Abstract
Introduction
Elderly patients represent the majority of lung cancer diagnoses but are poorly represented in clinical trials. We evaluated the overall survival (OS) of elderly patients with stage III non-small cell lung cancer (NSCLC) treated with definitive radiation compared to those treated with definitive chemoradiation.
Methods
We conducted a comparative effectiveness study of radiation therapy vs. chemoradiation in elderly (≥70 years old) patients with stage III NSCLC not treated surgically diagnosed from 2003–2014 using the National Cancer Database. Two cohorts were evaluated: patients treated with definitive (≥59.4 Gy) radiation (n=5,023) and patients treated with definitive chemoradiation (n=18,206). Chemoradiation was further defined as concurrent (radiation and chemotherapy started within 30 days of each other) or sequential (radiation started>30 days after chemotherapy). We compared OS between the treatment groups using the Kaplan-Meier method and Cox proportional hazards regression before and after propensity score matching (PSM).
Results
Treatment with chemoradiation was associated with improved OS compared to radiation before PSM (HR=0.66, 95%CI 0.64–0.68, p<.001) and after PSM (HR=0.67, 95%CI 0.64–0.70, p<.001). Relative to concurrent chemoradiation, sequential chemoradiation was associated with a 9% reduction in the risk of death (HR=0.91, 95%CI 0.85–0.96, p=.002).
Conclusions
We found that definitive chemoradiation resulted in a survival advantage compared to definitive radiation in elderly patients. Sequential chemotherapy and radiation was superior to concurrent chemoradiation. While prospective trials are needed, this analysis suggests that chemoradiation should strongly be considered for elderly patients and the optimal sequencing of chemotherapy and radiation remains an unanswered question for this patient population.
Keywords: elderly, stage III, non-small cell, chemotherapy, radiation therapy
Introduction
Stage III non-small cell lung cancer (NSCLC) represents 30% of all new lung cancer diagnoses and is a heterogeneous disease requiring a multi-disciplinary treatment approach.1 Nearly 70% of all lung cancer diagnoses and >70% of lung cancer deaths in the U.S. occur in patients ≥65 years of age.2 However, the elderly are under-represented in clinical trials making treatment decisions in this population challenging.3–5
Overall, the available data guiding decision-making in the elderly is limited. A multicenter retrospective review, based on the Netherlands Cancer Registry, reported no improvement in overall survival (OS) for patients ≥70 years old treated with concurrent chemoradiation (CRT) compared to sequential CRT or radiation therapy (RT) alone.6 In contrast, two additional studies, including subset analysis of 2 prospective trials and a Surveillance Epidemiology and End Results-Medicare (SEER) analysis demonstrated a survival benefit for CRT.7, 8 Given the conflicting and scant data in this patient population, the optimal treatment strategy for stage III NSCLC in the elderly needs to be further defined.
The objective of this study was to compare OS in elderly patients treated with RT alone compared to CRT using the National Cancer Database (NCDB). We hypothesized that patients that received CRT would have improved OS compared to those treated with RT alone.
Materials and Methods
The NCDB, a combined effort of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, is a nationwide hospital-based database that contains de-identified hospital registry data from more than 1,500 accredited facilities, representing more than 70% of newly diagnosed cancer cases in the U.S.9 The NCDB collects data on patient demographics and comorbidities, tumor characteristics and staging details, primary therapies administered, and OS. The CoC's NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data and have not verified and are not responsible for the statistical validity of the data analysis nor the conclusions presented in this study.
Patient Selection
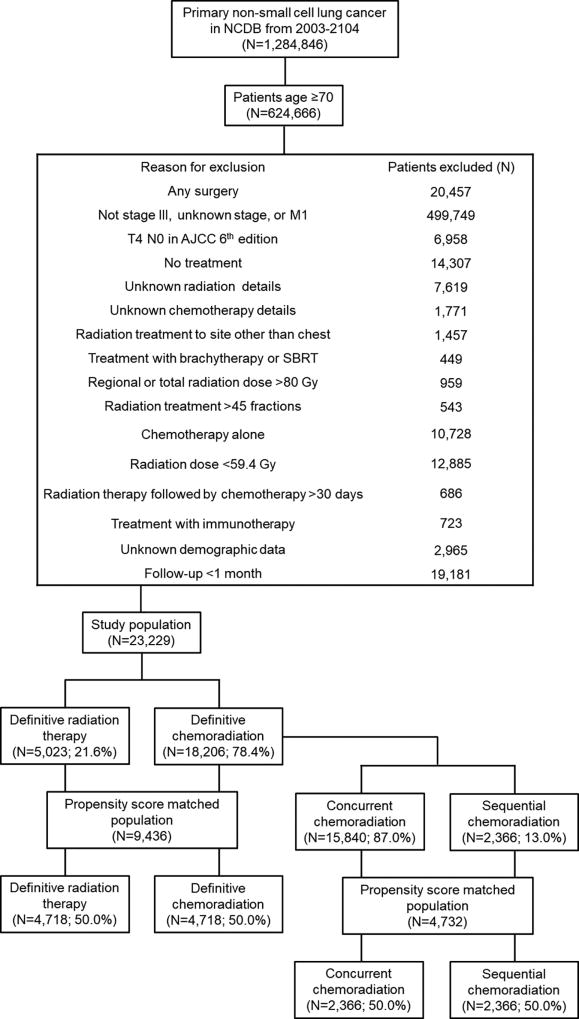
Patients diagnosed with stage III NSCLC from 2003–2014 were collected from the NCDB participant user file with additional inclusion and exclusion criteria summarized in Figure 1. We defined elderly as patients≥70 years old, as used previously in numerous studies.10–12 The transition to the American Joint Committee on Cancer (AJCC) 7th edition occurred in 2010, consequently, our patient cohort consisted of patients staged using both AJCC 6th and 7th editions. Patients with clinical T4N0M0 disease based on the AJCC 6th edition were excluded as these patients could be either clinical stage II (T3 N0 M0) or stage IV (M1a – malignant pleural or pericardial effusion) based on the AJCC 7th edition. Since the focus of this study was on patients not treated surgically, those treated with any type of surgical procedure were excluded. Additional exclusion criteria included: patients with unknown RT or chemotherapy details, with regional or total RT doses ≥80 Gy, treatment with more than 45 fractions of RT, treatment with immunotherapy, no treatment, treatment with chemotherapy alone, and treatment with palliative doses of radiation therapy (<59.4 Gy). After exclusion, patients were categorized as having received definitive RT alone (≥59.4 Gy) or definitive CRT (≥59.4 Gy). CRT patients were considered to have received concurrent CRT (CCRT) if chemotherapy was delivered within 30 days prior to or after initiation of RT while sequential CRT (SCRT) was defined as RT delivered >30 days after initiation of chemotherapy as defined in a prior study.13 Details regarding radiation treatment technique (e.g., 3D conformal radiation therapy versus intensity-modulated radiation therapy) were available for 35% of the patient population. Rather than exclude patients with unknown treatment technique, we chose not to include this variable in our analysis. Patients with unknown demographic data were excluded. For the urban/rural code, the 2013 classification codes were used while income was determined using the 2008–2012 code. Finally, patients with less than 1 month of follow-up were excluded from the analysis to limit immortal time bias.14
Figure 1.
Study flow diagram for analytic cohorts. NCDB, National Cancer Database; SBRT, stereotactic body radiation therapy.
Study Variables
We dichotomized the following baseline covariates: gender (male vs. female), race (white vs. non-white), median income (≥$48,000 vs. <$48,000), primary insurance payor (private vs. non-private), county location (metropolitan vs. urban/rural), facility type (academic vs. community/comprehensive community/integrated network programs), chemotherapy agents used (multi-agent regimen vs. single-agent regimen), and clinical stage group (IIIB vs. IIIA). The Charlson-Deyo score, a measure of comorbidity, was dichotomized as 0 (no comorbities) or 1 (≥1 comorbidity). The variables age and distance to the nearest facility were analyzed as continuous variables.
Statistical Methods
The primary objective of this study was to evaluate OS in elderly patients treated with CRT vs. RT alone. We also sought to evaluate the impact of number of chemotherapy agents used (multi-agent CRT vs. RT alone; single-agent CRT vs. RT; multi-agent CRT vs. single-agent CRT). Additional analysis included comparison of OS in CRT patients treated with CCRT vs. SCRT. Differences in patient characteristics between CRT and RT patients were tested using the χ2 test for categorical variables and the t-test for continuous variables. Survival was estimated using the Kaplan-Meier method and the log-rank test was used to compare survival curves. Cox regression analysis was used to test the association between treatment and demographic variables with OS on univariate analysis. Variables with p≤.10 on univariate analysis were included in the multivariate model.
Propensity score-matching (PSM) was performed to reduce potential selection bias. Logistic regression was used to identify predictors of treatment with definitive CRT vs. definitive RT alone. Patients treated with CRT were matched with those receiving radiation alone using a 1:1 nearest available neighbor match without replacement using an algorithm described by Coca-Perraillon.15 The caliper size was calculated as 0.20*standard deviation of the propensity score as described by Rosenbaum et al.16 Common support of the propensity score distributions was evaluated graphically and balance was evaluated by computing the standardized difference of the covariates across the two groups.17 Following PSM, OS was estimated using the Kaplan-Meier method and Cox regression was used to perform univariate and multivariate analysis. The exact same approach was used to perform PSM for patients receiving CCRT vs. SCRT. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
We identified 5,023 elderly patients treated with RT alone and 18,206 patients treated with CRT. The median radiation dose was 64.8 Gy (IQR, 61.2–66.6 Gy) over a median of 34 fractions (33–36) in the CRT group and 64.8 Gy (IQR, 60.0–66.6 Gy) over a median of 33 (30–35) in the RT group. The radiation was delivered with conventional fraction sizes with a median daily fraction size of 1.8 Gy (1.8–2.0 Gy) in the CRT group and 2.0 Gy (1.8–2.0 Gy) in the RT group. Several imbalances in characteristics existed between RT and CRT patients (Table 1). Most notably, CRT patients were more likely to have stage IIIB disease (36.7% vs. 32.5%, p<.001), but were also more likely to have no comorbidities (62.9% vs. 58.7%, p<.001) and be of younger age (75.8 years vs. 79.4 years, p<.001) compared to RT patients. Most of the CRT patients received multi-agent chemotherapy (86%, N=15,715). In the CRT cohort, the majority were treated with CCRT (87%, N=15,840) with a median time between the start of radiation and chemotherapy of 0 days (interquartile range (IQR), −1 [RT first] to 0 days). The median time from the start of chemotherapy to radiation in the SCRT group was 82 days (IQR, 56–112 days).
Table 1.
Patient Characteristics
| Radiation therapy alone (N=5,023) |
Chemoradiation (N=18,206) |
pa | |
|---|---|---|---|
| Age, mean (SD) | 79.4 (5.4) years | 75.8 (4.4) years | <.001 |
| Gender | <.001 | ||
| Male | 2750 (54.7%) | 10540 (57.9%) | |
| Female | 2273 (45.3%) | 7666 (42.1%) | |
| Race | .005 | ||
| White | 4398 (87.6%) | 16197 (89.0%) | |
| Non-white | 625 (12.4%) | 2009 (11.0%) | |
| Charlson-Deyo Score | <.001 | ||
| 0 | 2949 (58.7%) | 11450 (62.9%) | |
| >1 | 2074 (41.3%) | 6756 (37.1%) | |
| Median income | <.001 | ||
| ≥$48,000 | 2522 (50.2%) | 9825 (54.0%) | |
| <48,000 | 2501 (49.8%) | 8381 (46.0%) | |
| Primary insurance payor | .74 | ||
| Private | 491 (9.8%) | 1751 (9.6%) | |
| Non-private | 4532 (90.2%) | 16455 (90.4%) | |
| County location | .045 | ||
| Metropolitan | 4062 (80.9%) | 14489 (79.6%) | |
| Non-metropolitan | 961 (19.1%) | 3717 (20.4%) | |
| Distance to closest facility, mean (SD) | 15.9 (41.4) | 20.4 (74.7) | <.001 |
| Facility type | .98 | ||
| Academic | 1243 (24.8%) | 4502 (24.7%) | |
| Non-academic | 3780 (75.2%) | 13704 (75.3%) | |
| Clinical stage group | <.001 | ||
| IIIA | 3390 (67.5%) | 11523 (63.3%) | |
| IIIB | 1633 (32.5%) | 6683 (36.7%) | |
| Days to start of radiation, mean (SD) | 47.9 (40.2) | 55.5 (46.5) | <.001 |
| Radiation therapy dose, median (IQR) | 64.8 (60.0–66.6) Gy | 64.8 (61.2–66.6) Gy | .67 |
| Elapsed days of radiation, median (IQR) | 50 (45–55) days | 51 (47–56) days | .51 |
| Days to start of chemotherapy, median (IQR) | N/A | 36 (25–54) days | N/A |
| Type of chemotherapy | |||
| Multi-agent | N/A | 15715 (86.3%) | N/A |
| Single-agent/unknown | N/A | 2491 (13.7%) | N/A |
| Type of chemoradiation | |||
| Concurrent | N/A | 15840 (87.0%) | N/A |
| Sequential | N/A | 2366 (13.0%) | N/A |
The p values are from the χ2 test for categorical variables and the t-test for continuous variables.
SD, standard deviation; IQR, interquartile range; N/A, not applicable.
Predictors of Receiving CRT vs. RT
We identified both clinical and demographic variables associated with elderly patients receiving definitive CRT treatment (Supplementary Table 1). On univariate analysis, younger age, male sex, white race, higher income, living in a non-metropolitan county, stage IIIB, increased distance from the treating hospital, and a Charlson-Deyo score<1 were associated with higher odds of receiving CRT. All of these covariates, except for living in a metropolitan county, were independently associated with higher odds of receiving CRT on multivariate logistic regression analysis and were used to calculate propensity scores. Following PSM, the propensity score distributions between the two groups showed nearly ideal common support (Supplementary Figure 1A). The covariates were well-balanced between the two treatment groups following PSM with standardized differences between the covariates well below 10% (Supplementary Table 2).
Predictors of Receiving CCRT vs. SCRT
Supplementary Table 3 demonstrates that amongst the CRT patients, factors associated with receipt of CCRT compared to SCRT included: males, non-academic treatment facilities, stage IIIA disease, and higher comorbidity index. All of these factors remained significant on multivariate logistic regression analysis and were used to calculate propensity scores. Supplementary Figure 1B and Supplementary Table 4 demonstrate that PSM resulted in well balanced groups.
Survival Outcomes in the CRT vs. RT Cohorts
At the time of analysis, 19,041 of the 23,229 patients had died. The median follow-up for all elderly patients was 15.5 months (IQR 8.3–28.8 months) and the median follow-up for survivors was 30.7 months (IQR 19.1–49.8 months). We used Cox univariate and multivariate analyses to identify patient and treatment factors associated with OS (Supplementary Table 5). Factors independently associated with improved OS included younger age, female sex, non-white race, treatment at an academic facility, higher income, living in a metropolitan county, stage IIIA disease vs. IIIB, closer distance to the treatment hospital, Charlson-Deyo score<1, longer time to start of RT, and CRT vs. RT alone.
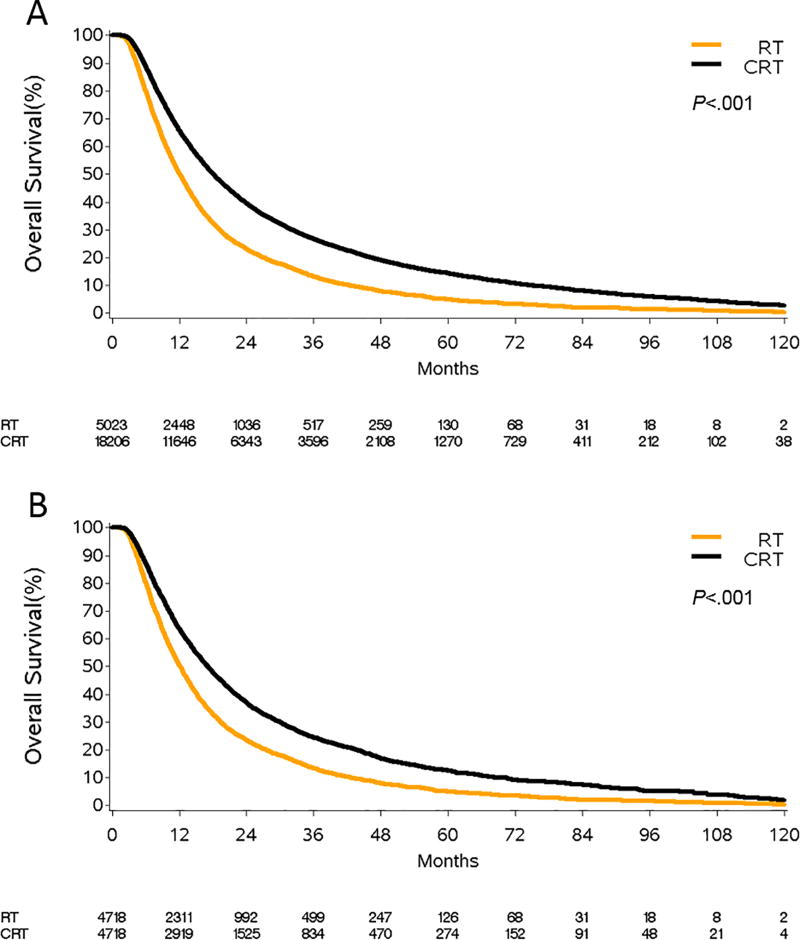
Prior to PSM, the median OS was 18.1 months (95%CI, 17.8–18.5 months) for patients treated with CRT and 12.2 months (95%CI, 11.7–12.6 months) for patients treated with RT (p<.001, Figure 2A). With PSM, a CRT therapy match was successfully identified for 4,718 of the 5,023 patients treated with RT (caliper size=0.03 based on propensity score SD=0.13). In the matched cohort, the survival advantage of CRT over RT alone persisted with a median OS of 17.2 months (95%CI, 16.6–17.8) and 12.2 months (95%CI, 11.8–12.6), respectively (p<.001; Figure 2B).
Figure 2.
Overall survival of patients treated with definitive chemoradiation (CRT) compared to those treated with definitive radiation therapy (RT) alone. Panel A represents the unmatched patient population while panel B represents propensity-score matched elderly patients. All curves represent actual survival as estimated by Kaplan-Meier.
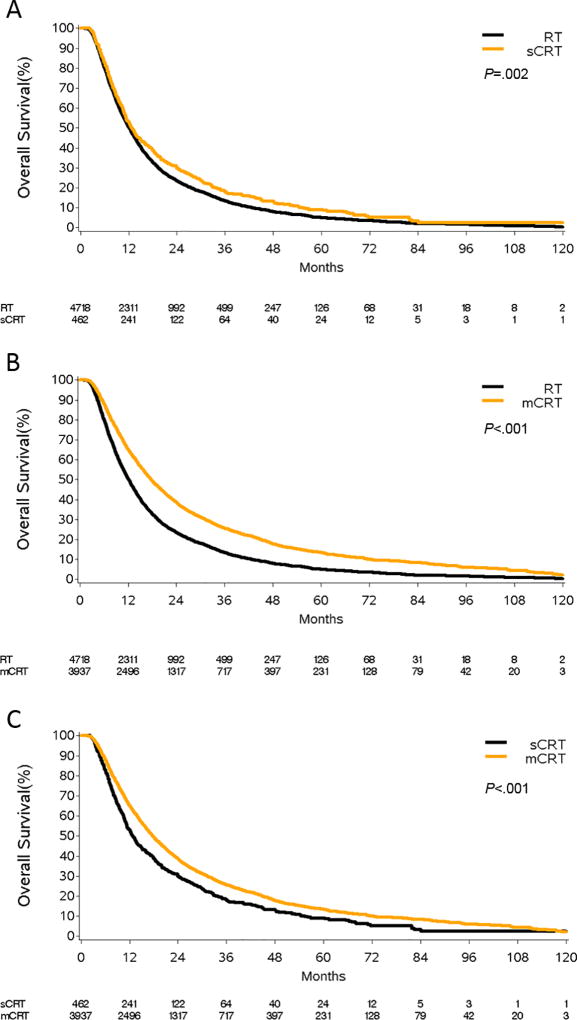
On univariate analysis in the matched cohort of elderly CRT vs. RT patients, factors associated with worse OS included: older age, male gender, white race, non-academic treatment facility, non-metropolitan location, stage IIIB, Charlson-Deyo score>0, and treatment with RT alone (Supplementary Table 6). After adjustment for confounders, CRT corresponded to a 33% reduction in the risk of death (HR=0.67, 95%CI 0.64–0.70, p<.001, Table 2). The benefit of CRT was greater for elderly patients treated with multi-agent chemotherapy (HR=0.64, 95%CI 0.61–0.67, p<.001, Figure 3B, Table 2) compared with single-agent chemotherapy (HR=0.83, 95%CI 0.75–0.92, p<.001, Figure 3A, Table 2). As demonstrated in Figure 3C and Table 2, for elderly patients treated with CRT, multi-agent chemotherapy resulted in a 21% decrease in the HR for death compared to single-agent chemotherapy (HR=0.79, 95%CI 0.71–0.88, p<.001).
Table 2.
Multivariate Analysis of Overall Survival for Chemoradiation vs. Radiation Therapy Alone and Concurrent Chemoradiation vs. Sequential Chemotherapy and Radiation after Propensity Score Matching
| CRT vs. RT Cohorts (N=9,436) |
CCRT vs. SCRT Cohorts (N=4,732) |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value |
| Age | 1.01 | 1.01–1.02 | <.001 | 1.02 | 1.01–1.02 | <.001 |
| Female vs. male | 0.84 | 0.81–0.88 | <.001 | 0.82 | 0.77–0.88 | <.001 |
| White vs. non-white | 1.18 | 1.10–1.26 | <.001 | 1.12 | 1.01–1.24 | .03 |
| Academic vs. non-academic | 0.95 | 0.90–1.00 | .05 | 0.90 | 0.84–0.97 | .005 |
| Private vs. non-private insurance | NS | NS | NS | NS | NS | NS |
| Median income (≥$48,000 vs. <$48,000) | 0.96 | 0.91–1.00 | .07 | 0.96 | 0.90–1.03 | .23 |
| County location (Metropolitan vs. non-metropolitan) | 0.97 | 0.91–1.02 | .23 | 0.98 | 0.90–1.06 | .58 |
| Clinical stage IIIB vs. stage IIIA | 1.18 | 1.12–1.23 | <.001 | 1.16 | 1.09–1.24 | <.001 |
| Distance to closest facilitya | NS | NS | NS | NS | NS | NS |
| Charlson-Deyo score (1 vs. 0) | 1.08 | 1.03–1.13 | <.001 | 1.15 | 1.08–1.23 | <.001 |
| CRT vs. RT alone | 0.67 | 0.64–0.70 | <.001 | N/A | N/A | N/A |
| SCRT vs. CCRT | N/A | N/A | N/A | 0.91 | 0.85–0.96 | .002 |
| Single-agent CRT vs. RT alone | 0.83 | 0.75–0.92 | <.001 | N/A | N/A | N/A |
| Multi-agent CRT vs. RT alone | 0.64 | 0.61–0.67 | <.001 | N/A | N/A | N/A |
| Multi-agent vs. Single-agent chemotherapyb | 0.79 | 0.71–0.88 | <.001 | 0.74 | 0.65–0.96 | .002 |
| Days to start of radiationc | 1.00 | 1.00–1.00 | <.001 | N/A | N/A | N/A |
Abbreviations: CRT, chemoradiation; RT, radiation therapy; CCRT, concurrent chemoradiation, SCRT, sequential chemotherapy and radiation; NS, p-value>.10 on univariate analysis; N/A, not applicable
log of distance (miles) used for analysis
in patients receiving chemoradiation
HR is 0.996 (0.995–0.997)
Figure 3.
Overall survival of patients treated with either single (sCRT) or multi-agent chemoradiation (mCRT) compared to those treated with radiation therapy alone (RT). Panel A is a comparison of sCRT vs. definitive RT alone and panel B represents mCRT vs. definitive RT alone. Panel C is a comparison of mCRT vs. sCRT. All analysis was performed using the propensity-score matched patients and all curves represent actual survival as estimated by Kaplan-Meier.
Survival Outcomes in CCRT vs. SCRT Patients
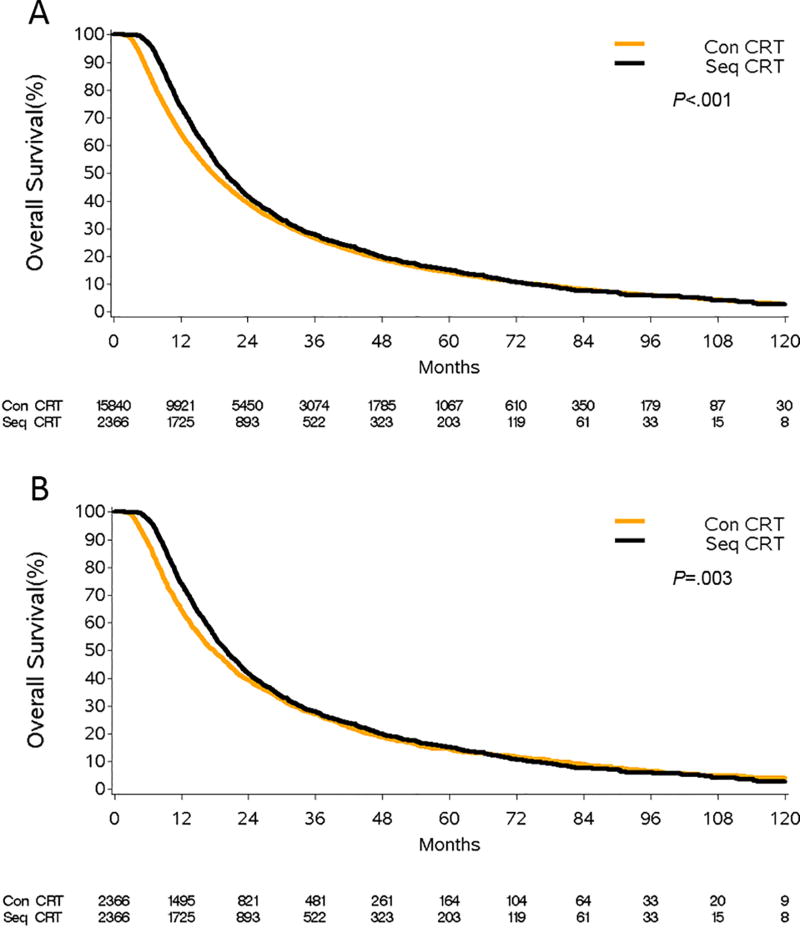
Elderly patients treated with definitive CRT were further subdivided into those treated with concurrent vs. sequential CRT based on the timing of initiation of radiation and chemotherapy. Of the 18,203 patients treated with definitive CRT, 15,840 received CCRT and 2,366 received SCRT. The median OS was significantly higher in patients treated with SCRT compared to CCRT: 20.0 months (95%CI, 19.1–20.9) vs. 17.8 months (95%CI, 17.4–18.2), p<.001 (Figure 4A). PSM identified a CCRT match for all 2,366 SCRT patients (caliper size=0.004 based on propensity score SD=0.02). Supplementary Table 6 demonstrates the univariate OS analysis in the matched CCRT vs. SCRT cohorts. On multivariate analysis, SCRT corresponded to a 9% reduction in the risk of death (HR=0.91, 95%CI 0.85–0.96, p=.002, Table 2, Figure 4B). We performed a sensitivity analysis where the definition of CCRT was restricted to patients who received chemotherapy within 14 days of the initiation of RT, and the survival benefit of SCRT over CCRT persisted.
Figure 4.
Overall survival of patients treated with definitive concurrent chemoradiation (Con CRT) and definitive sequential chemoradiation (Seq CRT). Panel A represents the unmatched patient population while panel B represents the propensity-score matched elderly patients. All curves represent actual survival as estimated by Kaplan-Meier.
Discussion
Treatment of the elderly with locally advanced NSCLC is challenging and, with an aging population, will remain an issue for the U.S. healthcare system for the foreseeable future.18 To our knowledge, our study represents the largest reported cohort of elderly stage III NSCLC patients not treated surgically. We found that combined modality therapy with radiation and chemotherapy results in improved OS compared to radiation alone in the elderly and that sequential therapy appears superior to concurrent therapy.
We found a significant OS benefit with the addition of chemotherapy to definitive RT with a 33% reduction in risk of death. Prior studies evaluating combined modality therapy in the elderly have reported conflicting results. For example, a retrospective study using the Netherlands Cancer Registry also evaluated elderly (≥70 years old) patients with unresectable stage III NSCLC and reported no survival benefit with concurrent CRT and increased toxicity.6 In contrast, a second analysis of patients aged ≥65 years compared concurrent CRT to RT alone and demonstrated a survival benefit (13.7 vs. 10.5 months, p=.05) for CRT compared to RT alone.8 However, the rates of grade ≥3 toxicity were significantly higher in the CRT group (89.9%) vs. RT alone group (32.4%). Similarly, Davidoff et al. found that CRT had a significant survival benefit when compared to RT alone (12.0 vs. 7.6 months) in patients ≥66 years old.7
In general, limited prospective data of CRT vs. RT alone in the elderly with stage III NSCLC exist. Two trials from Japan have evaluated CRT vs. RT in the elderly, the first of which was stopped early due to 4 deaths in the CRT arm.19 In a subsequent phase III trial that completed accrual, Atagi et al. found that CRT improved OS compared to RT alone in 197 patients >70 years of age (HR=0.68).10 The chemotherapy in this trial included single-agent low-dose carboplatin (30 mg/m2 × 20 days). There were higher rates of grade 3–4 hematologic toxicity and grade 3 infection in the CRT group, although rates of grade 3–4 radiation pneumonitis and late lung toxicity were similar between the groups. In a recent meta-analysis of the 243 patients treated on both Japanese trials and an additional 164 elderly patients from the Auperin et al. 2006 analysis of CRT using platinum compounds, the use of CRT was associated with a 34% reduction in the HR for death, similar to the 33% reduction seen in our study that included over 9,000 patients in the matched cohort.20 Additionally, we found a modest, but significant, 15% reduction in the risk of death when non-standard single agent chemotherapy is used.
In this NCDB analysis, we found that survival in elderly patients treated with SCRT had a 9% reduction in risk of death compared to CCRT. This finding is in contrast to the results of the 2010 Auperin et al. meta-analysis which demonstrated an OS benefit with concurrent vs. sequential CRT for patients with locally advanced NSCLC.21 The elderly were under-represented in that analysis with only 15% of patients ≥70 years old. Our results were similar to those from the Davidoff et al. SEER-Medicare database study that found an increased mortality risk with concurrent CRT compared to sequential CRT.7 However, this finding from our analysis must be taken with caution - the NCDB does not collect duration of chemotherapy treatment, so it is not possible to determine if patients in the sequential CRT group received combined modality therapy at the time of radiation.
The toxicity of combined modality therapy is a central issue in the management of elderly patients.12, 22–24 Several studies have found significantly higher rates of toxicity in patients receiving CRT vs. RT alone.6, 8, 10, 19, 25 In a recent pooled analysis of stage III NSCLC patients treated with CRT on 1 of 16 U.S. National Cancer Institute cooperative group studies, the 832 elderly patients (≥70 years old) experienced more toxicity, higher rate of treatment-related death, and worse OS compared to the 2,768 non-elderly patients.12 Given the strict eligibility criteria and close follow-up required on clinical trials, the rates of adverse-events/deaths and survival outcomes are likely worse in the non-clinical trial elderly population. Nonetheless, our results demonstrate that elderly patients treated with CRT had superior survival compared to those treated with RT alone. This underscores the importance of identifying and incorporating tools such as the comprehensive geriatric assessment (CGA) or Vulnerable Elders Survey-13 into the treatment decision-making process.18, 26, 27 Completion of the CGA can help predict risk of toxicity with treatment, can be used to fine-tune treatment recommendations,28 and its use has been endorsed by the International Society for Geriatric Oncology and the European Organization for Research and Treatment of Cancer.27, 29
This study has several limitations. The NCDB is a retrospective database with inherent weaknesses that include incomplete data, selection bias, and unmeasured confounders. One major limitation of the NCDB is that performance status is not captured. Instead, the Charlson-Deyo score, which measures the number of comorbidities each patient has, is collected. We fully recognize that there is no single variable or group of variables in any dataset that can accurately reflect the treatment decision for an individual patient. Therefore, while PSM was utilized to minimize treatment selection bias, imbalances likely remain in unmeasured variables between the treatment cohorts. The NCDB does not collect several key chemotherapy details, including specific agents used and the duration/number of cycles delivered. This makes it impossible to determine if patients received consolidation chemotherapy. Also, while an OS benefit was observed with definitive CRT compared to definitive RT alone, there is no available data in the NCDB regarding critically important endpoints of toxicity, quality of life, and cause of death.
Nonetheless, we feel that there are several strengths to be noted. This is by far the largest analysis of CRT vs. RT (and CCRT vs. SCRT) in elderly patients to date. While chemotherapy details are limited in the NCDB, the RT data is much more complete. The RT doses captured in the NCDB are those that were delivered, not intended, and we ensured patients received definitive doses of RT (≥59.4 Gy) to be included in this analysis. While the exact chemotherapy regimen is not reported, the NCDB does collect if 1 or >1 agent was used. We feel that the analysis of survival by number of chemotherapy agents used provides a useful framework for how to approach the elderly patient with stage III NSCLC.
Treatment of the elderly with stage III NSCLC should involve a multidisciplinary discussion. All patients not eligible for surgery should first be considered for CRT, with either concurrent or sequential radiation. Based on our findings, multi-agent chemotherapy is preferred over single-agent regimens in suitable patients. When multi-agent chemotherapy is not feasible, a single-agent regimen is supported by our results as well as those of Atagi et al.10 When chemotherapy is contraindicated or not recommended, then definitive RT alone should be considered.
In conclusion, we found that definitive CRT is superior to definitive RT in elderly patients with stage III NSCLC not treated surgically. We also found that in patients that received CRT, sequential chemotherapy and RT resulted in improved OS compared to concurrent CRT. While the optimal methodology for deciding on appropriate therapy in these patients is unknown, future clinical trials in stage III NSCLC should prioritize inclusion of the elderly in order to help further tailor therapeutic decisions for this expanding patient population.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health [grant P30 CA16058].
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: The authors have no conflicts of interest to disclose.
List of supplementary data:
Supplementary Table 1 – Supp Table 1.docx
Supplementary Table 2 – Supp Table 2.docx
Supplementary Table 3 – Supp Table 3.docx
Supplementary Table 4 – Supp Table 4.docx
Supplementary Table 5 – Supp Table 5.docx
Supplementary Table 6 – Supp Table 6.docx
Supplementary Figure 1 – Supp. Figure 1.docx
References
- 1.Crino L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. [Accessed June 13, 2017];SEER stat fact sheets: Lung and bronchus cancer. http://seer.Cancer.Gov/csr/1975_2014/
- 3.Sacher AG, Le LW, Leighl NB, et al. Elderly patients with advanced NSCLC in phase III clinical trials: Are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol. 2013;8:366–368. doi: 10.1097/JTO.0b013e31827e2145. [DOI] [PubMed] [Google Scholar]
- 4.Langer CJ. Neglected and underrepresented subpopulations: Elderly and performance status 2 patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer. 2006;7(Suppl 4):S126–137. doi: 10.3816/clc.2006.s.004. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Driessen EJ, Bootsma GP, Hendriks LE, et al. Stage III non-small cell lung cancer in the elderly: Patient characteristics predictive for tolerance and survival of chemoradiation in daily clinical practice. Radiother Oncol. 2016;121:26–31. doi: 10.1016/j.radonc.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Davidoff AJ, Gardner JF, Seal B, et al. Population-based estimates of survival benefit associated with combined modality therapy in elderly patients with locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:934–941. doi: 10.1097/JTO.0b013e31820eed00. [DOI] [PubMed] [Google Scholar]
- 8.Schild SE, Mandrekar SJ, Jatoi A, et al. The value of combined-modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer. 2007;110:363–368. doi: 10.1002/cncr.22780. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Database: A powerful initiative to improve cancer care in the united states. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the japan clinical oncology group (JCOG0301) Lancet Oncol. 2012;13:671–678. doi: 10.1016/S1470-2045(12)70139-0. [DOI] [PubMed] [Google Scholar]
- 11.Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378:1079–1088. doi: 10.1016/S0140-6736(11)60780-0. [DOI] [PubMed] [Google Scholar]
- 12.Stinchcombe TE, Zhang Y, Vokes EE, et al. Pooled analysis of individual patient data on concurrent chemoradiotherapy for stage III non-small-cell lung cancer in elderly patients compared with younger patients who participated in US National Cancer Institute cooperative group studies. J Clin Oncol. 2017;35:2885–2892. doi: 10.1200/JCO.2016.71.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang EH, Rutter CE, Corso CD, et al. Patients selected for definitive concurrent chemoradiation at high-volume facilities achieve improved survival in stage III non-small-cell lung cancer. J Thorac Oncol. 2015;10:937–943. doi: 10.1097/JTO.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 14.Park HS, Gross CP, Makarov DV, et al. Immortal time bias: A frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–1373. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Coca-Perraillon M. Local and global optimal propensity score matching; Presented at the SAS Global Forum 2007 Conference; Orlando, FL. April 16–19, 2007. [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Constructing a control-group using multivariate matched sampling methods that incorporate the propensity score. American Statistician. 1985;39:33–38. [Google Scholar]
- 17.Lanehart RE, Rodriguez de Gil P, Kim ES, et al. Propensity score analysis and assessment of propensity score approaches using SAS procedures; Presented at the SAS Global Forum 2012 Conference; Orlando, FL. April 22–25, 2012. [Google Scholar]
- 18.Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert's opinion paper: EORTC elderly task force, lung cancer group and international society for geriatric oncology. Ann Oncol. 2014;25:1270–1283. doi: 10.1093/annonc/mdu022. [DOI] [PubMed] [Google Scholar]
- 19.Atagi S, Kawahara M, Tamura T, et al. Standard thoracic radiotherapy with or without concurrent daily low-dose carboplatin in elderly patients with locally advanced non-small cell lung cancer: A phase III trial of the japan clinical oncology group (JCOG9812) Jpn J Clin Oncol. 2005;35:195–201. doi: 10.1093/jjco/hyi060. [DOI] [PubMed] [Google Scholar]
- 20.Dawe DE, Christiansen D, Swaminath A, et al. Chemoradiotherapy versus radiotherapy alone in elderly patients with stage III non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer. 2016;99:180–185. doi: 10.1016/j.lungcan.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 22.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang J, Li G, Ma L, et al. Predictors of grade >/= 2 and grade >/= 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol. 2013;52:1175–1180. doi: 10.3109/0284186X.2012.747696. [DOI] [PubMed] [Google Scholar]
- 24.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–483. doi: 10.1093/annonc/mdj117. [DOI] [PubMed] [Google Scholar]
- 26.Blanco R, Maestu I, de la Torre MG, et al. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol. 2015;26:451–463. doi: 10.1093/annonc/mdu268. [DOI] [PubMed] [Google Scholar]
- 27.Pallis AG, Fortpied C, Wedding U, et al. EORTC elderly task force position paper: Approach to the older cancer patient. Eur J Cancer. 2010;46:1502–1513. doi: 10.1016/j.ejca.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: An update on SIOG recommendations. Ann Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.