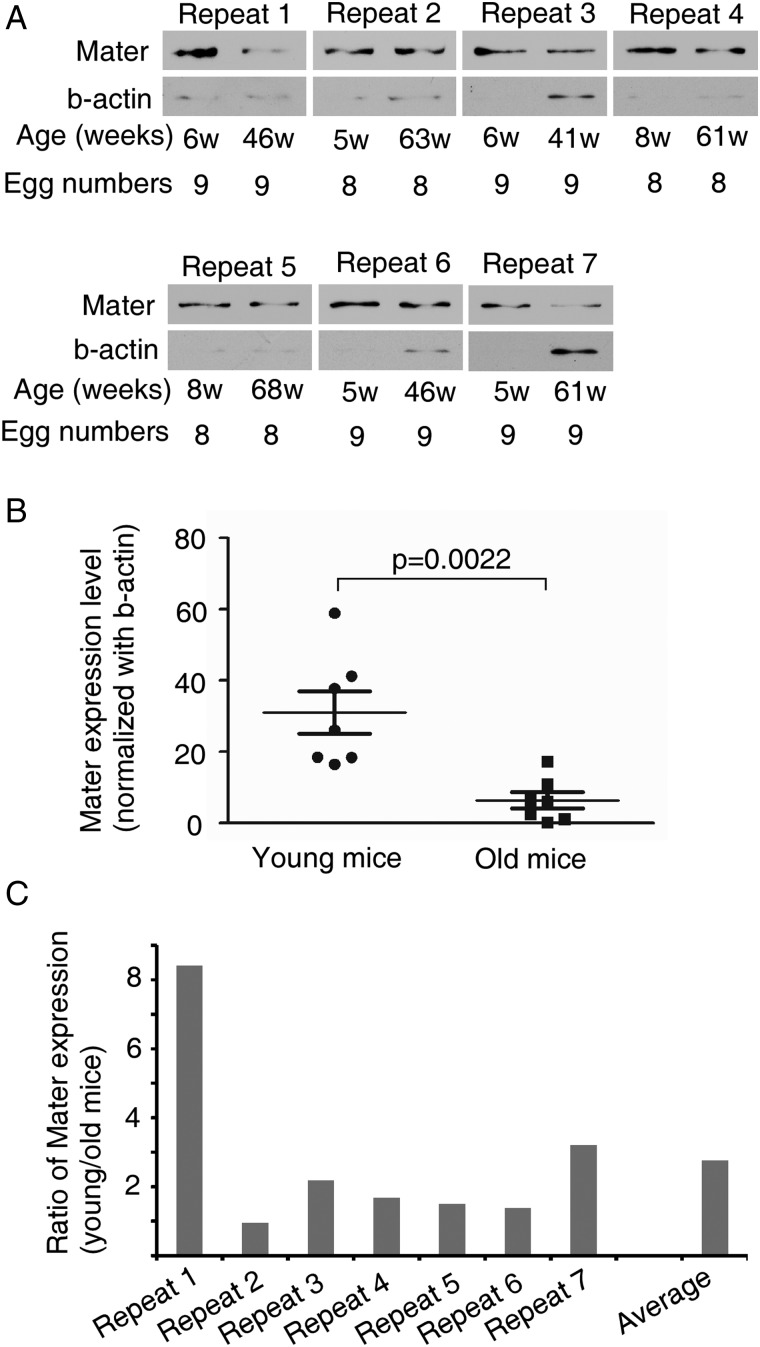
Previous study reported the decrease of Mater mRNA expression in oocytes from old (42–45 weeks old) versus young (5–6 weeks old) female mice (Hamatani et al., 2004). To validate the influence of maternal age on Mater expression, we examined the Mater protein level in MII oocytes collected from 7 young (5–8 weeks old) and 7 old (41–68 weeks old) female mice by immunoblotting analysis. These females were caged with fertile males all the time. The old females were fertile when young but stopped to give birth at >8 weeks before oocyte collection. The reduced or lost fecundity at the time of oocyte collection suggested that these mice had undergone maternal ageing. As shown (Fig. 1A and B), oocytes from old females contained 5-fold less Mater protein than oocytes from young females, when Mater expression was normalized with β-actin (P = 0.0022). To rule out the possibility that the aged oocytes accumulate more β-actin, we also normalized Mater expression against oocyte numbers. On average, the abundance of Mater protein in young oocytes was about 2.7 times higher than that in aged oocytes (Fig. 1A and C). These results were consistent with the previous mRNA expression data (Hamatani et al., 2004). Thus, Mater expression in oocytes decreases with maternal ageing.
Figure 1.
Mater protein expression in mouse oocytes decreases with maternal ageing. (A) Immunoblotting analysis of Mater protein expression in metaphase II (MII) oocytes from seven young and seven old females. Old females had lost fecundity before oocyte collection. (B) Mater protein level was normalized with β-actin. The average Mater expression level in old females was about five times lower than that in young mice (Student's T-test, P = 0.0022). (C) Mater expression was normalized against egg number and the ratios of protein level in young versus aged oocytes (young/old) were calculated for each experimental repeat.
Loss of Mater leads to centromere cohesion weakening
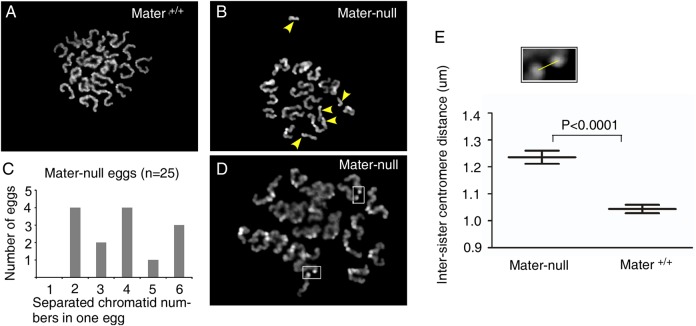
The Mater protein expression in oocytes decreases with maternal ageing, suggesting that its reduction might be related to age-dependent oocyte quality decline. To test this hypothesis, we took advantage of the Mater-null mouse line in which homozygous females do not express either Mater transcripts or proteins (Tong et al., 2000). Aneuploidy and, in particular, precocious sister chromatid separation are the hallmarks of age-dependent oocyte quality decline (Hunt and Hassold, 2008; Chiang et al., 2010), therefore we examined whether Mater depletion resulted in aneuploidy and especially premature sister chromatid separation. Metaphase chromosome spreads were prepared from in vivo matured MII oocytes of homozygous Mater-null and wild-type littermates at similar age (5–12 weeks old) and the chromosome numbers were examined. In wild-type counterparts, almost all examined MII oocytes (n = 25) had the normal chromosome number (96%, 24/25, Fig. 2A), except one which contained a prematurely separated single chromatid (4%, 1/25). In sharp contrast, a high proportion (56%, 14/25) of MII oocytes (n = 25) derived from homozygous Mater-null females displayed premature sister chromatid separation (Fig. 2B). The numbers of single chromatids in Mater-null oocytes ranged from two to six (Fig. 2C). We did not observe formal hyperploidy without separated single chromatids in Mater-null MII oocytes. In addition to the high incidence of precocious sister chromatid separation, sister chromatids in spreads of homozygous Mater-null MII oocytes appeared to be further apart than those in wild-type control (Fig. 2D). We thus measured the distance of 290 pairs of centromeres from 15 Mater-null MII eggs and of 280 pairs of centromeres from 14 Mater+/+ MII eggs (Fig. 2E). The average distance between the centres of two sister centromeres was increased in Mater-null eggs compared with that in Mater+/+ control MII oocytes (1.236 ± 0.243 µm versus 1.042 ± 0.202 µm, P < 0.0001, respectively) (mean ± SEM). Thus, the significantly higher frequency of precocious sister chromatid separation together with the increase in inter-centromere distance in Mater-null oocytes indicated that centromere cohesion, which holds the sister chromatids together until anaphase II, was remarkably weakened in the absence of Mater in oocytes. These defects mimicked the phenotypes of aged oocytes (Chiang et al., 2010; Lister et al., 2010).
Figure 2.
Oocytes from Mater-null female mice exhibit centromere cohesion reduction. (A) Representative chromosome spread of a MII egg from Mater+/+ mice containing normal chromosomes. (B) Representative chromosome spread of a MII egg from Mater-null females containing precociously separated sister chromatids (arrowheads). (C) Number of eggs containing different numbers of precociously separated sister chromatid in Mater-null mice. In total, 25 Mater-null eggs were examined. (D) Representative display of sister centromere distance enlargement (squares) in Mater-null MII eggs. (E) Inter-sister centromere distance was measured (square). The overall inter-sister centromere distance in Mater-null eggs was significantly larger than that in wild-type counterparts (Student's T-test, P < 0.0001). Data are mean ± SEM.
Loss of Mater does not compromise arm cohesion
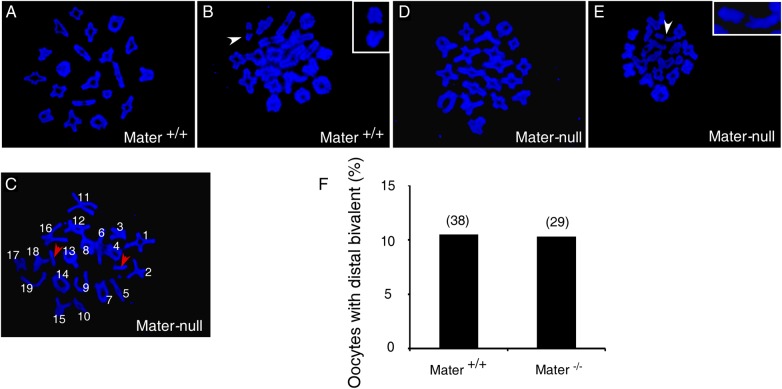
The cohesin complex mediates cohesion between sister chromatids. In germ cells, cohesin along a chromosomal arm and at the centromere performs distinct functions and undergoes sequential proteolytic degradation during the process of meiosis (Nasmyth and Haering, 2009; Sakuno and Watanabe, 2009; Lister et al., 2010; Wood et al., 2010). Mater-null oocytes display a severe centromere cohesion defect, therefore we wondered whether arm cohesion was also impaired. Severe reduction of arm cohesion below a critical threshold usually causes premature loss of physical linkage between homologs, which can be manifested cytologically by the appearance of univalents or the distally associated bivalent (Buonomo et al., 2000; Hodges et al., 2005; Chiang et al., 2010; Lister et al., 2010). We analysed the chromosome spreads of in vitro cultured oocytes during prometaphase I (∼4–5 h post germinal vesicle breakdown, GVBD). No univalent (defined as the entire separation of two homologs with distinct orientation in the chromosome spread) was observed in wild-type oocytes (n = 38) (Fig. 3A and B). Depletion of Mater did not increase the incidence of univalent, although 1 out of 29 Mater-null oocytes (n = 29) was occasionally found containing a univalent (Fig. 3C, red arrows). The percentage of oocytes with distally associated bivalents (defined as two homologs which are oriented as a unit but lack DAPI staining) in young Mater+/+ mice was similar to the previous report in young CD1 mice (Yun et al., 2014b) and did not differ from that in Mater-null counterparts (Fig. 3D, E and F, white arrow). These data suggested that arm cohesion was not severely impaired by the loss of Mater. Similarly, in a naturally ageing mouse model the arm cohesion is grossly normal and no premature separation of recombined homologous chromosomes occurs (Chiang et al., 2010; Lister et al., 2010).
Figure 3.
Mater depletion does not impair arm cohesion. Representative mouse metaphase I (MI) chromosome spread from Mater+/+oocyte containing normal bivalents (A) or one distal bivalent (B, arrowhead and inset). (C) MI chromosome spread from one Mater-null oocyte containing univalents (red arrowheads). Representative MI chromosome spread from Mater-null oocyte containing normal bivalents (D) or one distal bivalent (E, arrowhead and inset). (F) The percentages of oocytes containing distal bivalent were similar between Mater+/+and Mater-null mice. In total, 38 and 29 oocytes were examined in Mater+/+and Mater-null mice, respectively.
Loss of Mater protein causes abnormal chromosome congression during metaphases I and II
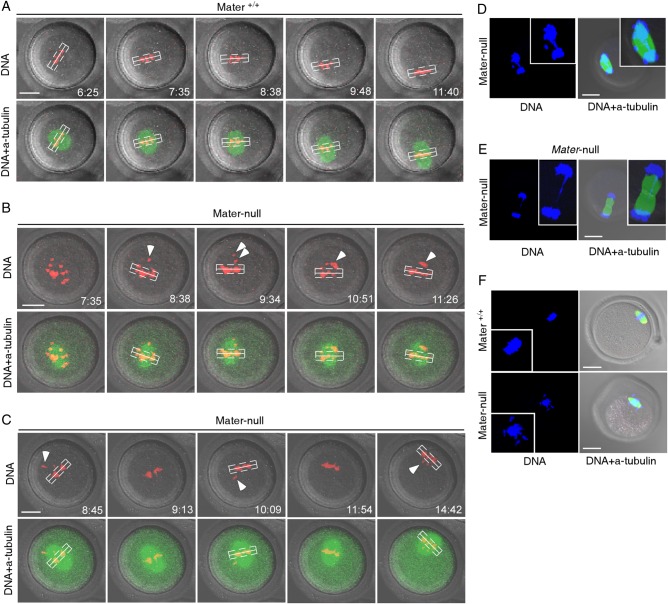
Defects in centromeric cohesion are closely associated with failure to establish or maintain bi-orientation of recombined homologs (bivalents) or sister chromatids, leading to chromosome misalignment at MI or MII (Chiang et al., 2010; Lister et al., 2010). Because the centromeric cohesion is weakened in Mater-null oocytes, we examined the chromosome behaviour at MI and MII. mRNAs encoding GFP tagged Eb1 (Eb1-GFP), a microtubule plus end binding protein which enabled monitoring of the spindle (Zheng et al., 2013), were injected into GV oocytes. DNA was simultaneously stained with Hoechst 33342. GV oocytes were matured in vitro and the process of first meiosis was filmed by time-lapse microscopy. A bivalent which is >4 µm away from the spindle equator was considered as displaced (or nonaligned) (Yun et al., 2014b). A total of 16 Mater+/+ and 20 Mater-null oocytes were filmed. The meiosis I spindle morphogenesis was grossly normal in Mater-null oocytes, excluding the possible relevance of spindle assembly to aneuploidy. However, chromosome alignment to the metaphase plate was impaired in Mater-null oocytes. In Mater+/+ control oocytes, chromosomes stably remained at the metaphase plate (Fig. 4A, Supplementary data, Movie S1). No oocyte had nonaligned bivalents (0%, n = 16). However, all Mater-null oocytes (100%, n = 20) contained nonaligned bivalent(s) exhibiting frequent displacement and continuous relocation (Fig. 4B and C, Supplementary data, Movie S2), which likely reflected that the bivalents could not form stable attachments to the spindle. Consistently, we observed a chromosome bridge or lagging at the 1st anaphase/telophase in Mater-null oocytes (58.3%, n = 24, Fig. 4D and E). MII oocytes from Mater-null females also displayed higher rates of chromosome misalignment (21/31, 67.7%) when compared with those from Mater+/+ females (2/37, 5.4%) (Fig. 4F).
Figure 4.
Abnormal chromosome alignment in mouse Mater-null oocytes. (A) Time-lapse microscopy showed that oocytes from Mater+/+ mice displayed normal chromosome alignment. (B, C) However, all oocytes (n = 20) from Mater-null mice contained nonaligned bivalent(s) (arrowheads) which was defined as >4 µm (dashed line) away from the spindle equator (solid line). DNA was labelled with Hoechst and the spindle was visualized by GFP (green fluorescent protein)-tagged Eb1 (microtubule-associated protein, RP/EB family, member 1). Images in panels (A–C) were taken from confocal projections at indicated scan time points (h:min). (D) Immunofluorescence staining detected the chromosome anaphase bridge in an oocyte from Mater-null females. (E) Lagging chromosome in telophase of oocytes from Mater-null mice. (F) Upper panel, alignment of chromosomes at the metaphase plate in the MII oocyte of Mater+/+ females. Lower panel, misalignment of chromosomes at the metaphase plate in a MII oocyte of Mater-null females. Scale bars, 20 µm.
Loss of Mater causes internalized distribution of CGs in cytoplasm
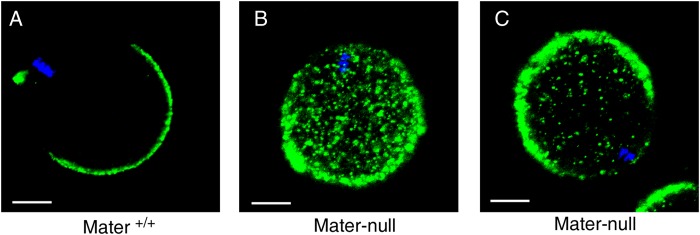
In addition to the centromere cohesion defect, we wondered whether there were other ageing-related cytoplasmic abnormalities in Mater-null oocytes. CG distribution to the cortex plays a critical role in preventing polyspermy and is generally considered as one of the criteria of oocyte developmental competence (Sun and Schatten, 2006). We therefore examined the impact of Mater depletion on CGs. As previously reported (Liu et al., 2005), all MII oocytes (n = 41) from Mater+/+ females displayed normal CG distribution and the CG-free domain was clearly visible (Fig. 5A). In sharp contrast, none of the Mater-null MII oocytes exhibited the normal CG distribution (n = 35). The majority of them (71.4%, 25/35) contained CGs uniformly distributed in the cytoplasm (Fig. 5B), and the rest (28.6%, 10/35) contained CGs in both the periphery and interior of the oocytes (Fig. 5C). Thus, without Mater protein in oocytes, the CG distribution exhibited a defect similar to that observed in old oocytes (Diaz and Esponda, 2004).
Figure 5.
Mater depletion in mice impairs normal distribution of cortical granules. (A) The normal distribution of cortical granules (CGs) in MII oocytes of Mater+/+mice. (B) Fully internalized distribution of CGs in MII oocytes of Mater-null mice. (C) Partially internalized distribution of CGs in MII oocytes of Mater-null mice. Scale bars, 20 µm.
Discussion
Maternal ageing (or reproductive ageing) is characterized by a decline in oocyte quality (Navot et al., 1991). Although aneuploidy and centromere cohesion weakening are recognized as the hallmarks of oocyte quality decline (Hunt and Hassold, 2008; Chiang et al., 2010; Lister et al., 2010; Nagaoka et al., 2012), the underlying molecular basis remains largely unclear. To understand the relevant factors, a previous study investigated the ageing associated genome-wide gene expression changes in mouse MII oocytes (Hamatani et al., 2004). They revealed that oocyte ageing was not accompanied by a global decline in transcript abundance. Rather, only ∼5% of detected genes exhibited a statistically significant expression change, and were relevant with oocyte ageing. Among these transcripts were a group of oocyte-specific NALP gene family members including Mater (Nalp5), Nalp4b, Nalp9a and Nalp14. Here, we present experimental data supporting that Mater is associated with the ageing-dependent oocyte quality decline. The mRNA transcripts of Mater decrease by about 3-fold in oocytes from old mice (42–45 weeks) compared with those from young mice (5–6 weeks) (Hamatani et al., 2004). We validated the Mater expression change at protein level and found a consistent decrease in aged versus young oocytes. Analysing in detail the phenotypes of Mater-null mouse oocytes revealed an elevated frequency of precocious sister chromatid separation, an increase in sister centromere distance and misalignment of chromosomes at the metaphase plate at meiosis I and II. These defects mimic the phenotypes of oocyte quality decline observed in naturally ageing mice (Chiang et al., 2010; Lister et al., 2010). Moreover, abnormal CG distribution, which is another phenotypic marker of quality decline in old oocytes (Diaz and Esponda, 2004), was also obvious in Mater-null oocytes. Taken together, these data support an association of Mater with ageing-dependent oocyte quality decline in mice. Mouse oocytes in antral follicles consist of two populations. One population contains surrounded-nucleolus (SN) and displays a high competence to develop to blastocyst after fertilization. The other population has not-surrounded nucleolus (NSN) and exhibits 2-cell developmental arrest. Interestingly, NSN oocytes expressed lower levels of Mater than SN oocytes (Monti et al., 2013). This association also supports the involvement of Mater in regulating oocyte quality. Mater is highly conserved among species. Knockdown of MATER by small interfering RNA in rhesus monkey oocytes reveals its essential role in preimplantation embryo development (Zou et al., 2009). In human, MATER mutation is associated with female reproductive wastage (for instance, recurrent abortion) and offspring multilocus imprinting disorders (Docherty et al., 2015). Thus, our results have implications for the molecular regulation of human maternal ageing.
Previous studies investigated the sub-cellular localization of Mater protein in mouse oocytes. For example, using transmission electron microscopy Tong et al. (2004) described the localization of Mater in mitochondria and the nucleolus . However, the nuclear localization of Mater is not clearly captured by regular immunofluroscence staining due to its low resolution. Rather, Mater is predominantly detected at the subcortical region of oocytes and preimplantation embryos (Tong et al., 2004; Li et al., 2008; Ohsugi et al., 2008; Zheng and Dean, 2009). At the subcortical region, Mater interacts with several other oocyte-specific proteins including Floped, Filia, Tle6 and Padi6 (Li et al., 2008), all of which play important but unclear roles to ensure successful preimplantation embryo development (Li et al., 2008; Yurttas et al., 2008; Zheng and Dean, 2009; Yu et al., 2014). Further investigation of Filia in embryonic stem cells, the surrogates of early embryo cells, uncovered its multiple functions in protecting genome stability through regulation of the DNA damage response (Zhao et al., 2015). Whether a fraction of Mater proteins can enter the oocyte nucleus needs further elucidation. In oocytes, Mater has been shown to play necessary roles in the formation of cytoplasmic lattices, which store maternally derived mRNAs and ribosomes. Under-expression of Mater affects ribosome biogenesis and protein translation (Yurttas et al., 2008; Kim et al., 2010). Therefore, it is possible that a decrease in Mater contributes to oocyte ageing partially through down-regulating the translation of relative proteins. A comprehensive understanding of the functional roles of Mater, and the mechanisms involved, could shed light on the age-dependent decline in oocyte quality.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
Y.-q.L., X.-c.H. and P.Z. performed the experiments and analysed the data. P.Z. designed the experiments and wrote the paper.
Funding
This work was supported by the research grants from Chinese NSFC to P.Z. (31071274).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Dr. Jurrien Dean in National Institutes of Health, USA for providing antibody against Mater and Mater-null mutation mice.
References
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996;10:2217–2222. [DOI] [PubMed] [Google Scholar]
- Buonomo SB, Clyne RK, Fuchs J, Loidl J, Uhlmann F, Nasmyth K. Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 2000;3:387–398. [DOI] [PubMed] [Google Scholar]
- Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 2010;17:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz H, Esponda P. Ageing-induced changes in the cortical granules of mouse eggs. Zygote 2004;2:95–103. [DOI] [PubMed] [Google Scholar]
- Docherty LE, Rezwan FI, Poole RL, Turner CL, Kivuva E, Maher ER, Smithson SF, Hamilton-Shield JP, Patalan M, Gizewska M et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun 2015;6:8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell 2012;6:1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R, Tsuda C, Perumalsamy AL, Naranian T, Chong J, Acton BM, Tong ZB, Nelson LM, Jurisicova A. NLRP5 mediates mitochondrial function in mouse oocytes and embryos. Biol Reprod 2012;5:131–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 2004;19:2263–2278. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 2007;16:R203–R208. [DOI] [PubMed] [Google Scholar]
- Hawkes K, Smith KR. Do women stop early? Similarities in fertility decline in humans and chimpanzees. Ann N Y Acad Sci 2010;1204:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CA, Hunt PA. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma 2002;3:165–169. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet 2005;12:1351–1355. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet 2008;2:86–93. [DOI] [PubMed] [Google Scholar]
- Jessberger R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep 2012;6:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kan R, Anguish L, Nelson LM, Coonrod SA. Potential role for MATER in cytoplasmic lattice formation in murine oocytes. PLoS One 2010;9:e12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Zhang X, Kan R, Cohen R, Mukai C, Travis AJ, Coonrod SA. The role of MATER in endoplasmic reticulum distribution and calcium homeostasis in mouse oocytes. Dev Biol 2014;2:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev Cell 2008;3:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 2010;17:1511–1521. [DOI] [PubMed] [Google Scholar]
- Liu XY, Mal SF, Miao DQ, Liu DJ, Bao S, Tan JH. Cortical granules behave differently in mouse oocytes matured under different conditions. Hum Reprod 2005;12:3402–3413. [DOI] [PubMed] [Google Scholar]
- Ma J, Milan D, Rocha D. Chromosomal assignment of the porcine NALP5 gene, a candidate gene for female reproductive traits. Anim Reprod Sci 2009;3–4:397–401. [DOI] [PubMed] [Google Scholar]
- Merriman JA, Lane SI, Holt JE, Jennings PC, Garcia-Higuera I, Moreno S, McLaughlin EA, Jones KT. Reduced chromosome cohesion measured by interkinetochore distance is associated with aneuploidy even in oocytes from young mice. Biol Reprod 2013;2:31. [DOI] [PubMed] [Google Scholar]
- Monti M, Zanoni M, Calligaro A, Ko MS, Mauri P, Redi CA. Developmental arrest and mouse antral not-surrounded nucleolus oocytes. Biol Reprod 2013;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 2012;7:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet 2009;43:525–558. [DOI] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 1991;8754:1375–1377. [DOI] [PubMed] [Google Scholar]
- Ohsugi M, Zheng P, Baibakov B, Li L, Dean J. Maternally derived FILIA-MATER complex localizes asymmetrically in cleavage-stage mouse embryos. Development 2008;2:259–269. [DOI] [PubMed] [Google Scholar]
- Pennetier S, Uzbekova S, Perreau C, Papillier P, Mermillod P, Dalbies-Tran R. Spatio-temporal expression of the germ cell marker genes MATER, ZAR1, GDF9, BMP15,andVASA in adult bovine tissues, oocytes, and preimplantation embryos. Biol Reprod 2004;4:1359–1366. [DOI] [PubMed] [Google Scholar]
- Pisani LF, Ramelli P, Lazzari B, Braglia S, Ceciliani F, Mariani P. Characterization of maternal antigen that embryos require (MATER/NLRP5) gene and protein in pig somatic tissues and germ cells. J Reprod Dev 2010;1:41–48. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Hoog C, Kitajima TS. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat Commun 2015;6:7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuno T, Watanabe Y. Studies of meiosis disclose distinct roles of cohesion in the core centromere and pericentromeric regions. Chromosome Res 2009;2:239–249. [DOI] [PubMed] [Google Scholar]
- Shomper M, Lappa C, FitzHarris G. Kinetochore microtubule establishment is defective in oocytes from aged mice. Cell Cycle 2014;7:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA. Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol Hum Reprod 2001;1:49–55. [DOI] [PubMed] [Google Scholar]
- Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006;2:193–205. [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010;4:685–695. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 2000;3:267–268. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Bondy CA, Zhou J, Nelson LM. A human homologue of mouse Mater, a maternal effect gene essential for early embryonic development. Hum Reprod 2002;4:903–911. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Gold L, De Pol A, Vanevski K, Dorward H, Sena P, Palumbo C, Bondy CA, Nelson LM. Developmental expression and subcellular localization of mouse MATER, an oocyte-specific protein essential for early development. Endocrinology 2004;3:1427–1434. [DOI] [PubMed] [Google Scholar]
- Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 2008;1–2:14–29. [DOI] [PubMed] [Google Scholar]
- Wood AJ, Severson AF, Meyer BJ. Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet 2010;6:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XJ, Yi Z, Gao Z, Qin D, Zhai Y, Chen X, Ou-Yang Y, Wang ZB, Zheng P, Zhu MS et al. The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics. Nat Commun 2014;5:4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y, Holt JE, Lane SI, McLaughlin EA, Merriman JA, Jones KT. Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice. Cell Cycle 2014a;12:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development 2014b;1:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurttas P, Vitale AM, Fitzhenry RJ, Cohen-Gould L, Wu W, Gossen JA, Coonrod SA. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development 2008;15:2627–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Zhang WD, Duan YL, Lu YQ, Cun YX, Li CH, Guo K, Nie WH, Li L, Zhang R et al. Filia is an ESC-specific regulator of DNA damage response and safeguards genomic stability. Cell Stem Cell 2015;6:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Dean J. Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc Natl Acad Sci USA 2009;18:7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Baibakov B, Wang XH, Dean J. PtdIns(3,4,5)P3 is constitutively synthesized and required for spindle translocation during meiosis in mouse oocytes. J Cell Sci 2013;(Pt 3):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 2009;5:631–636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.