Abstract
Analysis of mouse behavior often requires expensive equipment and transfer of the mice to new test en- vironments, which could trigger confounding behavior alterations. Here, we describe a system for track- ing mouse behavior in home cages using a low-cost USB webcam and free software (Fiji and wrMTrck). We demonstrate the effectiveness of this method by tracking differences in distance traveled, speed, and movement tracks between wild-type mice and amyotrophic lateral sclerosis (ALS) model mice (SOD1G93A).
METHOD SUMMARY
Here, we present a new method for tracking mouse behavior in home cages. Our approach allows quantification of distance traveled, average speed, and movement tracks using a significantly lower-cost system compared with commercial alternatives.
Online Protocols and Supplementary Materials for this article is available at www.BioTechniques.com/.
INTRODUCTION
Precise and accurate behavior analyses are essential for phenotyping disease-model mice and genetically modified mice (1). Although methods exist for measuring spontaneous behaviors of mice (actometers, running wheels, etc.), methods for quantifying distance traveled and tracking movement patterns in a home cage are limited. For example, running wheels allow measurement of the distance traveled in a home cage, but the results are complicated by the exercise effect of the activity in the wheel.
Commercial systems and custom systems can measure the distance traveled inside a cage and analyze mouse behaviors (Supplementary Table S1) (2–10); however, these systems use specialized cages, requiring animals to be transferred to a new environment for analysis. For some strains, altered behavior has been observed even after 3 weeks of habituation to a commercial metabolic cage system (11). Some commercial systems can analyze behavior in home cages, but one such system does not measure distance traveled (HomeCageScan; CleverSys Inc, Reston, VA), and another system monitors activity but does not accurately measure distance traveled (Infrared Motion Detector; Starr Life Sciences Corp., Oakmont, PA).
Here, we address these limitations by developing a system to track mouse behavior using free public domain software for video tracking: wrMTrck and Fiji. wrMTrck was developed to track the behavior of Caenorhabditis elegans and has also been applied successfully to track melanoblasts, mitochondria, Schistosoma mansoni, Xenopus tropicalis tadpoles, and yeast (12–17). Our method, which we named Mouse Behavior Tracker, measures distance traveled, average speed, and movement patterns/tracks in home cages using economical USB-webcams, free software, and a standard desktop computer. When Mouse Behavior Tracker results were compared with a commercial system that uses a video tracking method, EthoVision XT (Noldus Information Technology Inc., Leesburg, VA), very similar results were obtained.
MATERIALS AND METHODS
Animal models
Animal experiments were performed in accordance with the animal care and use protocol approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and the Guidelines for the Care and Use of Laboratory Animals. Three to six mice were analyzed for each genotype or coat color. Mice with black coat color: C57BL/6J wild-type male mice (Stock No: 000664) and SOD1G93A male mice (B6.Cg-Tg(SOD1*G93A) 1Gur/J, Stock No: 004435, high-copy number line on a pure B6 background) were purchased from the Jackson Laboratory (JAX, Bar Harbor, ME, USA) and analyzed in this study. Mice with brown coat color: Rosa tdTomato-EGFP, Wnt1-Cre male mice (Gt(ROSA)26Sor_tm4(ACTB-tdTomato,-EGFP)Luo_J (JAX stock No: 007576) X Tg(Wnt1-cre)11Rth Tg(Wnt1-GAL4)11Rth_J (JAX stock No: 003829)) and a littermate wild-type female mouse were analyzed in this study. White coat color mice: two female wild-type mice were analyzed in this study. The mice used in this study had been bred in our institutional animal facility, kept on a 14 hrs light and 10hrs dark cycle between 6am to 8pm, and had been fed ad libitum with normal chow (LabDiet, 53WU, PicoLab Rodent Diet 20). No statistical methods were used to predetermine sample size. A method of randomization was not used for Figure 3 because the animals were separated by genotype.
Method/Video recording
Mouse behavior was recorded using an economical webcam (Creative Webcam Instant Model VF 0040; Creative Technologies Ltd. Jurong East, Singapore) with the following settings: 320 × 240 pixels, 30 frames per second (fps), MJPEG compressor. Recordings were done between 12:00 PM and 4:00 PM under room ceiling fluorescent lighting as 20-min videos.
Tracking mouse behavior using Fiji
The analysis protocol described here was developed for macOS 10.12 using Fiji (version 1.51h, https://fiji.sc/) running on a Mac (Macbook Pro; CPU, Intel Core i7 2.8 GHz; Flash Storage 751 GB; RAM, 16GB 1.6 GHz DDR3; Graphics NVIDIA GeForce GT 650M 1024 MB; operating system, macOS 10.12.6). The RAM size of 16 GB was sufficient to process the 30 fps videos up to 30 minutes. The combination of Fiji, wrMTrck plugin, and the Mouse Behavior Tracker macro was confirmed to function properly on Mac OsX 10.9, 10.11, and macOS 10.12. Mouse behavior was tracked using the publicly available free software Fiji (https://fiji.sc/), wrMTrck plugin for ImageJ ("wrmtrck.zip" developed by Jesper Søndergaard Pedersen, http://www.phage.dk/plugins/wrmtrck.html), and our Mouse Behavior Tracker macro. The Mouse Behavior Tracker source codes for the AVI (MJPEG) file format and MP4 file format are included with the protocol in the Supplementary Material. The same video data files were analyzed using EthoVision XT (Noldus Information Technology Inc. Leesburg, VA, USA) for validation.
Statistics
All statistics were performed using GraphPad Prism software version 6. Significance was assessed by an unpaired t-test with a two-tailed p value. The p value and the n are described in the text, and the data are shown as the mean ± S.E.M.
A detailed protocol is available in the Online Protocols and Supplementary Materials for this article at www.BioTechniques.com/.
RESULTS AND DISCUSSION
Mouse behavior was recorded in a home cage with an inner lid without the filter tops using a webcam that was positioned above the cage with a chromatography column stand and adjustable clamps. An economical USB webcam provided sufficient sensitivity, resolution, and frame rate (Creative live webcam instant VF 0040, 320 × 240 pixels, 30 fps) to record mouse behavior in the home cage (Figure 1, Supplementary Video S1). File sizes for 20-min videos averaged 123 MB. Therefore, a desktop computer with standard hard disk storage space was sufficient for the recording (Dell Precision PWS390; CPU, Intel Core2 Duo 6600, 2.4 GHz; RAM, 2GB 2.3 GHz; internal hard disk, 148GB; operating system, Windows XP Professional Version 2002 service pack 3).
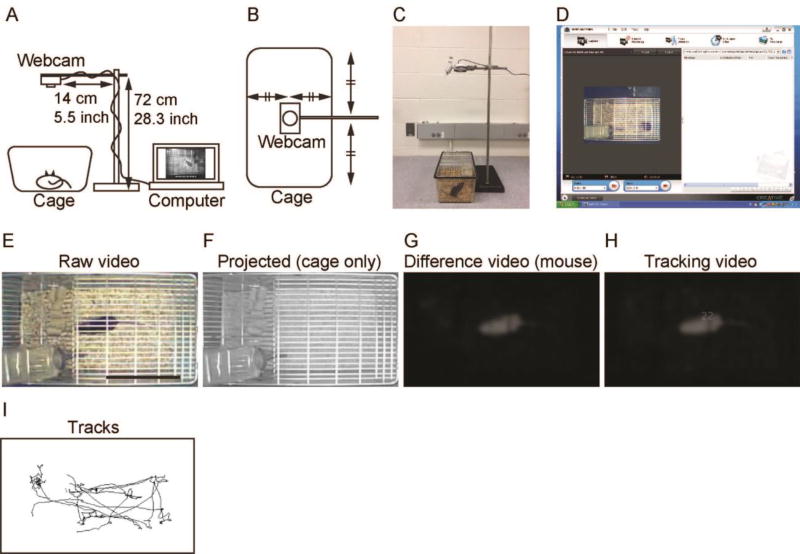
Figure 1. Mouse Behavior Tracker method.
Schematic diagram of the video recording setup showing the side view (A) and the top-down view (B), with dimensions. (C) A mouse being recorded by a Creative WebCam Instant Model VF 0040. (D) A screenshot of the Creative camera software showing a recording of a mouse. Representative images are shown from a raw video of a C57BL/6J wild-type mouse recorded at 30 frames per second (fps) (E), an average intensity-projected image showing the cage without the mouse (F), the differential binary-image video showing the mouse after subtracting the projected empty cage image from the raw video (G), the tracking video showing the mouse with a tracking number assigned by Mouse Behavior Tracker (H), and movement tracks of the mouse for a 1-min period (I). Scale bar in (E): 10 cm.
The 20-minute videos were analyzed using Fiji (ImageJ package software) running on an iMac with RAM size increased to 24GB (iMac 14.2, CPU, Intel Core i5 3.2 GHz; Hard disk size, 1TB; RAM, 24GB 1.6 GHz DDR3; operating system, Mac OsX 10.9.5). The videos were Z-projected in Fiji using the “Average Intensity Type” to generate an image of the cage without a mouse (Figure 1F). This cage image was subtracted from each frame of the 20-minute video to generate a video of a differential binary image of mouse behavior without the cage (Figure 1G). The differential binary image video was processed using the “Gaussian Blur” filter to remove shadows from the wire lid of the cage above the animal. The resulting time-lapse video was thresholded and tracked using Fiji and wrMTrck plugin for ImageJ. The wrMTrck plugin is based on the Object Tracker plugin developed by Dr. Wayne Rasband who developed ImageJ at NIH. The two plugins use the Pythagorean theorem to calculate the distance traveled of the tracked object. The 20-minute video was trimmed down to 1 minute to show representative results of a movement track and a tracking video (Figure 1H and Supplementary Video S2). The representative result of a movement track generated from a 20-minute video is shown in Figure 3C. These results suggest that Mouse Behavior Tracker is capable of tracking mouse behavior in videos ranging from one minute to 20 minutes. A Fiji macro was created for these processes (described in the Supplementary Material). Mouse Behavior Tracker generates the following data: (i) tracking data (distance traveled and average speed) as raw and summarized data in Microsoft Excel files, (ii) video of mouse tracking with the tracking object number on the tracked mouse, and (iii) movement tracks (Figure 1I). Mouse Behavior Tracker gave identical results when the same video of a mouse with black coat color was analyzed repeatedly three times. Thus, Mouse Behavior Tracker allowed us to quantify the mouse activity in a home cage.
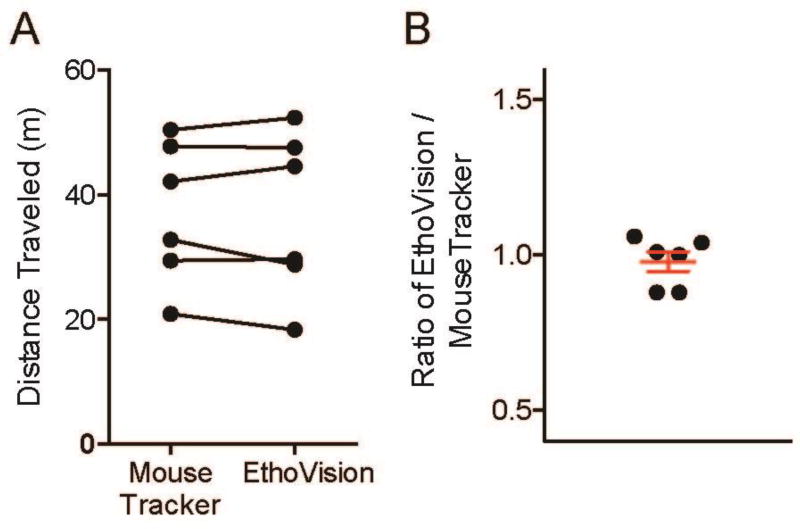
One commercial system, EthoVision XT (Noldus Information Technology Inc.), uses video tracking methods and quantifies distance traveled in home cages as in Mouse Behavior Tracker. Therefore, we compared Mouse Behavior Tracker and EthoVision XT by analyzing the distance traveled with the same video using the two programs. The distance traveled results were very similar, with the ratio of the results obtained from the 2 programs (EthoVision XT result divided by Mouse Behavior Tracker result) being 0.98 ± 0.03 (mean ± SEM, n = 6 wild-type C57BL/6J mice) (Figure 2); this demonstrates the accuracy of Mouse Behavior Tracker for distance traveled measurements.
Figure 2. Evaluation of Mouse Behavior Tracker method.
The distance traveled by mice in 20-min-long videos recorded at 30 frames-per-second (fps) was analyzed using the Mouse Behavior Tracker macro or the EthoVision XT software. (A) The graph shows the distance traveled of C57BL/6J wild-type mice, and each line represents one video analyzed using the two programs. (B) The ratio of distance traveled by dividing EthoVision XT results by Mouse Behavior Tracker results. The graph shows mean ± S.E.M. (red whiskers). These results indicate that very similar results were obtained from the two programs.
We also analyzed mice with brown or white coat color using these two software programs. Both programs could track mice with brown coat color. Mouse Behavior Tracker gave identical results when the same video of a mouse with brown coat color was analyzed three times. However, the Ethovision XT quantified 20% less distance traveled compared to the Mouse Behavior Tracker results using the settings for tracking mice with black coat color. The ratio of the results obtained from Ethovision XT over Mouse Behavior Tracker was 0.71 ± 0.1 (mean ± SEM, n = 3 mice). The reason for the difference in distance traveled quantified by the two software programs needs further investigation. Mice with white coat color were not trackable in either Mouse Behavior Tracker or Ethovision XT using the settings that tracked mice with black coat color. The inability to track white mice is likely due to the reduced contrast between the white coat color and the corn cob bedding in the cages.
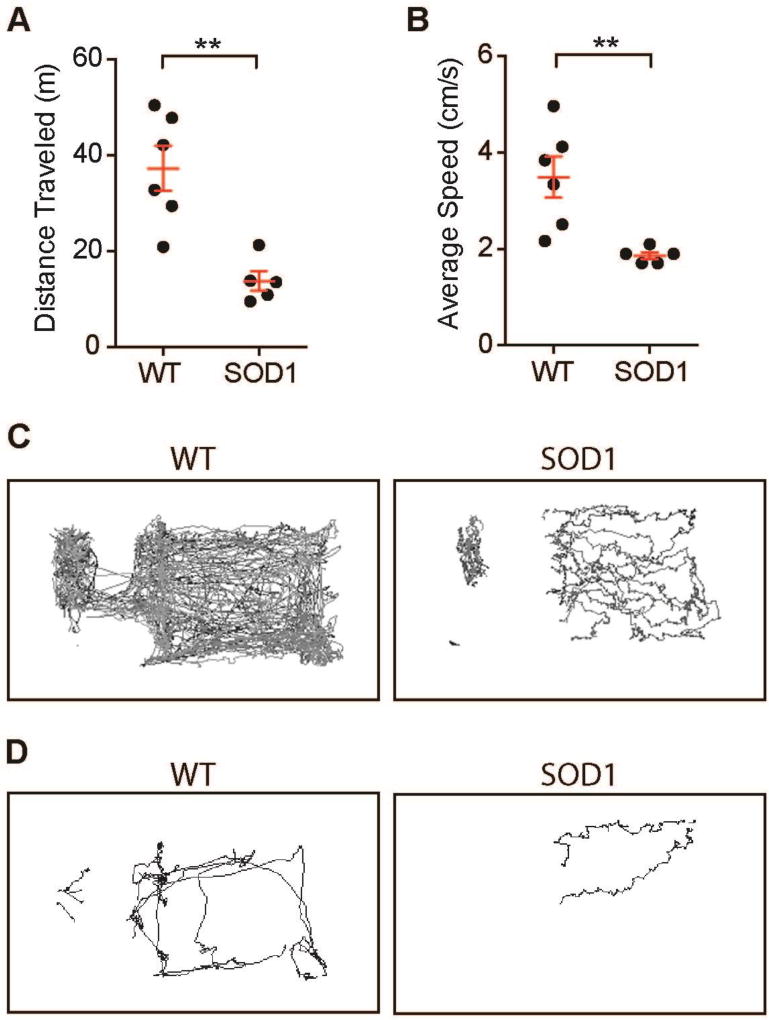
Finally, Mouse Behavior Tracker was used to analyze the behavior of amyotrophic lateral sclerosis (ALS) model mice (SOD1G93A, 139 – 155 days old) and age and sex matched wild-type controls (C57BL/6J, 141 – 168 days old) in their home cages. The mean distance traveled during a 20-minute recording was 13.79 ± 2.03 meters (mean ± SEM, n = 5 mice) for symptomatic stage SOD1G93A mice. However, wild-type mice traveled a significantly longer distance of 37.24 ± 4.68 meters (n = 6 mice, p = 0.0021 unpaired t-test; Figure 3A). In addition, the average speed of SOD1G93A mice movement was also significantly slower than that of wild-type mice (SOD1G93A: 1.86 ± 0.07 cm / sec, n = 5 mice; wild-type: 3.49 ± 0.43 cm / sec, n = 6 mice; p = 0.0076 unpaired t-test, Figure 3B). Furthermore, the movement pattern reflected the behavior differences of the two mouse strains. SOD1G93A mice moved slowly with alternating lateral displacements of the body, which were represented by characteristic zigzag tracks at the 30 fps video sampling rate (Figure 3D, Supplementary Videos 3, 4). This tottering movement of SOD1G93A mice may be related to the gait differences reported previously for this ALS animal model (18–21). Meanwhile, wild-type mice moved quickly and balanced, yielding smooth tracks without zigzag tracks. These results demonstrated that there are differences in the activity level (distance traveled and average speed) and movement pattern between SOD1G93A mice and wild-type mice in their home cages.
Figure 3. ALS model SOD1G93A mice are less active than wild-type mice and show tottering behavior.
Graphs show (A) distance traveled and (B) the average speed during the 20 minutes of recording time. Symptomatic stage SOD1G93A male mice (SOD1, 139 – 155 days old) traveled a shorter distance at a slower speed than age- and sex-matched wild-type mice (WT, 141 – 168 days old). A total of n = 5 to 6 mice were analyzed. The graph shows mean ± SEM (red whiskers), and each dot represents one mouse. Asterisks indicate significance by an unpaired t-test (p < 0.01). (C) Representative movement tracks indicated higher activity of wild-type mice than SOD1G93A mice during the 20-minute recordings at 30 fps. (D) Tracks of a wild-type mouse show a smooth and balanced movement pattern during a one-minute period. However, tracks of a SOD1G93A mouse indicated tottering behavior with a zigzag pattern during the same amount of time. These tracks are portions of the tracks shown in Figure 3C.
Measurement of spontaneous activity in non-exercised control animals is seldom undertaken; however, it is essential to compare activity of sedentary animals in home cages versus exercised animals with running wheels or forced exercise activities. This is partly due to a lack of a simple and economical method of measuring the distance traveled of sedentary animals in their home cages. Systems to analyze home-cage mouse behavior have been developed to quantify behavioral phenotypes (4) and social interactions (7), and mouse behavior has been analyzed using time-lapse VCR systems (22, 23). However, these systems do not measure the distance traveled in home cages. Mouse Behavior Tracker is an efficient and low-cost method that measures distance traveled, movement speed and displays movement tracks in home cages. Furthermore, this system generates tracks for detecting abnormal behavior, including tottering. Finally, a home cage based analysis is relevant in studies of aging, disease models, genetic manipulation, and pharmacological treatments. It should be noted that for long-term observation over many days or weeks, a commercially available system would be more suitable.
Supplementary Material
One-minute raw video (30 fps) of a C57BL/6J wild-type mouse shown in Figure 1E and the trajectory shown in Figure 1I.
One-minute tracking video (30 fps) of the tracked mouse shown in Figure 1H and the trajectory shown in Figure 1I. This tracking video shows the identical recording period as the video shown in Supplementary Video 1.
One-minute raw video (30fps) of the wild-type mouse. The trajectory of this mouse is shown in Figure 3D left.
One-minute raw video (30fps) of the SOD1G93A mouse. The trajectory of this mouse is shown in Figure 3D right.
Acknowledgments
We thank the following software developers for their work and for making their software publicly available: Jesper Søndergaard Pedersen for wrMTrck, and Fiji development team (24). We thank Robert. S. Rogers for technical assistance, Irfan Saadi for the resources, Kenneth McCarson and Michelle Winter for KUMC Rodent Behavior Facility support (NICHD HD 02528). This work was supported by grants from NIH 1R01NS078214 and 1R01AG051470 (H.N.). This paper is subject to the NIH Public Access Policy.
Footnotes
AUTHOR CONTRIBUTIONS
H.N. designed the experiment. S.T., N.N., and H.N. performed the experiments and analyzed the data. J.R. contributed essential technical expertise on video recording methods. S.T. and H.N. wrote the manuscript, and all authors reviewed the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing interests.
References
- 1.Fonio E, Golani I, Benjamini Y. Measuring behavior of animal models: faults and remedies. Nat Methods. 2012;9:1167–1170. doi: 10.1038/nmeth.2252. [DOI] [PubMed] [Google Scholar]
- 2.Pan WH, Lee CR, Lim LH. A new video path analyzer to monitor travel distance, rearing, and stereotypic movement of rats. Journal of neuroscience methods. 1996;70:39–43. doi: 10.1016/S0165-0270(96)00101-X. [DOI] [PubMed] [Google Scholar]
- 3.Quinn LP, Stean TO, Trail B, Duxon MS, Stratton SC, Billinton A, Upton N. LABORAS: Initial pharmacological validation of a system allowing continuous monitoring of laboratory rodent behaviour. Journal of neuroscience methods. 2003;130:83–92. doi: 10.1016/s0165-0270(03)00227-9. [DOI] [PubMed] [Google Scholar]
- 4.Jhuang H, Garrote E, Mutch J, Yu X, Khilnani V, Poggio T, Steele AD, Serre T. Automated home-cage behavioural phenotyping of mice. Nat Commun. 2010;1:68. doi: 10.1038/ncomms1064. [DOI] [PubMed] [Google Scholar]
- 5.Ou-Yang TH, Tsai ML, Yen CT, Lin TT. An infrared range camera-based approach for three-dimensional locomotion tracking and pose reconstruction in a rodent. Journal of neuroscience methods. 2011;201:116–123. doi: 10.1016/j.jneumeth.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Crispim Junior CF, Pederiva CN, Bose RC, Garcia VA, Lino-de-Oliveira C, Marino-Neto J. ETHOWATCHER: validation of a tool for behavioral and video-tracking analysis in laboratory animals. Comput Biol Med. 2012;42:257–264. doi: 10.1016/j.compbiomed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 7.de Chaumont F, Coura RD, Serreau P, Cressant A, Chabout J, Granon S, Olivo-Marin JC. Computerized video analysis of social interactions in mice. Nat Methods. 2012;9:410–417. doi: 10.1038/nmeth.1924. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrov V, Brunner D, Hanania T, Leahy E. High-throughput analysis of behavior for drug discovery. Eur J Pharmacol. 2015;750:82–89. doi: 10.1016/j.ejphar.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samson AL, Ju L, Ah Kim H, Zhang SR, Lee JA, Sturgeon SA, Sobey CG, Jackson SP, Schoenwaelder SM. MouseMove: an open source program for semi-automated analysis of movement and cognitive testing in rodents. Scientific reports. 2015;5:16171. doi: 10.1038/srep16171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt BM, Yap MH, Hodson-Tole EF, Kennerley AJ, Sharp PS, Grant RA. A novel automated rodent tracker (ART), demonstrated in a mouse model of amyotrophic lateral sclerosis. Journal of neuroscience methods. 2017 doi: 10.1016/j.jneumeth.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Kalliokoski O, Jacobsen KR, Darusman HS, Henriksen T, Weimann A, Poulsen HE, Hau J, Abelson KS. Mice do not habituate to metabolism cage housing--a three week study of male BALB/c mice. PLoS One. 2013;8:e58460. doi: 10.1371/journal.pone.0058460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beam M, Silva MC, Morimoto RI. Dynamic imaging by fluorescence correlation spectroscopy identifies diverse populations of polyglutamine oligomers formed in vivo. J Biol Chem. 2012;287:26136–26145. doi: 10.1074/jbc.M112.362764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mort RL, Keighren M, Hay L, Jackson IJ. Ex vivo culture of mouse embryonic skin and live-imaging of melanoblast migration. J Vis Exp. 2014 doi: 10.3791/51352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bros H, Hauser A, Paul F, Niesner R, Infante-Duarte C. Assessing Mitochondrial Movement Within Neurons: Manual Versus Automated Tracking Methods. Traffic. 2015;16:906–917. doi: 10.1111/tra.12291. [DOI] [PubMed] [Google Scholar]
- 15.de Saram PS, Ressurreicao M, Davies AJ, Rollinson D, Emery AM, Walker AJ. Functional mapping of protein kinase A reveals its importance in adult Schistosoma mansoni motor activity. PLoS Negl Trop Dis. 2013;7:e1988. doi: 10.1371/journal.pntd.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckelt K, Masanas H, Llobet A, Gorostiza P. Automated high-throughput measurement of body movements and cardiac activity of Xenopus tropicalis tadpoles. J Biol Methods 2014 [Google Scholar]
- 17.Ratcliff WC, Pentz JT, Travisano M. Tempo and mode of multicellular adaptation in experimentally evolved Saccharomyces cerevisiae. Evolution. 2013;67:1573–1581. doi: 10.1111/evo.12101. [DOI] [PubMed] [Google Scholar]
- 18.Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL. Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve. 2005;32:43–50. doi: 10.1002/mus.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knippenberg S, Thau N, Dengler R, Petri S. Significance of behavioural tests in a transgenic mouse model of amyotrophic lateral sclerosis (ALS) Behav Brain Res. 2010;213:82–87. doi: 10.1016/j.bbr.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Alves CJ, de Santana LP, dos Santos AJ, de Oliveira GP, Duobles T, Scorisa JM, Martins RS, Maximino JR, Chadi G. Early motor and electrophysiological changes in transgenic mouse model of amyotrophic lateral sclerosis and gender differences on clinical outcome. Brain Res. 2011;1394:90–104. doi: 10.1016/j.brainres.2011.02.060. [DOI] [PubMed] [Google Scholar]
- 21.Preisig DF, Kulic L, Kruger M, Wirth F, McAfoose J, Spani C, Gantenbein P, Derungs R, et al. High-speed video gait analysis reveals early and characteristic locomotor phenotypes in mouse models of neurodegenerative movement disorders. Behav Brain Res. 2016;311:340–353. doi: 10.1016/j.bbr.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Van de Weerd HA, Van Loo PLP, Van Zutphen LFM, Koolhaas JM, Baumans V. Strength of preference for nesting material as environmental enrichment for laboratory mice. Appl. Anim. Behav. Sci. 1998;55:369–382. doi: 10.1258/002367797780600152. [DOI] [PubMed] [Google Scholar]
- 23.Ambree O, Touma C, Gortz N, Keyvani K, Paulus W, Palme R, Sachser N. Activity changes and marked stereotypic behavior precede Abeta pathology in TgCRND8 Alzheimer mice. Neurobiol Aging. 2006;27:955–964. doi: 10.1016/j.neurobiolaging.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One-minute raw video (30 fps) of a C57BL/6J wild-type mouse shown in Figure 1E and the trajectory shown in Figure 1I.
One-minute tracking video (30 fps) of the tracked mouse shown in Figure 1H and the trajectory shown in Figure 1I. This tracking video shows the identical recording period as the video shown in Supplementary Video 1.
One-minute raw video (30fps) of the wild-type mouse. The trajectory of this mouse is shown in Figure 3D left.
One-minute raw video (30fps) of the SOD1G93A mouse. The trajectory of this mouse is shown in Figure 3D right.