Abstract
Beige adipocytes can dissipate energy as heat. Elaborate communication between metabolism and gene expression is important in the regulation of beige adipocytes. Although lipid droplet (LD) binding proteins play important roles in adipose tissue biology, it remains unknown whether perilipin 3 (Plin3) is involved in the regulation of beige adipocyte formation and thermogenic activities. In this study, we demonstrate that Plin3 ablation stimulates beige adipocytes and thermogenic gene expression in inguinal white adipose tissue (iWAT). Compared with wild-type mice, Plin3 knockout mice were cold tolerant and displayed enhanced basal and stimulated lipolysis in iWAT, inducing peroxisome proliferator–activated receptor α (PPARα) activation. In adipocytes, Plin3 deficiency promoted PPARα target gene and uncoupling protein 1 expression and multilocular LD formation upon cold stimulus. Moreover, fibroblast growth factor 21 expression and secretion were upregulated, which was attributable to activated PPARα in Plin3-deficient adipocytes. These data suggest that Plin3 acts as an intrinsic protective factor preventing futile beige adipocyte formation by limiting lipid metabolism and thermogenic gene expression.
Introduction
Adipose tissues are actively engaged in the regulation of energy homeostasis to respond to dynamic changes in obesity and cold acclimation (1,2). In mammals, adipose tissues have been traditionally classified into white adipose tissue (WAT) and brown adipose tissue (BAT). These two types of adipose tissues differ in various aspects, including anatomical locations, cellular morphologies, and metabolic characteristics. In WAT, adipocytes usually contain a large and unilocular lipid droplet (LD) and prominently maintain energy homeostasis not only by acting as a major energy depot but also by releasing various adipokines and lipid metabolites that have numerous effects on metabolic tissues (3,4). In contrast, BAT primarily governs nonshivering thermogenesis as well as energy expenditure in response to cold (2,3,5,6). BAT has uncoupling protein 1 (UCP1)–positive brown adipocytes that are packed with small and multilocular LDs and abundant mitochondria, leading to the dissipation of chemical energy in the form of heat (1,2,5,6). Recently, a new, distinct type of thermogenic adipocytes intermingled within WAT has been identified; these adipocytes were termed “beige” or “brite” adipocytes. In rodents, beige adipocytes are found in inguinal WAT (iWAT) upon cold or β-adrenergic stimuli and share several key characteristics with brown adipocytes including multilocular LDs, high mitochondrial density, and UCP1 expression (7,8). Nevertheless, brown and beige adipocytes arise from distinct developmental lineages with different features (7,9). Recent studies have shown that beige adipocytes appear to arise from transdifferentiation of mature white adipocytes (10,11) as well as de novo differentiation from beige adipocyte precursors (12,13). Upon cold exposure, hormones such as norepinephrine rewire transcriptional execution and metabolic regulation to induce beige/brite adipocyte differentiation in iWAT (1,14,15). In contrast, classic brown adipocytes originate from muscle-like cell lineages (9,16).
In eukaryotic cells, accumulated LDs contain neutral lipids, including triacylglycerides (TAGs) and cholesteryl esters, surrounded by a phospholipid monolayer. LDs are coated with LD-associated proteins such as perilipins (Plins) (17). In mammals, the Plin family is composed of five members, namely, Plin1 through 5. Among the five Plin isoforms, Plin1 has been identified as the major LD-coating protein in adipocytes (18). It has been suggested that Plins modulate intracellular lipid metabolism by regulating TAGs and cholesteryl esters within LDs in various cell types. For instance, Plin1 knockout (KO) mice have enhanced lipolysis in WAT and are resistant to diet-induced obesity (19,20). Moreover, adipose tissue–specific overexpression of Plin1 results in the reduction of LD size as well as WAT mass (21,22). Also, Plin2 KO mice are protected against hepatic lipid accumulation (17), whereas hepatic overexpression of Plin2 increases cellular TAGs and LD size, with reduced lipolysis (23). In addition, it has been reported that Plin5 plays a role in the regulation of LD hydrolysis in oxidative tissues (24,25). Thus, it appears that Plins stabilize and remodel intracellular LDs and influence lipid mobilization and utilization to regulate energy homeostasis. Nonetheless, the functional roles of Plin3 in adipocytes have not yet been thoroughly established.
In this study, we demonstrate that Plin3 deficiency enhances thermogenesis and beige adipocyte differentiation in iWAT by stimulating lipolysis and thermogenic gene expression. iWAT and differentiated adipocytes were examined for lipid metabolism and beige adipocyte gene index to gain mechanistic insights. In adipocytes, Plin3 deficiency stimulated fatty acid (FA) oxidation and the activity of peroxisome proliferator–activated receptor α (PPARα), one of the thermogenic transcription factors. To further investigate the roles of Plin3 in vivo, morphological changes and gene expression profiles in adipose tissues were scrutinized in Plin3 KO mice. In Plin3 KO mice, the expression of fibroblast growth factor 21 (FGF21) was augmented, at least partly, through PPARα activation. These data suggest that Plin3 might function as a negative regulator of thermogenesis in iWAT by limiting the availability of lipid metabolites.
Research Design and Methods
Animals and Metabolic Experiments
Plin3 KO mice were generated with the guidelines of the Animal Care and Use Committee of the National Institutes of Health. All animal experiments were conducted in compliance with protocols approved by the Institutional Animal Care and Use Committee at the Seoul National University (SNU-130508). Mice were maintained at 22–24°C in 12-h light/dark cycles and fed ad libitum with a standard rodent chow diet. For the cold tolerance test, 8–10-week-old male mice were placed in a cold room at 4°C (Testo Inc., Sparta, NJ) for 8 h. For thermoneutral and cold-exposure experiments, 8–10-week-old male mice were placed at 30°C for 7 days and then split into two groups: one group was exposed to thermoneutral condition and the other group to cold for 6 days. At the end of experiments, serum samples were collected. Free FAs (FFAs; Roche, Indianapolis, IN) and FGF21 (Antibody and Immunoassay Services, Pokfulam, Hong Kong) were measured according to the manufacturers’ protocols. Tissue samples were frozen for further analyses.
Thermal Imaging
The surface temperature of the mice was imaged using an infrared camera (CX320 Thermal Imaging Camera; COX Co., Seoul, Korea).
RNA Preparation and Reverse Transcription Quantitative PCR
RNA was extracted from cultured cells or frozen tissue samples using TRIzol (Ambion, Foster City, CA). For reverse transcription quantitative PCR (qRT-PCR), 1–3 µg total RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, Waltham, MA). SYBR Green reactions using the SYBR Green PCR Master mix (Enzynomics, Daejeon, South Korea) were assembled along with 10 pmol/L primers according to the manufacturer’s instructions. All primers used are listed with their sequences in Supplementary Table 1.
Adipocyte Differentiation and Cell Culture Experiments
Stromal vascular cells were prepared as previously reported (26) with minor modifications. Briefly, iWAT was dissected and washed with PBS, minced, and digested by collagenase I (Worthington Biochemical, Lakewood, NJ). Differentiation was initiated as described elsewhere (27). For small interfering RNA (siRNA) experiments to suppress PPARα or PPARγ, differentiated adipocytes were differentiated and transiently transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. siRNA duplexes were designed and purchased from Bioneer (Daejeon, South Korea; PPARα, 1411367; PPARγ, 1411392). At 24 h posttransfection, cells were treated with isoproterenol (Sigma-Aldrich, St. Louis, MO) or an equal amount of double-distilled H2O in serum-free DMEM. After incubation for 6–8 h, the cells were harvested for further analyses. To assess the effects of PPARα agonist and PPARα antagonist, cells pretreated with WY-14643 (Sigma-Aldrich) and GW-6471 (Sigma-Aldrich) for 48 h. Media were changed to DMEM containing 2% FA-free BSA, and cells were stimulated with 3 μmol/L isoproterenol or an equal amount of double-distilled H2O for 6–8 h.
Lipolysis
Differentiated mature adipocytes were chased with DMEM containing 2% FA-free BSA and treated with 50 μmol/L H-89 (Sigma-Aldrich) and/or 1 μmol/L isoproterenol. The levels of glycerol released into supernatants were quantified using a commercial kit (Sigma-Aldrich). Amounts of glycerol were normalized to the total protein content of the differentiated adipocytes using a Pierce BCA protein assay reagent (Thermo Fisher Scientific).
Immunoblotting and Histological Analysis
For immunoblotting, proteins were extracted and separated by SDS-PAGE and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were probed with primary antibodies against UCP1 (Abcam, Cambridge, MA) followed by horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). GAPDH was used as a loading control (Sigma-Aldrich). Band intensities were quantified using ImageJ (National Institutes of Health, Bethesda, MD). For immunohistochemistry, adipose tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Adipose tissues were stained with hematoxylin and eosin (H&E) or for UCP1. According to a previous report (28), quantitation of UCP1-positive adipocytes was carried out.
Mitochondrial Activity and Mitochondrial Nuclear DNA Quantification
For JC-1 staining, isolated mitochondria were incubated with 5 μg/mL JC-1 probe (Thermo Fisher Scientific) and then visualized under a Zeiss LSM 710 microscope (Carl Zeiss, Oberkochen, Germany). Total DNA from iWAT was extracted using the DNeasy blood and tissue kit (Qiagen, Germantown, MD), and the relative levels of mitochondrial DNA and nuclear DNA were quantified using primers specific for mitochondrial 16S rRNA and nuclear 18S rRNA genes. qRT-PCR primers are listed in Supplementary Table 1.
Cellular Oxygen Consumption
Cellular oxygen consumption rates (OCRs) of differentiated adipocytes were analyzed by Seahorse XFe24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) according to the manufacturer’s instruction. Mitochondrial proteins were isolated by the manufacturer’s protocol (Abcam). Values are normalized to average basal respiration.
Luciferase Assay
HEK293 cells were transiently transfected with various DNA plasmids (PPARα, retinoid X receptor α [RXRα], β-galactosidase, and DR-1) by the calcium-phosphate method, as described previously (29). Luciferase and β-galactosidase activities were measured according to the manufacturer’s protocol (Promega, Madison, WI). Relative luciferase activity was normalized to β-galactosidase activity in each sample.
Statistical Analysis
All values in graphs are presented as the mean ± SEM. Student t test was used for single comparisons. The error bars (SEM) shown for all results were derived from biological, not technical, replicates. Significant differences between two groups (*P < 0.05; **P < 0.01; ***P < 0.001) were evaluated by two-tailed unpaired t tests as the sample groups or by two-way ANOVA when two conditions were involved (GraphPad Software, La Jolla, CA).
Results
Plin3-Deficient Mice Are Cold Tolerant, With Increased Beige Adipocytes in iWAT
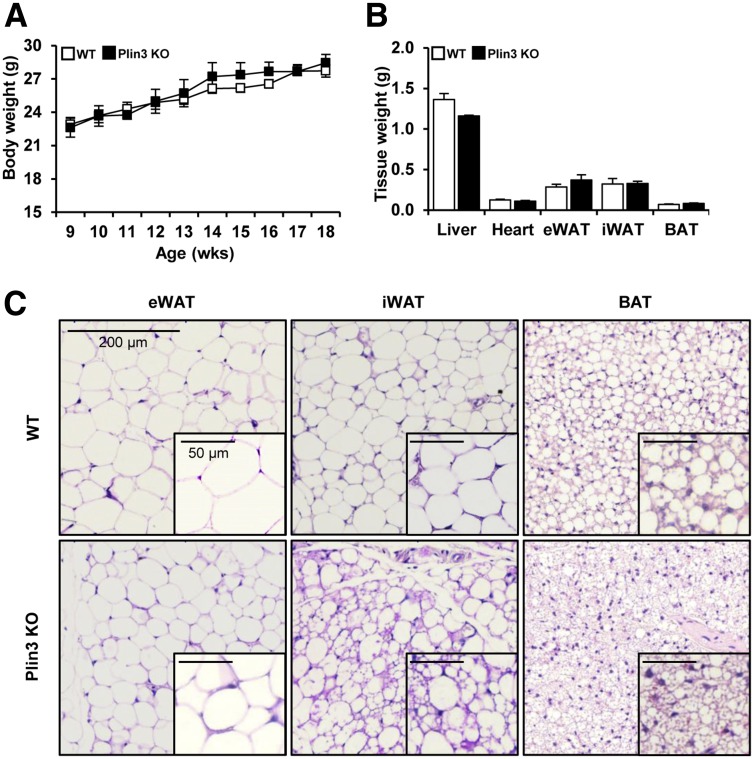
Unlike Plin1, Plin3 protein was ubiquitously expressed in various tissues, including WAT and BAT (Supplementary Fig. 1A). To further investigate the roles of Plin3 in lipid metabolism and fat tissue biology, we generated Plin3 KO mice with deletion of exons 3, 4, and 5 encoding the LD-binding domain of Plin3 (Supplementary Fig. 1B and C). Plin3 KO mice were born at Mendelian ratios and morphologically normal (Supplementary Fig. 1D). Even though body and various metabolic tissue weights of Plin3 KO mice were comparable to those of wild-type (WT) mice, LD morphologies in several adipose tissues including BAT, epididymal WAT, and iWAT of Plin3 KO mice were distinguishable from those of WT mice (Fig. 1).
Figure 1.
Plin3 deficiency stimulates the formation of small and multilocular LDs containing adipocytes. A: Body weights of WT and Plin3 KO mice at room temperature (n = 10 to 11 mice/group). B: Tissue weights in WT and Plin3 KO mice at 8–10 weeks of age (n = 6 mice/group). Mice were fed a normal chow diet. C: Representative images of H&E staining of adipose tissues of WT and Plin3 KO mice. Mice were fed a normal chow diet and grown at room temperature. Scale bars, 200 µm. The insets show LD morphology for each tissue at a higher magnification. Scale bars, 50 µm (n = 3 mice/group). Data represent the mean ± SEM. eWAT, epididymal WAT.
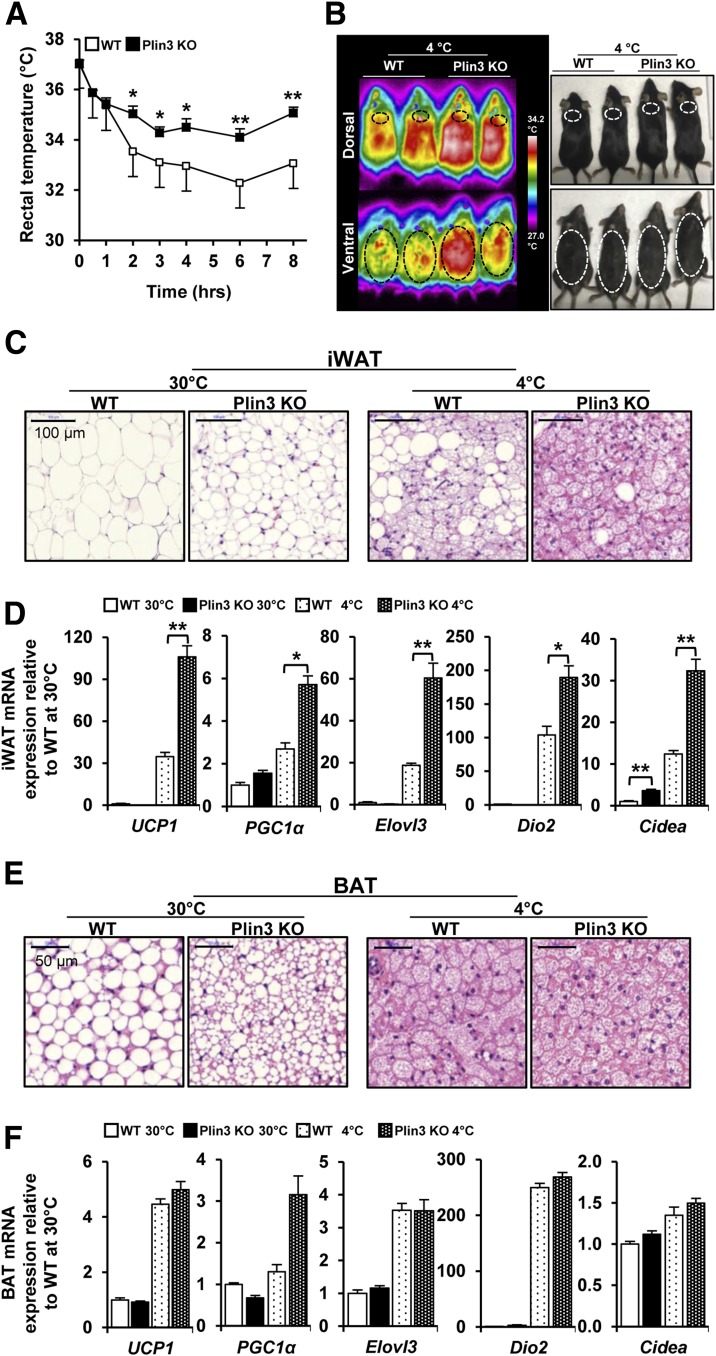
Given that the formation of multilocular LDs in WAT and BAT is one of the key phenotypic changes induced by thermogenic stimulation (7,30), we raised the question whether an increase in the number of adipocytes with multilocular LDs in adipose tissues of Plin3 KO might be associated with cold tolerance. Under thermoneutral (30°C) and cold temperature (4°C), body weight and the weights of several tissues including liver, epididymal WAT, iWAT, and BAT were not different between WT and Plin3 KO mice (Supplementary Fig. 2A). Interestingly, Plin3 KO mice were more cold tolerant than WT mice (Fig. 2A). Also, infrared imaging analysis revealed that cold-exposed Plin3 KO mice generated higher body temperature (Fig. 2B). At thermoneutral temperature, iWAT of Plin3 KO mice showed smaller adipocytes, with multilocular LDs, than iWAT of WT mice (Fig. 2C). Under cold conditions, the formation of small and multilocular LDs in iWAT was greatly increased in Plin3 KO mice as compared with WT mice (Fig. 2C). At thermoneutral temperature, mRNA of UCP1, a surrogate thermogenic marker, was barely detectable in iWAT from WT and Plin3 KO mice (Fig. 2D). On the contrary, compared with WT mice, UCP1 mRNA expression was markedly increased in iWAT from Plin3 KO mice upon cold stimulation. In addition, mRNA levels of other thermogenic genes including PGC1α, Elovl3, Dio2, and Cidea were greatly elevated in iWAT of Plin3 KO mice (Fig. 2D). Moreover, mRNA levels of beige adipocyte–specific genes including CD137, Tbx1, TMEM26, and Slc27a1 were further elevated in iWAT of Plin3 KO mice upon cold stimulation (Supplementary Fig. 2B). In BAT, Plin3 deficiency led to a reduction in LD size at thermoneutral temperature, whereas the size and shape of LDs were comparable between WT and Plin3 KO mice under cold stimulation (Fig. 2E). Compared with BAT of WT mice, mRNA levels of the thermogenic genes such as UCP1, PGC1α, Elovl3, Dio2, and Cidea were not significantly altered in BAT of Plin3 KO mice under both thermoneutral and cold temperatures (Fig. 2F). In accordance with these, Plin3 KO mice exhibited higher body temperature and energy expenditure than WT mice in the presence of CL-316,243, β3-adrenergic receptor agonist (Supplementary Fig. 2C and D). At room temperature, mRNA levels of thermogenesis, FA oxidation, and mitochondrial-related genes were slightly but not dramatically increased in Plin3 KO in iWAT (Supplementary Fig. 2E). Also, Plin3 KO mice appeared to exhibit elevated whole-body energy metabolism parameters at room temperature (Supplementary Fig. 2F). Taken together, these data indicate that Plin3 deletion would induce cold resistance and potentiates thermogenic gene expression in iWAT.
Figure 2.
Plin3 KO mice are cold tolerant and contain multilocular LDs in adipose tissues. A: Changes in rectal temperature during cold exposure (n = 6 mice/group, three independent experiments). B: Infrared (left) and photographic (right) images of surface body temperature of WT and Plin3 KO mice after cold exposure (n = 2 mice/group). C: Representative images of H&E staining of iWAT at 30°C or 4°C for 6 days (n = 3 mice/group). Scale bars, 100 µm. D: qRT-PCR analysis for thermogenic gene expression in iWAT of WT and Plin3 KO mice (n = 6 mice/group). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to WT at 30°C. E: Representative images of H&E staining of BAT at 30°C or 4°C for 6 days (n = 3 mice/group). Scale bars, 50 µm. F: qRT-PCR analysis for thermogenic gene expression in BAT of WT and Plin3 KO mice (n = 6 mice/group). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to WT at 30°C. Data represent the mean ± SEM. *P < 0.05; **P < 0.01 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control.
Plin3 Deficiency Activates UCP1 Expression in iWAT Upon Cold Exposure
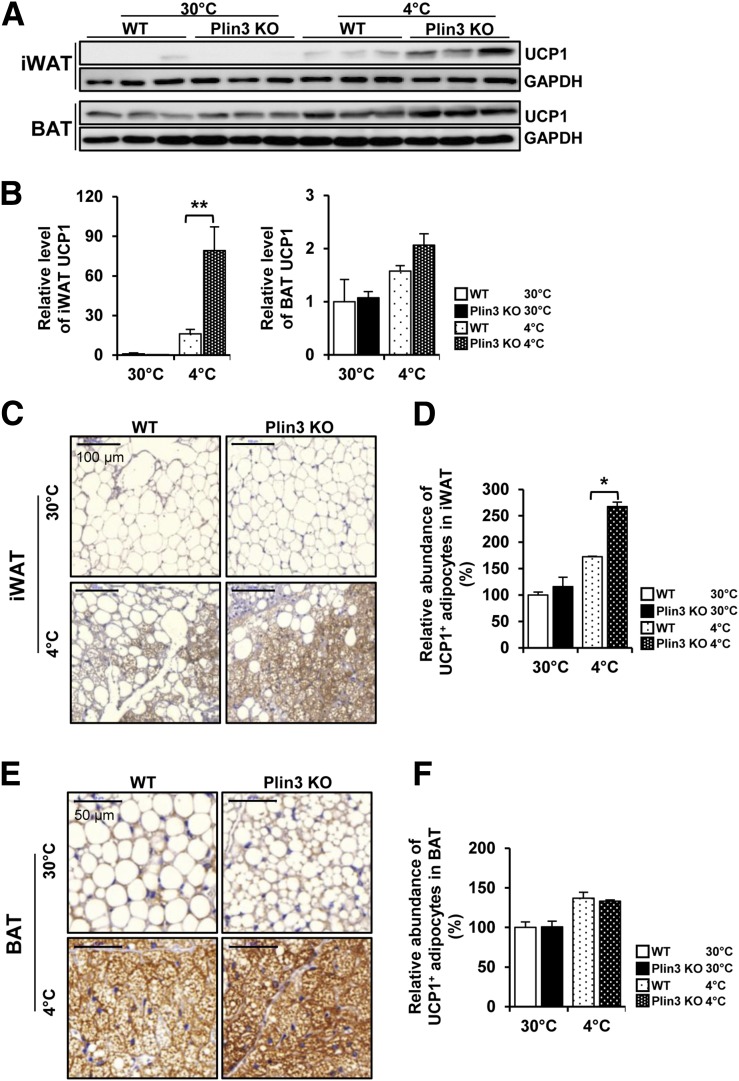
As beige adipocytes are characterized by enhanced UCP1 expression and thus contribute to thermogenic activity (8,11), we examined the expression level of UCP1 protein in iWAT. Under thermoneutral conditions, UCP1 protein was hardly detected in iWATs from both WT and Plin3 KO mice, whereas UCP1 protein was greatly upregulated in cold-exposed iWAT from Plin3 KO mice (Fig. 3A and B). Even though UCP1 protein was abundantly expressed in BAT under thermoneutral and cold conditions, the extent of UCP1 protein induction in BAT was lower than that in iWAT at cold conditions. At thermoneutral temperatures, the number of UCP1-positive adipocytes in iWAT did not differ between WT and Plin3 KO mice (Fig. 3C and D). In contrast, compared with cold-stimulated iWAT of WT mice, cold-stimulated iWAT of Plin3 KO mice showed more UCP1-positive adipocytes (Fig. 3C and D). However, BAT from WT or Plin3 KO mice exhibited similar abundances of UCP1-positive adipocytes at both thermoneutral and cold temperatures (Fig. 3E and F). As cold condition increased UCP1 expression in BAT from WT and Plin3 KO to a comparable extent, it appeared that iWAT, rather than BAT, might primarily contribute to the augmented thermogenic activity in Plin3 KO mice upon cold exposure. Collectively, these results suggest that deletion of Plin3 could stimulate UCP1-positive beige adipocytes in iWAT in response to cold exposure.
Figure 3.
Plin3 deficiency increases UCP1 expression in iWAT upon cold exposure. A: Immunoblots for UCP1 protein in iWAT and BAT of WT and Plin3 KO mice exposed to 30°C or 4°C for 6 days (n = 3 mice/group). GAPDH was used as a loading control. B: Quantitation of UCP1 protein levels in A and normalized to GAPDH protein. UCP1 levels are relative to WT at 30°C. C: Immunohistochemical staining of UCP1 in iWAT of WT and Plin3 KO mice exposed to 30°C or 4°C for 6 days (n = 3 mice/group). Scale bars, 100 µm. D: Quantitation of UCP1-positive adipocytes in C. UCP1 levels are relative to WT at 30°C. E: Immunohistochemical staining of UCP1 in BAT of WT and Plin3 KO mice exposed to 30°C or 4°C for 6 days (n = 3 mice/group). Scale bars, 50 μm. F: Quantitation of UCP1-positive adipocytes in E. UCP1 levels are relative to WT at 30°C. Data represent the mean ± SEM. *P < 0.05; **P < 0.01 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control.
Plin3 Deficiency Leads to an Increase in Lipolysis
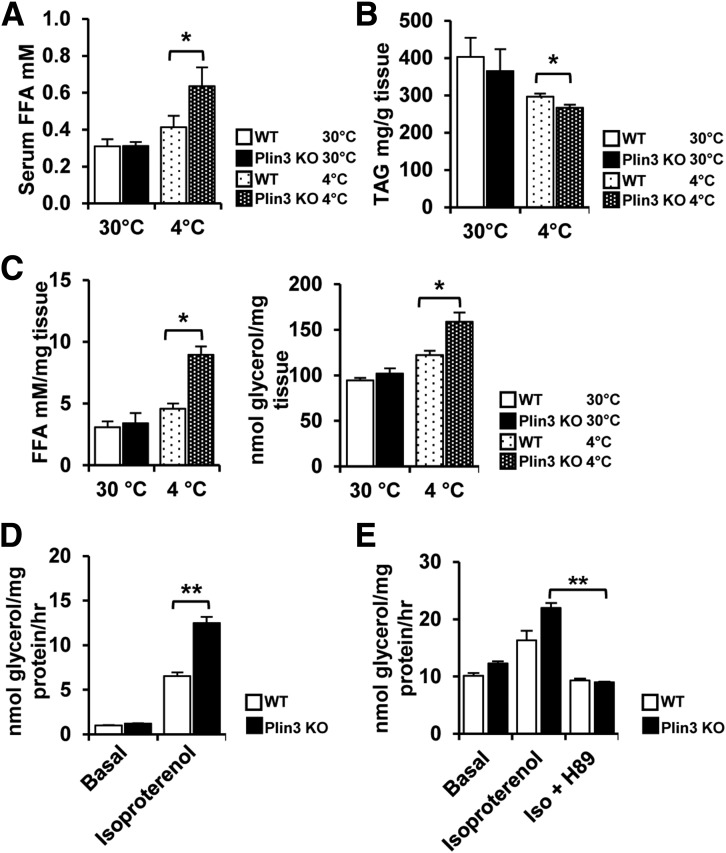
Plins have been implicated in the regulation of LD either through engaging in lipid storage or utilization (19,20,24). This led us to test whether TAG hydrolysis might be involved in small and multilocular LD formation in iWAT of Plin3 KO mice. When we analyzed the serum level of FFAs, it was not different in WT and Plin3 KO mice at thermoneutral temperature. However, after cold exposure, serum FFAs were significantly higher in Plin3 KO than in WT mice (Fig. 4A). In addition, the level of TAG in iWAT was decreased to a further extent in Plin3 KO mice upon cold stimulation (Fig. 4B). Next, we examined lipolytic activities by measuring FFAs and glycerol. Although the levels of FFAs and glycerol in iWAT from WT mice were similar to those of Plin3 KO mice at thermoneutral temperatures, both metabolites were further upregulated in cold-exposed iWAT of Plin3 KO mice (Fig. 4C), implying that iWAT of Plin3 KO mice appeared to be readily lipolytic in response to cold stimulation. Then, to determine whether increased lipolysis in Plin3-ablated iWAT might be cell autonomous, differentiated adipocytes from WT and Plin3 KO mice were subjected to measurement of lipolytic activities. To mimic cold exposure through β-adrenergic activation, differentiated adipocytes were treated with isoproterenol, a β-adrenergic activator. As shown in Fig. 4D, the level of released glycerol was further elevated in isoproterenol-treated Plin3 KO adipocytes, but not in Plin3 KO brown adipocytes (Supplementary Fig. 3). To confirm the increased lipolytic capacity of Plin3-deficient adipocytes, we examined downstream signaling cascade of protein kinase A (PKA), which is the key factor to mediate lipolysis. Compared with WT adipocytes, the phosphorylation levels of PKA substrates (Ser/Thr) and hormone-sensitive lipase (Ser563) were further increased in Plin3-deficient adipocytes upon isoproterenol (Supplementary Fig. 4A). In the LD fraction of Plin3-deficient adipocytes, the levels of ATGL and CGI58 proteins seemed to be further enhanced upon isoproterenol (Supplementary Fig. 4B). When PKA was inhibited by H-89, increased lipolytic activity in Plin3 KO adipocytes was nullified (Fig. 4E), implying that PKA might mediate enhanced lipolysis in Plin3-deficient adipocytes. Therefore, these data suggest that Plin3 deficiency would potentiate lipolysis in iWAT upon cold stimulus.
Figure 4.
Plin3 deficiency promotes lipolysis upon cold or isoproterenol stimulus. A: Serum FFA levels in WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3–5 mice/group). B: TAG levels in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 4 to 5 mice/group). C: FFA and glycerol levels in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3–5 mice/group). D: Stromal vascular cells were isolated from iWAT of WT and Plin3 KO mice and fully differentiated into adipocytes. For basal and stimulated lipolysis, differentiated adipocytes were treated with or without isoproterenol (1 μmol/L) for 3 h. The levels of glycerol were measured from conditional media. E: Differentiated adipocytes pretreated with or without H-89 (50 μmol/L; an inhibitor of PKA) followed by isoproterenol (Iso; 1 μmol/L) for 3 h. The levels of glycerol were measured from conditional media. Data represent the mean ± SEM. *P < 0.05; **P < 0.01 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control.
Plin3 Deficiency Promotes Mitochondrial Activity and FA Oxidation Upon Cold Stimulus
As Plin3 KO mice showed an enhanced induction of UCP1-positive beige adipocytes with elevated lipolytic activities during cold stimuli, we asked the question whether these cold-induced beige adipocytes in Plin3 KO mice might have altered mitochondrial activity and biogenesis. Compared with WT iWAT, Plin3 KO iWAT showed stronger increases in mRNA levels of the mitochondrial oxidative phosphorylation genes including ATPase, CytC, and Cox8b upon cold stimulation (Fig. 5A). To confirm this, mitochondrial OCRs were determined in differentiated adipocytes from WT and Plin3 KO mice. As shown in Fig. 5B, OCRs were slightly but significantly higher in Plin3 KO adipocytes not only in the basal state but also after stimulation with isoproterenol. The maximum respiratory capacity with carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone was further elevated in Plin3 KO adipocytes as compared with WT adipocytes. In addition, FA oxidation rate was significantly higher in cold-exposed Plin3 KO iWAT than in WT iWAT (Fig. 5C). To further investigate mitochondrial respiration capacity of iWAT, OCRs were measured in isolated mitochondria from WT iWAT and Plin3 KO iWAT. As shown in Fig. 5D, Plin3 deficiency showed elevated mitochondrial OCRs. Moreover, mitochondrial membrane potentials of isolated mitochondria from Plin3 KO iWAT were higher than those from WT iWAT (Fig. 5E). On the contrary, both WT and Plin3-deficient brown adipocytes exhibited similar degrees of OCRs (Supplementary Fig. 5A). Given that PKA activation enhanced lipolytic capacity in Plin3-deficient adipocytes (Fig. 4E), we decided to test whether mitochondrial activity in Plin3-deficient adipocytes might be regulated by PKA. As shown in Supplementary Fig. 5B, mitochondrial activity was augmented by forskolin in Plin3 KO adipocytes, whereas H-89 suppressed mitochondrial membrane potentials. Nonetheless, mRNA expression of the mitochondrial biogenesis genes such as Tfam and Nrf1 was not different between WT and Plin3 KO iWATs (Fig. 5F), which was confirmed by mitochondrial DNA measurements (Fig. 5G). Together, these results propose that Plin3 deficiency would promote mitochondrial activity and FA oxidation in cold-exposed iWAT.
Figure 5.
Plin3 deficiency promotes mitochondrial activity and FA oxidation upon cold exposure. A: qRT-PCR analysis of mitochondrial gene expression in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3 mice/group). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to WT at 30°C. B: OCRs of differentiated adipocytes from iWAT of WT or Plin3 KO mice. C: FA oxidation (FAO) rates in iWAT from WT or Plin3 KO mice exposed to 30°C or 4°C (n = 4 mice/group). D: OCRs of isolated mitochondria from iWAT of WT or Plin3 KO mice (n = 5 mice/group). E: JC-1 staining and fluorescence intensity of isolated mitochondria from iWAT of WT or Plin3 KO mice (n = 5 mice/group). Scale bars, 50 μm. F: qRT-PCR analysis of mitochondrial gene expression in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3 mice/group). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to WT at 30°C. G: qRT-PCR analysis of adipose tissue mitochondrial DNA (mtDNA) contents (mtDNA/nuclear [n]DNA ratio) in iWAT from WT and Plin3 KO mice (n = 5 mice/group). Data represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control. FCCP, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone.
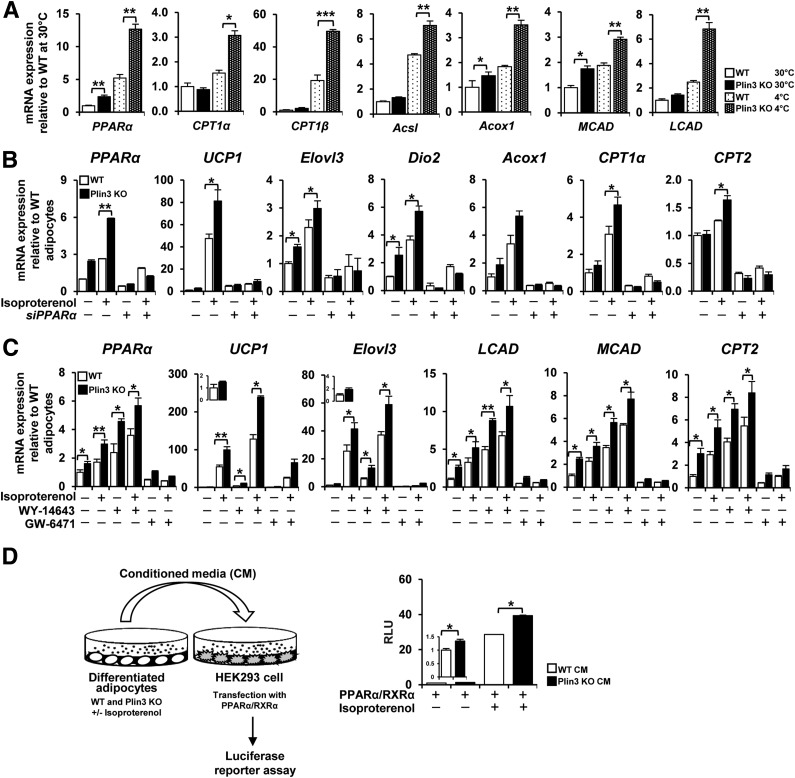
PPARα Stimulates Thermogenic Beige Adipocyte Formation in Plin3 KO Mice
To understand the molecular mechanisms underlying the promotion of beige adipocyte formation and thermogenic activation in Plin3 KO mice, we profiled gene expression, particularly, of genes associated with lipid metabolism. Transcript levels of PPARα and its target genes were higher in Plin3 KO iWAT than in WT iWAT, which was further boosted upon cold exposure (Fig. 6A). Then, to test whether PPARα would be indeed involved in the regulation of thermogenic gene expression in Plin3 KO iWAT, differentiated adipocytes were treated with or without isoproterenol in the absence or presence of PPARα siRNA. Similar to the data for iWAT, adipocytes from Plin3 KO mice expressed higher mRNA levels of PPARα and its target genes (Fig. 6B). Moreover, mRNA levels of the thermogenic genes including UCP1, Elovl3, and Dio2 were further elevated in isoproterenol-treated Plin3 KO adipocytes. In both WT and Plin3 KO adipocytes, mRNA levels of thermogenic genes and PPARα target genes were potently attenuated by PPARα suppression via siRNA. To confirm the above findings, we examined the effects of activated PPARα with WY-14643, a synthetic agonist of PPARα, on the expression of thermogenic and PPARα target genes. Activation of PPARα with WY-14643 augmented the expression of thermogenic genes and PPARα target genes, which were more strongly elevated in Plin3 KO adipocytes (Fig. 6C). Then, to evaluate the role of PPARα activation in Plin3 KO adipocytes, GW-6471, a synthetic antagonist of PPARα, was tested in differentiated adipocytes. Similar to the results obtained with PPARα siRNA, inactivation of PPARα with GW-6471 suppressed the mRNA levels of thermogenic genes and PPARα target genes (Fig. 6C). These results indicate that PPARα activation would play crucial roles in upregulating thermogenic gene expression in Plin3-deficient adipocytes. To further investigate whether elevated PPARα activity in Plin3-deficient adipocytes might be regulated by PKA, we examined mRNA levels of PPARα target genes with or without forskolin and/or H-89. As shown in Supplementary Fig. 6, the mRNA levels of PPARα target genes were elevated by forskolin, whereas such effects were abolished by H-89, indicating that increased PPARα activity in Plin3 KO adipocytes might potentiate thermogenic programing upon PKA activation. Next, to examine the possibility whether beige adipocyte formation in Plin3 KO mice might be due to Plin3-deficient adipocytes in a cell-autonomous manner, we tested the effects of suppression and overexpression of Plin3 in differentiated adipocytes. When Plin3 was suppressed via siRNA in adipocytes, their phenotypes and gene expression pattern were similar to Plin3 KO adipocytes (Supplementary Fig. 7). On the contrary, ectopic expression of Plin3 would reverse overall phenotypes of Plin3-deficient adipocytes (Supplementary Fig. 8). These results imply that beige adipocyte formation in Plin3 KO mice might be primarily due to Plin3 deficiency in adipocytes.
Figure 6.
PPARα plays a crucial role to stimulate thermogenic beige adipocytes in Plin3-deficient iWAT. A: qRT-PCR analysis of FA oxidation gene expression in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3 mice/group). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to WT controls. B: qRT-PCR analysis of PPARα and thermogenic marker genes in differentiated adipocytes with or without PPARα siRNA (siPPARα). Differentiated adipocytes were treated with or without isoproterenol (3 μmol/L) for 8 h. Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to untreated WT controls. C: PPARα and thermogenic gene expression by qRT-PCR analysis in differentiated adipocytes. Differentiated adipocytes were treated with PPARα agonist (WY-14643, 20 μmol/L) or PPARα antagonist (GW-6471, 10 μmol/L) for 48 h followed by 8-h incubation in the presence or absence of isoproterenol (3 μmol/L). D: PPARα activity by luciferase reporter assay with conditioned media (CM) from WT and Plin3 KO adipocytes by 6-h incubation in the absence or presence of isoproterenol (3 μmol/L). Relative luciferase activity was normalized to β-galactosidase activity in each sample. Data represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control. RLU, relative luminescence units.
To understand potential mechanism(s) how PPARα activity might be upregulated in Plin3-deficient adipocytes, we performed luciferase reporter assays (31,32). As shown in Fig. 6D, conditioned media from Plin3-deficient adipocytes promoted the transcriptional activity of PPARα with or without isoproterenol, implying that transcriptional activity of PPARα might be stimulated by secretory factor(s) from Plin3 KO adipocytes. Moreover, ectopic expression of Plin3 in Plin3 KO adipocytes did not alter mRNA levels of PPARα (Supplementary Fig. 8C), implying that Plin3 might not directly regulate PPARα expression in adipocytes. Taken together, these data propose that Plin3-deficient adipocytes might release unknown metabolite(s) that could stimulate PPARα activity.
In contrast, mRNA levels of several adipogenic and lipogenic genes under control of PPARγ were not different between WT and Plin3 KO, regardless of temperature (Supplementary Fig. 9A–C). Furthermore, although the expression of these genes was equally sensitive to PPARγ suppression through siRNA in both WT and Plin3 KO adipocytes in the thermogenic genes (e.g., UCP1, Elovl3, and Dio2), Plin3 KO adipocytes exhibited more responsive to β-adrenergic stimulation than in WT adipocytes upon PPARγ suppression (Supplementary Fig. 9D). Collectively, these results propose that PPARα activation would play an important role in promoting thermogenic gene expression in Plin3 KO adipocytes.
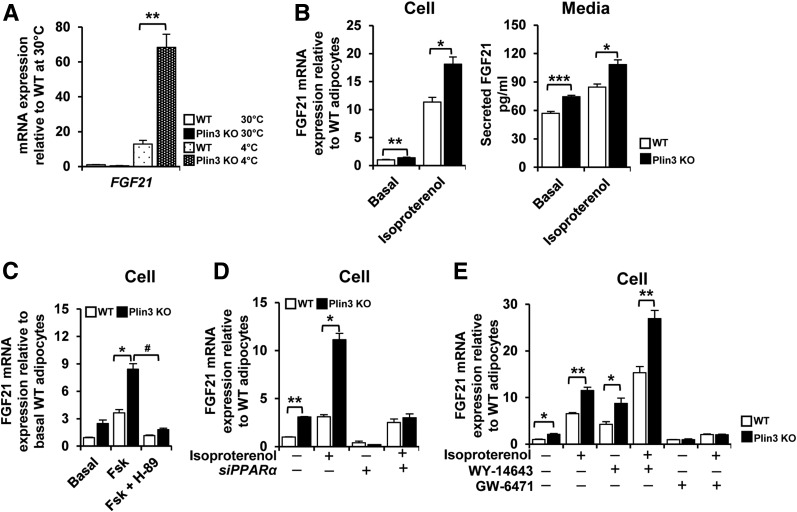
Plin3 Deficiency Results in Elevated FGF21 Expression
As FGF21 contributes to stimulation of beige adipocyte differentiation (33,34), we decided to test whether FGF21 might be associated with increased beige adipocytes in Plin3 KO mice. Upon cold exposure, the level of FGF21 mRNA was more highly elevated in iWAT of Plin3 KO mice than in that of WT mice (Fig. 7A). Compared with WT adipocytes, the levels of FGF21 mRNA and secreted FGF21 protein were enhanced in Plin3 KO adipocytes under basal and stimulated states (Fig. 7B). In Plin3-deficient adipocytes, forskolin-induced FGF21 expression was suppressed by H-89 (Fig. 7C). Next, to test whether elevated FGF21 expression in Plin3 KO adipocytes might be linked to PPARα activation, the level of FGF21 mRNA was examined in adipocytes with or without PPARα suppression. Although the levels of FGF21 mRNA were higher in Plin3 KO than in WT adipocytes with or without isoproterenol, these were nullified by PPARα suppression via siRNA (Fig. 7D). To investigate whether PPARα activation was directly involved in FGF21 expression, the effects of WY-14643 and GW-6471 on FGF21 expression were examined. In Plin3 KO adipocytes, FGF21 mRNA expression was promoted by WY-14643, whereas it was abolished by GW-6471 (Fig. 7E). Together, it is likely that PPARα would be one of the key factors to upregulate FGF21 expression in Plin3 KO adipocytes, which would, at least partly, contribute to induce beige adipocyte differentiation.
Figure 7.
Plin3 deficiency upregulates FGF21 through PPARα activation. A: qRT-PCR analysis of FGF21 gene expression in iWAT from WT and Plin3 KO mice exposed to 30°C or 4°C (n = 3 mice/group). FGF21 mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to untreated WT controls. B: Differentiated adipocytes from WT and Plin3 KO mice were treated with or without isoproterenol (1 μmol/L) for 3 h. FGF21 mRNA and secreted FGF21 proteins were determined. FGF21 mRNA level was normalized to cyclophilin mRNA, and FGF21 expression levels are relative to untreated WT controls. C: Differentiated adipocytes pretreated with or without H-89 (50 μmol/L) followed by forskolin (Fsk; 10 μmol/L; an activator of adenylyl cyclase) for 3 h. FGF21 mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to untreated WT controls. D: qRT-PCR analysis of FGF21 mRNA in differentiated adipocytes with or without PPARα siRNA (siPPARα) in the presence or absence of isoproterenol (3 μmol/L) for 8 h. E: qRT-PCR analysis of FGF21 mRNA in differentiated adipocytes treated with or without PPARα agonist (WY-14643, 20 μmol/L) or PPARα antagonist (GW-6471, 10 μmol/L) for 48 h followed by 8-h incubation in the presence or absence of isoproterenol (3 μmol/L). Each mRNA level was normalized to cyclophilin mRNA, and mRNA expression levels are relative to untreated WT controls. Data represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 indicate significant differences between groups as determined by either two-tailed unpaired Student t tests or two-way ANOVA versus control; #P < 0.05 indicates significant differences between Plin3 KO + Fsk vs. Plin3 KO with Fsk + H-89, as determined by two-tailed unpaired Student t tests.
In order to investigate whether PPARα activity would indeed be crucial in Plin3-deficient adipocytes, the effects of GW-6471 on thermogenic activity and FGF21 expression were examined. In Plin3-deficient adipocytes, PPARα antagonist GW-6471 downregulated OCRs (Supplementary Fig. 10A). Moreover, GW-6471 reduced rectal temperature in Plin3 KO mice (Supplementary Fig. 10B). In accordance with these, mRNA levels of PPARα and its target genes including Acox1, Acsl, UCP1, and FGF21 were abolished by GW-6471 in Plin3 KO iWAT (Supplementary Fig. 10C). These results imply that PPARα activation would be important for thermogenic capacity of Plin3 KO mice.
Discussion
Recent studies have focused on the regulation of nonshivering thermogenesis in BAT and iWAT. However, it is largely unknown whether LD-associated proteins such as Plin3 might contribute to modulating thermogenic beige adipocytes. In this study, we showed that Plin3 deficiency promoted beige adipocyte formation through PPARα activation. Plin3 KO mice exhibited small and multilocular LDs in iWAT and BAT, accompanied with cold tolerance. In Plin3 KO iWAT, LD remodeling appeared to be associated with augmented thermogenic activities such as upregulated UCP1 expression and enhanced lipolysis and mitochondrial activity. Interestingly, Plin3 KO adipocytes showed enhanced PPARα activation, leading to thermogenic gene expression. Therefore, these findings uncover a novel role of Plin3 as a protective factor inhibiting unnecessary beige adipocyte formation in iWAT.
LDs are involved in lipid metabolism by storing and releasing lipid metabolites (17). Accumulating evidence suggests that Plin-mediated lipid metabolism might influence whole-body metabolism. For instance, it has been reported that Plin3 is involved in obesity-induced dysregulation of lipid metabolism and insulin sensitivity in liver and skeletal muscle, respectively (35,36). In WAT, Plin1 overexpression promotes beige adipocyte-like phenotypes through suppressing lipogenesis and FSP27 expression (21). High-fat diet (HFD)–fed Plin2 KO mice showed suppression of hepatic steatosis and increased small LD-containing adipocytes, accompanied with increased UCP1 expression in iWAT (37). To date, it has not been investigated whether Plin3 might be involved in beige adipocyte formation as well as lipid metabolism in adipose tissue. We provided several lines of evidence that deletion of Plin3 promoted beige adipocytes in iWAT upon cold stimuli. Firstly, Plin3 KO mice harbored more small and multilocular LDs in iWAT at cold temperature. Secondly, mRNA levels of thermogenic genes were elevated in iWAT of Plin3 KO mice upon cold stimuli. Moreover, Plin3 KO mice were cold tolerant during cold exposure, implying that Plin3 deficiency potentiated multilocular LD formation with execution of thermogenic gene reprograming to maintain body temperature under cold. Lastly, cold-stimulated UCP1 expression was elevated to a higher level in iWAT of Plin3 KO mice than in that of WT mice. As the proportion of iWAT is greater than that of BAT in the whole body, it is plausible to speculate that iWAT of Plin3 KO mice might primarily contribute to cold tolerance by enhancing beige adipocytes.
It has been reported that stimulation by β3-adrenergic receptor agonists or low-temperature exposure promotes lipolysis and oxidative metabolism in adipose tissue and thereby boosts thermoregulatory responses (38,39). During lipolysis, FAs are released from hydrolysis of TAG and serve as both activators and metabolic substrates for thermogenic fuels (1). Although ATGL KO mice and adipose-specific CPT2 KO mice are defective for induced thermogenic activity (40,41), it remains largely unknown whether lipolysis in WAT is indeed crucial for beige adipocytes and thermogenesis for cold adaptation. In this study, we demonstrated that lipolysis and mitochondrial FA oxidation were elevated in iWAT of Plin3 KO mice upon cold exposure, potentially, resulting in elevated thermogenic responses. Compared with WT mice, Plin3 KO mice displayed elevated serum FFAs and reduced TAG amounts in iWAT after cold stimuli. In addition, the levels of released FFAs and glycerol were increased in iWAT of Plin3 KO mice upon cold stimuli. Isoproterenol, as a β-adrenergic activator, mimics the changes induced by cold stimulation. Indeed, isoproterenol increased the level of released glycerol in Plin3-deficient adipocytes, concomitantly with elevated small and multilocular LDs. In Plin3 KO mice, there are at least two possible pathways to promote thermogenesis in beige adipocytes upon cold: 1) lipid metabolites might activate PPARα and 2) increased FAs might augment mitochondrial activity.
Compared with other nuclear hormone receptors, PPARs have relatively large ligand-binding domains (42,43). Although endogenous ligands of PPARs have not been clearly elucidated, it has been reported that long-chain FAs are able to activate PPARs including PPARα by binding to their ligand-binding domains (44). Various lipid metabolites are able to promote thermogenic programing in adipocytes (45,46). For example, activated PPARs upregulate expression of UCP1 and FA oxidation genes that are required for thermogenic activation (47,48). Moreover, it has been shown that activated PPARα potentiates beige adipocytes (44,49). In this study, we demonstrated that mRNA levels of PPARα and its target genes in iWAT of Plin3 KO mice were promoted under cold conditions. In this regard, our following data suggested that activated PPARα plays a key role in potentiating beige adipocytes in Plin3 KO mice upon cold stimulation. Firstly, suppression of PPARα expression downregulated expression of thermogenic genes and PPARα target genes in Plin3-deficient adipocytes. Secondly, PPARα agonists stimulated the expression of thermogenic genes as well as PPARα target genes, whereas PPARα antagonist abolished these responses in Plin3-deficient adipocytes. Lastly, in Plin3 KO adipocytes, the level of FGF21 expression was upregulated by activated PPARα, whereas inhibition of PPARα with either siRNA or GW-6471 nullified FGF21 expression in Plin3 KO adipocytes. Although PPARγ is a master adipogenic transcription factor and plays a crucial role in inducing beige adipocytes (50,51), it is likely that PPARγ is not involved in the regulation of thermogenesis or beige adipocyte differentiation in iWAT of Plin3 KO mice. Together, these data propose that Plin3 deletion could augment thermogenic reprograming in iWAT through PPARα activation.
As lipolytic products, increased FAs released from iWAT contribute to the enhancement of mitochondrial activity and UCP1 activity for thermogenic regulation. In addition, it has been reported that FAs promptly stimulate mitochondrial UCP1 activation and provide fuel for heat generation rather than ATP production (46,52). Given that iWAT of Plin3 KO mice showed increased lipolysis, it seems that elevated lipid metabolites in iWAT of Plin3 KO might induce UCP1-dependent thermogenesis, accompanied by enhanced mitochondrial activity and FA oxidation. In accordance with this, we observed that Plin3 KO adipocytes exhibited increased mitochondrial activity and gene expression. We also observed that iWAT of cold-exposed Plin3 KO mice boosted lipolysis, mitochondrial activity, and FA oxidation without altering mitochondrial biogenesis. These data imply that Plin3 deficiency could contribute to nonshivering thermogenesis, at least partly, by upregulating mitochondrial activity and FA oxidation in iWAT. Although WAT has less oxidative capacity and lower ability to oxidize FA than BAT, rodent WAT is capable to stimulate FA oxidation in response to adrenergic activation (53). Collectively, it is likely that Plin3-deficient iWAT could enhance mitochondrial respiration and FA oxidation upon adrenergic activation or cold stimulus, leading to further thermogenic activities through activated PPARα. Compared with HFD-fed WT mice, HFD-fed Plin3 KO mice exhibited improved metabolic phenotypes assessed by glucose tolerance test, insulin tolerance test, and lipolytic activity (Supplementary Fig. 11). Although it remains unclear how Plin3 ablation could affect metabolic phenotypes in obesity, pathophysiological roles of Plin3 need to be further investigated.
In summary, we demonstrate that Plin3 deficiency in iWAT could increase lipolysis, which would activate PPARα to stimulate thermogenic reprograming in beige adipocytes under cold conditions (Fig. 8). Therefore, our data suggest that Plin3 in adipose tissue might act as a barrier protein to reserve LD as an energy reservoir, which might prevent unnecessary energy burning for beige adipocyte induction.
Figure 8.
Schematic proposed model. In Plin3 KO iWAT upon cold stimulation, LDs are remodeled to small and multilocular LDs that promote lipolysis and FA oxidation, leading to PPARα activation, to induce thermogenesis.
Supplementary Material
Article Information
Acknowledgments. The authors thank the members of the Laboratory of Adipocyte and Metabolism Research for helpful discussion.
Funding. This work was supported by grants from the National Creative Research Initiative Program (2011-0018312) funded by the Ministry of Education, Science and Technology. This work was also partly supported by Korea Mouse Phenotyping Project (2013M3A9D5072550) of the Ministry of Science and ICT through the National Research Foundation. This work was also supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.K.L. designed and conducted the study, performed experiments, and wrote the manuscript. J.H.S., J.S.H., Y.G.J., and Y.J. contributed to performing animal experiments and discussed the data. Y.J.P. discussed the data and contributed to writing the manuscript. K.T.D. and C.S. contributed to the design and generation of the perilipin 3 KO animals and discussion. A.R.K. participated in the design, generation, and propagation of the perilipin 3 KO animals and in the editing and discussion of the manuscript. J.B.K. supervised the whole project, discussed the data, and edited the manuscript. J.B.K. is the guarantor of this work and, as such, had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0983/-/DC1.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 2011;214:242–253 [DOI] [PubMed] [Google Scholar]
- 3.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19:1252–1263 [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006;444:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 2012;109:10001–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidossis LS, Porter C, Saraf MK, et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab 2015;22:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbatelli G, Murano I, Madsen L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010;298:E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 11.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000;279:C670–C681 [DOI] [PubMed] [Google Scholar]
- 12.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013;19:1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QA, Scherer PE. The AdipoChaser mouse: a model tracking adipogenesis in vivo. Adipocyte 2014;3:146–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao W, Medvedev AV, Daniel KW, Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 2001;276:27077–27082 [DOI] [PubMed] [Google Scholar]
- 15.Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 2015;26:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol 2016;17:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmel AR, Sztalryd C. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu Rev Nutr 2016;36:471–509 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 1991;266:11341–11346 [PubMed] [Google Scholar]
- 19.Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 2000;26:474–479 [DOI] [PubMed] [Google Scholar]
- 20.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A 2001;98:6494–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawada T, Miyoshi H, Shimada K, et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS One 2010;5:e14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi H, Souza SC, Endo M, et al. Perilipin overexpression in mice protects against diet-induced obesity. J Lipid Res 2010;51:975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura M, Inoguchi T, Ikuyama S, et al. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab 2002;283:E775–E783 [DOI] [PubMed] [Google Scholar]
- 24.Kuramoto K, Okamura T, Yamaguchi T, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem 2012;287:23852–23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Sreenivasan U, Hu H, et al. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria [published correction appears in J Lipid Res. 2013;54:3539]. J Lipid Res 2011;52:2159–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huh JY, Kim JI, Park YJ, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol 2013;33:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013;504:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei H, Chiba S, Moriwaki C, et al. A clinical approach to brown adipose tissue in the para-aortic area of the human thorax. PLoS One 2015;10:e0122594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995;83:803–812 [DOI] [PubMed] [Google Scholar]
- 30.Okamatsu-Ogura Y, Fukano K, Tsubota A, et al. Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PLoS One 2013;8:e84229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARalpha promoter: identification of a functional nuclear receptor response element. Mol Endocrinol 2002;16:1013–1028 [DOI] [PubMed] [Google Scholar]
- 32.Liu GH, Qu J, Shen X. Thioredoxin-mediated negative autoregulation of peroxisome proliferator-activated receptor alpha transcriptional activity. Mol Biol Cell 2006;17:1822–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher FM, Kleiner S, Douris N, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012;26:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen BM, Ding X, Morgan DA, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 2014;20:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr RM, Patel RT, Rao V, et al. Reduction of TIP47 improves hepatic steatosis and glucose homeostasis in mice. Am J Physiol Regul Integr Comp Physiol 2012;302:R996–R1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinert M, Parker BL, Chaudhuri R, et al. mTORC2 and AMPK differentially regulate muscle triglyceride content via Perilipin 3. Mol Metab 2016;5:646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McManaman JL, Bales ES, Orlicky DJ, et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J Lipid Res 2013;54:1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cypess AM, Weiner LS, Roberts-Toler C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 2015;21:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res 2014;55:2276–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006;312:734–737 [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Reports 2015;10:266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem 2010;285:40409–40415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 1998;395:137–143 [DOI] [PubMed] [Google Scholar]
- 44.Hondares E, Rosell M, Díaz-Delfín J, et al. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem 2011;286:43112–43122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J Biol Chem 2012;287:25038–25048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012;151:400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 2011;1812:1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012;15:395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem 2001;276:1486–1493 [DOI] [PubMed] [Google Scholar]
- 50.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 1994;8:1224–1234 [DOI] [PubMed] [Google Scholar]
- 52.Shu L, Hoo RL, Wu X, et al. A-FABP mediates adaptive thermogenesis by promoting intracellular activation of thyroid hormones in brown adipocytes. Nat Commun 2017;8:14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am J Physiol Endocrinol Metab 2005;289:E617–E626 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.