Abstract
Adherence to recommendations for monitoring of metabolic side effects of antipsychotic medications has been historically low. This randomized controlled trial tested whether a computerized, patient-centered intervention that educated Veterans with serious mental illness about these side effects and encouraged them to advocate for receipt of monitoring would increase rates of monitoring compared to enhanced treatment as usual. The mean proportion of days adherent to monitoring guidelines over the one-year study was similarly high and did not differ between the intervention (range: 0.81 – 0.98) and comparison (range: 0.76 – 0.96) groups. Many individuals in both groups had persistent abnormal metabolic parameter values despite high rates of monitoring, contact with medical providers, and receipt of cardiometabolic medications. Participants exposed to the intervention were interested in receiving personalized information about their cardiometabolic status, demonstrating the preliminary feasibility of brief interventions for enhancing involvement of individuals with serious mental illness in health care decision making.
Keywords: antipsychotic medications, metabolic side effects, monitoring, randomized controlled trial
Introduction
Individuals with serious mental illness experience higher rates of morbidity and premature mortality than the general population (Colton and Manderscheid 2006; Angst et al. 2002; Saha et al. 2007), which has been shown to be largely attributable to the increased prevalence of cardiovascular disease (Brown et al. 2000; Osby et al. 2000) conferred by high rates of overweight and obesity (Allison et al. 2009; Kilbourne et al. 2009; Morden et al. 2012), Type 2 diabetes (Dixon et al. 2000), and metabolic syndrome (McEvoy et al. 2005; Mitchell et al. 2013). While these medical conditions may be linked to lifestyle-related behaviors including physical inactivity (Brown et al. 1999), poor nutrition (Brown et al. 1999), and high rates of cigarette smoking (Dixon et al. 2007), several lines of evidence suggest that weight gain and other metabolic side effects including glucose dysregulation and lipid abnormalities associated with antipsychotic treatment may also increase cardiovascular risk in these individuals (Lieberman et al. 2005; Newcomer et al. 2007; Rummel-Kluge et al. 2010).
Despite the proliferation of clinical guidelines that recommend regular monitoring of weight and other metabolic parameters in all patients prescribed antipsychotic medications (American Diabetes Association 2004; Marder et al. 2004), a review of 48 studies in five countries showed adherence to these recommendations to be low (Mitchell et al. 2012), going above 50% only for monitoring of blood pressure (70%) and triglycerides (60%) before the guidelines were released. After the guidelines were published, monitoring for glucose (56%) and lipids (29%), in particular, remained suboptimal (Mitchell et al. 2012). In the Department of Veterans Affairs (VA) healthcare system, physical and mental health services are largely integrated, laboratory services are mostly available on-site, test results are accessible by all health providers via the electronic medical record, and recommendations for metabolic monitoring are included in VA-specific clinical guidelines; yet, rates of monitoring still fall short. For example, a recent study in 32 VA facilities showed that while 67% of Veterans had their weight measured within 30 days of receipt of a new antipsychotic prescription, only 50% received follow-up monitoring 60–120 days later. Provision of baseline and follow-up monitoring was much lower for glucose/hemoglobin A1c (46% and 27%) and LDL cholesterol (32% and 16%) (Mittal et al. 2013). Efforts to improve monitoring of metabolic side effects of antipsychotic medications that go beyond passive dissemination of guidelines, which has been shown to be ineffective in changing physician behavior more generally (Giguere et al. 2012), are clearly needed.
The burgeoning recovery movement transforming mental health services in the U.S. emphasizes consumer and family involvement in mental health care (New Freedom Commission on Mental Health. 2003), and the Institute of Medicine’s report, “Crossing the Quality Chasm” included patient-centeredness as an essential component of quality care (Institute of Medicine. 2001). Two aspects of patient-centered care have been linked to positive health outcomes. The first involves development of knowledge and skills for self-management of chronic illnesses that facilitate patients’ active participation in their own care (Mead and Bower 2000). Evidence suggests that efforts to incorporate patients’ perspectives and to encourage greater involvement in care (patient activation) results in greater adherence to treatment regimens, more effective disease self-management, better disease control, and greater patient satisfaction (Stewart et al. 2000). The nature of the clinician-patient relationship or therapeutic alliance is a second key dimension of patient-centered care (Stewart et al. 2014). Many studies document the role of patient-physician communication in improving patient outcomes, with patient-centered communication having positive effects on satisfaction, adherence to recommended treatment, and health status (Stewart 1995).
A number of programs to improve monitoring for the metabolic adverse effects of antipsychotic medications that go beyond passive dissemination of guidelines have been described (Barnes et al. 2008; Schneiderhan et al. 2009; Nicol et al. 2011; Thompson et al. 2011; DelMonte et al. 2012; Ramanuj 2013; Velligan et al. 2013). These programs included interventions for clinicians or clinics comprised of one, and often multiple, components including educational sessions (Barnes et al. 2008; Nicol et al. 2011; Thompson et al. 2011; Ramanuj 2013; Velligan et al. 2013), posting of printed educational materials (Barnes et al. 2008; Thompson et al. 2011; Ramanuj 2013), audit and feedback on monitoring practices (Barnes et al. 2008; Nicol et al. 2011; Ramanuj 2013), paper reminders about monitoring placed in medical charts (Nicol et al. 2011; Thompson et al. 2011), computerized reminders about monitoring at the time of antipsychotic prescribing (DelMonte et al. 2012), and provision of implementation tools (e.g., monitoring equipment) (Thompson et al. 2011) and other delivery system and procedural interventions (e.g., hiring of a medical assistant charged with ensuring labs were drawn and results presented on a metabolic tracking form, implementation of a pharmacist or nurse-led metabolic monitoring clinic) (Schneiderhan et al. 2009; Velligan et al 2013). Although shown to be effective in increasing rates of metabolic monitoring, these interventions consisted predominately of quality improvement programs evaluated with non-randomized designs in small samples, only some of which included comparison groups (Nicol et al. 2011; DelMonte et al. 2012; Velligan et al. 2013). In addition, none of these interventions targeted individuals with serious mental illness as potential agents of change in improving rates of metabolic monitoring within a patient-centered care framework. The objective of the current study was to conduct a randomized controlled trial of a computerized, patient-centered intervention aimed at educating Veterans with serious mental illness about the metabolic side effects of antipsychotics and encouraging them to advocate for receipt of guideline-recommended side effect monitoring. It was hypothesized that rates of metabolic monitoring would increase in Veterans exposed to the intervention relative to a comparison condition. It was further hypothesized that exposure to the intervention would lead to follow-up metabolic monitoring and receipt of medical services in Veterans identified as having abnormal metabolic parameter values.
Methods
Setting and Design
The study was a randomized controlled trial conducted at two VA outpatient mental health clinics in the U.S. Mid-Atlantic region, one serving a predominately metropolitan area and its surrounding suburbs and the other serving a relatively rural area. Veteran and prescriber participants provided written informed consent and were enrolled in the study between March 2010 and October 2011. The Institutional Review Board of the University of Maryland School of Medicine approved the study.
Participants
Veteran-participants were eligible for the study if they were 18 to 70 years of age, diagnosed with a psychotic disorder (schizophrenia, schizoaffective disorder, psychosis disorder not otherwise specified (NOS)), bipolar disorder, major depression, or post-traumatic stress disorder (PTSD), were currently prescribed one or more oral or injectable second-generation antipsychotic (SGA) medications available at baseline (aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone) by a psychiatrist or nurse practitioner (NP), had at least two outpatient visits with the prescribing psychiatrist/NP in the past year, were deemed by the prescriber to be clinically stable to participate in the study, and had at least a fourth grade reading level. Additionally, Veterans were only eligible if they and their prescribers both agreed to have one research visit audio taped for the evaluation of patterns of patient-clinician communication around screening for metabolic side effects. Veterans with diagnoses of dementia or other organic brain syndrome or traumatic brain injury were excluded from participating.
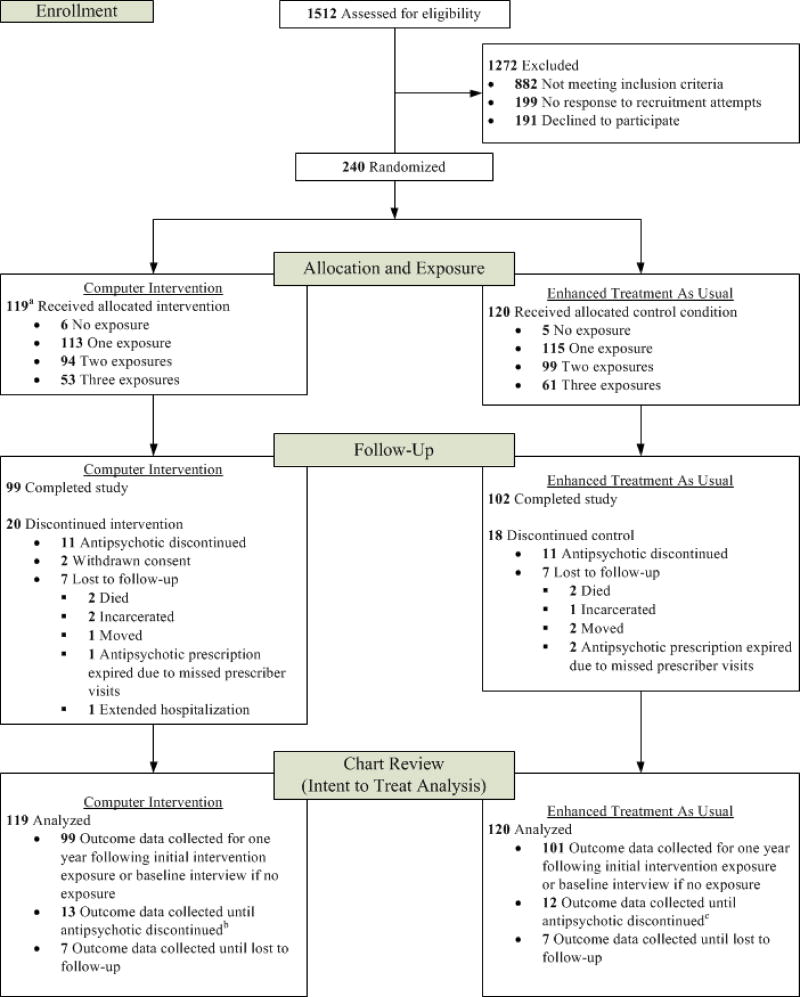
Figure 1 shows the screening and enrollment process as specified by the Consolidated Standards of Reporting Trials guidelines (Schulz et al. 2010). Among 1512 Veterans screened for eligibility, 630 (42%) met inclusion criteria and were approached for participation in the mental health clinic or via recruitment letters mailed to their homes. A total of 431 (68%) responded to a letter or were contacted in the mental health clinic; compared to those who could not be reached (n=199), those who responded were significantly older (55 ± 9 vs. 53 ± 11 years of age; p=0.013) but did not differ by gender (p=0.11).
Figure 1. Flow of Study Participants.
a I participant did not receive intervention (received control in error)
b The antipsychotic was discontinued for two of these participants between the end of their study participation and the chart review end date
c The antipsychotic was discontinued for one of these participants between the end of their study participation and the chart review end date
Among those who responded to the letter, 191 refused to participate in the study or attempts to schedule an enrollment interview were unsuccessful. A total of 240 (56%) provided written informed consent after receiving a complete study description. Veterans who consented to participate were significantly younger (54 ± 8 years) than those who refused to participate or were unreachable (56 ± 9 years) (p=0.023) but did not differ by gender (p=0.38). Reasons for refusing to participate included not being interested (81%), not having time (6%), not being comfortable (2%), and other reasons (11%) such as lack of transportation, distance, and medical issues. One participant randomized to the intervention condition was administratively withdrawn from the study and analyses due to inadvertent exposure to the control condition, leading to a final sample size of 239. All 21 prescribers (13 psychiatrists, 8 NPs) who were approached for the study agreed to participate and provided written informed consent. On average, each prescriber had 11 patients participating in the study (range 3–21).
Randomization
To balance the effect of prescribers on outcomes, the study sample was stratified by prescriber (21 levels). Random allocation to the two conditions was in blocks of size two or four within prescriber with block size determined randomly. Further stratification by more variables would have led to sparse strata.
Description of intervention
The intervention for this study was an educational program on the metabolic side effects of antipsychotic medications and the recommended frequency of monitoring presented to the participant on a touch screen laptop computer immediately prior to a visit with the psychiatrist or NP prescribing their antipsychotic medication. The computer intervention contained several attributes consistent with the principles of patient-centered care. First, it educated participants about six metabolic parameters potentially affected by antipsychotic medications (weight, blood pressure, blood sugar, LDL cholesterol, HDL cholesterol, and triglycerides) and the potential health impact of aberrant values using information derived from widely available patient education materials from the American Diabetes Association and the American College of Cardiology.
Second, the program provided the participant with personalized feedback on the extent to which their care adhered to metabolic monitoring recommendations (American Diabetes Association 2004; Marder et al. 2004). In this study, we evaluated whether monitoring of weight and blood pressure occurred every 3 months, whether monitoring of fasting blood glucose or glycosylated hemoglobin (HbA1c) occurred once yearly, and whether a lipid panel (LDL cholesterol, HDL cholesterol, triglycerides) was drawn every 2 years, consistent with expert recommendation for frequency of metabolic monitoring. Each participant’s monitoring history was obtained from the electronic medical record and the most recent date of monitoring (if any) ordered by any VA clinician for each parameter was inputted into the computer program before each antipsychotic prescriber visit.
For those individuals who had received metabolic monitoring at the recommended interval, the program displayed the results of the most recent test along with a brief interpretation of the results. For those who had both fasting blood glucose and HbA1c measured on the (same) most recent date of monitoring, the HbA1c value was displayed and interpreted. Abnormal values for each metabolic parameter are described in the footnote for Table 1; consistent with clinical guidelines, abnormal values for blood pressure, fasting blood glucose/HbA1c, and LDL cholesterol differed for participants with vs. without diabetes, which was ascertained prior to their first exposure to the intervention.
Table 1.
Baseline Characteristics of Study Participants in the Computer Intervention and Enhanced Treatment as Usual (ETAU) Groups
| Total (N=239) | Computer Intervention (N=119) | ETAU (N=120) | ||||
|---|---|---|---|---|---|---|
| Mean±SD /n | % | Mean±SD /n | % | Mean±SD /n | % | |
| Demographic Characteristicsa | ||||||
| Age | 54.3 ± 8.3 | 55.1 ± 8.0 | 53.5 ± 8.6 | |||
| Male | 213 | 89 | 107 | 90 | 106 | 88 |
| White race | 113 | 47 | 59 | 50 | 54 | 45 |
| Currently married | 61 | 26 | 24 | 20 | 37 | 31 |
| Some college or higher education | 134 | 56 | 64 | 54 | 70 | 58 |
| Currently work for pay (part or full time) | 49 | 21 | 28 | 24 | 21 | 18 |
| Mental health characteristics/services utilization | ||||||
| Diagnosisa | ||||||
| Schizophrenia/schizoaffective disorder/unspecified psychosis/psychosis disorder NOS | 72 | 30 | 36 | 30 | 36 | 30 |
| Bipolar disorder | 76 | 32 | 36 | 30 | 40 | 33 |
| Major depression | 63 | 26 | 33 | 28 | 30 | 25 |
| Post-traumatic stress disorder | 28 | 12 | 14 | 12 | 14 | 12 |
| Antipsychotic medicationb,d | ||||||
| Aripiprazole | 61 | 26 | 29 | 24 | 32 | 27 |
| Clozapine | 3 | 1 | 3 | 3 | 0 | 0 |
| Olanzapine | 29 | 12 | 12 | 10 | 17 | 14 |
| Quetiapine | 79 | 33 | 39 | 33 | 40 | 33 |
| Risperidone | 70 | 29 | 37 | 31 | 33 | 28 |
| Ziprasidone | 23 | 10 | 11 | 9 | 12 | 10 |
| Psychiatric symptomsa | ||||||
| BASIS-24 overall score | 1.3 ± .7 | 1.3 ± .7 | 1.4 ± .7 | |||
| BASIS-24 emotional liability subscale score | 1.8 ± 1.1 | 1.9 ± 1.1 | 1.8 ± 1.1 | |||
| BASIS-24 psychosis subscale score | 1.0 ± 1.0 | 1.0 ± 1.0 | .9 ± 1.0 | |||
| BASIS-24 substance abuse subscale score | .4 ± .7 | .4 ± .6 | .4 ± .7 | |||
| Number of psychiatrist/nurse practitioner (prescriber) visits, past yearc | 7.4 ± 4.5 | 7.8 ± 5.3 | 7.1 ± 3.5 | |||
| Inpatient psychiatric hospitalization, past yearc | 28 | 12 | 12 | 11 | 15 | 13 |
| Somatic health characteristics/service utilization | ||||||
| Diagnosis of diabetesb | 70 | 29 | 39 | 33 | 31 | 26 |
| Diagnosis of hypertensionb | 148 | 62 | 78 | 66 | 70 | 58 |
| Diagnosis of dyslipidemiab,* | 110 | 46 | 63 | 53 | 47 | 39 |
| Diagnosis of coronary artery diseaseb,** | 28 | 12 | 19 | 16 | 9 | 8 |
| Prescribed diabetes medicationa*** | 42 | 18 | 27 | 23 | 15 | 13 |
| Prescribed blood pressure medicationa | 177 | 74 | 84 | 71 | 93 | 78 |
| Prescribed dyslipidemia medicationa | 119 | 50 | 60 | 50 | 59 | 49 |
| Number of primary care visits, past yearc | 3.3 ± 2.6 | 3.2 ± 2.3 | 3.3 ± 2.9 | |||
| Number of cardiometabolic-related care visits, past yearc | .5 ± 1.8 | .6 ± 1.6 | .4 ± 2.0 | |||
| Inpatient medical hospitalization, past yearc | 33 | 14 | 14 | 12 | 19 | 16 |
| Weight/metabolic parameter valuesb | ||||||
| Body mass index ≥25 (overweight/obese) | 206 | 86 | 99 | 83 | 107 | 89 |
| Elevated fasting blood glucose or glycosylated hemoglobin (HbA1c)e | 118 | 51 | 63 | 55 | 55 | 47 |
| Elevated blood pressuref | 93 | 39 | 45 | 38 | 48 | 40 |
| Elevated LDL cholesterolg | 48 | 22 | 23 | 21 | 25 | 23 |
| Low HDL cholesterolh | 118 | 53 | 62 | 55 | 56 | 50 |
| Elevated triglyceridesi | 84 | 37 | 41 | 37 | 43 | 38 |
Assessed at baseline interview
Diagnoses, prescriptions, and most recent metabolic lab results from chart review were defined as occurring up to 365 days prior to participants’ date of first intervention exposure (or baseline interview date if the participant had no exposure to their intervention).
Frequency counts of service utilization from chart review were defined over the 365-day period prior to the date of first intervention exposure (or baseline if participant had no exposure to their intervention).
Categories not mutually exclusive
Fasting blood sugar (without diabetes: > 100 mg/dL; with diabetes: > 130 mg/dL) and/or HbA1c (without diabetes: > 6%; with diabetes: > 7%)
Without diabetes: systolic blood pressure (bp) ≥ 140 mm Hg and/or diastolic bp ≥ 90 mm Hg; with diabetes: systolic bp ≥ 130 mm Hg and/or diastolic bp ≥ 80 mm Hg
Without diabetes: ≥ 130 mg/dL; with diabetes: ≥ 100 mg/dL
With or without diabetes: men: < 50 mg/dL; women: < 40 mg/dL
With or without diabetes: ≥ 150 mg/dL
X2=4.56, df=1, p=0.033,
X2=4.14, df=1, p=0.042,
X2=4.28, df=1,p=0.039
Third, the program encouraged participants to begin a dialogue with their prescribers about getting monitored or talking about the results of monitoring, regardless of whether they had received the recommended tests or not, and even if their test results were normal. To further activate participants to speak with their prescribers, at the end of the program they were given the option of printing out a summary of their monitoring status and the test results that were reviewed with them in the program. This summary included education about the frequency of recommended monitoring, personalized information on their monitoring status, and a message encouraging them to speak with their prescriber about monitoring. Two copies of the report were provided so that they could keep one and give one to their prescribers in order to facilitate a conversation.
The intervention was developed using web-design principles shown in previous research to reduce cognitive burden and enhance usability in individuals with serious mental illness (Rotondi et al. 2015). The program was audio-assisted so all of the text on the screen was read aloud to the participant. It was delivered on a touch-screen tablet computer so that participants did not need to have experience using a computer or a mouse to navigate through it. The program required very few navigational decisions, except to progress forward or backward through pages, and with audio prompts to advance to the next slide. Each page presented one idea using language that was simple, but explicit, and contained no distracting graphics. To further reduce cognitive load, pages that provided additional educational information about the metabolic side effects and recommended frequency of monitoring were optional only and appeared only one level below the main pages.
Description of comparison condition
Individuals in the comparison condition, enhanced treatment as usual (ETAU), received a printed educational pamphlet, which, like the computer program, provided a brief explanation of each of the metabolic side effects and the recommended frequency of monitoring for each. Unlike the computer program, the pamphlet did not provide participants with any personalized information on their monitoring status or the results of any metabolic monitoring tests.
Study Participation
In both conditions, participants continued to receive their mental health and medical care as usual at the VA. The computer intervention or ETAU was delivered to participants up to 3 times over a one-year exposure period, but no more frequently than every 4 months and only at times that corresponded with regularly scheduled visits with their prescriber. These visits occurred in a private room adjacent to the prescriber’s waiting room immediately prior to the visit with the prescriber. Over the one-year period, 113 (95%) and 115 (96%) participants had at least one exposure, 94 (79%) and 99 (83%) had at least two exposures, and 53 (45%) and 61 (51%) had three exposures to the computer intervention or ETAU, respectively. Six participants in the computer condition (5%) and 5 (4%) in the control condition had no exposures (see Figure 1).
For those in the intervention condition, the computer program compiled a report on participants’ use of the program. This report included the length of viewing for the entire session as well as for each individual page, which enabled us to characterize whether participants viewed any of the optional pages with additional educational information on each side effect. The program also recorded whether the participant chose to print out the optional summary report of their monitoring status and test results at the end of each session. Because of infrequent malfunctions in the system, one or more computer use reports were obtained for 111/113 (98%) participants with at least one exposure to the computer intervention.
Completion of the Study
A total of 38 participants did not complete the study (Figure 1). Twenty-two (11 in each condition) participants became ineligible when their antipsychotic medication was discontinued by their prescriber while they remained in treatment. Ten participants became ineligible because they were no longer participating in outpatient care: 3 (2 intervention, 1 control) were incarcerated,1 had an extended inpatient hospitalization (intervention), 3 moved (1 intervention, 2 control), and 3 (1 intervention, 2 control) missed visits with their prescriber and the antipsychotic prescription expired. Two participants voluntarily withdrew (intervention condition) and 4 died (2 in each study condition). All available data from these participants including that from the chart review up until their antipsychotic prescription was discontinued or expired or until they were lost to follow-up, as described in Figure 1, were used in the intent-to-treat analyses.
Assessments and Measures
After consent was obtained, and prior to randomization, all participants completed a baseline interview, during which demographic characteristics (age, gender, race, marital status, education level, work status) and psychiatric symptom severity were ascertained. Psychiatric symptom severity over the past week was measured by the average score of the 24-item, self-report revised Behavior and Symptom Identification Scale (BASIS-24), which has demonstrated adequate validity within individuals with serious mental illness (Eisen et al. 2004). Responses are rated on a 5-point scale (0 to 4), with higher scores representing greater symptom severity.
Chart reviews were conducted to capture information on dates and results of metabolic monitoring for the six aforementioned metabolic parameters, number of outpatient visits with the antipsychotic prescriber, numbers of outpatient visits with primary care and cardiometabolic-related specialty care providers, numbers of psychiatric and medical hospitalizations, primary psychiatric diagnosis, diagnoses of selected cardiometabolic medical conditions (diabetes, hypertension, dyslipidemia, coronary artery disease), and information on the type and dates of prescribing for all antipsychotic, diabetes, antihypertensive, and cholesterol medications. Outcome variables constructed from the chart review data such as proportion of days adherent to metabolic monitoring guidelines and frequency counts of service utilization were defined over a 365-day ‘observation’ period beginning with the participant’s date of first exposure to the computer intervention or ETAU. Observation periods were anchored to the date of the baseline interview for participants never exposed to the computer intervention or ETAU. For 39 participants, observation periods were truncated to less than 365 days due to their antipsychotic being discontinued/the prescription expiring or they were lost to follow-up (see Figure 1 under “Chart Review”).
Data Analysis
Frequency tables and histograms were used to examine variable distributions and check for potential errors. Participants randomized to the two treatment groups were described and compared on selected baseline variables. Chi-square or Fisher’s exact tests and t-tests were used to test for imbalances in these variables that may have occurred between treatment groups despite randomization. Variables with significant (p<.05) treatment group differences were entered into primary analysis models for covariate adjustment.
To test the first hypothesis that there would be greater adherence to metabolic monitoring guidelines in the computer intervention group versus the ETAU group, the proportion of days adherent to guidelines was calculated for each of the six metabolic parameters (see Description of Intervention for guidelines) for each participant during their observation period. For each metabolic parameter, the mean proportion of days adherent between the two treatment groups was compared using generalized estimating equations (GEE) (Liang and Zeger 1993) with identity link function and Huber-White (Huber 1967) standard errors to account for clustering of observations due to patients having common prescribers. Following the intent-to-treat principle, all randomized participants were included in these analyses (and in the analyses below for the second hypothesis) including participants who were never exposed to their assigned intervention. In sensitivity analyses, the models were re-fit after excluding the 39 individuals whose observation period was less than 365 days after their first exposure to the computer intervention or ETAU.
The second hypothesis specified that participants in the computer intervention group with abnormal metabolic values would be more likely than those in the ETAU group to receive follow-up metabolic monitoring and selected medical services during the observation period. To test this hypothesis, participants with abnormal metabolic parameter values (excluding BMI) that occurred up to one year prior to their first exposure to the computer intervention or ETAU were identified. For those participants with abnormal values, the following 4 variables were defined over their observation period and constructed from the chart review data (as discussed in the Assessments and Measures section): (1) an indicator of whether any follow-up monitoring was conducted; (2) an indicator of whether the result of the most recent follow-up monitoring was abnormal; (3) an indicator of whether the participant had any primary care or cardiometabolic-related outpatient visits; and (4) the number of primary care or cardiometabolic-related outpatient visits. Logistic regression was used to compare the treatment groups on the first three indicators, and negative binomial regression was used for the fourth indicator to account for extra-Poisson variance in this count variable. These analyses were performed on only subsets of the full sample who had metabolic monitoring prior to their first exposure to the intervention/ETAU recorded and whose values were abnormal, resulting in smaller sample sizes that precluded the use of GEE. Instead ordinary generalized linear models were used. In sensitivity analyses, the models were re-fit after excluding the 39 individuals who had less than 365 days of observation after their first exposure to the computer intervention or ETAU.
Results
Patient characteristics of the total sample and by treatment group are provided in Table 1. Overall, the sample had a mean age of 54.3 years, was 89% male, and 47% white. Primary psychiatric diagnoses were: 30% with schizophrenia/schizoaffective disorder/other psychosis, 32% with bipolar disorder, 26% with major depression, and 12% with post-traumatic stress disorder (PTSD). The percentage of participants prescribed the six SGAs available at baseline ranged from 1% for clozapine to 33% for quetiapine (some were prescribed more than one SGA and some received a first-generation antipsychotic concurrently). Participants averaged 7 visits with their prescribers over the past year and had relatively low levels of psychiatric symptoms, with only 12% having had an inpatient psychiatric hospitalization.
Participants averaged 3 primary care visits over the prior year. Many had diagnoses of diabetes (29%), hypertension (62%), and dyslipidemia (46%) and were prescribed medications for these conditions. Treatment groups differed on the percentage with dyslipidemia (p=.033), coronary artery disease (p=.042), and prescribed a diabetes medication (p=.039), all higher in the computer intervention group. These variables were therefore adjusted in all regression analyses comparing the two treatment groups.
Table 2 presents the mean proportion of days adherent to monitoring guidelines for the six metabolic parameters in the total sample and each of the treatment groups. The mean proportion of days adherent in the total sample was high, ranging from .94 to .97 for LDL and HDL cholesterol, triglycerides, and blood glucose/HbA1c. Mean proportion of days adherent was somewhat lower for weight (.79) and blood pressure (.81) monitoring. No significant differences were found between the two treatment groups in the proportion of days adherent to monitoring guidelines for any of the six metabolic parameters. After excluding the 39 individuals without the full 365-day observation period, the results were unchanged. In addition, there was no effect of the intervention (all p’s ranging from 0.448–0.640) in the subsample of 50 individuals without co-occurring diagnoses of Type 2 diabetes, hypertension, or dyslipidemia for which monitoring of the same metabolic parameters that were the target of the intervention is routinely completed.
Table 2.
Mean Proportion of Days Adherent to Monitoring Guidelines for Six Metabolic Parameters
| Total (N=239) | Computer Intervention (N=119) | ETAU (N=120) | |
|---|---|---|---|
| Mean ± SD | Mean ± SDa | Mean ± SDa | |
| METABOLIC PARAMETER | |||
| Weight | .787 ± .245 | .814 ± .225 | .761 ± .262 |
| Blood Glucose/HbA1c | .970 ± .129 | .977 ± .107 | .962 ± .148 |
| Blood Pressure | .810 ± .249 | .835 ± .218 | .785 ± .275 |
| LDL Cholesterol | .944 ± .157 | .937 ± .170 | .951 ± .143 |
| HDL Cholesterol | .947 ± .155 | .941 ± .166 | .952 ± .143 |
| Triglycerides | .943 ± .159 | .941 ± .166 | .945 ± .151 |
In analyses adjusted for diagnosis of dyslipidemia, diagnosis of coronary artery disease, and prescription of diabetes medication, there were no significant difference between the groups.
Substantial numbers of participants had abnormal values for each of the metabolic parameters at the monitoring nearest in time to their first exposure to the computer intervention/ETAU (or baseline for those with no exposures) (Table 1). The median (interquartile range) number of days between the date of the most recent monitoring and the date of first computer intervention/ETAU exposure (or baseline) was: weight: 4 (0–42) days, blood pressure: 11(0–42) days, fasting blood glucose/HbA1c: 63 (21–132) days, and lipid profile (LDL and HDL cholesterol/triglycerides): 97 (35–182) days. Overall, 86% (206/239) of the sample was overweight or obese, 53% (118/223) had low HDL cholesterol, 51% (118/232) had increased fasting blood glucose/HbA1c, 39% (93/238) had elevated blood pressure, 37% (84/225) had elevated triglycerides, and 22% (48/220) had increased LDL cholesterol.
For each metabolic parameter except weight, Table 3 presents summary statistics for the four indicators of follow-up monitoring and care for the participants in each treatment group who had an abnormal value for that parameter on or prior to their first exposure to the computer intervention/ETAU (or baseline). In the overall sample, 82–94% of participants with abnormal values for any of the 5 parameters received follow-up metabolic monitoring, and these values continued to be abnormal in 48–76% of participants. Overall, 83–85% of participants with abnormal values had at least one outpatient visit with a primary care or cardiometabolic care-related provider, averaging approximately 3 visits over their observation period after the initial abnormal finding/s. There were no significant differences between the treatment group subsamples on any of these outcomes. Results were unchanged in sensitivity analyses after removing any of the 39 participants without the full 365-day observation period from each subset of participants with abnormal metabolic values.
Table 3.
Follow-up Monitoring and Care among Those with Abnormal Values for 5 Metabolic Parametersa
| Sample with elevated fasting blood sugar or HbA1cb | |||||||||
| Total (N=118) | Computer Int. (N=63) | ETAU (N=55) | |||||||
| N | M ± SD/n | % | N | M ± SD/n | % | N | M ± SD/n | % | |
|
| |||||||||
| Received follow-up fasting blood glucose or HbA1c monitoring | 118 | 107 | 91 | 63 | 56 | 89 | 55 | 51 | 93 |
| Elevated follow-up fasting blood glucose or HbA1c value | 107 | 70 | 65 | 56 | 34 | 61 | 51 | 36 | 71 |
| Any primary care or cardiometabolic-related care outpatient visit | 118 | 99 | 84 | 63 | 51 | 81 | 55 | 48 | 87 |
| No. of primary care or cardiometabolic-related care outpatient visits | 118 | 3.1 ± 3.2 | 63 | 3.4 ± 3.9 | 55 | 2.8 ± 2.0 | |||
|
| |||||||||
| Sample with elevated blood pressureb | |||||||||
| Total (N=93) | Computer Int. (N=45) | ETAU (N=48) | |||||||
| N | M ± SD/n | % | N | M ± SD/n | % | N | M ± SD/n | % | |
|
| |||||||||
| Received follow-up blood pressure monitoring | 93 | 87 | 94 | 45 | 43 | 96 | 48 | 44 | 92 |
| Elevated follow-up blood pressure value | 87 | 50 | 58 | 43 | 24 | 56 | 44 | 26 | 59 |
| Any primary care or cardiometabolic-related care outpatient visit | 93 | 80 | 86 | 45 | 40 | 89 | 48 | 40 | 83 |
| No. of primary care or cardiometabolic-related care outpatient visits | 93 | 3.3 ± 3.1 | 45 | 3.7 ± 3.5 | 48 | 2.9 ± 2.7 | |||
|
| |||||||||
| Sample with elevated LDL Cholesterolb | |||||||||
| Total (N=48) | Computer Int. (N=23) | ETAU (N=25) | |||||||
| N | M ± SD/n | % | N | M ± SD/n | % | N | M ± SD/n | % | |
|
| |||||||||
| Received follow-up LDL cholesterol monitoring | 48 | 40 | 83 | 23 | 18 | 78 | 25 | 22 | 88 |
| Elevated follow-up LDL cholesterol value | 40 | 19 | 48 | 18 | 11 | 61 | 22 | 8 | 36 |
| Any primary care or cardiometabolic-related care outpatient visit | 48 | 41 | 85 | 23 | 19 | 83 | 25 | 22 | 88 |
| No. of primary care or cardiometabolic-related care outpatient visits | 48 | 3.3 ± 3.6 | 23 | 4.2 ± 4.8 | 25 | 2.4 ± 1.8 | |||
|
| |||||||||
| Sample with low HDL Cholesterolb | |||||||||
| Total (N=118) | Computer Int. (N=62) | ETAU (N=56) | |||||||
| N | M ± SD/n | % | N | M ± SD/n | % | N | M ± SD/n | % | |
|
| |||||||||
| Received follow-up HDL cholesterol monitoring | 118 | 97 | 82 | 62 | 52 | 84 | 56 | 45 | 80 |
| Low follow-up HDL cholesterol value | 97 | 74 | 76 | 52 | 41 | 79 | 45 | 33 | 73 |
| Any primary care or cardiometabolic-related care outpatient visit | 118 | 100 | 85 | 62 | 49 | 79 | 56 | 51 | 91 |
| No. of primary care or cardiometabolic-related care outpatient visits | 118 | 2.9 ± 2.7 | 62 | 3.1 ± 3.3 | 56 | 2.8 ± 1.8 | |||
|
| |||||||||
| Sample with elevated triglyceridesb | |||||||||
| Total (N=84) | Computer Int. (N=41) | ETAU (N=43) | |||||||
| N | M ± SD/n | % | N | M ± SD/n | % | N | M ± SD/n | % | |
|
| |||||||||
| Received follow-up triglycerides monitoring | 84 | 69 | 82 | 41 | 34 | 83 | 43 | 35 | 81 |
| Elevated follow-up triglycerides value | 69 | 50 | 73 | 34 | 28 | 82 | 35 | 22 | 63 |
| Any primary care or cardiometabolic-related care outpatient visit | 84 | 70 | 83 | 41 | 35 | 85 | 43 | 35 | 81 |
| No. of primary care or cardiometabolic-related care outpatient visits | 84 | 3.2 ± 3.3 | 41 | 3.4 ± 3.8 | 43 | 3.0 ± 2.9 | |||
Within up to 1 year after first exposure to computer intervention or ETAU.
In analyses adjusted for diagnosis of dyslipidemia, diagnosis of coronary artery disease, and prescription of diabetes medication, there were no significant differences between the sub-samples with elevated metabolic parameters.
With regard to usage of the computer intervention, the average duration of total viewing time was 11.3 ± 5.6 minutes per session. Among the n=111 individuals with at least one computer use log available for analysis, the percentage who viewed optional supplemental information on the six metabolic parameters ranged from 34% for blood sugar/HbA1c to 44% for obesity. The overall percentage of visits in which participants printed out their metabolic monitoring summary reports was 63%.
Discussion
This paper reports the results of the first randomized controlled trial of an intervention developed to increase rates of monitoring for the metabolic side effects of SGAs in individuals with serious mental illness. The brief, patient-centered, computer-delivered intervention informed patients about the extent to which their care was consistent with expert recommendations for side effects monitoring and prompted them to communicate with their prescribers about either receiving monitoring or discussing the results of such monitoring. Contrary to the first study hypothesis, the intervention did not increase the proportion of days participants’ care adhered to metabolic monitoring recommendations relative to a comparison group provided educational pamphlets. However, in the two VA clinics in which the study was conducted, rates of monitoring were notably higher than that previously reported in both VA and non-VA settings (Mitchell et al. 2012; Mittal et al. 2013), exceeding 75% for weight and blood pressure and 90% for blood glucose and lipids in both treatment groups. While not in place when the study was proposed, local quality improvement efforts, including prescribers receiving automatic reminders about monitoring at the time of SGA prescribing as well as personalized feedback on their rates of monitoring, were subsequently implemented in both clinics independent of the study and were in effect throughout its duration, thereby contributing to a significant ceiling effect for this outcome. This suggests that activating patients regarding prescriber activities (i.e. ordering monitoring labs, providing follow up) may be less necessary in settings in which clinicians and systems that support them are fully activated. Whether or not this intervention can enhance rates of metabolic monitoring in other VA or non-VA treatment settings that lack such programs and that do not already have high rates of monitoring merits further investigation. The impact of the intervention on other facets of patient-centered care, including communication patterns between patients and prescribers, will be addressed in a separate report.
Consistent with the growing number of reports of increased cardiovascular risk in individuals with serious mental illness, including major depression (Rethorst et al. 2014) and PTSD (Heppner et al. 2012), most participants in this study were overweight or obese, and anywhere from one quarter to one half of them possessed abnormalities in blood pressure, blood glucose, and lipids suggestive of an increased likelihood of metabolic syndrome. With regard to the second study hypothesis, the computer intervention did not differentially impact receipt of or the results of follow-up metabolic monitoring, or receipt of relevant medical services, in Veterans identified as having abnormal metabolic parameter values. While it is encouraging that the vast majority of participants in both groups received additional metabolic monitoring following an abnormal result, close to half or more of these follow-up tests remained abnormal. This occurred despite Veterans in both groups having a relatively high level of contact with both mental health and medical providers, including prescription of medications for diabetes, hypertension, and dyslipidemia. These findings suggest several avenues of additional research. Because such high rates of monitoring did not translate into more favorable metabolic profiles as might have been expected, more work is needed to better understand the utility of metabolic side effect monitoring of SGAs as an indicator of quality care. Further, because signficant cardiovascular risk persisted in study participants despite relatively frequent contact with mental health and medical care providers and receipt of medication treatments for cardiometabolic conditions, future research should investigate the quality of the medical care being provided, whether other cardiovascular risks prevalent in these patients (e.g., cigarette smoking, poor nutrition, sedentary lifestyle) are being properly addressed, and the extent to which patients understand and adhere to prescribed treatments. Subsequent versions of the patient-focused intervention described herein are likely to require going beyond prompting about receipt of metabolic monitoring to include strategies for improving abnormal metabolic parameters and addressing other cardiometabolic risks.
Study findings suggest that individuals with serious mental illness are interested in obtaining personalized information about their cardiometabolic status and thus appear receptive to such interventions. Up to 40% of study participants elected to view optional educational information on one or more of the metabolic parameters addressed in the program. Further, when given the option, over 60% of participants chose to print summary reports of their metabolic profile that was reviewed with them during the program. However, whether participants provided copies of the report to their mental health prescribers as suggested by the program could not be determined. Nevertheless, it was encouraging that so many participants made the choice on their own to print such a report. Instances when participants discussed a report with their mental health prescribers during one of their study visits will be described in a separate report.
Despite concerns raised that cognitive impairments experienced by some with serious mental illness could interfere with their use of computers, this study demonstrated that few individuals experienced difficulties in navigating the program; in fact, many had had prior experience using computers. This study further demonstrated the feasibility of providing brief patient-focused interventions to individuals with serious mental illnesses within the mental healthcare setting. The computerized intervention was delivered in less than 15 minutes and could be made available to patients via kiosks in waiting areas prior to prescriber visits or alternatively, on secure websites on computers or smartphones for viewing at the patient’s convenience.
This study had limitations, including the aforementioned ceiling effect imparted by higher than anticipated baseline rates of metabolic monitoring in study clinics. While a limitation, this afforded one of the first opportunities to evaluate providers’ responses to abnormal metabolic profiles of individuals with serious mental illness in the context of near optimal levels of metabolic monitoring. In addition, randomization occurred at the level of the patient rather than the prescriber which may have led to contamination, in that having patients randomized to the computer intervention may have affected prescribers’ metabolic monitoring practices in their patients randomized to the comparison condition. Although prescribers were not explicitly informed by study staff regarding how interventions were assigned to their patients, they may have been able to determine this during the course of the study (e.g., if their patients in the computer intervention group shared their summary monitoring report with them as encouraged by the program). However, any resultant bias would have been conservative as it could have affected both groups. Further, three (and often fewer) exposures to the intervention over the course of a one-year period may not have been adequate to affect change in participants’ metabolic parameters, in particular. In addition, since the educational pamphlet that could have been shared with prescribers was provided to all participants in the comparison group immediately prior to a visit with the prescriber, it may have had more effect than expected, particularly since only 60% of participants in the intervention group printed their summary monitoring report that they could have shared with their prescribers. Finally, the feasibility of the intervention outside of an integrated healthcare system such as that in the VA in which metabolic monitoring can be conducted on site, and the results can be easily accessed by both mental health and medical providers via an electronic medical record, is not known.
In contrast to other programs that have intervened directly on prescribers or instituted system level changes to enhance metabolic monitoring, the intervention tested in this study enlisted patients as agents of change. This report contributes to the growing body of work (Bartels et al. 2013; Alegria et al. 2014) involving the development and testing of such interventions that encurage individuals with serious mental illnesses to participate more fully in both mental health and medical encounters in order to increase engagement in treatment, enhance quality of care, and improve health outcomes.
Acknowledgments
Funding: This study was funded by a U.S. Department of Veterans Affairs Health Services Research and Development Merit Award (IIR-07-256) to Dr. Kreyenbuhl. It is the result of work supported with resources and the use of facilities at the VISN 5 Mental Illness Research, Education, and Clinical Center (MIRECC). Dr. Klingaman was also supported by the U.S. Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment. This work reflects the authors’ personal views and in no way represents the official view of the Department of Veterans Affairs of the U.S. Government.
Footnotes
Ethical approval: All procedures performed in this study were in accordance with the ethical standards of the institutional research committee of the University of Maryland, Baltimore and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: The authors declare no conflicts of interest, and all authors certify responsibility for this manuscript.
References
- Alegría M, Carson N, Flores M, Li X, Shi P, Lessios AS, Polo A, Allen M, Fierro M, Interian A, Jimenez A, La Roche M, Lee C, Lewis-Fernández R, Livas-Stein G, Safar L, Schuman C, Storey J, Shrout PE. Activation, self-management, engagement, and retention in behavioral health care: a randomized clinical trial of the DECIDE intervention. JAMA Psychiatry. 2014;71(5):557–565. doi: 10.1001/jamapsychiatry.2013.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. American Journal of Preventive Medicine. 2009;36(4):341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders. 2002;68(2–3):167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Barnes TR, Paton C, Hancock E, Cavanagh MR, Taylor D, Lelliott P UK Prescribing Observatory for Mental Health. Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: a quality improvement programme. Acta Psychiatrica Scandinavica. 2008;118(1):26–33. doi: 10.1111/j.1600-0447.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Aschbrenner KA, Rolin SA, Hendrick DC, Naslund JA, Faber MJ. Activating older adults with serious mental illness for collaborative primary care visits. Psychiatric Rehabilitation Journal. 2013;36(4):278–288. doi: 10.1037/prj0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychological Medicine. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. British Journal of Psychiatry. 2000;177:212–217. doi: 10.1192/bjp.177.3.212. [DOI] [PubMed] [Google Scholar]
- Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing Chronic Disease. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- DelMonte MT, Bostwick JR, Bess JD, Dalack GW. Evaluation of a computer-based intervention to enhance metabolic monitoring in psychiatry in patients treated with second-generation antipsychotics. Journal of Clinical Pharmacy and Therapeutics. 2012;37(6):668–673. doi: 10.1111/j.1365-2710.2012.01369.x. [DOI] [PubMed] [Google Scholar]
- Dixon L, Medoff D, Wohlheiter K, DiClemente C, Goldberg R, Kreyenbuhl J, Adams C, Lucksted A, Davin C. Correlates of severity of smoking among persons with severe mental illness. American Journal on Addictions. 2007;16(2):101–110. doi: 10.1080/10550490601184415. [DOI] [PubMed] [Google Scholar]
- Dixon L, Weiden P, Delahanty J, Goldberg R, Postrado L, Lucksted A, Lehman A. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophrenia Bulletin. 2000;26(4):903–912. doi: 10.1093/oxfordjournals.schbul.a033504. [DOI] [PubMed] [Google Scholar]
- Eisen SV, Normand S, Belanger AJ, Spiro A, Esch D. The Revised Behavior and Symptom Identification Scale (BASIS-R): Reliability and validity. Medical Care. 2004;42(12):1230–1241. doi: 10.1097/00005650-200412000-00010. [DOI] [PubMed] [Google Scholar]
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, Makosso-Kallyth S, Wolf FM, Farmer AP, Gagnon MP. Printed educational materials: effects on professional practice and healthcare outcomes. The Cochrane Database of Systematic Reviews. 2012;10:CD004398. doi: 10.1002/14651858.CD004398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner PS, Lohr JB, Kash TP, Jin H, Wang H, Baker DG. Metabolic syndrome: relative risk associated with post-traumatic stress disorder (PTSD) severity and antipsychotic medication use. Psychosomatics. 2012;53(6):550–558. doi: 10.1016/j.psym.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. In: Le Cam LM, Neyman J, editors. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. Berkeley: University of California Press; 1967. [Google Scholar]
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- Kilbourne AM, Morden NE, Austin K, Ilgen M, McCarthy JF, Dalack G, Blow FC. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. General Hospital Psychiatry. 2009;31(6):555–563. doi: 10.1016/j.genhosppsych.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K-Y, Zeger SL. Regression analysis for correlated data. Annual Review of Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT, Jr, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S. Physical health monitoring of patients with schizophrenia. American Journal of Psychiatry. 2004;161(8):1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Stroup S, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia Research. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Mead N, Bower P. Patient-centeredness: a conceptual framework and review of the empirical literature. Social Science and Medicine. 2000;51(7):1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychological Medicine. 2012;42(1):125–147. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophrenia Bulletin. 2013;39(2):306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Li C, Williams JS, Viverito K, Landes RD, Owen RR. Monitoring veterans for metabolic side effects when prescribing antipsychotics. Psychiatric Services. 2013;64(1):28–35. doi: 10.1176/appi.ps.201100445. [DOI] [PubMed] [Google Scholar]
- Morden NE, Lai Z, Goodrich DE, MacKenzie T, McCarthy JF, Austin K, Welsh DE, Bartels S, Kilbourne AM. Eight-year trends of cardiometabolic morbidity and mortality in patients with schizophrenia. General Hospital Psychiatry. 2012;34(4):368–379. doi: 10.1016/j.genhosppsych.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. Journal of Clinical Psychiatry. 2007;68(Suppl 1):20–27. [PubMed] [Google Scholar]
- New Freedom Commission on Mental Health. Achieving the Promise: Transforming Mental Health Care in America. Rockville, MD: 2003. Final Report. DHHS Publication No. SMA-03-3832. [Google Scholar]
- Nicol GE, Morrato EH, Johnson MC, Campagna E, Yingling MD, Pham V, Newcomer JW. Best practices: implementation of a glucose screening program based on diffusion of innovation theory methods. Psychiatric Services. 2011;62(1):12–14. doi: 10.1176/ps.62.1.pss6201_0012. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophrenia Research. 2000;45(1–2):21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- Ramanuj PP. Improving blood and ECG monitoring among patients prescribed regular antipsychotic medications. Mental Health in Family Medicine. 2013;10(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009–2010 National Health and Nutrition Examination Survey (NHANES) Journal of Clinical Psychiatry. 2014;75(12):e1428–32. doi: 10.4088/JCP.14m09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondi AJ, Eack SM, Hanusa BH, Spring MB, Haas GL. Critical design elements of e-health applications for users with severe mental illness: singular focus, simple architecture, prominent contents, explicit navigation, and inclusive hyperlinks. Schizophrenia Bulletin. 2015;41(2):440–448. doi: 10.1093/schbul/sbt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, Kissling W, Davis JM, Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2010;123(2–3):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8):975–987. doi: 10.1592/phco.29.8.975. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- Stewart MA. Effective physician-patient communication and health outcomes: A review. CMAJ: Canadian Medical Association Journal. 1995;152(9):1423–1433. [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Brown JB, Donner A, McWhinney IR, Oates J, Weston WW, Jordan J. The impact of patient-centered care on patient outcomes. Journal of Family Practice. 2000;49(9):796–804. [PubMed] [Google Scholar]
- Stewart M, Brown JB, Weston WW, McWhinney IR, McWilliam CL, Freeman CR. Patient-Centered Medicine: Transforming the Clinical Method, Third Edition. London: Radcliffe Publishing Ltd.; 2014. [Google Scholar]
- Thompson A, Hetrick SE, Alvarez-Jiménez M, Parker AG, Willet M, Hughes F, Gariup M, Gomez DL, McGorry PD. Targeted intervention to improve monitoring of antipsychotic-induced weight gain and metabolic disturbance in first episode psychosis. The Australian and New Zealand Journal of Psychiatry. 2011z;45(9):740–748. doi: 10.3109/00048674.2011.595370. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Castillo D, Lopez L, Manaugh B, Davis C, Rodriguez J, Milam AC, Dassori A, Miller AL. A case control study of the implementation of change model versus passive dissemination of practice guidelines for compliance in monitoring for metabolic syndrome. Community Mental Health Journal. 2013;49(2):141–149. doi: 10.1007/s10597-011-9472-z. [DOI] [PubMed] [Google Scholar]