Abstract
While aging is generally associated with episodic memory decline, not all older adults exhibit memory loss. Furthermore, emotional memories are not subject to the same extent of forgetting and appear preserved in aging. We conducted high-resolution fMRI during a task involving pattern separation of emotional information in older adults with and without age-related memory impairment (characterized by performance on a word-list learning task: low performers: LP vs. high performers: HP). We found signals consistent with emotional pattern separation in hippocampal dentate (DG)/CA3 in HP but not in LP individuals, suggesting a deficit in emotional pattern separation. During false recognition, we found increased DG/CA3 activity in LP individuals, suggesting that hyperactivity may be associated with overgeneralization. We additionally observed a selective deficit in basolateral amygdala—lateral entorhinal cortex—DG/CA3 functional connectivity in LP individuals during pattern separation of negative information. During negative false recognition, LP individuals showed increased medial temporal lobe functional connectivity, consistent with overgeneralization. Overall, these results suggest a novel mechanistic account of individual differences in emotional memory alterations exhibited in aging.
Keywords: Memory, Aging, Emotion, Hippocampus, Amygdala, Lateral entorhinal cortex
1. Introduction
While episodic memory deficits are a hallmark characteristic of aging, emotion’s modulatory influence on memory remains less well characterized in aging. While some studies show no age-related differences, others have reported alterations with aging (Foster et al., 2012). Several human studies have shown that memory for emotional experiences is preserved with age (Denburg et al., 2003; Kensinger et al., 2002). Some studies have suggested that there is a “positivity effect,” where older adults may be more likely to attend to positive information in the environment (Mather and Carstensen, 2005). In contrast, some have suggested that memory for detailed information in older adults reveals no such positivity effect and may actually be biased toward remembering negative details (Kensinger et al., 2007). This suggests that the relationship between aging and emotional modulation of memory is complex and requires a more thorough investigation.
Decades of research in animal models have shown that experiences associated with physiological arousal are better remembered than nonarousing experiences (McGaugh, 2004). The basolateral amygdala (BLA) and the hippocampus have been implicated in the storage of emotional memories (McGaugh, 2004). The BLA projects to the hippocampus via: (1) indirect connections through the lateral entorhinal cortex (LEC); (2) indirect connections through the hypothalamus and medial septum; and (3) direct connections mainly to CA1 and subiculum (Petrovich et al., 2001; Pitkänen et al., 2000). These connections are thought to modulate the strength of emotional memories (LeDoux, 2007; McGaugh, 2004). In contrast to the medial entorhinal cortex (MEC), the LEC is more strongly connected to the BLA (Agster et al., 2016; Canto et al., 2008; McDonald and Mascagni, 1997). Thus, assessing neural activity in the BLA, LEC, and the hippocampus may elucidate the mechanisms underlying the emotional modulation of memory.
The amygdala and its relationship with the hippocampus is also altered in aging. In normal aging, there is a reduction in amygdala volume of about 4% compared with young adults (Whalen and Phelps, 2009). A reduction of noradrenergic input to the hippocampus (Kubanis and Zornetzer, 1981) as well as changes in peripheral epinephrine levels (Sternberg et al., 1985) have been reported in animal models of aging. Age-related alterations in synaptic plasticity in the amygdala-hippocampal network have also been reported (Almaguer et al., 2002). St Jacques et al. (2009) suggested that an age-related reduction in the contribution of amygdala-hippocampal mechanisms may be compensated by enhanced contribution of amygdala-prefrontal mechanisms to the formation of emotional memories (Murty et al., 2009; St Jacques et al., 2009).
Recently, we have used an emotional mnemonic discrimination task to investigate the emotional modulation of memory, rooting our hypotheses in computational models of hippocampal functioning (Leal et al., 2014a,b). Mnemonic discrimination, or the ability to discriminate highly similar items, has been used as a proxy for hippocampal pattern separation (Bakker et al., 2008; Lacy et al., 2011). Hippocampal pattern separation, or the process of disambiguating previously stored representations as distinct from one another, is one mechanism for reducing interference of similar experiences (Marr, 1971; Yassa and Stark, 2011). For the emotional mnemonic discrimination task, participants are shown negative, neutral, and positive scenes and are asked to rate the emotional valence of the images. Participants are then tested on their memory of the images (old/new recognition test) and are shown the same images (targets), similar images (lures), and new images (foils). We have previously reported in young adults that the hippocampal dentate (DG)/CA3 showed signals consistent with pattern separation during mnemonic discrimination of negative images, whereas the amygdala showed emotional modulation signals regardless of memory performance (i.e., during both correct rejections [CRs] and false alarms [FAs]; Leal et al., 2014a). This suggests that the DG/CA3 subregion of the hippocampus is involved in being able to discriminate highly similar emotional information in young adults and that DG/CA3 may work in concert with the amygdala to aid in emotional discrimination.
Several studies have shown that older adults show deficits on mnemonic discrimination of object identity (Holden et al., 2013; Stark et al., 2013; Toner et al., 2009), spatial location (Holden and Gilbert, 2012; Reagh et al., 2014a; Stark et al., 2010), and temporal order (Roberts et al., 2014; Tolentino et al., 2012). High-resolution fMRI studies have shown that increased DG/CA3 activity in older adults is associated with deficits in mnemonic discrimination (Yassa et al., 2011a) as well as “representational rigidity,” where older adults require more dissimilarity for successful discrimination (Leal and Yassa, 2015; Yassa, et al., 2011b). However, these studies did not vary and assess the influence of emotion on the event to be remembered or consider the increased variability in age-related memory impairment (Gallagher et al., 2006).
We have previously developed an individual differences approach, inspired by studies of spatial learning in animals (Gallagher et al., 1993), that characterizes individual differences in older adults according to their performance on the Rey Auditory Verbal Learning Test (RAVLT)—delayed recall component (Reagh et al., 2014a; Stark et al., 2010). Examining episodic memory performance for emotional events through the lens of an individual differences approach may reveal emotional memory deficits selective to low-performing older adults. The present study used the emotional mnemonic discrimination task to investigate emotional pattern separation in aging. We hypothesized that healthy older adults who perform well on the RAVLT (high performers, [HPs]) would show signals consistent with emotional pattern separation, similar to that of young adults, while older adults who perform worse on the RAVLT (low performers, [LP]) would show deficits in emotional pattern separation signals that may be associated with amygdala-hippocampal connectivity deficits. To determine the specificity of connectivity deficits, we planned to investigate 3 subnetworks within the medial temporal lobe (MTL): (1) BLA-CA1 connectivity (direct); (2) BLA-LEC-DG/CA3 connectivity (indirect); and (3) DG/CA3-CA1 connectivity (intrahippocampal).
2. Materials and methods
2.1. Participants
Twenty-seven participants (N = 27, 18 female; mean age 72.2 + 7.6SD, age range = 60–91) were recruited from the local Orange County community via local campus announcements, flyers, and ads in local newspapers. Participants received monetary remuneration for their participation. Informed consent was obtained from all participants, with all procedures approved by the University of California, Irvine Institutional Review Board.
2.2. Inclusion/exclusion criteria
All participants were screened against major medical or psychiatric morbidities as well as substance abuse history. Participants received a neuropsychological evaluation during their visit. The battery was designed to examine memory function, as well as other aspects of general cognitive ability. The assessment included the following: (1) Mini-Mental State Examination (MMSE) to test global cognitive status; (2) RAVLT to assess verbal learning and memory; (3) Digit Span Backward and Forward to assess working memory; (4) Trail Making tests A and B to assess attention, visual search, and mental processing speed; (5) Beck Depression Inventory-II to assess depressive symptoms; (6) Geriatric Depression Scale to assess depressive symptoms more specific to older adults; (7) Letter-Number Sequencing to assess executive function; (8) Stroop Color and Word Test to assess executive function; (9) Beck Anxiety Inventory to assess symptoms of anxiety; and (10) a modified version of the Pittsburgh Sleep Quality Index to assess sleep quality and recent stress (see Table 1 for results). Participants included were healthy older adults with no diagnosed disorders associated with memory impairments such as mild cognitive impairment or Alzheimer’s disease (AD). We split participants into 2 groups: HP (N = 15, 11 female; mean age 70.9 + 6.6SD) and LP (N = 12, 7 female; mean age 73.9 + 8.6SD), based on a median split of performance on the RAVLT-delayed test (≤10 in the LP group). It is important to note that none of the older adults were “clinically impaired” compared with “normal”, as no older adults were diagnosed with dementia, however, the distinction between HP and LP groups is a helpful way to characterize subtle neurobiological differences between those with intact memory compared with those who are showing signs of subtle memory deficits. This method of splitting groups into HP and LP has been used previously (Reagh et al., 2014a, 2015; Stark et al., 2013). Importantly, the LP group did not present with memory complaints, nor did they present with memory deficits sufficient for a diagnosis of clinical impairment. The particular selection of the RAVLT was motivated by the fact that it is a hippocampus-sensitive and a highly standardized neuropsychological test (Estévez-González et al., 2003). Furthermore, recent modeling work suggests that changes in RAVLT performance are found very early in the clinical/pathological progression of AD, even before detectable changes in amyloid pathology (Jedynak et al., 2012). There were no significant differences between the older adult groups in age, education, and cognitive status (based on the MMSE) (p’s > 0.05). All participants had normal or corrected-to-normal vision.
Table 1.
Participant characteristics
| Groups | High performance (HP) | Low performance (LP) | ||
|---|---|---|---|---|
| N | 15 | 12 | ||
| Sex (M:F) | 4:11 | 5:7 | ||
| Age | 70.9 | 73.9 | ||
| Education | 16.5 | 16.8 | ||
|
| ||||
| Variables | Mean | SEM | Mean | SEM |
|
| ||||
| Digit span forward | 10.2 | 0.5 | 10.0 | 0.5 |
| Digit span backward | 6.8 | 0.5 | 6.0 | 0.6 |
| Letter-number sequencing | 18.9 | 0.7 | 19.0 | 0.5 |
| Geriatric Depression Scale | 0.7 | 0.3 | 0.7 | 0.4 |
| Mini-Mental State Exam | 28.9 | 0.3 | 28.9 | 0.3 |
| RAVLT immediate recalla | 12.9 | 0.5 | 8.7 | 0.9 |
| RAVLT-delayed recalla | 13.1 | 0.4 | 7.5 | 0.7 |
| RAVLT recognition recalla | 14.5 | 0.3 | 13.1 | 0.6 |
| Trail Making Test A | 28.4 | 1.5 | 31.4 | 1.8 |
| Trail Making Test Ba | 61.6 | 4.2 | 89.1 | 10.3 |
| Stroop test (Word-Color)a | 36.5 | 2.1 | 28.3 | 2.3 |
| Beck Anxiety Inventory | 3.8 | 0.8 | 4.1 | 1.2 |
| Beck Depression Inventory-II | 2.9 | 0.9 | 3.1 | 1.2 |
| Hours of sleep (night before) | 8.0 | 0.4 | 7.9 | 0.3 |
| Level of stress (past month) | 2.7 | 0.3 | 1.9 | 0.3 |
Key: RAVLT, Rey Auditory Verbal Learning Test; SEM, standard error of the mean.
Significantly different between groups; RAVLT immediate recall (t[25] = –4.2, p < 0.001), RAVLT-delayed recall (t[25] = –7.7, p < 0.001), RAVLT recognition (t[25] = –2.2, p= 0.04), Trail Making Test B (t[25] = 2.7, p = 0.01), Stroop test (Word-Color; t[25] = –2.6, p= 0.02).
2.3. Imaging data collection
Functional MRI data were collected using a 3-Tesla Philips scanner equipped with a SENSE head coil using both higher-order shims and SENSE imaging techniques. Functional images were collected using a high-speed EPI single-shot pulse sequence (1.5-mm isotropic resolution, 19 oblique axial slices parallel to the principal axis of the hippocampus, field of view = 180 × 28.3 mm × 180 mm, flip angle = 70°, SENSE parallel reduction factor = 2, TR/TE = 2200/26 ms, matrix size = 128 × 128). We collected a high-resolution structural MPRAGE scan that we developed for accurate delineation of hippocampal subfields (Leal et al., 2014a) and high-resolution diffeomorphic alignment (0.65-mm isotropic resolution; 231 sagittal slices, field of view = 232 mm × 240 mm × 150 mm, flip angle = 18°, TR/TE = 11/ 5.03 ms, matrix size = 448 × 448). SENSE parallel imaging was used in 2 directions (2×1.5). The specific absorption rate (<10%) and peripheral nerve stimulation (<75%) were within required limits based on the scanner-calculated values. Total scan time for the volume was 5 minutes 44 seconds.
2.4. Emotional mnemonic discrimination task
An Apple iMac equipped with MATLAB (Version R2013a, Natick, MA, USA) software and PsychToolbox version 3.0 was used to present the stimuli and record responses. Each trial consisted of 2 displays: an image display (2500 ms) and a fixation display (500 ms). During both study and test phases, images (negative, neutral, or positive) were presented on the center of the screen with a black background for 2500 ms. The fixation display consisted of a white fixation cross on the center of the screen with a black background for 500 ms. Images were rated a priori by separate groups of participants for emotional valence on a scale 1–9(1 being the most negative, 9 being the most positive, and 5 being neutral). Images rated 1–3.5 were called negative, images rated 3.6–6 were called neutral, and images rated 6.1–9 were called positive. Another sample rated the images for emotional arousal on a scale 1–9 (1 being the least arousing, 9 being the most arousing). Negative and positive images received higher arousal ratings than neutral, although negative images were more arousing than positive images. A third sample was used to examine relative similarity of negative, neutral, and positive images as measured by number of FAs/total responses during task performance, which we categorized into low- and high-similarity bins. In addition, we independently collected subjective similarity ratings on pairs of stimuli presented simultaneously in a fourth sample. We found that both FA/total responses and subjective similarity ratings resulted in a significant difference between the 2 similarity bins when stimuli were collapsed across all emotional categories as well as within each emotional category. Together, these experiments show that high-and low-similarity stimuli were both perceived subjectively by participants as such and influenced behavioral responses in the predicted manner (Leal et al., 2014b).
Participants underwent an incidental encoding phase in the scanner (7.5 minutes) where they were shown emotional and nonemotional images, presented in randomized order, and were asked to rate the images for emotional valence via button press (negative, neutral, and positive). Participants were given a subsequent surprise test, broken up into 2 sessions (7.3 minutes each), 5 minutes after the encoding phase where they were presented with exact repeats of the scenes presented during encoding (targets), some scenes that were similar but not identical to ones seen during encoding (lures), and some scenes were completely new (foils; Fig. 1A). Participants were asked to indicate whether items were “old” or “new” via button press. Participants were explicitly told that in order for an image to be called “old,” it had to be the exact same image they saw before. The experiment consisted of 149 images during the study phase and 291 images during the test phase. Targets, lures, and foils were evenly distributed across emotion.
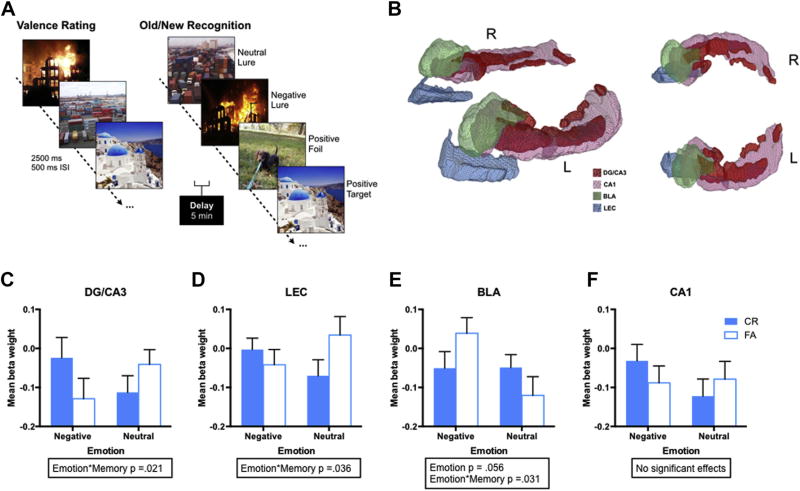
Fig. 1.
Task design, regions of interest, and overall activity profiles. (A) Participants performed an emotional mnemonic discrimination task. During encoding, participants rated images according to their emotional valence as negative, neutral, or positive via button press. Each image was presented for 2500 ms with a 500-ms interstimulus interval. After a 5-min delay, participants underwent a surprise recognition test where they viewed negative, neutral, and positive targets, foils, and lures varying in similarity and were asked to indicate whether items were “old” or “new”, (B) The schematic shows a 3D rendering of our high-resolution anatomical template (0.65-mm isotropic) of the dentate gyrus (DG)/CA3 (red), CA1 (pink), subiculum (SUB, purple), basolateral amygdala (BLA, green), and lateral entorhinal cortex (LEC, blue), (C) mean beta weight in the DG/CA3 for negative and neutral stimuli during correct rejections (CRs) and FAs, (D) mean beta weight in the LEC for negative and neutral stimuli during CRs and FAs, (E) mean beta weight in the BLA for negative and neutral stimuli during CRs and FAs, (F) Mean beta weight in the CA1 for negative and neutral stimuli during CRs and FAs. Data are represented as mean ± SEM. Abbreviations: BLA, basolateral amygdala; LEC, lateral entorhinal cortex; SEM, standard error of the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.5. Image analysis
All data analyses were conducted using Analysis of Functional NeuroImages (Cox, 1996). Images were corrected for slice timing and subject motion. Time points in which significant motion events occurred (movement exceeded 3 degrees of rotation or 2 mm of translation in any direction relative to prior acquisition ±1 time point) were censored from further analyses. Functional images were then coregistered to the structural scans acquired in the same session using Analysis of Functional NeuroImages’s align_epi_anat.py algorithm. High-resolution structural scans (0.55-mm isotropic) were aligned to an anatomical template developed in our lab (Leal et al., 2014a) using Advanced Normalization Tools (Avants et al., 2011), which uses a powerful diffeomorphic algorithm (SyN; Klein et al., 2009) to warp individual participants into the template space. The template was then used to manually trace hippocampal subfields, amygdala nuclei, and surrounding cortical regions including LEC and MEC. This template has been used in past studies investigating hippocampal sub-fields and surrounding cortical nuclei (Leal et al., 2014a; Reagh and Yassa, 2014; Reagh et al., 2014b). We used the same template to add amygdala subnuclei. Amygdala segmentation procedures were based on (Entis et al., 2012), but were modified to define 3 regions: BLA, CEA, and CORT. To label these subregions of the amygdala, 3 key points were identified: (1) the medial tip of the alveus (up to where optic tract is); (2) the most lateral point of the entorhinal sulcus; and (3) bottom of the circular sulcus. These 3 points are easily observable and provide a reliable landmarking system for segmenting the amygdala subregions. After identifying the 3 points, lines are drawn to connect the 3 points to each other, creating 4 quadrants (basomedial and basolateral were combined to form the basolateral complex). We collapsed across CEA and CORT regions, as these were quite small. Region of interest (ROIs) included in the analysis are shown in Fig. 1B and the full set of ROIs are shown in Supplementary Fig. S1.
Behavioral vectors based on trial type (classified according to emotion and behavioral decision) were used to model the data using a deconvolution approach based on multiple linear regression. The resultant fit coefficients (betas) estimated activity versus an implicit baseline (novel foils) for a given time point and trial type in a voxel. The sum of the fit coefficients over the expected hemodynamic response (3–12 seconds after trial onset) was taken as the model’s estimate of the response to each trial type (relative to novel foils).
2.6. Extracting ROI voxels
We selected voxels for subsequent analyses based on combining the voxels that changed with any of the task conditions. Active voxels were selected based on the overall F, agnostic to specific condition or contrast so as not to bias subsequent analyses and remove concerns regarding circularity and double-dipping (Kriegeskorte et al., 2009). This served to remove voxels that did not respond to any of the task conditions so that the analyses can be more sensitive to subtle changes across conditions. This voxel mask was then combined with anatomical ROI masks for the amygdala subnuclei, hippocampal subfields, and surrounding cortices. Voxel betas from the resulting hybrid functional/structural ROIs were averaged and all subsequent statistical analyses were conducted on these averages.
2.7. Statistical analyses
All statistical analyses of behavioral variables and ROI activation means were conducted in SPSS v. 21 (IBM Corp, released 2012, Armonk, NY, USA). Planned comparisons were conducted using repeated-measure ANOVAs. Post hoc contrasts were conducted where appropriate and critical Scheffe’s were reported during the first instance of reporting. All tests used the General Linear Model (ANOVA and correlations). Normality assumptions were investigated using Kolmogorov-Smirnov tests and all distributions investigated did not significantly deviate from the normal distribution. Repeated measures tests were corrected for error nonsphericity using Greenhouse-Geisser correction where appropriate. Partial η2 values were reported where appropriate. Statistical values were considered significant at a final corrected alpha level of 0.05, which appropriately controls for type I error.
Correlation matrices (correlograms) were generated in MATLAB across the networks of interest (DG/CA3, LEC, BLA, CA1) using pairwise r2 values (2-tailed) for all conditions and groups (negative and neutral, CRs and FAs, HP and LP). The mean beta weight for each ROI was correlated with one another for each participant for every condition (e.g., correlation of beta weights for BLA and CA1 for each participant during Negative CRs). Pearson’s r values were then used to define 3 MTL networks: (1) BLA-CA1 connectivity; (2) BLA-LEC-DG/CA3 connectivity, which was calculated by averaging the pair-wise BLA-LEC and LEC-DG/CA3 pairwise correlations; and (3) DG/CA3-CA1 connectivity. The differences between pairwise dependent correlation coefficients were then calculated using z-scores (2-tailed) within groups (Lee and Preacher, 2013) as well as the differences between correlation coefficients between groups (VassarStats.net).
Moderation analyses were conducted in SPSS using the PROCESS module (Hayes, 2013). The module employs hierarchical regression analysis to first test the relationships between the predictor and the outcome and the moderator and the outcome (conditional effects) and to then test the interaction between the predictor and the moderator (i.e., how the effect of the predictor on the outcome changes as the moderator changes). It also provides a table of estimates of the outcome for various combinations of the predictor and the moderator, which can be used to visualize the moderation effect. Furthermore, it provides data to probe the interaction further by determining where in the distribution of the moderator the predictor is related to the outcome to better discern the interpretation of the interaction (i.e., Johnson-Neyman technique).
3. Results
Older adults (N = 27, 18 female; mean age 72.2 + 7.6SD) performed an emotional mnemonic discrimination task (Fig. 1A) during high-resolution fMRI. During encoding, participants saw emotional or nonemotional scenes and were asked to rate the valence of the scenes as negative, neutral, or positive. After a 5-minute delay, participants were tested in an old/new recognition task with some of the same scenes they saw before (targets), novel scenes they had not seen yet (foils), and scenes that were similar but not identical to ones they previously saw during encoding (lures). We calculated a lure discrimination index (LDI) for each emotional condition as the probability of a “New” response to a lure (p[“New”|Lure]) minus the probability of a “New” response to a target (p[“New”|Target]) to correct for response bias. The LDI measures the participant’s ability to discriminate highly similar items (i.e., lures) from those they saw before, which allows us to investigate the computational properties of the hippocampus, namely, pattern separation. While a standard d prime measure (p [“Old”|Target]-p[“Old”|Foil]) also gives us a measure of memory, d prime has been shown to be a more general measure of memory, whereas LDI is a more sensitive measure for picking up on subtle memory deficits that may occur with aging (Stark et al., 2013) due to its hypothesized reliance on hippocampal pattern separation.
3.1. Emotional modulation of lure discrimination behavior
To examine lure discrimination behavior, we conducted a repeated measures ANOVA across emotion (negative, neutral, positive) and hypothesized to find differences in performance between neutral and emotional discrimination. We found a marginally significant effect of emotion (F(2,52) = 3.26, p = 0.052, partial η2 = 0.11; Table 2). Our a priori contrast of interest comparing neutral against emotional discrimination showed a significant difference (emotional < neutral) (F[1,26] = 5.28, p = 0.03, partial η2 = 0.17). Reaction time data are shown in Supplementary Table S1. Consistent with our prior results using this task in young adults, we found no significant effect of sex (F[1,25] = 0.436, p = 0.515) or sex by emotion interaction (F[2,50] = 2.42, p = 0.1), although it is likely that sample sizes were not large enough to fully examine sex differences.
Table 2.
Performance on emotional mnemonic discrimination task
| Measure | Overall (mean, SEM) | HP (mean, SEM) | LP (mean, SEM) |
|---|---|---|---|
| Negative LDI | 0.39, 0.04 | 0.38, 0.06 | 0.42, 0.03 |
| Neutral LDI | 0.47, 0.04 | 0.47, 0.05 | 0.47, 0.06 |
| Positive LDI | 0.40, 0.03 | 0.41, 0.04 | 0.40, 0.05 |
Key: HP, high performance; LDI, lure discrimination index; LP, low performance; SEM, standard error of the mean.
3.2. Emotional discrimination in DG/CA3 versus false recognition in LEC and BLA
We focused our fMRI analysis on hippocampal subfields DG/CA3 and CA1 and inputs to the hippocampus from the BLA, either directly or through the LEC (Fig. 1B). The central and cortical nuclei of the amygdala (CEA/CORT) and the MEC were used as control regions to ensure specificity of the amygdala-hippocampal network subject to analysis. Supplementary Fig. S1 demonstrates the template with all ROIs. To examine emotional pattern separation, our analyses focused on the following comparisons: (1) retrieval trials where similar lures were presented and either correctly rejected or falsely recognized; and (2) comparison of negative and neutral items (we excluded positive items from analysis, as these items were not matched to negative items for arousal; Leal et al., 2014b). We defined “emotional pattern separation” signals as showing (1) increases in BOLD activity during correct discrimination of lures relative to false recognition of lures; and (2) during correct discrimination of lures, signals would be modulated by emotion, that is, emotional responses would be significantly different from neutral responses (Leal et al., 2014a). The mean beta weights reported for each ROI are relative to novel foils, but importantly, the contrast between the conditions (e.g., CRs vs. FAs) is informative in determining if signals are consistent with hippocampal pattern separation. We collapsed across left and right hemispheres, as the patterns were largely similar.
We conducted region-based ANOVAs with emotion (negative vs. neutral) and memory (CRs vs. FAs) as within-subject factors. In DG/CA3, we found a significant emotion × memory interaction (F[1,26] = 6.03, p = 0.021, partial η2 = 0.19; Fig. 1C), which was driven by greater activity during negative CRs compared with neutral CRs (t[26] = 2.19, p = 0.038), with no difference during FAs. This interaction is consistent with an emotional pattern separation signal in the DG/CA3.
In the LEC, we found a significant emotion × memory interaction, where activity was greater for negative versus neutral CRs, but the pattern was reversed during FAs (F[1,26] = 4.91, p = 0.036, partial η2 = 0.16; Fig. 1D). Post hoc contrasts revealed no significant pairwise effects, suggesting the interaction was entirely dependent on the opposing relationships between negative and neutral stimuli when comparing CRs and FAs. In the BLA, we found a significant emotion × memory interaction (F[1,26] = 5.23, p = 0.031, partial η2 = 0.17; Fig. 1E), where activity was the opposite of the DG/CA3 pattern with greater activity during negative FAs compared with neutral FAs (t[26] = 2.49, p = 0.020) with no difference during the CRs. In CA1, we found no significant effects (all p’s > 0.05; Fig. 1F). We did not find any significant emotion × memory interactions in either the CEA/CORT or MEC (all p’s > 0.05). Data across all ROIs and conditions are in Supplementary Fig. S2.
3.3. Reduced DG/CA3 emotional pattern separation and enhanced generalized false recognition signals in low-performing adults
To examine individual variability in emotional mnemonic discrimination, we stratified participants into HP and LP groups based on the RAVLT-delayed recall (see methods for more details). Importantly, the LP group did not present with memory complaints, nor did they present with memory deficits sufficient for a diagnosis of clinical impairment. Neuropsychological testing revealed significant group differences on the RAVLT (immediate, delayed, and recognition), Trail Making Test B, and the Stroop Test (Word-Color; see Table 1 for statistics). We examined LDI performance across the groups and conducted a repeated measures ANOVA and found no significant differences in LDI for emotion (F[2,50] = 3.02, p = 0.064, partial η2 = 0.11), group (F[1,25] = 0.2, p = 0.662, partial η2 = 0.01), or emotion × group (F[2,50] = 0.1, p = 0.889, partial η2 = 0.004). We hypothesized that while behavioral performance was matched across groups, the underlying neural signals during correct discrimination and false recognition may be different across groups.
We performed a 3-way ANOVA to directly compare the effects of memory in each ROI followed by separate repeated measures ANOVAs for CRs and FAs. When directly comparing CRs and FAs in the DG/CA3, we found a significant memory × group interaction (F[1,25] = 6.25, p = 0.019, partial η2 = 0.20), where there was increased activity during FAs versus CRs in the LP group compared with the HP group.
To examine effects during accurate discrimination, we conducted an ANOVA for DG/CA3 CRs with emotion (negative, neutral) and group (HP, LP) and found a significant effect of emotion (F[1,25] = 4.375, p = 0.047, partial η2 = 0.15; Fig. 2A) where there was greater activity for negative compared with neutral trials. We also found a significant emotion × group interaction (F[1,25] = 5.31, p = 0.030, partial η2 = 0.18), where the HP group showed greater activity during negative compared with neutral CRs (F[1,25] = 10.87, critical Scheffé = 8.48, p < 0.05), whereas the LP group showed no difference between negative and neutral activity during CRs (F[1,25] = 0.02, p > 0.05). No significant effects were observed for CA1 (there was a marginal group × memory interaction, p = 0.052), and there were no significant group differences or interactions with group in the LEC and BLA (Fig. 2B and C). Data across all ROIs, conditions, and groups are in Supplementary Fig. S3. These results suggest that the DG/CA3 emotional discrimination signal identified previously in healthy young adults (Leal et al., 2014a) is similar to that in HP adults. It is important to note that these groups cannot be directly compared, as the current investigation focused on within age group comparisons but suggests that both young adults and older adults with little memory impairment show signals consistent with emotional pattern separation. DG/CA3 activity in LP adults did not appear to discriminate between negative and neutral items (i.e., showed a reduced emotional discrimination signal).
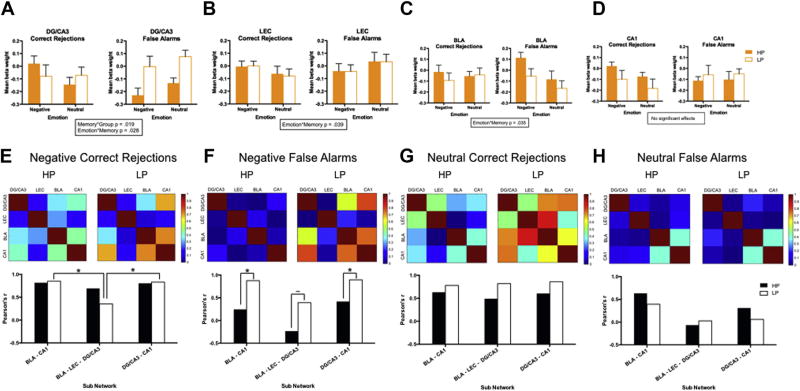
Fig. 2.
High-performing (HP) versus low-performing (LP) individuals’ activity during emotional discrimination and false recognition. (A) Mean beta weight in the dentate gyrus (DG)/CA3 for negative and neutral stimuli during correct rejections (CRs) and false alarms (FAs), (B) mean beta weight in the lateral entorhinal cortex (LEC) for negative and neutral stimuli during CRs and FAs, (C) mean beta weight in the basolateral amygdala (BLA) for negative and neutral stimuli during CRs and FAs, (D) mean beta weight in the CA1 for negative and neutral stimuli during CRs and FAs, (EeH) correlation matrices for pairwise r2 values across ROIs in HP and LP individuals (upper panel). We then compared 3 subnetwork functional connectivity scores based on known anatomical connectivity of the amygdala to the hippocampus and intra-hippocampal connectivity: (1) BLA-CA1 connectivity (direct), (2) BLA-LEC-DG/CA3 connectivity (indirect—via the perforant path), and (3) DG/CA3-CA1 connectivity (intrahippocampal–Schaffer collaterals). We calculated a subnetwork functional connectivity score for 3 pathways (lower panel). For the indirect pathway, this was done by averaging the pairwise BLA-LEC and LEC-DG/CA3 pairwise correlations. All correlations were normalized using a Fisher’s r to z transform and then compared using a z(difference) test. Data are represented as mean ± SEM. * indicates a significant effect, ~ indicates a marginal effect See text for further details. Abbreviations: ROI, region of interest; SEM, standard error of the mean.
To examine effects during false recognition, we conducted an ANOVA for DG/CA3 during FAs, we observed a significant group effect (F[1,25] = 14.37, p < 0.001, partial η2 = 0.37; Fig. 2A), where the LP group showed increased activity in DG/CA3 compared with the HP group regardless of emotion. There were no significant effects for ANOVAs in CA1, LEC, or BLA.
3.4. Selective deficit of BLA-LEC-DG/CA3 connectivity during negative discrimination in low-performing adults
To investigate context-dependent amygdala-hippocampal connectivity, we generated correlation matrices (correlograms) using pairwise r2 values for each participant across ROI pairs for each condition (negative and neutral FAs and CRs; Fig. 2E–H, upper panel). The full matrices (including control regions) are in Supplementary Fig. S4. We then compared 3 subnetwork functional connectivity scores based on known anatomical connectivity of the amygdala to the hippocampus and intrahippocampal connectivity: (1) BLA-CA1 connectivity (direct); (2) BLA-LEC-DG/CA3 connectivity (indirect—via the perforant path); and (3) DG/CA3-CA1 connectivity (intrahippocampal–Schaffer collaterals). We calculated a subnetwork functional connectivity score for each of the 3 pathways (Fig. 2E–H, lower panel). For the indirect pathway, this was done by averaging the pairwise BLA-LEC and LEC-DG/CA3 correlations. All correlations were normalized using a Fisher’s r to z transform and then compared using a z(difference) test.
We hypothesized that the LP group would exhibit selective deficits in the indirect pathway but not in the direct or intra-hippocampal pathways, as perforant path deficits have been shown in age-related cognitive decline (Barnes, 1979; Barnes et al., 2000; Smith et al., 2000; Yassa et al., 2010). Consistent with this hypothesis, during negative CRs, the LP group showed a selective impairment in connectivity within the indirect subnetwork (BLA-LEC-DG/CA3) compared to the direct subnetwork (BLA-CA1; z = 4.92, p < 0.001; Fig. 2E) and the intrahippocampal network (DG/ CA3-CA1; z = −4.8, p < 0.001). This was not the case in the HP group (p’s > 0.05). There were no significant between group differences for BLA-LEC-DG/CA3 connectivity (z = 1.09, p = 0.276), BLA-CA1 connectivity (z = −0.24, p = 0.81), or DG/CA1-CA1 connectivity (z = −0.19, p = 0.849). These results suggest that LP older adults show deficits in functional communication through the indirect amygdala-hippocampal network, which is consistent with the impaired emotional pattern separation noted earlier.
3.5. Enhanced connectivity during negative false recognition across the MTL in low-performing individuals
During negative FAs, there was increased connectivity across 2 subnetworks in the LP group compared with the HP group (BLA-CA1 z = −2.53, p = 0.01, DG/CA3-CA1 z = −2.27, p = 0.023; Fig. 2F). This difference was only marginal in the BLA-LEC-DG/CA3 network but trended in the same direction (z = −1.49, p = 0.136). This suggests that the LP group is overgeneralizing across the hippocampal-amygdala network during negative false recognition. This is in line with our finding of hippocampal hyperactivity during FAs but appears to be selective to negative FAs. While the BLA did not show any group differences in mean beta weight activity, there are clear differences in network connectivity between BLA and the hippocampus in the HP and LP groups. We also found significant within-subject effects in the LP group, in which they again showed a selective impairment in connectivity within the indirect subnetwork (BLA-LEC-DG/CA3) compared to the direct subnetwork (BLA-CA1; z = 5.73, p < 0.001; Fig. 2E) and the intrahippocampal network (DG/CA3-CA1; z = −5.84, p < 0.001), which further supports a deficit in connectivity in the indirect amygdala-hippocampal network. There were no significant differences during neutral FAs and CRs (p’s > 0.05; Fig. 2G and H), signifying the specificity of these effects for processing significant emotional stimuli.
3.6. The influence of BLA on DG/CA3 activity during false recognition depends on individual differences in memory performance
While the formation of HP and LP groups has shown to be a fruitful means to examine the increased variability in memory performance in older adults, we additionally applied a continuous approach to investigate individual differences in memory abilities. We wanted to determine whether there were links between the amount of memory performance and amygdala-hippocampal connectivity. To test this, we correlated activity in the DG/CA3 and BLA during CRs and FAs with RAVLT-delayed scores. Since the LP group showed increased DG/CA3 activity during both negative and neutral FAs (Fig. 2A), we decided to collapse across emotion to gain power. We found a marginal correlation between RAVLT and DG/ CA3 FA activity (Pearson’s r = −0.375, p = 0.054; Fig. 3A), where higher RAVLT performance was correlated with less DG/CA3 activity during false recognition. We expected to see a correlation with BLA as well, however, RAVLT performance was not significantly correlated with BLA activity during false recognition and only trended toward significance (Pearson’s r = 0.322, p = 0.102). We hypothesized that there might be a more complex interaction between the variables, such that the effect of BLA on DG/CA3 activity may depend on individual differences in RAVLT performance during false recognition. To test this hypothesis, we conducted a moderation analysis using hierarchical linear regression (PROCESS module in SPSS, Hayes, 2013) to determine if the influence of BLA activity on DG/CA3 activity during false recognition depends on RAVLT performance. The 2 predictors (RAVLT and BLA activity) were first entered into the regression analysis to determine each predictor’s effect DG/CA3 activity and then the interaction term was added. Results indicated that RAVLT performance (b = −0.038, p = 0.003) and BLA activity during false recognition (b = 2.01, p = 0.014) were both associated with DG/CA3 activity and accounted for a significant amount of variance in DG/CA3 activity (R2 = 0.346, F [3,23] = 4.05, p = 0.02). The interaction between BLA activity and RAVLT performance explained a significant increase in variance in DG/CA3 activity (ΔR2 = 0.178, F[1,23] = 6.26, p = 0.02; Fig. 3B). Thus, the influence of BLA activity on DG/CA3 activity during false recognition depends on memory performance as quantified by the RAVLT-delayed recall component.
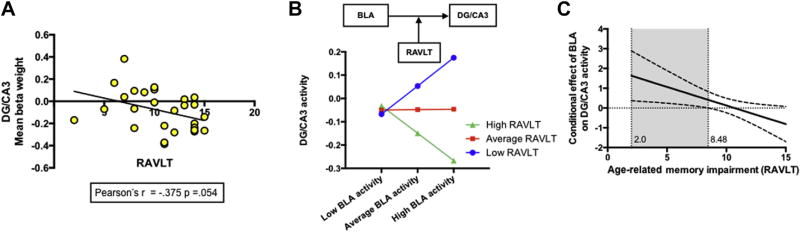
Fig. 3.
Relationship between BLA and DG/CA3 activity during false recognition is moderated by individual differences in memory performance (A) negative correlation between the delayed Rey Auditory Verbal Learning Test (RAVLT) and mean beta weight in DG/CA3 during false recognition, (B) visualization of moderation analysis showing the influence of the BLA on DG/CA3 is moderated by age-related memory impairment such that low RAVLT scores were associated with high BLA activity, but low DG/CA3 activity, (C) visualization of the specificity of the conditional effect of BLA on DG/CA3 activity, showing that the confidence band is above 0 at less than or equal to 8.48 on the RAVLT (shaded gray). Abbreviations: BLA, basolateral amygdala; DG, dentate gyrus.
The unstandardized simple slopes were tested for low (−1 standard deviation [SD] below the mean), moderate (mean), and high (+1 SD above the mean) levels of the RAVLT (Fig. 3B). Different patterns in the slope of the regression line with varying levels of RAVLT performance show that the relation between BLA and DG/CA3 activity is unique and depends on the level of RAVLT performance. Results suggest that the conditional effect of BLA activity on DG/CA3 activity was present for low RAVLT scores (1 SD below the mean; b = 0.648, p = 0.02; Fig. 3B) but not for moderate or high RAVLT scores (p’s > 0.05). To further characterize the nature of this interaction, we used the Johnson-Neyman technique. This technique identifies points in the range of the moderator variable where the effect of the predictor on the outcome transitions from being statistically significant to nonsignificant (Hayes, 2013). We found that when the RAVLT score was less than or equal to 8.48, higher BLA activity led to higher DG/CA3 activity during false recognition (region of significance: RAVLT ≤ 8.48, Fig. 3C). Fig. 3C shows a plot of the conditional effect of BLA on DG/CA3 activity during false recognition as a function of RAVLT performance with confidence bands. The region of significance is depicted as the values of the RAVLT corresponding to points where a conditional effect of 0 is outside of the confidence band. As can been seen, when the RAVLT is 8.48 or less, the confidence bands are entirely above zero (shaded gray). Thus, individual differences in memory abilities moderate the impact of the BLA on DG/CA3 activity, and this conditional relationship generally manifested in those with the lowest memory scores.
4. Discussion
While it has been repeatedly shown that older adults exhibit memory impairments in aging, not all memories are treated equally. Some information may be preferentially processed due to the emotional significance of the experience. Emotionally arousing events are typically better remembered than neutral events; however, there have been mixed findings of the impact of emotion in aging. The present study makes a contribution in improving our understanding of the complex impact of emotion on memory in the context of aging.
Age-related variability in memory performance has been shown in both rats and humans (Gallagher et al., 2006; Hilborn et al., 2009; Robitsek et al., 2008). Gallagher et al. have examined this increased variability in memory performance by dichotomizing older rats into 2 groups (Gallagher et al., 1993). One group of older rats performs on par with young rats on the Morris water maze, whereas another group performs below young rat performance. They have found that these 2 groups show differences in medial temporal lobe functioning, where age-impaired rats show synaptic loss in the perforant path (Smith et al., 2000), loss of inhibitory tone (Spiegel et al., 2013), hyperexcitability in the CA3 subregion of the hippocampus (Wilson et al., 2004, 2005), as well as loss of reelin and increased phosphorylated tau expression in the LEC (Stranahan et al., 2011). Previous studies have shown that LP older adults show deficits in the ability to discriminate highly similar experiences (Holden et al., 2013; Reagh et al., 2014a; Stark et al., 2010). Age-related individual differences in memory performance have been associated with perforant path loss (Yassa et al., 2010), hyperactivation in the dentate/CA3 network (Yassa et al., 2011a), loss of entorhinal-hippocampal functional connectivity (Yassa et al., 2011b), and reduced dendritic integrity in the DG/CA3 region (Yassa et al., 2011b). These cross-species findings suggest that this approach is fruitful and validated by neurobiological evidence in understanding individual differences in aging.
In the present study, we used an emotional mnemonic discrimination task that is sensitive to emotional versus neutral memory processing via the amygdala-hippocampal network. Based on the entire sample of older adults, the data suggest that the emotional pattern separation signal is selective to the DG/CA3 subregion of the hippocampus, with the BLA expressing an emotional false recognition signal. This is consistent with a role for the DG/CA3 in driving discrimination responses that may be dependent on pattern separation and a role for the BLA in driving overgeneralized responses that may be dependent on pattern completion.
Interestingly, the LEC expressed a false recognition signal that was also modulated by emotion but was in the opposite direction from the BLA (increased activity during neutral compared with negative stimuli). Furthermore, the LEC also expressed a discrimination signal that was modulated by emotion similar to DG/CA3. Increases and decreases in BOLD activity should not be evidence of increased and decreased involvement of a region in a particular computation per se (e.g., decreased BOLD activity could be due to increased precision). Furthermore, the mean beta weights are negative or positive with respect to the novel foils, thus the directionality of individual conditions may not be meaningful. Our comparisons of interest contrast 2 conditions against each other (e.g., negative vs. neutral, CRs vs. FAs), which eliminate the effect of novel foils. In other words, we contrast “CR minus novel foils” versus “FA minus novel foils”, where novel foils cancel each other out and we are left with a comparison of CR versus FA. The role of the LEC has not been previously examined in detail in emotional tasks; however, it is an anatomical gateway by which input from the BLA reaches the DG/CA3, thus, its involvement in this task showing a mixture of signals similar to BLA and DG/CA3 is not surprising. Importantly, the effects noted above tended to manifest only in the regions of interest and not in our a priori control regions (MEC and CEA/CORT). Based on these results, we conclude that the DG/CA3 tends to be involved in discrimination of emotional information in old and young adults (Leal et al., 2014a), whereas false recognition of emotional information tends to involve the BLA and LEC. Further, we find that these processes are altered in some but not all older adults.
LP older adults exhibited deficits in emotional pattern separation, whereas HP older adults maintained this capability. Furthermore, the BLA-LEC-DG/CA3 pathway was selectively altered in LP older adults, providing further evidence of dysfunctional amygdala-hippocampal connectivity through a key hippocampal input pathway involved in pattern separation. The hippocampus receives input from the entorhinal cortex via the perforant path, which is degraded in aging (Barnes, 1979; Barnes et al., 2000; Smith et al., 2000; Yassa et al., 2010). Evidence from electrophysiological studies suggests that plasticity in the lateral perforant path to the dentate gyrus is compromised with age (Froc et al., 2003). The LEC also expresses increases in phosphorylated tau, decreased reelin expression, and decreased synaptophysin expression in aged-impaired outbred rats (Stranahan et al., 2011). Taken together, these data are consistent with the apparent deficits in BLA-LEC-DG/ CA3 connectivity observed here. Interestingly, deficits in this pathway occur in the absence of deficits in the other 2 pathways we examined, the direct BLA-CA1 pathway as well as the intra-hippocampal pathway from DG/CA3 to CA1. It is possible that the intact communications among those pathways accounts for the absence of behavioral performance differences between the LP and HP individuals.
Our findings of altered emotional pattern separation in aging are a significant addition to what is known about pattern separation impairment in aging and further characterize MTL dysfunction in aging. We find that (1) emotional pattern separation is preserved in HP older adults, similar to findings in young adults; (2) emotional pattern separation is impaired in LP older adults; and (3) decreased connectivity in the BLA-LEC-DG/CA3 indirect pathway is specific to LP older adults. Previous work from our laboratory has suggested that LP older adults are more likely to show degradation in perforant path integrity (Yassa et al., 2010), DG/CA3 representational rigidity (i.e., failure of pattern separation; Yassa et al., 2011b) as well as DG/CA3 hyperactivity (Yassa et al., 2010) when discriminating similar object stimuli compared with their healthy young counterparts, who show a pattern separation signal that is nonlinear across stimulus interference bins (Yassa et al., 2011a). In the present work, we complement these findings by demonstrating that emotional pattern separation in the DG/CA3 is impaired in LP older adults and that the indirect pathway from BLA to LEC and from LEC to DG/CA3 appears to be compromised in the same individuals. The latter could be stemming at least in part from the previously reported deficits in perforant path integrity but additionally implicates connectivity with the BLA.
Previous studies in rodents found elevated firing rates in hippocampal CA3 (Wilson et al., 2005) and human studies have also found evidence of hyperactivity in hippocampal DG/CA3 which predicted memory impairment (Yassa et al., 2011a). Furthermore, interventions reducing the hyperactivity in older rats (Koh et al., 2010), Alzheimer’s transgenic mice (Sanchez et al., 2012), as well as humans with amnestic mild cognitive impairment (Bakker et al., 2012), have all shown evidence of cognitive rescue, suggesting that such hyperactivity is dysfunctional rather than compensatory. In human clinical work, the reduced hyperactivity was associated with reduced rate of false recognition of similar items on a pattern separation task (Bakker et al., 2015). Here, we see evidence of hyperactivity in DG/CA3, that is, selective to false recognition and limited only to LP older adults. This is consistent with the notion that hyperactivity may be an index of dysfunction and an indicator of age-related cognitive decline. Moreover, the hyperactivity here was not specific to negative or neutral FAs, suggesting a broad overgeneralization of information during false recognition. Our findings add 3 major contributions: (1) the effect seems to be generalized such that hyperactivity exists for negative and neutral information; (2) hyperactivity is only evident in those with LP older adults; and (3) hyperconnectivity between MTL regions occurs only during negative false recognition, consistent with faulty overgeneralization. This further supports the notion that DG/CA3 hyperactivity in aging is a dysfunctional condition.
In the neuropsychological battery, we note significant differences between the HP and LP groups on the Stroop Word-Color interference task as well as the Trail Making Test B, both of which measure executive function and mental processing speed. Differences on these measures may signify frontal lobe alterations in LP older adults. Due to the nature of our high-resolution scan protocols, the frontal lobes were beyond our field of view; however, future studies with whole brain fMRI (perhaps using our same task) should examine alterations frontal lobe alterations, as well as connectivity between the MTL and the prefrontal cortex. A particular limitation of the study is the absence of characterization of AD pathology. It is possible that LP individuals are more likely to be those harboring AD pathology and thus more likely to exhibit cognitive decline in the future; however, this could not be tested in the present study. This is an ongoing topic of interest in the field (Jagust, 2013), and we hope that our findings and our task may enable additional research to address these relationships.
Another significant limitation of the present study is that there is no direct comparison to young adults, so it is difficult to make claims about age-related impairment relative to young adults. This makes it difficult to distinguish weather the HP older adult group performs similarly to young adults and truly have no age-related memory alterations or if they may also show age-related deficits but potentially not as great as the LP group. It also raises the question of whether individual variability in memory performance in young adults would also be associated with neural alterations such as those observed in this study. In past work, we have observed that the range of performance on RAVLT in young adults is much more restricted which suggests either a lack of significant individual variability in memory performance or an insensitivity of the RAVLT as an assay of memory function in young adults. We suggest that future studies should attempt to identify assays that are capable of detecting individual differences in young adults to address this question.
Furthermore, we excluded analysis of the positive stimuli for the present study, as positive stimuli were not as arousing as negative stimuli. However, a positivity effect in aging has been shown, in which older adults remember more positive information compared with negative or neutral information (Mather and Carstensen, 2005). Future studies that attempt to match arousal levels of negative and positive stimuli could be more informative in determining how positive stimuli are processed by the MTL in aging.
It is also worth noting that the current behavioral results (reduced emotional compared with neutral discrimination) were similar to previous findings in young adults showing reduced emotional versus neutral discrimination (Leal et al., 2014a, b). However, previous behavioral data in a different sample of older adults in our laboratory suggested that emotional discrimination may be preserved in aging (Leal and Yassa, 2014). It is possible that performance inside the scanner and out of the scanner may be slightly different. For example, it is possible that older adults tested during scanning might exhibit some additional arousal due to scanner anxiety that allows for their performance on the emotional task to look like that of young adults (norepinephrine production is reduced with age; Gutchess and Park, 2006; Leal and Yassa, 2015). Absent this arousal in the scanner, it is possible that older adults do not exhibit the arousal-mediated emotional tradeoff. It is also possible that there is a cohort or geographical effect on sampling, as the previous sample was collected in Baltimore City while at the Johns Hopkins University, whereas the current sample was collected in Orange County while at the University of California, Irvine. There was also an age difference where the mean age for the UCI group (mean age = 72.2, SD = 7.6) was higher than the JHU group (mean age = 66.7, SD = 4.3). Future comparisons with independent samples will be necessary to determine how emotional discrimination behavior changes with age under different arousal conditions. Analyses were conducted during specific behavioral conditions (CRs or FAs), which allows us to make inferences directly about the computational processes engaged during those conditions and prevents the analyses from being biased by participant performance. The fact that there were no behavioral differences in discrimination or recognition of lure items, while somewhat surprising, given past reported behavioral results, allows for cleaner analyses that are not affected by group-level performance differences. In addition, our sample sizes were relatively small in the present study as well as in the previous article from JHU, so it is possible that the behavioral effects are variable such that the effects can be significant in one case and not another.
5. Conclusions
Our results shed light on the neural mechanisms underlying the process of discriminating emotional memories in older adults and identify important individual variability in this neurocognitive construct with age. Using a novel paradigm, coupled with state-of-the-art high-resolution imaging techniques, we are able to identify several biomarkers for these memory alterations that can be used to further examine emotional and mnemonic processing during aging.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 MH102392, R21 AG049220, and P50 AG16573 as well as NIA training grant AG027668 support to Stephanie L. Leal (PI: M. Albert). The authors would like to thank the study participants, Amanda Chun for assistance with recruitment and study procedures, and Jared Roberts and Zachariah Reagh for assistance with imaging analysis and statistical procedures.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neurobiolaging.2016.08.018.
References
- Agster KL, Tomás Pereira I, Saddoris MP, Burwell RD. Subcortical connections of the perirhinal, postrhinal, and entorhinal cortices of the rat. II. efferents. Hippocampus. 2016;26:1213–1230. doi: 10.1002/hipo.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaguer W, Estupiñán B, Uwe Frey J, Bergado JA. Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiol. Aging. 2002;23:319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science (New York, N.Y.) 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophys-iological and behavioral study in the rat. J. Comp. Physiol. Psych. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path—granule cell synapse. Neurobiol. Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, Witter MP. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Entis JJ, Doerga P, Barrett LF, Dickerson BC. A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. NeuroImage. 2012;60:1226–1235. doi: 10.1016/j.neuroimage.2011.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez-González A, Kulisevsky J, Boltes A, Otermín P, García-Sánchez C. Rey verbal learning test is a useful tool for differential diagnosis in the pre-clinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int. J. Geriatr. Psychiatry. 2003;18:1021–1028. doi: 10.1002/gps.1010. [DOI] [PubMed] [Google Scholar]
- Foster TC, DeFazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Front. Aging Neurosci. 2012;4:12. doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froc DJ, Eadie B, Li AM, Wodtke K, Tse M, Christie BR. Reduced synaptic plasticity in the lateral perforant path input to the dentate gyrus of aged C57BL/6 mice. J. Neurophysiol. 2003;90:32–38. doi: 10.1152/jn.00105.2003. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age (Dordrecht, Netherlands) 2006;28:221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Park DC. fMRI environment can impair memory performance in young and elderly adults. Brain Res. 2006;1009:133–140. doi: 10.1016/j.brainres.2006.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. Guilford Press; New York, NY: 2013. pp. 1–507. [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, Hunter MA. Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. J. Clin. Exp. Neuropsychol. 2009;31:412–424. doi: 10.1080/13803390802232659. [DOI] [PubMed] [Google Scholar]
- Holden HM, Gilbert PE. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front. Aging Neurosci. 2012;4:9. doi: 10.3389/fnagi.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden HM, Toner C, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation varies in older adults. Learn. Mem. 2013;20:358–362. doi: 10.1101/lm.030171.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Review vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 2013;77:219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT, Raunig D, Jedynak CP, Caffo B, Prince JL. A computational neurodegenerative disease progression score: method and results with the Alzheimer’s disease neuroimaging initiative cohort. NeuroImage. 2012;63:1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2007;62:P208–P215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanis P, Zornetzer SF. Age-related behavioral and neurobiological changes: a review with an emphasis on memory. Behav. Neural Biol. 1981;31:115–172. doi: 10.1016/s0163-1047(81)91195-x. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Jones CK, Yassa MA. Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus. 2014a;24:1146–1155. doi: 10.1002/hipo.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Yassa MA. Asymmetric effects of emotion on mnemonic interference. Neurobiol. Learn. Mem. 2014b;111:41–48. doi: 10.1016/j.nlm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Yassa MA. Effects of aging on mnemonic discrimination of emotional information. Behav. Neurosci. 2014;128:539–547. doi: 10.1037/bne0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Yassa MA. Neurocognitive aging and the Hippocampus across species. Trends Neurosci. 2015;38:200–812. doi: 10.1016/j.tins.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17:868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee IA, Preacher KJ. [Accessed April 11, 2016];Calculation for the test of the difference between two dependent correlations with one variable in common. 2013 Available at: http://quantpsy.org/corrtest/corrtest2.htm.
- Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn. Sci. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McDonald A, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan H-Y, Callicott JH, Goldberg TE, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. J. Cogn. Neurosci. 2009;21:1920–1933. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res. Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, Yassa MA. Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus. 2015;26:417–422. doi: 10.1002/hipo.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Roberts JM, Ly M, Diprospero N, Murray E, Yassa MA. Spatial discrimination deficits as a function of mnemonic interference in aged adults with and without memory impairment. Hippocampus. 2014a;24:303–314. doi: 10.1002/hipo.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Watabe J, Ly M, Murray E, Yassa MA. Dissociated signals in human dentate gyrus and CA3 predict different facets of recognition memory. J. Neurosci. 2014b;34:13301–13313. doi: 10.1523/JNEUROSCI.2779-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Yassa MA. Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4264–E4273. doi: 10.1073/pnas.1411250111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, Ly M, Murray E, Yassa MA. Temporal discrimination deficits as a function of lag interference in older adults. Hippocampus. 2014;24:1189–1196. doi: 10.1002/hipo.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J. Neurosci. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J. Comp. Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013;51:2442–2449. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn. Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DB, Martinez JL, Gold PE, McGaugh JL. Age-related memory deficits in rats and mice: enhancement with peripheral injections of epinephrine. Behav. Neural Biol. 1985;44:213–220. doi: 10.1016/s0163-1047(85)90212-2. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychol. Sci. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Haberman RP, Gallagher M. Cognitive decline is associated with reduced reelin expression in the entorhinal cortex of aged rats. Cereb. Cortex. 2011;21:392–400. doi: 10.1093/cercor/bhq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino JC, Pirogovsky E, Luu T, Toner CK, Gilbert PE. The effect of interference on temporal order memory for random and fixed sequences in nondemented older adults. Learn. Mem. 2012;19:251–255. doi: 10.1101/lm.026062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn. Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Cognitive aging and the hippocampus: how old rats represent new environments. J. Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Phelps EA. The Human Amygdala. Guilford Press; New York, NY: 2009. [Google Scholar]
- Yassa MA, Muftuler LT, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. PNAS. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. PNAS. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.