Abstract
The global burden of peripheral artery disease (PAD) is significant. This has led to numerous recent advances in magnetic resonance imaging (MRI) techniques in PAD. Older techniques such as time of flight MRI or phase contrast MRI are burdened by long acquisition times and significant issues with artifacts. In addition, the most used MRI modality, contrast-enhanced MR angiography (CE-MRA) is limited by the use of gadolinium contrast and its potential toxicity. Novel MRI techniques such as arterial spin labeling (ASL), blood-oxygen-level dependent imaging (BOLD), and first-pass perfusion gadolinium enhancement are advancing the field by providing skeletal muscle perfusion/oxygenation data while maintaining excellent spatial and temporal resolution. Perfusion data can be critical to providing objective clinical data of a visualized stenosis. In addition, there are a number of new MRI sequences assessing plaque composition and lesion severity in the absence of contrast. These approaches used in combination can provide useful clinical and prognostic data and provide critical endpoints in PAD research.
Keywords: arterial spin labeling, blood-oxygen-level dependent imaging, first-pass gadolinium enhanced imaging, magnetic resonance angiography (MRA), magnetic resonance imaging (MRI), magnetic resonance spectroscopy, peripheral artery disease (PAD), peripheral artery plaque, phosphocreatine
Introduction
The global burden of peripheral artery disease (PAD) is enormous, with an estimated 202 million people afflicted by the disease worldwide.1 In the United States, approximately 8 million people are affected by PAD, involving 12% to 20% of Americans >64 years of age.2,3 The morbidity and mortality associated with PAD is well described. Pooled data from 11 studies revealed an ankle–brachial index (ABI) of <0.9 was associated with increased risk of all-cause mortality, cardiovascular mortality, coronary artery disease, and stroke.4
The diagnosis of PAD can be established by an initial history and physical exam. However, non-invasive imaging is critical in determining the degree of disease or the lack of perfusion, in addition to providing anatomical detail of the atherosclerotic plaque.
Current non-invasive imaging methods
The ABI is a critical screening tool for suspected PAD. Its overall accuracy to diagnose PAD is well established and it is dependent on the severity of the lesion.5–7 For a stenosis of >50%, the ABI has a sensitivity and specificity of 91% and 86%, respectively.6,7 Pulse volume recordings (PVR), segmental pressures, and post-exercise ABIs can improve the ability to provide a generalized location of a stenosis, to evaluate the degree of symptoms, and to assess for small vessel disease. However, ABI is unable to specify exact lesion locations and can be limited by non-compressible arteries, such as in diabetic patients.5,8 In those patient populations, toe–brachial indices can be of significant benefit.8
Duplex ultrasonography is useful in determining the location and degree of stenosis. For a stenosis of >50%, duplex ultrasonography has a sensitivity and specificity of >90% when used with color doppler.9 There are technical difficulties with imaging iliac vessels due to bowel gas and tortuosity, and arterial calcification can cause shadowing artifact obscuring flow.10 Despite its limitations, duplex ultrasound is a safe and relatively inexpensive modality and is commonly used in post-intervention surveillance.
Computed tomography angiography (CTA) has improved spatial resolution compared to duplex sonography. The use of CTA has grown in recent years with the advent of multidetector scanners and the ability to obtain multiple cross-sectional images with faster acquisition times. Several small studies of multidetector scanning for PAD has shown sensitivities of 89% to 100% and specificities of 92% to 100% for lesions of >50% stenosis.5,11,12 CTA still has issues regarding post-processing of raw data or severe calcifications that can lead to significant artifacts.13–16
Current MRA techniques
Contrast-enhanced magnetic resonance angiography (MRA) is an excellent method for diagnosing the anatomic location and degree of stenosis in PAD.5 Many studies show significant correlation between MRA and catheter angiography when compared to other imaging modalities. Collins et al. performed a meta-analysis comparing contrast-enhanced magnetic resonance imaging (MRI), duplex sonography, and CTA and showed superior sensitivity and specificity with MRA for the detection of lesions of >50% stenosis.17 There is controversial evidence of MRA superiority to catheter angiography in identifying patent vessels, diseased pedal vessels, and vessel targets for bypass.18–22
The type of MRA technique used is critical in the evaluation of PAD as each technique differs in advantages and disadvantages and will affect the overall accuracy of the test (Tables 1 and 2). The general goal is to maximize spatial resolution and, at the same time, obtain dynamic data.23,24
Table 1.
Current MRA imaging techniques for peripheral artery disease.
| Technique | Contrast | Advantages | Limitations | Clinically available |
|---|---|---|---|---|
| Time of flight MRA | No | High spatial resolution; imaging of distal pedal vessels | Prolonged imaging times; sensitive to turbulent flow, patient motion, in-plane flow direction, retrograde blood flow; artifacts | Yes |
| Phase contrast MRA | No | Reduced saturation-related artifacts; flow quantification | Difficult to image very slow flow and significant aliasing with very high flow | Yes |
| 3D half Fourier spin echo | No | Excellent spatial resolution; gated imaging | Significant artifacts if unable to gate properly, such as poor ECG quality or arrhythmias | Yes |
| Balanced steady state free procession MRA | No | Excellent signal to noise ratio; gated imaging | Artifacts related to tissue interference, metallic objects or tissue homogeneity; long acquisition times | Yes |
| Quiescent-interval single-shot MRA | No | Less acquisition time; excellent spatial resolution and image quality; reduced sensitivity to patient motion | Reduced image quality related to in-plane vessels | Yes |
| Contrast-enhanced MRA | Yes | Excellent image quality | Contrast-related side effects; requires coordination between contrast bolus and image acquisition; limited use in kidney disease | Yes |
MRA, magnetic resonance angiography; ECG, electrocardiography.
Table 2.
Novel MRI techniques for peripheral artery disease.
| Technique | Contrast | Advantages | Limitations | Clinically available |
|---|---|---|---|---|
| First-pass gadolinium enhanced perfusion | Yes | Semi-quantitative perfusion analysis; rapid imaging time | Use of contrast and contrast-related adverse effects | Yes; primarily used for myocardial perfusion |
| Arterial spin labeling | No | Quantifiable perfusion; excellent spatial and temporal resolution | Low signal to noise ratio; affected by partial volume effects and arterial transit times | Yes; not widely available, used in brain imaging for cerebral blood flow |
| Blood-oxygen-level dependent MRI | No | High signal to noise ratio; high temporal resolution | Susceptibility artifacts | Yes; not widely available, used in cerebral imaging |
| Phosphorus-31 magnetic resonance spectroscopy | No | Reproducible and reliable; assessment of energetics | Lack of availability; not spatially localized; prolonged acquisition times | No |
| Creatinine chemical exchange saturation transfer | No | Spatial localization; assessment of energetics | Lack of validation studies; not widely available | No |
MRI, magnetic resonance imaging.
Non-contrast techniques
Time of flight
Time of flight MRA (TOF-MRA) is a non-contrast MRI technique that creates vessel and background contrast by detecting flow in vessels. Fully magnetized (or unsaturated) blood flowing into a perpendicular imaging plane has relatively higher signal intensity than the stationary, progressively saturated background spins.23 The main disadvantage of TOF-MRA is prolonged imaging acquisition times making it difficult for patients, in addition to significant artifacts related to saturation bands, turbulent flow, patient motion, or ghosting artifacts, which can limit diagnostic accuracy.23 TOF-MRA is also not sensitive to flow in the in-plane direction and in situations of retrograde blood flow.
Phase contrast
Phase contrast MRA (PC-MRA) was one of the first MRA techniques utilized for peripheral arterial imaging.25 PC-MRA is a non-contrast technique that separates blood flow from the background stationary tissue by observing the phase difference between non-zero phase protons of blood and the zero phase protons of stationary background tissue (Figure 1).25,26 It has some advantages compared to TOF-MRA, such as reduced acquisition times and fewer saturation-related artifacts.23 Both TOF-MRA and PC-MRA are rarely used today with the availability of more advanced techniques. TOF-MRA is occasionally used in situations to image the very distal lower extremities while PC-MRA is primarily involved with obtaining dynamic flow data and quantifying arterial flow.27
Figure 1.
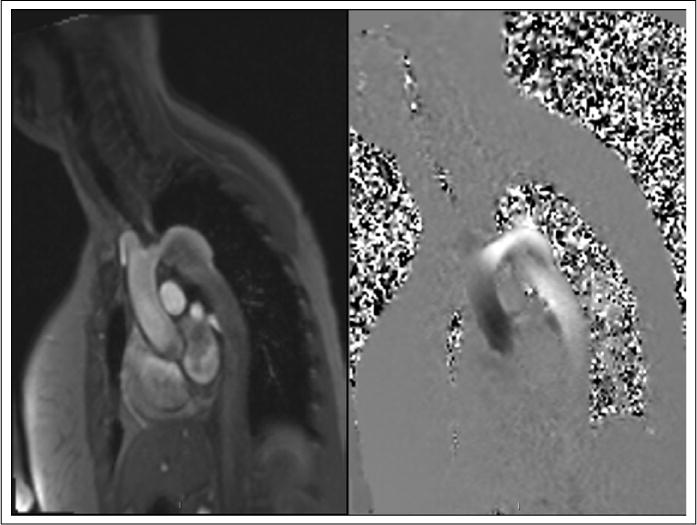
Left panel: Phase contrast MRI of the aortic arch. Note the coarctation and aneurysmal dilation. Right panel: Velocity encoding performed on phase contrast MRI. Post-processing analysis can be performed to determine velocities.
3D half-Fourier fast spin echo
Electrocardiographic-triggered three-dimensional half-Fourier fast spin echo (3D FSE) imaging is another non-contrast technique that acquires electrocardiographic (ECG) gated images.28 This technique contrasts the difference between fast flowing blood on the arterial side and slow moving blood on the venous side during systole. The systolic images are subtracted from diastolic images to separate the arteries from veins.29 The limitations of this technique are primarily related to gating. Arrhythmias, patient motion or various timing issues can cause significant artifacts and significantly disrupt image quality.29
Balanced steady state free procession MRA
Balanced steady state free procession MRA (b-SSFP) is another commonly used peripheral arterial imaging technique. It uses T2/T1 weighted imaging ratios to contrast blood from the surrounding tissue.30 This technique has an excellent signal to noise ratio particularly if fat is suppressed from the images or there is ECG gating.31 The technique has a few disadvantages, including prolonged acquisition times.32, 33 There can also be artifacts related to blood flow, tissue interference, metallic objects, or tissue homogeneity.32,33
Quiescent-interval single-shot MRA
Quiescent-interval single-shot (QISS) MRA is a non-contrast ECG gated b-SSFP that has been recently developed for the imaging of PAD.34 QISS is a 2D technique that first uses a pre-saturation radio frequency pulse to suppress the stationary tissue in a slice followed by another pulse to suppress the venous signal. This is followed by the quiescent inflow period where magnetized blood enters into the imaging slice.35 Finally, data are acquired during diastole at the time of slow arterial flow using b-SSFP sequences.34,35 This process is then repeated for every slice. Benefits of this method include shorter acquisition time and total imaging time compared to TOF-MRA sequences.35 In addition, QISS uses non-subtractive single-shot image acquisition, which reduces sensitivity to patient motion.36–38
Contrast-enhanced technique
All the previous techniques described do not require contrast, but currently the most often used modality is contrast-enhanced MRA (CE-MRA). Older CE-MRA techniques had several limitations with complete imaging of the peripheral arteries.33 However, current methods have significantly reduced imaging times. Initially, scout images in multiple axes with the use of TOF-MRA or b-SSFP are obtained and reviewed to make sure all arterial structures are included in the imaging volume.23 Prior to contrast administration, accurate planning for image acquisition at peak arterial contrast enhancement needs to be determined, and can be tested by the use of a small test bolus. Real time bolus monitoring software on current magnets allow the operator or software to detect contrast signal enhancement in a target arterial bed that then triggers image acquisition.39 Pre and post-processing strategies are then employed to enhance arterial structures and suppress venous structures. Planning is critical to ensure best image quality (Figure 2B). A major issue with CE-MRA is the need for gadolinium-based contrast agents that have a rare association with nephrogenic systemic fibrosis (NSF) in patients with stage IV or V chronic kidney disease on dialysis. However, the incidence of NSF has declined dramatically due to avoidance in these patients as well as the use of cyclic rather than linear agents that reduce the possibility of the gadolinium becoming unchelated.39 Large studies of >20,000 patients show an acute adverse reaction rate of 0.76% with a reported severe acute reaction rate of 0.03%, which is less than that reported with iodinated contrast used in computed tomography.40
Figure 2.
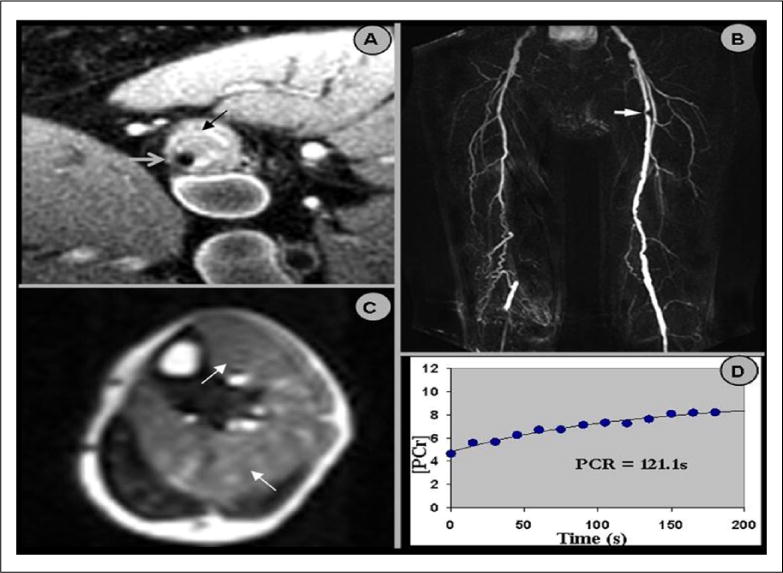
(A) High resolution black blood magnetic resonance imaging (MRI) of the superficial femoral artery (SFA) in cross section. The vessel lumen is denoted by the gray arrow and the vessel wall by the black arrow. Note the large amount of atherosclerotic plaque in the vessel wall. (B) Magnetic resonance angiography (MRA) of the SFA. The arrow denotes a severe stenosis in the proximal left SFA, which corresponds to the vessel wall image in (A). (C) Post-exercise contrast-enhanced peak perfusion of the calf muscle in cross section. Note the heterogeneous signal intensity. The arrows depict the muscle groups (anterior tibialis and soleus muscles) with the greatest contrast enhancement (i.e. perfusion). (D) A plot of phosphocreatine recovery. The phosphocreatine recovery time constant (PCr) is calculated using a mono-exponential fit of these data. Reprinted from [76] with permission from Elsevier.
Recent advances in MRI techniques
Recent advances have maintained a focus on either non-contrast MRI techniques or hybrid approaches that use both CE-MRA and other non-contrast techniques. Current research continues to advance the principle goals in PAD imaging of improving spatial resolution, limiting contrast use, obtaining accurate dynamic data, and performing analysis of skeletal muscle perfusion. The gold standard for skeletal muscle perfusion assessment is the highly invasive microsphere method which requires arterial and tissue sampling.41 Many of the recent advances in MRI techniques are advancements on previous MRI techniques or development of non-contrast image acquisition.
First-pass gadolinium enhanced skeletal muscle perfusion imaging
First-pass gadolinium enhanced MRI of skeletal muscle perfusion with use of a plantar flexion ergometer can be performed on patients with PAD (Figure 2C). The technique, which is analogous to MRI myocardial perfusion studies, is performed using T1-weighted sequences to visualize the gadolinium-based contrast agents’ uptake in tissue.42 The applied technique can be used along with the arterial input function to obtain a semi-quantitative peak calf perfusion index. This ratio correlated to the 6-minute-walk test and was able to diagnose mild to moderate PAD in patients.42,43 There are some limitations, including the requirement of semi-quantitation to correct for arterial input, which may be proximal to a stenosis, as well as the use of gadolinium-based contrast.42,43 Further improvements with absolute quantitation of perfusion may be possible in the future.
Arterial spin labeling
Arterial spin labeling (ASL) is a non-contrast technique.44,45 In ASL, the protons in arterial blood are imparted with a magnetic tag, which differs in magnetization from that of the surrounding soft tissue. Another ‘control’ scan is obtained without tagging of the arterial blood. This allows for measurement of the signal difference of the tagged blood flow from the untagged image.44,45 Recently, two forms of ASL have successfully demonstrated perfusion abnormalities in PAD patients: continuous ASL (cASL)46 and pulsed ASL (pASL).47 Perfusion can be measured after plantar flexion ergometry or with cuff occlusion hyperemia (Figure 3). The latter is more reproducible.48 The optimal method of ASL for this application remains to be determined and whether the best endpoint is peak perfusion or time to peak perfusion.
Figure 3.
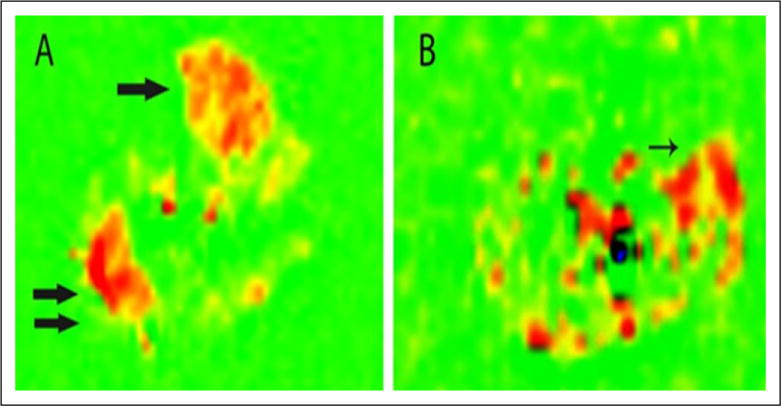
Pulsed arterial spin-labeling of a calf in a normal patient (A) and patient with peripheral artery disease (B) after peak exercise. Flow is increased in the anterior tibialis (single arrow) and lateral gastrocnemius (double arrow) muscles of the normal patient. The patient with peripheral artery disease has the highest flow signal in the peroneus longus muscle (arrow). Reprinted from [48] with permission from Elsevier.
Blood-oxygen-level dependent MRI
Another novel method of PAD imaging is based on a relative measure of tissue oxygenation also called blood-oxygen-level dependent MRI (BOLD MRI).49 BOLD MRI has been used to assess brain activation and oxygenation of skeletal muscle and the kidneys in previous studies.50–53 Englund et al. performed a study on 96 patients with varying degrees of PAD and assessed perfusion using pASL, measuring mixed venous saturation (SVO2) using MRI susceptometry, and using BOLD MRI, calling the method PIVOT.54 They found abnormalities in the time to peak perfusion and time to peak BOLD MRI assessments in PAD patients compared to normal patients.53 It is likely that tissue oxygenation and perfusion are closely linked, although this has not been definitively demonstrated as of yet.
Phosphorus-31 magnetic resonance spectroscopy
Phosphorus-31 magnetic resonance spectroscopy (31P-MRS) has been used to measure skeletal muscle metabolism and can be used to measure phosphocreatine (PCr) recovery after exercise as an accurate measure of tissue ischemia and a surrogate for mitochondrial function.54–56 Prolonged recovery time is a proven and reproducible marker of skeletal muscle ischemia in PAD patients after exercise (Figure 2D).56 However, it is limited by the availability of this technology, prolonged acquisition times and poor spatial resolution.57
Creatine chemical exchange saturation transfer
A recent concept has been to use creatine chemical exchange saturation transfer (crCEST) as a non-spectroscopic imaging technique to measure localized creatine kinetics in skeletal muscle of PAD patients. This imaging method has been studied in myocardial tissue and skeletal muscle (Figure 4) and correlated with 31P-MRS in a handful of normal subjects and PAD patients.57,58 Further studies validating this technique are needed. Potential advantages are spatial localization to individual muscle groups and matching with perfusion studies.
Figure 4.
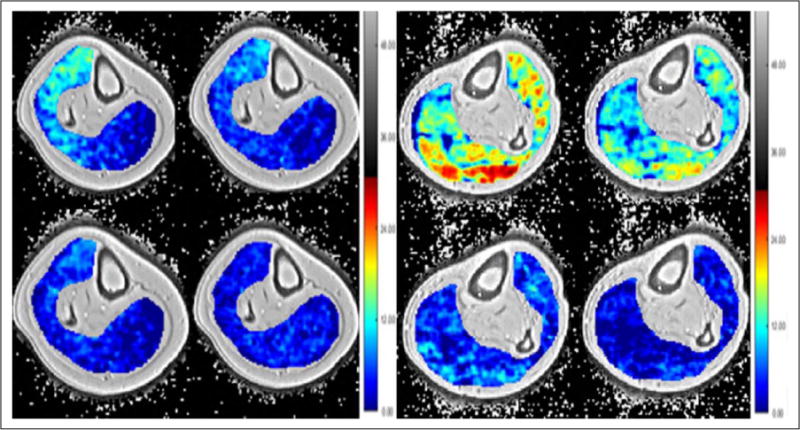
Left panel: creatine chemical exchange saturation transfer (CrCEST) of the calf of a normal subject imaged immediately after exercise then 1, 3, and 5 minutes post-exercise. Right panel: crCEST of the calf of a patient with peripheral artery disease immediately after exercise with images 3, 7, and 10 minutes post-exercise. Note the delay in reaching baseline deep blue in the patient with peripheral artery disease compared to the normal subject.
MRI assessment of peripheral arterial plaque
There have been significant advances in improving visualization and characterization of atherosclerotic plaque by MRI (Figure 5). Accurate and reliable plaque assessment provides objective data for research applications. The assessment of plaque volume imaged by high resolution multi-slice turbo-spin-echo pulse sequences with fat presaturation and suppression of flowing blood has been proven to have excellent intra-observer (r=0.997), inter-observer (r=0.987), and test-retest (r=0.996) reliability and reproducibility.59 The use of statins and ezetimibe to lower low-density lipoprotein cholesterol (LDL-C) showed no progression of plaque volume in previously statin-naïve patients by using MRI plaque volume assessments as a primary endpoint.60
Figure 5.
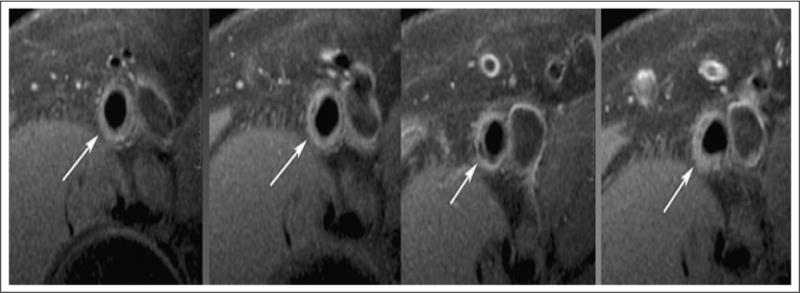
Representative sequential images (left to right) from the superficial femoral artery (arrow) of a subject with mild to moderate peripheral artery disease with both the luminal and adventitial border clearly delineated. Note the slice-to-slice variation in plaque morphology. The third slice from left demonstrates plaque with calcification (area of low signal).
Plaque composition also plays a role in patient outcomes. Plaque calcification assessed by CT is associated with increased risk of amputations in PAD patients.61 The adverse effects of particular features related to plaque composition in coronary and carotid arteries have been well studied and include high risk plaque characteristics such as intra-plaque hemorrhage (IPH), lipid rich necrotic core (LRNC), or thinned fibrous caps.62–64 The clinical impact of these characteristics in the peripheral arteries is still being studied. Polonsky et al. assessed superficial femoral artery (SFA) plaque by MRI on 300 patients with PAD and found 22.4% to have LRNC.65 Also, plaque lesion eccentricity, defined as [(maximum wall thickness – minimum wall thickness)/maximum wall thickness] ≥ 0.5, shows increased high risk characteristics that include larger plaque burden, increased calcification, and more lipid content compared to concentric lesions.65 However the prognostic and clinical impact of these morphological features in PAD patients is not well understood. MRI visualization of SFA occlusion and SFA plaque burden (measured by increased plaque area and decreased percent luminal area) are associated with reduced ABIs and 6-minute walk distance.66–68 In addition, increased collateralization associated with SFA occlusion is associated with improved functional performance.68 However, LRNC and calcium were not associated with mobility loss in patients with PAD.69
An alternative 3D approach to plaque visualization is ‘sampling perfection with application optimized contrast using different flip angle evolution’ or SPACE. The latter is a 3D FSE, ‘black blood’ T1 weighted imaging technique (3D-T1w SPACE) that improves efficiency and allowed for imaging large vascular territories.70–74 Mihai et al. performed a feasibility study of 3D-T1w SPACE and compared the method to CE-MRA in the setting of PAD.75 They found the technique to be comparable to CE-MRA in calculating luminal area. In addition, 3D-T1w SPACE compared to CE-MRA identified more significant lesions in patients due to improved visualization of the arterial wall and ability to assess vascular thickening and remodeling.74
Novel MRI and MRA techniques used in clinical studies
Combinations of novel MRI techniques are currently being used in PAD research (Figure 2). Anderson et al. performed first-pass gadolinium calf muscle perfusion and 31P-MRS on 85 patients with mild to moderate PAD. Lesion severity was determined by MRA and symptoms were assessed by treadmill testing with VO2 max and a 6-minute walk test. They demonstrated multifactorial contributions to claudication. They found symptomatic PAD to be related to the severity of macrovascular obstruction along with atherosclerotic plaque burden, reduced tissue perfusion and abnormal energy metabolism.76 Interestingly, 31P-MRS measurement of prolonged phosphocreatine recovery time did not correlate with calf muscle perfusion but correlated with the 6-minute walk test,76 suggesting uncoupling of metabolism and perfusion in claudicants.
Furthermore, these techniques can be used to determine endpoints after medical intervention. Sixty-eight patients with mild to moderate PAD were treated with LDL lowering agents (i.e. statins and ezetimibe) for 2 years and assessed for improvements in plaque regression, perfusion, metabolism, and exercise capacity using CE-MRA, first-pass gadolinium perfusion, 31P-MRS, and exercise treadmill with the 6-minute walk test. Other than improvement in resting ABI, all other parameters did not improve with LDL lowering therapy.77 A pilot study of 10 patients with symptomatic PAD underwent similar imaging techniques 2 months prior and 10 months after percutaneous intervention of the lower extremity. Improvement in phosphocreatine recovery time and ABI was noted.78
Conclusion
Although catheter angiography still remains the gold standard for diagnosis, awareness of the impact of PAD has led to a growth in the role of non-invasive modalities.5 The goal of non-invasive imaging is to obtain images with excellent spatial resolution and to provide dynamic data in regards to anatomy and physiology. Though ABI and duplex sonography will always have some role in screening and diagnosis, their limitations will always lend favor to CTA, MRA, and catheter angiography for more accurate assessment of disease. Major advances have been made in recent years in MRI approaches that have the benefit of a lack of ionizing radiation. Current research is directed toward non-contrast techniques to avoid the risk of contrast. Novel approaches for measuring skeletal muscle tissue perfusion, oxygenation, and energetics are emerging. However, MRI still has some limitations such as prolonged acquisition times, limited availability of advanced MRI sequences and technology, and cost that will hopefully be overcome in the years to come.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by R01 HL075792 (CMK). Roshin Mathew has no financial relationship or interest with any proprietary entity producing healthcare goods or services.
Footnotes
CME Accreditation Statement
The University of Virginia School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™ per article. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learners are expected to read this article along with any references and supporting material as appropriate, and complete the online post-test questions with an 80% pass rate to receive credit. Post-test questions are accessed through the Society for Vascular Medicine (SVM) website (www.vascularmed.org/VMJ-CME). Please note that CME credits are only available to members of the SVM. This activity expires 2 years after the publication date, on April 1, 2020.
Disclosures:
The faculty, staff and planning committee of the University of Virginia Office of Continuing Medical Education have no financial affiliations to disclose.
The CME planning committee of the Vascular Medicine Editorial Office disclosed the following:
Heather Gornik has disclosed research support from AstraZeneca and CVR Global, intellectual property rights from Summit Doppler Systems, Inc., and intellectual property rights and stock/ownership from FlexLife Health (proceeds donated). Aditya Sharma has disclosed research support from National Institute of Health Sciences, AstraZeneca, Biomet Biologics, and Portola Pharmaceuticals. Valerie Clark has no financial affiliations to disclose.
The authors have disclosed the following: Christopher Kramer is supported by a research grant from Regeneron in this area; this work was supported in part by R01 HL075792 (CMK). Roshin Mathew has no financial relationship or interest with any proprietary entity producing healthcare goods or services.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christopher Kramer is supported by a research grant from Regeneron in this area.
ORCID iD
Roshin C Mathew https://orcid.org/0000-0002-7927-4607
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison MA, Ho E, Denenberg JO, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Ostchega Y, Paulose-Ram R, Dillon CF, et al. Prevalence of peripheral arterial disease and risk factors in people aged 60 and older: Data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2007;55:583–589. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 4.Heald CL, Fowkes FG, Murray GD, et al. Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: Systematic review. Atherosclerosis. 2006;189:61–69. doi: 10.1016/j.atherosclerosis.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:e71–e126. doi: 10.1016/j.jacc.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Lijmer JG, Hunink MG, van den Dungen JJ, et al. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–398. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Li J, Pang W, et al. Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ J. 2008;72:605–610. doi: 10.1253/circj.72.605. [DOI] [PubMed] [Google Scholar]
- 8.Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med. 2016;21:382–389. doi: 10.1177/1358863X16645854. [DOI] [PubMed] [Google Scholar]
- 9.De Vries SO, Hunink MG, Polak JF. Summary receiver operating characteristic curves as a technique for meta-analysis of the diagnostic performance of duplex ultrasonography in peripheral arterial disease. Acad Radiol. 1996;3:361–369. doi: 10.1016/s1076-6332(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 10.Sacks D, Robinson ML, Marinelli DL, et al. Peripheral arterial Doppler ultrasonography: Diagnostic criteria. J Ultrasound Med. 1992;11:95–103. doi: 10.7863/jum.1992.11.3.95. [DOI] [PubMed] [Google Scholar]
- 11.Martin ML, Tay KH, Flak B, et al. Multidetector CT angiography of the aortoiliac system and lower extremities: A prospective comparison with digital subtraction angiography. AJR Am J Roentgenol. 2003;180:1085–1091. doi: 10.2214/ajr.180.4.1801085. [DOI] [PubMed] [Google Scholar]
- 12.Catalano C, Fraioli F, Laghi A, et al. Infrarenal aortic and lower extremity arterial disease: Diagnostic performance of multi-detector row CT angiography. Radiology. 2004;231:555–563. doi: 10.1148/radiol.2312020920. [DOI] [PubMed] [Google Scholar]
- 13.Schernthaner R, Stadler A, Lomoschitz F, et al. Multidetector CT angiography in the assessment of peripheral arterial occlusive disease: Accuracy in detecting the severity, number, and length of stenoses. Eur Radiol. 2008;18:665–671. doi: 10.1007/s00330-007-0822-8. [DOI] [PubMed] [Google Scholar]
- 14.Met R, Bipat S, Legemate DA, et al. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA. 2009;301:415–424. doi: 10.1001/jama.301.4.415. [DOI] [PubMed] [Google Scholar]
- 15.Varga-Szemes A, Wichman JL, Schoepf UJ, et al. Accuracy of non-contrast quiescent single-shot lower extremity MR angiography versus CT angiography for diagnosis of peripheral artery disease: Comparison with digital subtraction angiogrpahy. JACC Cardiovasc Imaging. 2017;10:1116–1124. doi: 10.1016/j.jcmg.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Lau JF, Weinberg MD, Olin JW. Peripheral artery disease Part I. Clinical evaluation and noninvasive diagnosis. Nat Rev Cardiol. 2011;8:405–418. doi: 10.1038/nrcardio.2011.66. [DOI] [PubMed] [Google Scholar]
- 17.Collins R, Burch J, Cranny G, et al. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: Systematic review. BMJ. 2007;334:1257. doi: 10.1136/bmj.39217.473275.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelemans PJ, Leiner T, de Vet HC, et al. Peripheral arterial disease: Meta-analysis of the diagnostic performance of MR angiography. Radiology. 2000;217:105–114. doi: 10.1148/radiology.217.1.r00oc11105. [DOI] [PubMed] [Google Scholar]
- 19.Baum RA, Rutter CM, Sunshine JH, et al. Multicenter trial to evaluate vascular magnetic resonance angiography of the lower extremity. American College of Radiology Rapid Technology Assessment Group. JAMA. 1995;274:875–880. [PubMed] [Google Scholar]
- 20.Owen RS, Carpenter JP, Baum RA, et al. Magnetic resonance imaging of angiographically occult runoff vessels in peripheral arterial occlusive disease. N Engl J Med. 1992;326:1577–1581. doi: 10.1056/nejm199206113262428. [DOI] [PubMed] [Google Scholar]
- 21.Dorweiler B, Neufang A, Kreitner KF, et al. Magnetic resonance angiography unmasks reliable target vessels for pedal bypass grafting in patients with diabetes mellitus. J Vasc Surg. 2002;35:766–772. doi: 10.1067/mva.2002.119505. [DOI] [PubMed] [Google Scholar]
- 22.Hartnell G. MR angiography compared with digital subtraction angiography. AJR Am J Roentgenol. 2000;175:1188–1189. doi: 10.2214/ajr.175.4.1751188. [DOI] [PubMed] [Google Scholar]
- 23.Leiner T. Magnetic resonance angiography of abdominal and lower extremity vasculature. Top Magn Reson Imaging. 2005;16:21–66. doi: 10.1097/01.rmr.0000185431.50535.d7. [DOI] [PubMed] [Google Scholar]
- 24.Kramer H, Nikolaou K, Sommer W, et al. Peripheral MR angiography. Magn Reson Imaging Clin N Am. 2009;17:91–100. doi: 10.1016/j.mric.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Nesbit GM, DeMarco JK. 2D time-of-flight MR angiography using concatenated saturation bands for determining direction of flow in the intracranial vessels. Neuroradiology. 1997;39:461–468. doi: 10.1007/s002340050446. [DOI] [PubMed] [Google Scholar]
- 26.Wedeen VJ, Meuli RA, Edelman RR, et al. Projective imaging of pulsatile flow with magnetic resonance. Science. 1985;230:946–948. doi: 10.1126/science.4059917. [DOI] [PubMed] [Google Scholar]
- 27.Korosec FR, Mistretta CA. MR angiography: Basic principles and theory. Magn Reson Imaging Clin N Am. 1998;6:223–256. [PubMed] [Google Scholar]
- 28.Frydrychowicz A, Winterer JT, Zaitsev M, et al. Visualization of iliac and proximal femoral artery hemodynamics using time-resolved 3D phase contrast MRI at 3T. J Magn Reson Imaging. 2007;25:1085–1092. doi: 10.1002/jmri.20900. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki M, Takai H, Sugiura S, et al. Peripheral MR angiography: Separation of arteries from veins with flow-spoiled gradient pulses in electrocardiography-triggered three-dimensional half-Fourier fast spin echo imaging. Radiology. 2003;227:890–896. doi: 10.1148/radiol.2273020227. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki M, Sugiura S, Tateishi F, et al. Non-contrast-enhanced MR angiography using 3D ECG synchronized half-Fourier fast spin echo. J Magn Reson Imaging. 2000;12:776–783. doi: 10.1002/1522-2586(200011)12:5<776::aid-jmri17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Foo TK, Ho VB, Marcos HB, et al. MR angiography using steady-state free precession. Magn Reson Med. 2002;48:699–706. doi: 10.1002/mrm.10278. [DOI] [PubMed] [Google Scholar]
- 32.Katoh M, Spuentrup E, Stuber M, et al. Free breathing renal magnetic resonance angiography with steady-state free-precession and slab selective spin inversion combined with radial k-space sampling and water-selective excitation. Magn Reson Med. 2005;53:1228–1233. doi: 10.1002/mrm.20467. [DOI] [PubMed] [Google Scholar]
- 33.Scheffler K, Lehnhardt S. Principles and applications of balanced SSFP techniques. Eur Radiol. 2003;13:2409–2418. doi: 10.1007/s00330-003-1957-x. [DOI] [PubMed] [Google Scholar]
- 34.Mihai G, Simonetti SP, Thavendiranathan P. Noncontrast MRA for the diagnosis of vascular diseases. Cardiol Clin. 2011;29:341–350. doi: 10.1016/j.ccl.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Hodnett PA, Koktzoglou I, Davarpanah AH, et al. Evaluation of peripheral arterial disease with nonenhanced quiescent-interval single-shot MR angiography. Radiology. 2011;260:282–293. doi: 10.1148/radiol.11101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodnett PA, Ward EV, Davarpanah AH, et al. Peripheral arterial disease in a symptomatic diabetic population: Prospective comparison of rapid unenhanced MR angiography (MRA) with contrast-enhanced MRA. AJR Am J Roentgenol. 2011;197:1466–1467. doi: 10.2214/AJR.10.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward EV, Galizia MS, Usman A, et al. Comparison of quiescent inflow single-shot and native space for nonenhanced peripheral MR angiography. J Magn Reson Imaging. 2013;38:1531–1538. doi: 10.1002/jmri.24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelman RR, Silvers RI, Thakrar KH, et al. Nonenhanced MR angiography of the pulmonary arteries using single-shot radial quiescent-interval slice-selective (QISS): A technical feasibility study. J Cardiovasc Magn Reson. 2017;19:48. doi: 10.1186/s12968-017-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prince MR. Contrast-enhanced MR angiography: Theory and optimization. Magn Reson Imaging Clin N Am. 1998;6:257–267. [PubMed] [Google Scholar]
- 40.Costello JR, Kalb B, Martin DR. Incidence and risk factors of gadolinium-based contrast agent immediate reactions. Top Magn Reson Imaging. 2016;25:257–263. doi: 10.1097/RMR.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 41.Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: Review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol. 2008;191:W307–W311. doi: 10.2214/AJR.07.3951. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin MH, Korthuis RJ, Sexton WL, et al. Regional muscle blood flow capacity and exercise hyperemia in high-intensity trained rats. J Appl Physiol. 1988;64:2420–2427. doi: 10.1152/jappl.1988.64.6.2420. [DOI] [PubMed] [Google Scholar]
- 43.Isbell DC, Epstein FH, Zhong X, et al. Calf muscle perfusion at peak exercise in peripheral arterial disease: Measurement by first-pass contrast enhanced magnetic resonance imaging. J Magn Reson Imaging. 2007;25:1013–1020. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kramer CM. Novel magnetic resonance imaging end points for physiological studies in peripheral arterial disease: Elegance versus practicality. Circ Cardiovasc Imaging. 2015;8:e003360. doi: 10.1161/CIRCIMAGING.115.003360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Detre JA, Zhang W, Roberts DA, et al. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7:75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 46.Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu W-C, Mohler E, Ratcliffe SJ, et al. Skeletal muscle microvascular flow in progressive peripheral arterial disease: Assessment with continuous arterial spin-labeling perfusion magnetic resonance imaging. J Am Coll Cardiol. 2012;53:2372–2377. doi: 10.1016/j.jacc.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollak AW, Meyer CH, Epstein FH, et al. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging. 2012;5:1224–1230. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez D, Pollak AW, Meyer CH, et al. Arterial spin labeling perfusion cardiovascular magnetic resonance of the calf in peripheral arterial disease: Cuff occlusion hyperemia vs exercise. J Cardiovasc Magn Reson. 2015;17:23. doi: 10.1186/s12968-015-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langham MC, Floyd TF, Mohler ER, III, et al. Evaluation of cuff-induced ischemia in the lower extremity by magnetic resonance oximetry. J Am Coll Cardiol. 2010;55:598–606. doi: 10.1016/j.jacc.2009.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ledermann HP, Heidecker H-G, Schulte A-C, et al. Calf muscles imaged at BOLD MR: Correlation with TcPO2 and flowmetry measurements during ischemia and reactive hyperemia – Initial experience. Radiology. 2006;241:477–484. doi: 10.1148/radiol.2412050701. [DOI] [PubMed] [Google Scholar]
- 54.Englund EK, Langham MC, Li C, et al. Combined measurement of perfusion, venous oxygen saturation, and skeletal muscle T2* during reactive hyperemia in the leg. J Cardiovasc Magn Reson. 2013;15:70. doi: 10.1186/1532-429X-15-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chance B. Applications of 31P NMR to clinical biochemistry. Ann N Y Acad Sci. 1984;428:318–332. doi: 10.1111/j.1749-6632.1984.tb12307.x. [DOI] [PubMed] [Google Scholar]
- 56.Bendahan D, Giannesini B, Cozzone PJ. Functional investigations of exercising muscle: A noninvasive magnetic resonance spectroscopy magnetic resonance imaging approach. Cell Mol Life Sci. 2004;61:1001–1015. doi: 10.1007/s00018-004-3345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isbell DC, Berr SS, Toledano AY, et al. Delayed calf muscle phosphocreatinine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47:22892295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kogan F, Harris M, Debrosse C, et al. In vivio chemical exchange saturation transfer imaging of creatinine (crCEST) in skeletal muscle at 3T. J Magn Reson Imaging. 2014;40:596–602. doi: 10.1002/jmri.24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haris M, Singh A, Kejia C, et al. A technique for in vivo mapping of myocardial creatinine kinase metabolism. Nat Med. 2014;20:209–215. doi: 10.1038/nm.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isbell DC, Meyer CH, Rogers WJ, et al. Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:71–76. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West AM, Anderson JD, Meyer CH, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon timing of statin initiation. Atherosclerosis. 2011;218:156–162. doi: 10.1016/j.atherosclerosis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guzman RJ, Brinkley DM, Schumacher PM, et al. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–1974. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with MRI—Initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 64.Davies MJ, Thomas AC. Plaque Assuring—The cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polonsky TS, Liu K, Tian L, et al. High-risk plaque in the superficial femoral artery of people with peripheral arterial disease: Prevalence and associated clinical characteristics. Atherosclerosis. 2014;237:169–176. doi: 10.1016/j.atherosclerosis.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li F, McDermott MM, Li D, et al. The association of lesion eccentricity with plaque morphology and plaque components in the superficial femoral artery: A high-spatial-resolution, multi-contrast weighted MRI study. J Cardiovasc Magn Reson. 2010;12:37. doi: 10.1186/1532-429X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDermott MM, Liu K, Li D, et al. Superficial femoral artery plaque, the Ankle Brachial Index, and leg symptoms in peripheral arterial disease: The Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging. 2011;4:246–252. doi: 10.1161/CIRCIMAGING.110.962183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDermott MM, Liu K, Carroll TJ, et al. Superficial femoral artery plaque and functional performance in peripheral arterial disease: The Walking and Leg Circulation Study (WALCS) III. JACC Cardiovasc Imaging. 2011;4:730–739. doi: 10.1016/j.jcmg.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDermott MM, Carroll TH, Kibbe M, et al. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–694. doi: 10.1016/j.jcmg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDermott MM, Carroll T, Carr J, et al. Femoral artery plaque characteristics, lower extremity collaterals, and mobility loss in peripheral artery disease. Vasc Med. 2017;22:473–481. doi: 10.1177/1358863X17729030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Botnar RM, Stuber M, Kissinger KV, et al. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 72.Simonetti OP, Finn JP, White RD, et al. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 73.Crowe LA, Gatehouse P, Yang GZ, et al. Volume-selective 3D turbospin echo imaging for vascular wall imaging and distensibility measurement. J Magn Reson Imaging. 2003;17:572–580. doi: 10.1002/jmri.10294. [DOI] [PubMed] [Google Scholar]
- 74.Mugler JP, III, Bao S, Mulkern RV, et al. Optimized singleslab three-dimensional spin-echo MR imaging of the brain. Radiology. 2000;216:891–899. doi: 10.1148/radiology.216.3.r00au46891. [DOI] [PubMed] [Google Scholar]
- 75.Mihai G, Chung YC, Kariisa M, et al. Initial feasibility of a multi-station high resolution three-dimensional dark blood angiography protocol for the assessment of peripheral arterial disease. J Magn Reson Imaging. 2009;30:785–793. doi: 10.1002/jmri.21923. [DOI] [PubMed] [Google Scholar]
- 76.Anderson JD, Epstein FH, Meyer CH, et al. Multifactorial determinants of functional capacity in peripheral arterial disease: Uncoupling of calf muscle perfusion and metabolism. J Am Coll Cardiol. 2009;54:628–635. doi: 10.1016/j.jacc.2009.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West AM, Anderson JD, Epstein FH, et al. Low-density lipoprotein lowering does not improve calf muscle perfusion, energetics, or exercise performance in peripheral arterial disease. J Am Coll Cardiol. 2011;58:1068–1076. doi: 10.1016/j.jacc.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.West AM, Anderson JD, Epstein FH, et al. Percutaneous intervention in peripheral artery disease improves calf muscle phosphocreatine recovery kinetics: A pilot study. Vasc Med. 2012;17:3–9. doi: 10.1177/1358863X11431837. [DOI] [PMC free article] [PubMed] [Google Scholar]


