Abstract
Clinical management of primary and secondary central nervous system (CNS) malignancies frequently includes radiotherapy to forestall tumor growth and recurrence after surgical resection. While cranial radiotherapy remains beneficial, adult and pediatric brain tumor survivors suffer from a wide range of debilitating and progressive cognitive deficits. Although this has been recognized as a significant problem for decades, there remains no clinical recourse for the unintended neurocognitive sequelae associated with these types of cancer treatments. In previous work, multiple mechanisms have been identified that contribute to radiation-induced cognitive dysfunction, including the inhibition of neurogenesis caused by the depletion of radiosensitive populations of stem and progenitor cells in the hippocampus. To explore the potential neuroprotective properties of a pro-neurogenic compound NSI-189, Long-Evans rats were subjected to a clinically relevant fractionated irradiation protocol followed by four weeks of NSI-189 administered daily by oral gavage. Animals were then subjected to five different behavioral tasks followed by an analysis of neurogenesis, hippocampal volume and neuroinflammation. Irradiated cohorts manifested significant behavioral decrements on all four spontaneous exploration tasks. Importantly, NSI-189 treatment resulted in significantly improved performance in four of these tasks: novel place recognition, novel object recognition, object in place and temporal order. In addition, there was a trend of improved performance in the contextual phase of the fear conditioning task. Importantly, enhanced cognition in the NSI-189-treated cohort was found to persist one month after the cessation of drug treatment. These neurocognitive benefits of NSI-189 coincided with a significant increase in neurogenesis and a significant decrease in the numbers of activated microglia compared to the irradiated cohort that was given vehicle alone. The foregoing changes were not accompanied by major changes in hippocampal volume. These data demonstrate that oral administration of a pro-neurogenic compound exhibiting anti-inflammatory indications could impart long-term neurocognitive benefits in the irradiated brain.
Introduction
Primary and secondary malignancies of the brain are deadly forms of cancer that are notoriously difficult to treat, largely due to the sensitive anatomical site in which they reside and because they are protected behind the blood-brain barrier. These realities have led to the use of carefully designed cranial radiotherapy protocols to improve progression-free survival (1, 2) after surgical resection. While technological advancements in beam delivery and imaging have helped limit overt normal tissue injury, such as radionecrosis and/or edema, a progressive onset of cognitive dysfunction invariably occurs (3–5). Importantly, neurocognitive sequelae transpire at much lower dose thresholds than those required to elicit morphologically distinguishable changes in the brain. The resultant cognitive decrements are multifaceted and manifest as hippocampal-dependent (and independent) deficits in learning and memory, and often include disruptions in attention, concentration and executive functions, such as planning and multitasking (6, 7). Since there are no long-term, satisfactory solutions for the treatment of radiation-induced cognitive impairments, surviving patients have little clinical recourse in managing the debilitating cognitive decrements that compromise their quality of life.
Considerable evidence now points to the depletion of neural stem and progenitor cells as one of the contributory mechanisms underlying radiation-induced cognitive dysfunction (8). These radiosensitive cells reside in the neurogenic subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), and their elimination after irradiation leads to a marked and persistent inhibition of neurogenesis (9). Exposure to ionizing radiation also triggers a persistent oxidative stress and inflammatory footprint that further compromise the neurogenic niche, thereby leading to more chronic impairments of neurogenesis and cognition (10, 11). While disruptions to neurogenesis cannot account for all radiation-induced neurocognitive sequelae, the temporal coincidence linking the attrition of these multipotent cells to the inhibition of neurogenesis and onset of hippocampal-dependent learning and memory deficits is striking, and points to the importance of the neurogenic process in the preservation and maintenance of cognitive health (12).
The persistence of radiation-induced oxidative stress and inflammation can interfere with the proliferation and differentiation of stem cells in the brain. These secondary reactive processes also contribute to the degradation of the microvasculature and neuronal structure that are believed to be contributory, if not causal, to radiation-induced cognitive dysfunction. While antioxidant and anti-inflammatory strategies have been used to restore neurogenesis after irradiation (13, 14), these often lead to mixed results when scrutinized for their capability to prevent or reverse cognitive deficits caused by prior irradiation (15). The foregoing provides much of the backdrop for investigating whether alternative pro-neurogenic compounds might hold promise for ameliorating certain adverse neurocognitive complications associated with cranial radiation exposure. NSI-189 is a benzylpiperazine-aminopyridine, a novel small molecule that stimulates neurogenesis of cultured hippocampus-derived human neural stem cells (data on file, Neuralstem, non-peer-reviewed). Importantly, mouse brains treated with NSI-189 exhibited a significant increase in hippocampal cell proliferation and hippocampal volume, with no observable off-target effects in other brain regions [data on file, Neuralstem, non-peer-reviewed; and Supplementary Information (http://dx.doi.org/10.1667/RR14879.1.S1)]. Furthermore, NSI-189 treatment has shown behavioral efficacy in a mouse model of depression, and has shown considerable promise in a recent phase 1B, randomized, double-blind dose escalation study in humans for the treatment of major depressive disorder (16). In the current study, we explored whether NSI-189, administered orally after a clinically relevant, fractionated cranial irradiation protocol, could ameliorate neurocognitive complications one month later.
Materials and Methods
Animals and Irradiation
All animal procedures were performed in accordance with NIH guidelines and were approved by the University of California Institutional Animal Care and Use Committee. Ten-week-old Long-Evans (LE) rats (Crl:LE, strain code 006; Charles River Laboratories, Wilmington, MA) were divided randomly into three groups (n = 15– 16/group): controls receiving oral gavage (vehicle only) and sham irradiation; cohorts receiving oral gavage (vehicle only) and 27 Gy head-only fractionated exposure; and cohorts receiving oral gavage (NSI-189, 30 mg/kg) and 27 Gy head-only fractionated exposure. Since administration of NSI-189 alone (i.e., in the absence of irradiation) was deemed clinically irrelevant, it was not included in the study. For cranial irradiation, animals were anesthetized [2.5% (vol/ vol) isoflurane/oxygen], placed ventrally on the treatment table (X-RAD 320 irradiator; Precision X-ray Inc., North Branford, CT) without restraint and positioned under a collimated (1.0 cm2 diameter) beam for head-only irradiation delivered at a dose rate of 1.0 Gy/min. Fractionation of 27 Gy was delivered over 3 separate doses of 8.67 Gy, which were administered 48 h apart.
NSI-189 and BrdU Administration
Animals were administered drug starting from 24 h after the final dose of radiation, and continued on a standard daily regimen for a total of four weeks. Stock concentrations of NSI-189 were prepared by Neuralstem Inc., and delivered to University of California, Irvine (UCI) staff every three weeks. NSI-189 is prepared in sterile vehicle (normal saline) as a 1× solution (15 mg/ml). The drug was administered by daily oral gavage at a concentration adjusted to the weight of the animals. The daily dosing was set at 2 ml/kg, setting the target daily dose of 30 mg/kg. Thus, the daily volume of the drug typically varied between 0.6–1.0 ml/rat. The exact volume of the NSI at the indicated concentration was recorded for each cohort. Animals were weighed weekly, which was used to adjust all dosing for that week. All drug administration transpired between 12:00–2:00 p.m. each day. For administration, sterile gavage needles with protected tips were used for each animal. The entire gavage procedure for each animal lasted less than 1 min. During the last three days of oral gavage (four weeks prior to sacrifice), animals were injected three times daily with BrdU (50 mg/kg) prepared fresh daily at UCI.
Cognitive Testing
To evaluate the outcome of NSI-189 treatment on cognitive function, all rats from each treatment group (control, irradiation only, irradiation with NSI-189) were subjected to cognitive testing one week after termination of oral gavage (five weeks postirradiation). Cognitive testing was performed over the course of three weeks and included four different spontaneous exploration tasks followed by contextual and cued fear conditioning, as described in detail elsewhere (13, 17). All data were collected using EthoVision® version 8.5 (Noldus Information Technology, Sterling, VA), and subsequently hand-scored by a technician blind to the treatment groups. All spontaneous exploration test data are presented in exploration ratio format [(tnovel/tnovel + tfamiliar)].
Assessment of Neurogenesis
Immunohistochemical analysis was performed on each cohort of animals subjected to sequential BrdU injections one month prior to sacrifice to determine the percentage of BrdU-positive (BrdU+) cells co-expressing the mature neuronal marker NeuN (i.e., BrdU+/NeuN+ cells). At least 50–100 BrdU+ cells were scored for each animal and all data were derived from 6–8 serial sections from 4–6 individual animals/group. Further details of this analysis have been described in detail elsewhere (18).
Determination of Hippocampal Volume
Rat brains across the groups were leveled and multiply embedded into gelatin blocks so that all groups were treated equally. Group codes were blinded during histology and quantification. The blocks were fixed in formalin, quickly frozen in a dry ice bath, and then sectioned coronally by using a freezing microtome at 35–40 lm thickness. Floating brain sections (1-in-6 series) were stained with Nissl. The slides were imaged on the Nikont® AZ100 macroscope along with NIS-Elements AR software (Nikon Corp.) equipped with X-Y motorized stage to perform image stitching at 10× magnification across the entire anterior-posterior (A-P) axis of the hippocampal subfields (DG, CA1 and CA3). After converting into 8-bit grayscale images, the evaluators contoured the molecular layer of DG and molecular zones across the stratum lacunosum-moleculare, stratum radiatum, stratum lucidum and stratum oriens, sparing the pyramidal layer of CA3 and CA1, respectively, based on the rat brain atlas. Volumetric measurements of the subfields were performed on blind-coded slides using commercial software (Stereo Investigator®; MBF Science, Williston, VT). Thereafter, the data were extracted to Microsoft Excel for data documentation, and the statistical analysis was performed using GraphPad Prism, version 6 (LaJolla, CA) with one-way analysis of variance (ANOVA) with post hoc analysis.
Assessment of Activated Microglia
To quantify the number of activated (ED-1+) microglia in the brain, immunohistochemical analysis was performed as described elsewhere (19). The number of ED-1+ microglia (per mm2) was quantified visually using the Nikon Eclipse TE-2000 brightfield microscope from 4–5 animals per group (3 sections per brain, every 15th section). Data are presented as the number of ED-1+ cells per hippocampal slice.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism version 6. One-way ANOVA was used to assess significance between control and irradiated groups receiving either vehicle or NSI-189. When overall group effects were found to be statistically significant, a Bonferroni's multiple comparisons test was used to compare the individual experimental groups. All data are expressed as the mean ± SEM and a value of P ≤ 0.05 was considered statistically significant.
For four of the tasks that were tested (novel place recognition, novel object recognition, object in place and temporal order), the exploration ratio, or the proportion of total time spent exploring the novel spatial location [tnovel/(tnovel + tfamiliar)], was used as the main dependent measure. The behavior of the animals during the entire 5-min test was analyzed. The number of animals used in all exploration task analyses were: control (n = 16); irradiation only (n = 16); and irradiation with NSI-189 (n = 15).
For the fear-conditioning task, percentage of time spent freezing was used as the main dependent measure. Freezing was assessed during the final minute of the baseline (i.e., before tone-shock pairings were administered) and post-training (i.e., after tone-shock pairings) phases. For the context test, freezing was assessed over the entire 5-min trial. For the pre-cue test, freezing was assessed during the first minute, in which no tone was sounded; and for the cue test, freezing was assessed immediately after the tone was sounded for the final minute of the trial. Repeated measures ANOVA was used to assess group (between-subjects factor) effects on freezing behavior followed by Bonferroni's multiple comparison tests where appropriate.
Results
Cognitive Testing
Testing was done to assess the functional consequences of radiation exposure on the brain, and whether oral supplementation of NSI-189 could impart any neurocognitive benefits. At 4–6 weeks postirradiation, Long-Evans rats were administered a series of spontaneous exploration tasks that quantify the innate tendency of rats to explore novelty. These cognitive tests were then followed by a fear-conditioning task not reliant on spontaneous exploration. Administration of NSI-189 by oral gavage was started 24 h postirradiation and discontinued 1–4 weeks prior to and over the duration of behavioral assessments.
Novel Place Recognition
One month after irradiation, animals were habituated and tested on the novel place recognition task (Fig. 1). First, rats were exposed to two identical objects in specific spatial locations within a test arena. Rats that remember the previous spatial arrangement of the objects will spend more time exploring the object that has been moved to the novel spatial location. Successful performance of the task has been shown to rely on intact hippocampal function (20). The total exploration of objects during the familiarization and test phases was indistinguishable among all cohorts. The means and 95% confidence intervals (CI) for the exploration ratios of each group are as follows: controls (mean = 63.3, 95% CI = 57.09, 69.51); irradiation only (mean = 45.93, 95% CI = 35.39, 56.82); and irradiation with NSI-189 (mean = 60.69, 95% CI = 52.96, 68.41). A significant overall group effect was found [F(2,44) = 5.357; P = 0.0083] for the exploration ratio to differ among groups. After a 1-h retention interval between familiarization and test phases, the irradiation only cohort animals spent significantly less time exploring the novel place compared to the control and irradiation with NSI-189 cohorts (P < 0.05 for both) (Fig. 1). In contrast, the irradiation with NSI-189 cohort did not differ significantly from controls. These data indicate that in irradiated animals, NSI-189 treatment ameliorated the decrement of spatial exploration behavior on the novel place recognition task, compared to those irradiated animals that were not given NSI-189.
Fig. 1.
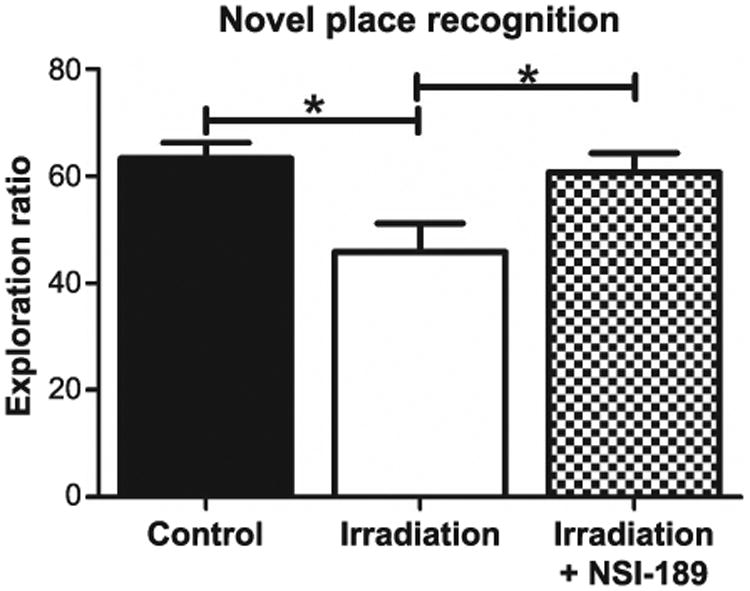
Testing for spatial cognitive impairments in the novel place recognition task. Rats were introduced to two identical objects placed within an open arena for 5 min. After a 60-min rest, animals were returned to the open arena for 3 min where the same objects were present with one placed in a novel location. Controls spent a significantly greater amount of time exploring the novel place (*P < 0.05) compared to irradiated animals. Irradiated animals treated with NSI-189 spent significantly more time exploring the novel place (*P < 0.05) compared to irradiated only animals and there was no significant difference (P > 0.05) compared to controls.
Novel Object Recognition
The novel object recognition task tests the functional connectivity of the hippocampus and the medial prefrontal cortex (mPFC), and requires the animal to distinguish a familiar object from a novel object (21, 22). As before, rats explored familiar and novel objects equally during this task. The group means and 95% CI for the DI are: control (mean = 68.30, 95% CI = 61.94, 74.66); irradiation only (mean = 55.14, 95% CI = 48.18, 62.09); and irradiation with NSI-189 (mean = 71.28, 95% CI = 63.50, 79.06). Again, a significant overall group effect was found [F(2,44) = 6.37; P = 0.0038] for the DI to differ among groups. After a 5-min retention interval between familiarization and test phases, the irradiation only cohort spent significantly less time exploring the novel object compared to the control (P < 0.05) and irradiation with NSI-189 (P < 0.01) groups (Fig. 2). For the novel object recognition, the control and irradiation with NSI-189 groups were statistically indistinguishable, demonstrating that drug treatment ameliorated radiation-induced deficits in novel object exploration behavior.
Fig. 2.
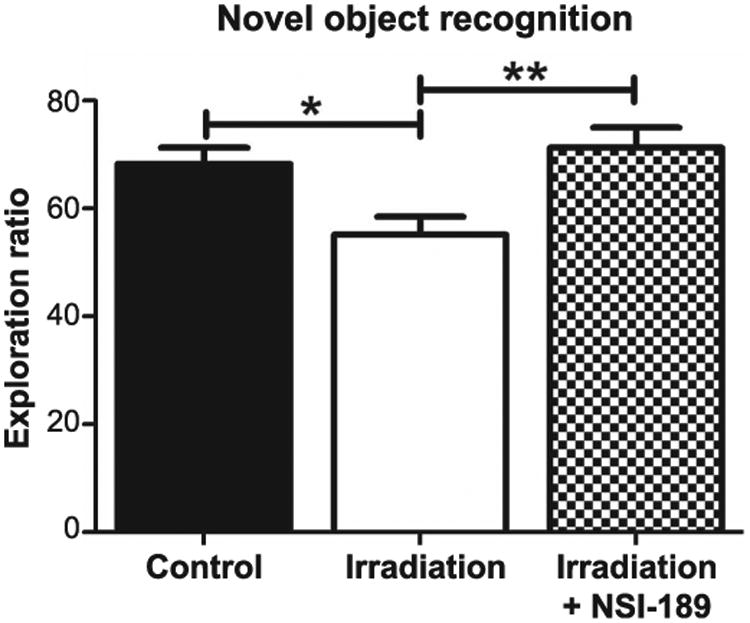
Testing for impairments of episodic memory retention in the novel object recognition task. Rats were allowed to freely explore two identical objects in an open arena for 5 min. After a 5-min rest, animals were returned to the arena, where one of the objects was replaced with a novel object, and were allowed to explore for an additional 5 min. Controls spent significantly more time exploring the novel object (*P < 0.05) compared to irradiated only animals. Irradiated animals treated with NSI-189 spent significantly more time exploring the novel object (**P < 0.01) compared to irradiated only animals and there was no significant difference (P > 0.05) compared to controls.
Object in Place
Rats were then habituated and tested on the object-in-place task, which is also dependent on intact hippocampal, prefrontal and perirhinal cortical functions (21, 22). In this case, functionally intact rats exhibit a preference towards objects that have been moved to a novel location. Total exploration times for the objects followed similar trends as those observed for the previous tests. The group means and CI for the DI in each cohort are: control (mean = 64.75, 95% CI = 59.98, 69.52); irradiation only (mean = 54.85, 95% CI = 49.47, 60.23); and irradiation with NSI-189 (mean = 64.60, 95% CI = 59.17, 70.02). The overall group effect for DI among the three cohorts was again found to differ significantly [F(2,44) = 5.146; P < 0.01]. After familiarization, test phases showed that the irradiation only cohort spent significantly less time exploring the novel placement of objects compared to the control (P < 0.05) and irradiation with NSI-189 (P < 0.05) groups (Fig. 3). The control and irradiation with NSI-189 cohorts showed no significant differences, demonstrating that drug treatment ameliorated the impairment of spatial memory of animals on the object-in-place task after irradiation.
Fig. 3.
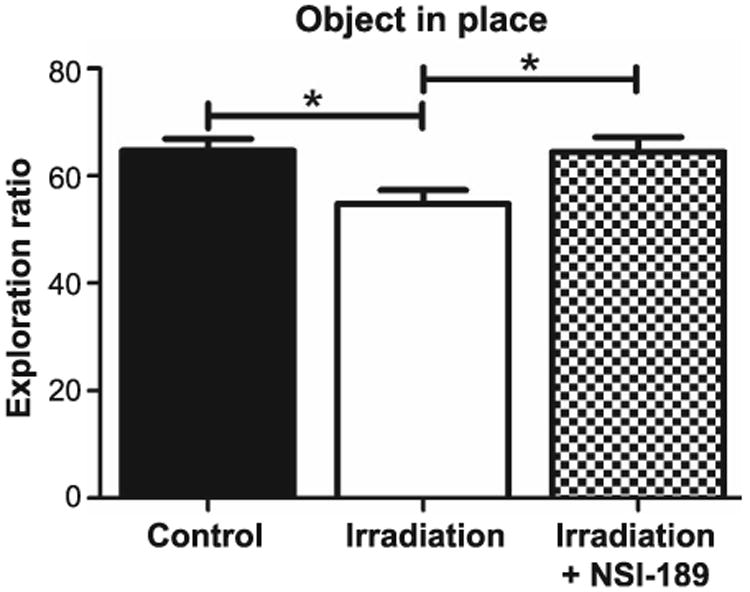
Testing for spatial memory retention impairments with the object-in-place task. Rats were allowed to explore an open arena that contained four distinct objects near the corners for 5 min. After a 5-min rest, animals were returned to the arena where two objects had switched locations. They were allowed to explore for an additional 5 min. Controls spent significantly more time exploring the shifted objects (*P < 0.05) compared to irradiated only animals. Irradiated animals treated with NSI-189 spent significantly more time exploring objects that had shifted compared to irradiated only animals (*P < 0.05), but there was no significant difference compared to controls.
Temporal Order
For the temporal order task, rats were familiarized with two sets of objects, 3 h apart, and after an additional 1 h, were subjected to a test phase using one of the same objects presented in each of the earlier phases. Rats with intact hippocampal function show a preference for the first rather than the more recent object explored. Total exploration times of the objects during the familiarization and test phases were not found to be statistically different. Group means and CI for the DI in each cohort are: control (mean = 64.68, 95% CI = 59.71, 69.64); irradiation only (mean = 46.44, 95% CI = 36.55, 56.33); and irradiation with NSI-189 (mean = 67.95, 95% CI = 61.15, 74.75). Analysis of recency discrimination in the temporal order task revealed an overall group effect [F(2,44) = 10.63; P = 0.0002] and showed that radiation exposure impaired recency memory, as reflected by a reduced preference for the object presented prior to the last (Fig. 4). Importantly, the control and irradiation with NSI-189 cohorts were not found to differ statistically, demonstrating again that drug treatment ameliorated radiation-induced decrements in recency memory, as assessed by the temporal order task (21, 22).
Fig. 4.
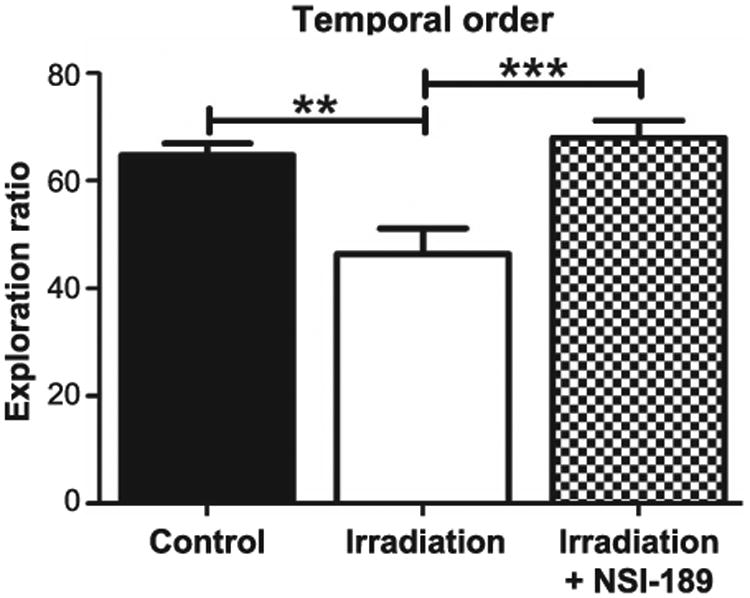
Spatiotemporal episodic memory impairments measured with the temporal order task. During this task, rats were allowed to freely explore two identical objects for 5 min (phase 1). After a 4-h rest period, rats were placed back into the arena with two new identical objects and were allowed to explore for an additional 5 min (phase 2). One hour later, rats were allowed to explore one of each object they previously encountered for an additional 5 min (testing phase shown). Controls spent a significantly greater percentage of time with the phase 1 object during testing (**P < 0.01) compared to irradiated only animals. Irradiated animals treated with NSI-189 showed a significant difference (**P < 0.01) compared to irradiated only animals. Controls and irradiated animals treated with NSI-189 showed no significant difference (P > 0.05).
In summary, findings from each of the four open-field, spontaneous exploration tasks indicate that oral administration of the small molecule NSI-189 was able to significantly ameliorate radiation-induced behavioral deficits.
Fear Conditioning
To determine how animals would perform on a task not dependent on locomotor activity, rats were then subjected to a fear condition paradigm. Each phase (training, context and cue) of the fear-conditioning task was administered over a three-day period. Group means for the phases of this task were not found to differ using repeated measures ANOVA (Fig. 5), despite an interesting trend found for the contextual phase of this task, which tracked prior data sets showing improved performance of irradiated cohorts given NSI-189 compared to those irradiated alone. As in all other behavioral tasks, drug-treated cohorts were again not found to differ significantly from unirradiated controls treated with vehicle. All groups showed significant increases in freezing behavior after the tone-shock pairing (post-training phase), indicating that irradiation did not impair motor or sensory function. Moreover, the fact that cued memory was intact also demonstrates that the acquisition of the tone-shock pairing was not impaired and that the radiation-induced deficit (trend) was specific to the memory of the context in which the pairing had been learned.
Fig. 5.
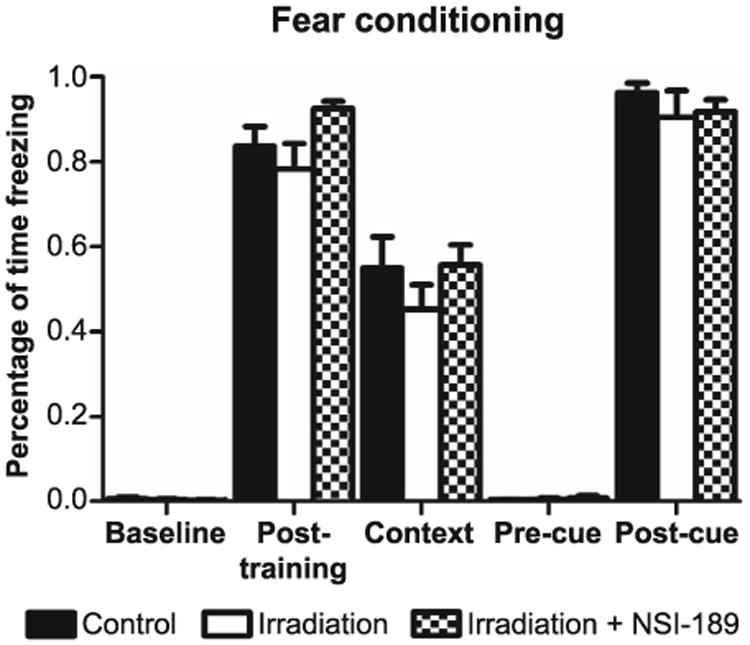
Hippocampal- and amygdala-dependent learning and memory assessed using contextual and cued versions of the fear-conditioning task. Animals were trained to associate a tone that is paired with a mild foot shock on day 1 training. On day 2, animals were returned to the same training arena (context) for 5 min and freezing was measured in the absence of shock or tone. On day 3, animals were placed in a new novel environment but were exposed to the same tone (cue), which they had been exposed to in training, in the absence of shock. Freezing was measured for 1 min after the tone. During context, no significant difference was measured among all groups (P = 0.19).
Neurogenesis
Prior studies conducted in mice have indicated the pro-proliferative properties of this small molecule compound (Supplementary Information; http://dx.doi.org/10.1667/RR14879.1.S1). Following a similar administration protocol, oral dosing of NSI-189 increased hippocampal cell proliferation and volume after a 28-day regimen in unirradiated control mice (see Supplementary Information). Recently published work in a preclinical stroke model using rats has shown that NSI-189 treatment can enhance cell proliferation in the peri-infarct cortex and increased hippocampal MAP2 immunoreactivity (23). To determine whether similar effects would operate in a radiation model, animals were subjected to a BrdU paradigm designed to label newly born cells one month before the onset of cognitive testing. A significant overall group effect was found [F(2,13) = 11.27; P = 0.0014] for the means to differ between the cohorts. Multiple comparisons analysis showed that radiation caused a significant (P < 0.01) reduction (47%) in the number of recently born granule cell neurons (BrdU+/NeuN+) cells present in the hippocampal dentate gyrus compared to controls (Fig. 6). Importantly, this radiation-induced deficit was ameliorated significantly (P < 0.05) by NSI-189 treatment, resulting in an increase (55%) of dual stained granule cell neurons compared to the irradiated cohort (Fig. 6). These data corroborated past data derived from mice (see Supplementary Information) and demonstrate that the neurocognitive benefits of NSI-189 may in part, be attributed to an enhancement of neurogenesis that persisted one month after the cessation of drug treatment.
Fig. 6.
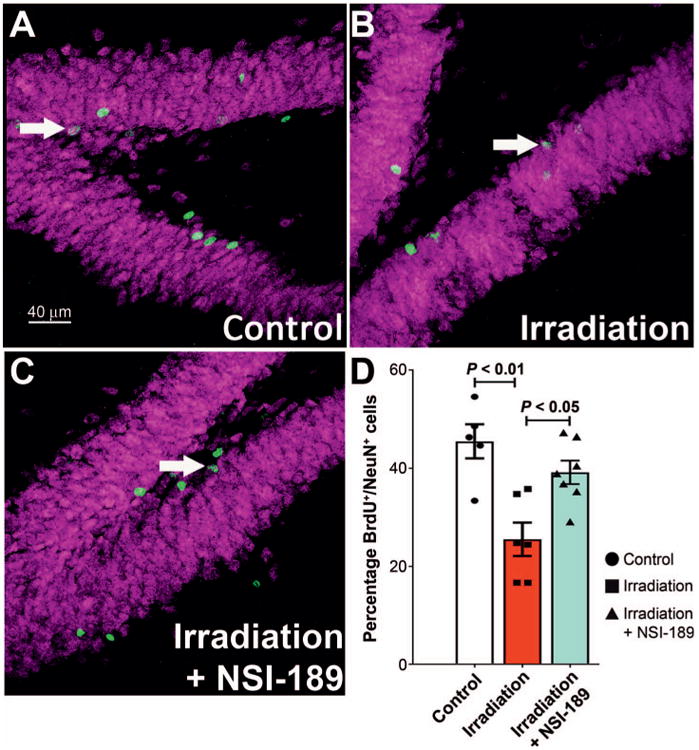
NSI-189 treatment resolves the inhibition of neurogenesis after irradiation. Panels A–C: Dual-immunofluorescence staining for BrdU (green) and NeuN (magenta) in the hippocampus of control, irradiated only and irradiated animals treated with NSI-189 groups, respectively. Fractionated cranial irradiation significantly reduced (P < 0.01) the percentage of BrdU+ cells differentiating into the mature neurons (BrdU+/NeuN+, arrow) compared to the unirradiated control group. Importantly, NSI-189 treatment was found to significantly increase (P < 0.05) neurogenesis in the irradiated brain. Panel D: Data are shown as the mean 6 SEM. (n = 5–6 animals per group). Scale bars = 40 lm (panels A–C).
Hippocampal Volume
To ascertain whether enhanced neurogenesis was associated with increased hippocampal volume, the various subfields of the hippocampus were assessed using a series of volumetric measurements through the entire A-P axis. Despite the pro-neurogenic effects of NSI-189, fractionated irradiation did not impart significant change, as volumetric data among the experimental cohorts was not found to be statistically different (Fig. 7). These data suggest that increased neurogenesis after irradiation and NSI-189 administration is not sufficient to enhance hippocampal volume, or at least, not at this relatively early post-treatment time.
Fig. 7.
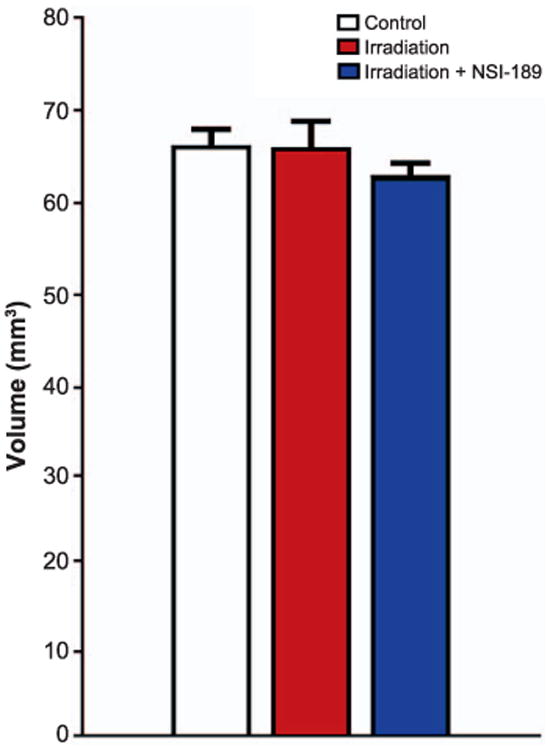
NSI-189 treatment does not elicit changes in hippocampal volume after irradiation. Volumetric measurements of the hippocampal subfields through histological assessments of Nissl stained tissues revealed no significant differences among the cohorts. Data are shown as the mean 6 SEM (n = 4–6 animals per group).
Neuroinflammation
Recent work from our laboratory has shown a strong correlation between radiation-induced cognitive dysfunction and the levels of activated microglia in the brain (24). To ascertain whether some of the beneficial neurocognitive effects of NSI-189 could be explained by changes in the number of activated microglia, we quantified the number of ED-1+ cells in the dentate hilus of control and irradiated animals. For these data, a significant overall group effect was again found [F(2,10) = 9.48; P = 0.0049]. Consistent with past results, multiple comparisons analysis revealed that irradiation caused a significant (P = 0.05) increase (95%) in the number of activated (ED-1+) microglia compared to unirradiated controls. Similar analysis indicated that after irradiation and NSI-189 treatment, the numbers of activated microglia were reduced (56%) significantly (P < 0.01) (Fig. 8) compared to the irradiated cohort. These data suggest that a certain fraction of the cognitive benefits afforded by prior NSI-189 treatment after irradiation may be linked to an attenuation of neuroinflammation.
Fig. 8.
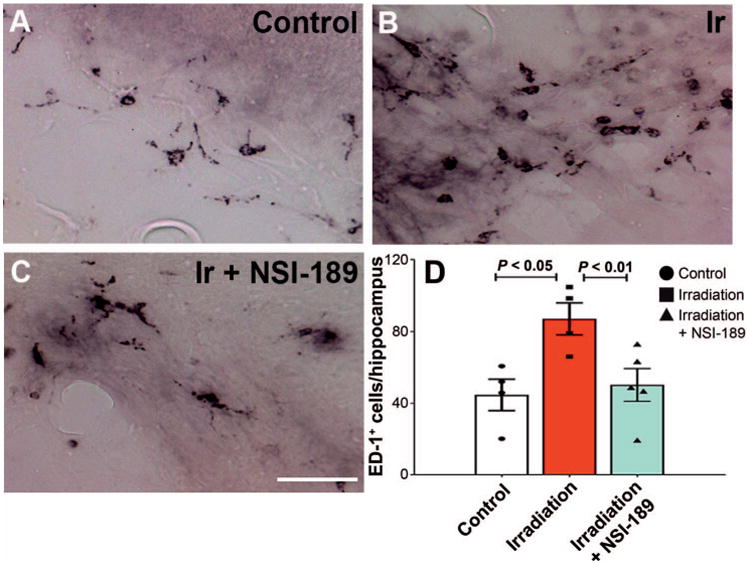
NSI-189 treatment attenuates radiation-induced neuroinflammation. Panels A–C: Immunohistochemistry for activated microglia (black, ED-1+) as shown in the hippocampal dentate hilus of control, irradiated only (Ir) and irradiated with NSI-189 treatment groups, respectively. Quantification of ED-1+ microglia at three months postirradiation (per hippocampal section), indicates that the irradiation only group had significantly elevated numbers of microglia compared to unirradiated controls (P < 0.05). Notably, treatment with NSI-189 was found to significantly attenuate the numbers of activated microglia in the irradiated brain (P < 0.01). Panel D: Data are derived from the overall hippocampus (DH, DG, CA1/3) and are shown as the mean ± SEM (n=4– 5 animals per group). Scale bars = 20 lm (panels A–C).
Discussion
Cognitive decrements associated with the radiotherapeutic management of brain cancer are both progressive and debilitating, adversely affecting quality of life. With improved diagnosis and longer-term survival continually on the rise, critical survivorship issues such as neurocognitive health are becoming increasingly important. Acknowledgment of these problems has stimulated increased efforts to resolve them. However, the majority of these adverse indications associated with the clinical management of cancer currently remain an unmet medical need. This prompted the current investigation of NSI-189, a well-tolerated neurogenic small molecule compound showing considerable promise in a phase 1B, randomized, double-blind, placebo controlled, dose escalation study for the treatment of major depressive disorder in humans (16).
In rodents, NSI-189 exhibits excellent brain penetration (data on file, Neuralstem, non-peer-reviewed), and exhibits anti-depressant activity in preclinical mouse models of depression (data on file, Neuralstem, non-peer-reviewed). A recently published study demonstrated significant amelioration of stroke-induced motor and neurological deficits that were accompanied by enhanced structural plasticity, which was maintained for six months after a three-month NSI-189 treatment regimen (24). Humans surviving cranial radiotherapy often manifest anxiety-related disorders including signs of depression (6). These observations suggest that the efficacy of NSI-189 might extend to radiation-induced neurocognitive effects. As a result, current studies were designed to approximate the clinical treatment of brain cancer patients, and incorporated a fractionated irradiation protocol coupled with the oral administration (via gavage) of NSI-189 over one month immediately after cessation of irradiation in adult Long-Evans rats.
To determine the neuroprotective role of NSI-189 in a well-characterized model of radiation injury, rats were subjected to a rigorous series of behavioral tasks designed to interrogate both hippocampal and cortical function. In contrast to irradiated animals given vehicle alone, the performance of NSI-189-treated animals was indistinguishable from unirradiated controls in each of the four spontaneous exploration tasks administered (Figs. 1–4), with both controls and NSI-189-treated animals showing significant preference for the exploration of prior novelty unique to each task. Data were remarkably consistent, in that overall exploration (locomotor activity) between the cohorts was similar for each of the tasks. Impairments in episodic, spatial and recognition memory involving hippocampal, mPFC and perirhinal circuitry were clearly evident by one month postirradiation, and each of these deficits was resolved by administration of NSI-189.
Rats were also administered an additional behavioral paradigm involving a contextual fear-conditioning task that does not rely on spontaneous exploration but is known to engage the hippocampus (25). Data derived from this task did not uncover any differences that were statistically significant (Fig. 5). Despite a trend toward reduced freezing in the hippocampal-dependent context phase of fear-conditioning, irradiation did not elicit marked deficits in any of the other phases of this task. Regardless, the fact that the NSI-189-treated and control groups were statistically indistinguishable in contextual fear-conditioning provides evidence that all cohorts exhibited the same behavioral trends on each of the behavioral testing paradigms administered in this study.
Collectively, our behavioral studies provide compelling evidence for the neurocognitive benefits of NSI-189 after a clinically relevant irradiation paradigm. Furthermore, we sought to determine whether certain neurocognitive benefits associated with NSI-189 treatment might be related to changes in neurogenesis and/or neuroinflammation after irradiation. Additional support for this possibility comes from studies in mice, where a similar administration regimen improved hippocampal cell proliferation and volume, albeit in an unirradiated brain (see Supplementary Information; http://dx.doi.org/10.1667/RR14879.1.S1). In cohorts subjected to irradiation, NSI-189 treatment was found to enhance neurogenesis significantly, although this was not found to be associated with any noticeable changes in hippocampal volume. While the reasons for the latter are uncertain, changes in hippocampal volume after irradiation may not track temporally with more rapid decreases or increases in neurogenesis, or may simply take longer to manifest in rats. Analysis of similarly treated cohorts showed that the numbers of activated microglia were reduced significantly after treatment with NSI-189 compared to irradiated but untreated animals one month after exposure. These findings corroborate past data showing that reductions in microglia correlated with improved neurocognitive outcomes (19, 26), and points to a previously unrecognized anti-inflammatory property of this small molecule.
While our current treatment paradigm has shown beneficial cognitive effects associated with improved neurogenesis and reduced inflammation, there are additional mechanisms that may be responsible for these effects, such as changes in myelination (27), the blood-brain barrier (28) and/or altered cerebral blood flow (29). It is also important to note that our NSI treatment regimen was specifically designed to optimize cognition. This necessitated the termination of NSI-189 via oral gavage, since this would have produced undesirable stress and confounded our behavioral measurements. Regardless of the mechanisms, the lack of suitable and efficacious pharmacologic treatment options for the devastating side effects of cranial radiotherapy underscore the urgency for developing satisfactory interventions for this long-term mental health problem. Our efforts to thwart cognitive dysfunction through a pharmacologic approach employing the small molecule compound NSI-189 may provide the experimental foundation for a potential solution.
Supplementary Material
Fig. S1. A comparison of cell proliferation (BrdU+ cells) in the subgranular zone of the hippocampal dentate gyrus (DG) at various doses of oral NSI-189 administration. Compared to controls, a significant increase was only found at a dose of 30 mg/kg. P < 0.05, Bonferroni's multiple comparison test.
Fig. S2. A comparison of overall hippocampal volume after various doses of oral NSI-189 administration. Compared to controls, a significant increase was found at NSI-189 doses of 10 and 30 mg/kg. P < 0.05, Bonferroni's multiple comparison test.
Acknowledgments
This work was supported by Neuralstem Inc. and the NIH-NINDS (grant no. 5R01 NS074388 to CLL).
Footnotes
Editor's note. The online version of this article (DOI: 10.1667/ RR14879.1) contains supplementary information that is available to all authorized users.
Supplementary Information: Assessment of histological changes in mice treated with NSI-189.
References
- 1.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48:1176–84. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–63. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 4.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–23. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–86. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 6.Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park) 2000;14:75–9. discussion 79, 81–2, 85. [PubMed] [Google Scholar]
- 7.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 8.Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–32. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer T, Fike J. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–7. [PubMed] [Google Scholar]
- 10.Liao AC, Craver BM, Tseng BP, Tran KK, Parihar VK, Acharya MM, et al. Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat Res. 2013;180:1–6. doi: 10.1667/RR3339.1. [DOI] [PubMed] [Google Scholar]
- 11.Tseng BP, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, et al. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal. 2013;20:1410–22. doi: 10.1089/ars.2012.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parihar VK, Allen BD, Tran KK, Chmielewski NN, Craver BM, Martirosian V, et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Anti-oxid Redox Signal. 2015;22:78–91. doi: 10.1089/ars.2014.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 15.Greene-Schloesser D, Moore E, Robbins ME. Molecular path-ways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fava M, Johe K, Ereshefsky L, Gertsik LG, English BA, Bilello JA, et al. A phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study of NSI-189 phosphate, a neurogenic compound, in depressed patients. Mol Psychiatry. 2016;21:1483–4. doi: 10.1038/mp.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie LA, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–65. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 18.Parihar VK, Acharya MM, Roa DE, Bosch O, Christie LA, Limoli CL. Defining functional changes in the brain caused by targeted stereotaxic radiosurgery. Transl Cancer Res. 2014;3:124–37. doi: 10.3978/j.issn.2218-676X.2013.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acharya MM, Martirosian V, Christie LA, Riparip L, Strnadel J, Parihar VK, et al. Defining the optimal window for cranial transplantation of human induced pluripotent stem cell-derived cells to ameliorate radiation-induced cognitive impairment. Stem Cells Transl Med. 2015;4:74–83. doi: 10.5966/sctm.2014-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–31. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, et al. NSI-189, a small molecule with neurogenic properties, exerts behavioral, and neurostructural benefits in stroke rats. J Cell Physiol. 2017;232:2731–40. doi: 10.1002/jcp.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. doi: 10.1038/srep31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 26.Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A. 2016;113:4836–41. doi: 10.1073/pnas.1521668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Qiu D, So KF, Wu EX, Leung LH, Gu J, et al. Radiation induced brain injury: assessment of white matter tracts in a pre-clinical animal model using diffusion tensor MR imaging. J Neurooncol. 2013;112:9–15. doi: 10.1007/s11060-012-1031-0. [DOI] [PubMed] [Google Scholar]
- 28.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn CA, Zhou SM, Raynor R, Tisch A, Light K, Shafman T, et al. Dose-dependent effects of radiation therapy on cerebral blood flow, metabolism, and neurocognitive dysfunction. Int J Radiat Oncol Biol Phys. 2009;73:1082–7. doi: 10.1016/j.ijrobp.2008.05.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. A comparison of cell proliferation (BrdU+ cells) in the subgranular zone of the hippocampal dentate gyrus (DG) at various doses of oral NSI-189 administration. Compared to controls, a significant increase was only found at a dose of 30 mg/kg. P < 0.05, Bonferroni's multiple comparison test.
Fig. S2. A comparison of overall hippocampal volume after various doses of oral NSI-189 administration. Compared to controls, a significant increase was found at NSI-189 doses of 10 and 30 mg/kg. P < 0.05, Bonferroni's multiple comparison test.


