Case 1 is a 50-year-old female never smoker with recurrent small-cell lung cancer who visited our institution to discuss further treatment plan in 2015. She had a family history of pancreatic cancer in a sibling and leukemia in another sibling. She underwent left upper lobe wedge resection of a primary lesion, adjuvant cisplatin plus etoposide, and topotecan at another institution. She had a past history of breast cancer. which was diagnosed in 2006. She underwent lumpectomy, and pathological findings from her breast cancer showed it to be ER positive, PR negative, and HER2 negative. Postoperatively, she was treated with adjuvant chemotherapy and radiotherapy followed by Tamoxifen. In 2010, she had an isolated lung lesion, and underwent left upper lobe lobectomy after needle biopsy. Final pathology showed low grade, moderately differentiated adenocarcinoma, ER + (15%), PR −, HER2 IHC 1+, HER2 FISH −, CK AE1/AE3 +, CK7 +, mammaglobin +, CK20 −, TTF1 −, and was consistent with metastatic breast carcinoma. She again received adjuvant chemotherapy. In 2015, CT scan showed 18×10×16 mm subpleural nodule in the left lower lung, and she underwent wedge resection. Final pathology on this specimen showed small cell lung cancer, synaptophysin +, CD56 +, TTF-1 +, HMWK focally +, CK5 −, GATA − (Figure 1). Both the small cell lung cancer specimen and metastatic breast cancer specimen were sent for NGS testing (FoundationOne™), and TP53 Y236* mutation and PARK2 Q347* mutation were detected in both specimens (Table 1) [1]. Germline mutations in TP53 and PARK2 were suspected since these same mutations were present in both tumor specimens, and allele frequencies for these were requested. Allele frequencies of the TP53 Y236* mutation and the PARK2 Q347* mutation in the small cell lung cancer specimen were 83% and 47%, respectively. She was recommended to see genetics and to undergo germline genetic testing, but she declined. As exactly the same mutations in TP53 and PARK2 were detected in two separate cancer specimens (both the metastatic breast cancer specimen and the primary lung cancer specimen), and their allele frequencies were significantly increased in the lung specimen, we clinically diagnosed the patient with germline mutations in TP53 and PARK2. Targeted therapy directed against FGFR2 amplification was planned in a clinical trial setting, but unfortunately she did not meet the eligibility criteria of the clinical trial due to elevated liver enzymes.
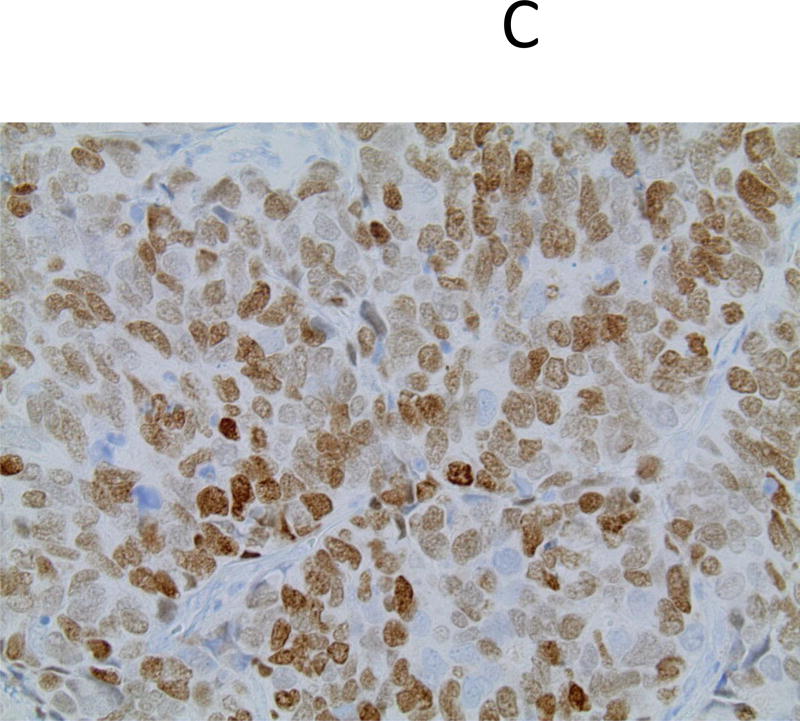
Figure 1.
Pathological features of small cell lung carcinoma in a 50-year-old female never smoker, left upper lobe wedge resection; hematoxylin-eosin (A), immunohistochemistry for synaptophysin (B) and thyroid transcription factor-1 (C); original magnification ×400.
Table 1.
Results of FoundationOne™ in a 50-year-old female never smoker with recurrent small-cell lung cancer
| Lung Cancer Specimen (collected and submitted in 2015) | |
| Alterations | Mutation allele frequency |
| TP53 Y236* | 83% |
| PARK2 Q347* | 47% |
| FGFR2 Amplification | NA |
| Breast Cancer Specimen (collected in 2010, submitted in 2015) | |
| Alterations | Mutation allele frequency |
| TP53 Y236* | NA |
| PARK2 Q347* | NA |
| STK11 truncation exon8 | NA |
| CCND3 amplification | NA |
| VEGFA amplification | NA |
Abbreviation: NA, not available.
Case 2 is a 34-year-old female former smoker with advanced lung adenocarcinoma (metastatic pleural effusion) who was referred to our institution for further treatment in 2016. She received pleural catheter placement, four cycles of carboplatin plus pemetrexed followed by two cycles of maintenance pemetrexed, and four doses of nivolumab. A blood sample was sent for cfDNA testing (FoundationACT™) and MET 3028+2T>C (splice site mutation) and BRCA2 L3061* mutations were detected (Table 2) [1]. Allele frequencies for these mutations were 0.19% and 50.7%, respectively, and a germline mutation in BRCA2 was suspected. She was referred to genetics, and underwent testing for mutations in the BRCA2 gene. The result showed that she had a germline BRCA2 L3061* mutation. Of note, this patient had no personal or family history of breast or ovarian cancer that would have otherwise prompted germline genetic testing of the BRCA2 gene. She received crizotinib, and had a partial response.
Table 2.
Result of FoundationACT™ in a 34-year-old female former smoker with advanced lung adenocarcinoma
| Blood (collected and submitted in 2016) | |
| Alterations | Mutation allele frequency |
| MET 3028+2T>C (splice site mutation) | 0.19% |
| BRCA2 L3061* | 50.7% |
These two cases suggest that practicing oncologists should be aware that detecting significant germline mutations in lung cancer patients is possible with currently available and routine technologies.
Germline mutations predisposing to lung cancer are rare, but germline mutations in EGFR, HER2, BRCA2, CDKN2A, BAP1, SFTPA2, and PARK2 are associated with an increased risk of lung cancer [2]. We retrospectively investigated 3,869 lung cancer patients seen at our institution between February 2012 and January 2017, and seven patients including two patients presented here were found to have potentially predisposing germline mutations (Three had BRCA2 germline mutations, two had germline TP53 mutations [of which one patient also had a PARK2 mutation], one had a BRCA1 mutation, and one had an EGFR T790M mutation.).
Introduction of NGS technology and liquid biopsies to clinical practice can raise the probability of detecting germline mutations in lung cancer patients [1, 3]. As in our patient from Case 2, this can have implications for screening and preventative measures for cancers besides lung cancer, as well as implications for cancer risks for other relatives. Clinicians should be alert to the potential existence and importance of germline mutations in their lung cancer patients.
Acknowledgments
Funding sources
This work was supported by The Lilly Oncology Fellowship from The Japanese Respiratory Society, alumni scholarship from the Juntendo University School of Medicine, a research fellowship from Uehara Memorial Foundation, Pelotonia postdoctoral fellowship (to TS) and the Blue Beautiful Skies Fund (to DPC).
Conflicts of interest
TS reports grants and personal fees from Chugai Pharmaceutical, grants from Boehringer Ingelheim, personal fees from AstraZeneca, Ono Pharmaceutical, Nichi-iko Pharmaceutical, Sanofi, and Pfizer, outside the submitted work. KSC reports grants from Addario Lung Cancer Foundation, outside the submitted work. GAO reports grants and other from Pfizer, and Genentech, grants from Pfizer, BMS, Ignyta, and Merck, other from Novartis, and Amgen, outside the submitted work. DPC reports personal fees from Abbvie, Adaptimmune, Amgen, Ariad, AstraZeneca, Bayer Health Care, Biocept, Biothera, Boehringer Ingelheim, Cancer Support Community, Celgene, Clovis Oncology, Foundation Medicine, Genentech/Roche, Genoptix, GlaxoSmithKline, Gritstone, Guardant Health, ImmuneDesign, Janssen Diagnostics, MedImmune, Merck, MSD, Novartis, Palobiofarma, Peregrine Pharmaceuticals, Inc., Pfizer, prIME Oncology, Stemcentrx, Synta Pharmaceuticals Corp., Takeda, Teva Pharmaceuticals, Verastem, grants and personal fees from Bristol Myers-Squibb, outside the submitted work.
References
- 1. [last accessed on 27/Jun/2017];Foundation Medicine. http://www.foundationmedicine.com/
- 2.Clamon GH, Bossler AD, Abu Hejleh T, et al. Germline mutations predisposing to non-small cell lung cancer. Fam Cancer. 2015 Sep;14(3):463–9. doi: 10.1007/s10689-015-9796-x. [DOI] [PubMed] [Google Scholar]
- 3. [last accessed on 27/Jun/2017];Guardant Health. https://www.guardanthealth.com/guardant360/