Abstract
Significance: Oxidative stress contributes to numerous pathophysiological conditions such as development of cancer, neurodegenerative, and cardiovascular diseases. A variety of measurements of oxidative stress markers in biological systems have been developed; however, many of these methods are not specific, can produce artifacts, and do not directly detect the free radicals and reactive oxygen species (ROS) that cause oxidative stress. Electron paramagnetic resonance (EPR) is a unique tool that allows direct measurements of free radical species. Cyclic hydroxylamines are useful and convenient molecular probes that readily react with ROS to produce stable nitroxide radicals, which can be quantitatively measured by EPR. In this work, we critically review recent applications of various cyclic hydroxylamine spin probes in biology to study oxidative stress, their advantages, and the shortcomings.
Recent Advances: In the past decade, a number of new cyclic hydroxylamine spin probes have been developed and their successful application for ROS measurement using EPR has been published. These new state-of-the-art methods provide improved selectivity and sensitivity for in vitro and in vivo studies.
Critical Issues: Although cyclic hydroxylamine spin probes EPR application has been previously described, there has been lack of translation of these new methods into biomedical research, limiting their widespread use. This work summarizes “best practice” in applications of cyclic hydroxylamine spin probes to assist with EPR studies of oxidative stress.
Future Directions: Additional studies to advance hydroxylamine spin probes from the “basic science” to biomedical applications are needed and could lead to better understanding of pathological conditions associated with oxidative stress. Antioxid. Redox Signal. 28, 1433–1443.
Keywords: : electron paramagnetic resonance, reactive oxygen species, superoxide, hydroxylamine spin probes, electron spin resonance
Introduction
Reactive oxygen species (ROS) is a general term for oxygen-centered radicals (superoxide [O2•−], HO2•, RO•, RO2•) or chemically reactive peroxide oxidants (hydrogen peroxide [H2O2], ROOH, HOONO, peroxynitrite [ONOO−]) produced from molecular oxygen in biochemical reactions. The main sources of ROS in animal cells include nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, xanthine oxidase, uncoupled nitric oxide synthase, and mitochondria. ROS can cause cellular damage, but they also play an important role in cell signaling networks. Living cells constantly produce ROS, which are scavenged by low-molecular antioxidants and specific enzymes, to form certain balance between oxidants and antioxidants that determine redox status of cellular thiols. Overproduction of ROS or depletion of antioxidant capacity leads to imbalance between oxidants and antioxidants, leading to an oxidative stress, which is implicated in cellular damage and development of various pathological conditions. The lifetime of ROS in biological systems ranges from nanoseconds to seconds depending on their reactivity and the level of cellular antioxidants, and this makes their detection and measurement difficult (9). Moreover, different ROS have very distinct reactivity and play very different roles in cellular redox signaling and oxidative stress. As a consequence, no single method can be offered for measurement of all varieties of ROS. The imbalance between ROS overproduction and antioxidant activity leads to accumulation of oxidative damage to biomolecules such as DNA, lipids, and proteins, which can be measured by “markers of oxidative stress”: oxidatively modified nucleotides (i.e., 8-hydroxyguanine), lipid peroxidation products (i.e., malondialdehyde and F2-isoprostanes), and protein carbonyl formation. The specific role of these products in various pathological conditions, however, is not clear. Many cellular dysfunctions are related to changes in redox status or cellular redox signaling and do not show correlation with accumulations of specific “markers of oxidative stress.” Therefore, measurement of markers of oxidative stress does not provide specific molecular mechanisms for investigation of oxidative stress and cellular redox signaling, and this gives rise to the demand for development of specific and sensitive methods for ROS detection.
Current Methods for ROS Detection
Various methods for ROS measurement have been recently reviewed in references 20, 21, 32, 72, 82. It is important to note that there is no method for ROS detection that is currently accepted as a “gold standard,” since every sensor or reagent has advantages and disadvantages including cyclic hydroxylamine spin probes.
Spin trapping is the most unambiguous method for detection of free radicals (36, 66). Spin traps react with the short-lived free radical via covalent bond formation (radical addition reaction) to produce persistent nitroxide called spin adduct, which would have a “signature” electron paramagnetic resonance (EPR) spectrum specific for each trapped radical. Regretfully, spin trapping of many free radicals proceeds with much lower rates as compared with their scavenging with cellular antioxidants. For instance, superoxide dismutase (SOD) and ascorbate may prevent O2•−detection in cells and tissues (96). Moreover, intracellular reductants such as ascorbate and flavin proteins reduce radical adducts to EPR-silent diamagnetic products, severely limiting the use of spin traps in biological systems (99).
Fluorescent probes produce stable products upon reactions with ROS and provide high sensitivity detection. Unfortunately, applications of fluorescent probes in ROS studies are hindered by serious issues: (i) formation of multiple nonspecific oxidation products, (ii) light sensitivity, and (iii) redox cycling of the probes (41). For example, dihydroethidium and mitoSOX form two fluorescent products, ethidium produced by nonspecific redox reactions, and 2-hydroxyethidium, a specific adduct of O2•− (98). The fluorescent spectra of ethidium and 2-hydroxyethidium overlap making fluorescence detection nonspecific, therefore, it is necessary to use high performance liquid chromatography analysis of samples containing O2•− specific products and nonspecific oxidation products such as ethidium to correctly analyze the fluorescence signal (97). Dichlorodihydrofluorescein diacetate (DCFH-DA) is a commonly used oxidative stress marker. Despite the popularity of this assay, it cannot be reliably used to measure intracellular H2O2 and other ROS due to indirect oxidation and redox cycling of 2,7-dichlorodihydrofluorescein (DCF) (41). For these reasons, the editorial board of Free Radicals in Biology and Medicine journal stated that DCFH should not be used.
What Are Hydroxylamine Spin Probes?
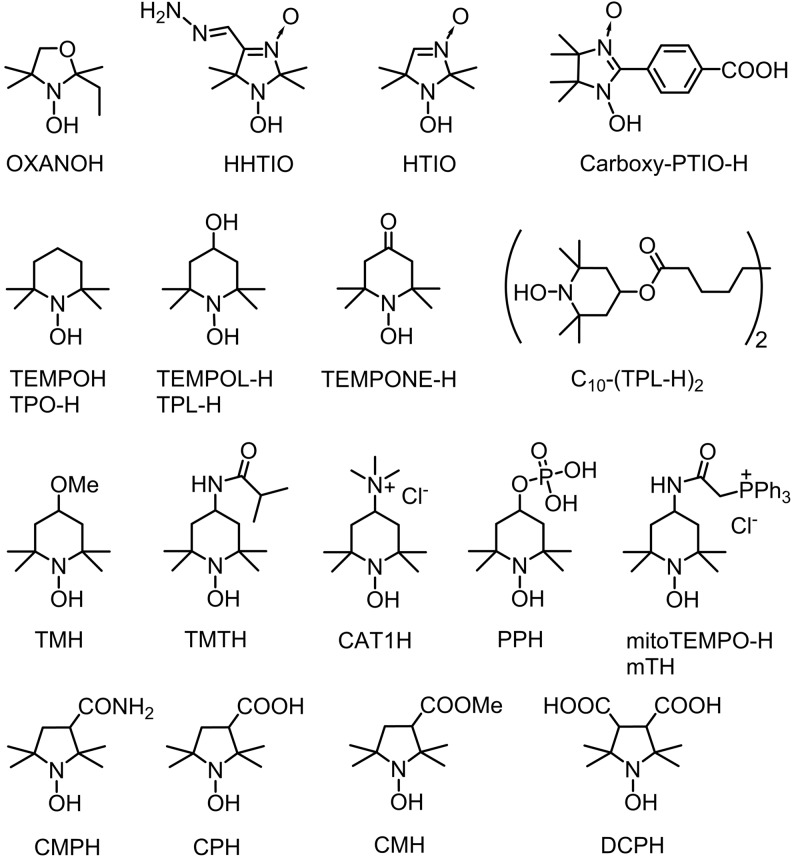
The terms “cyclic hydroxylamines” and “hydroxylamine spin probes” are actually used for primary products of stable nitroxides reduction. The hydroxylamine spin probes previously used for ROS measurements are listed in Figure 1.
FIG. 1.
Chemical structures of hydroxylamine spin probes.
Most of the probes are commercially available or can be prepared from simple commercially available nitroxides (4, 15, 33, 51, 70, 75, 79, 87, 91, 93). Nitroxides are certainly the broadest family of stable free radicals. Overwhelming number of various structures have been synthesized with variable physical and chemical properties. Well-developed chemistry of nitroxides offers great possibilities for design of hydroxylamine spin probes. Hydrolytically stable hydroxylamines of pyrrolidine and piperidine series can form hydrochlorides that are fairly stable crystalline compounds and may be stored for a long time in dry atmosphere. These samples are usually free from background EPR signal of corresponding nitroxide. Both hydroxylamines and corresponding nitroxides demonstrate little or no cytotoxicity; therefore, application of cyclic hydroxylamine spin probes normally produce negligible disturbance to the cellular metabolic processes.
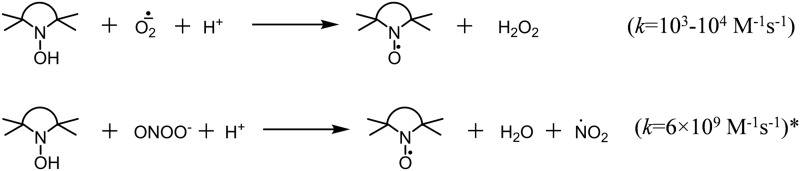
It is important to distinguish spin traps from hydroxylamine spin probes. Hydroxylamines are not spin traps: they cannot bind free radicals. They can undergo oxidation to stable nitroxides, and the nitroxide accumulation can be followed by EPR. Transformation of hydroxylamine to nitroxide requires one-electron oxidation, thus, the reagents, which can accept one electron to give thermodynamically stable products, provide higher oxidation rates. Hydroxylamines rapidly react with oxygen-centered free radicals, including superoxide (Fig. 2). Fast oxidation can occur with some molecular oxidants, such as peroxynitrite, if stable radical species are released in the reaction.
FIG. 2.
Reactions of cyclic hydroxylamines with ROS. *The constant for reaction of TEMPONE-H with peroxynitrite is given (17). ROS, reactive oxygen species.
In contrast, reaction with oxygen, H2O2, and organic peroxides is very slow in the absence of mediator capable of one-electron transfer (22, 84). Transition metal ions can increase spontaneous oxidation of hydroxylamines in oxygen-containing solutions (65, 75, 88). To decrease the spontaneous oxidation, stock solutions and buffers should be treated with Chelex and Fe/Cu chelators such as diethylenetriaminepentaacetic acid (DTPA) or ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) should be added. These reagents should also be added to tissue cellular and subcellular samples where natural iron- and copper-chelating structures are partly removed or diluted.
Reaction of hydroxylamine spin probe with different oxidants leads to accumulation of essentially the same nitroxide. Specific scavengers of certain ROS have to be used to identify particular ROS or particular enzymatic system responsible for nitroxide accumulation since hydroxylamine spin probes neither show specific reactivity to certain ROS type nor allow for EPR spectral identification of different ROS. Specificity of superoxide detection is often confirmed by inhibition of EPR signal with SOD or cell-permeable polyethylene glycol conjugated SOD while peroxynitrite detection can be confirmed by inhibition of EPR signal with urate supplementation (22). Alternatively, specific stimulation or inhibition of ROS-generating enzymes (e.g., Fas ligand for stimulation of NADPH-dependent superoxide production, Nox2 inhibitor gp91ds, or addition/removal of the enzyme substrate can be used to clarify the role of this enzymatic system (65).
Factors Influencing Efficacy of ROS Detection Using Hydroxylamine Spin Probes
Reactivity
Hydroxylamines are bases, for most of them equilibrium is observed between free base and protonated form in neutral solutions. The electron-withdrawing substituents expectedly decrease the pKa of protonated forms, shifting the equilibrium toward the free base. It is likely that hydroxylammonium cations have diminished reactivity with ROS (37). The apparent rate constants of reaction with superoxide and pKa values of some hydroxylamines are listed in Table 1. The data show that many of hydroxylamine spin probes have pKa close to 7 and, therefore, are at least partly converted into nonreactive protonated form at physiological pH. This factor hides other structural effects, such as influence of ring size and electronic effects of substituents upon the rate of oxidation. However, five-membered-ring pyrrolidine hydroxylamines generally are stronger reductants than those of piperidine series and, therefore, they are more disposed to artifacts. For example, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) was found to react directly with components of photosynthetic electron transport chain (PETC) to form corresponding nitroxide, whereas 1-hydroxy-4-isobutyramido-2,2,6,6-tetramethylpiperidine (TMTH) did not react (51).
Table 1.
Properties of Various Hydroxylamine Spin Probes
| Hydroxylamine spin probe | pKa | k × 10−3, M−1s−1[pH] | Kp, (21) |
|---|---|---|---|
| OXANOH | 1.7 [7.8] (75) | ||
| TEMPOH (TPO-H) | 7.5 (37), 7.96 (43) | 1.1 (95) | |
| TEMPOL-H (TPL-H) | 7.1 (37), 5.18 (43) | 2.1 (95), 0.4 [7.8] (53) | |
| TEMPONE-H | 12 [7.4] (16) | ||
| CPH | 7.7 (48) | 3.2 (17) | 0.05 |
| CMH | 6.7 (unpublished) | 12 (27) | 27 |
| PPH | 0.84 (15) | 0.005 | |
| TMH | 7.1 (48) | 4.2 (22) | 43 |
| TMTH | 7.7 (48) | 4.9 (22) | 35 |
| mitoTEMPO-H (mTH) | 7.8 (22) | 8 | |
| CAT1H | ∼4 (51), 4.8 (48) | 6.4 (22) | |
| DCPH | 8.3 (48) | 0.55 (48) | |
| 3-Carbamoyl-proxyl-H | 5.85 (43) | 4.9 (95) |
pKa values, rate constants of reaction with superoxide, partition coefficients in octanol–water mixtures (Kp).
CAT1H, 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium; CMH, 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine; CPH, 3-carboxy-1-hydroxy-2,2,5,5-tetramethylpyrrolidine; DCPH, 3,4-dicarboxy-1-hydroxy-2,2,5,5-tetramethylpyrrolidine; mTH, mitoTEMPO-H, 1-hydroxy-4-[2-triphenylphosphonio)-acetamido]-2,2,6,6-tetramethylpiperidine; OXANOH, 2-ethyl-1-hydroxy-2,5,5-trimethyl-3-oxazolidine; PPH, 1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine; TMH, 1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine; TMTH, 1-hydroxy-4-isobutyramido-2,2,6,6-tetramethylpiperidine.
Nitroxide stability
Efficacy of ROS measurement using hydroxylamine spin probes depends not only on the rate of hydroxylamine accumulation but also on stability of resulting nitroxide. Half-life of some nitroxides in biological samples may exceed hours. The nitroxides are known to undergo bioreduction with low-molecular biogenic antioxidants (ascorbate, glutathione, etc.) and with enzymatic systems, the primary products being hydroxylamines. Factors influencing nitroxide decay rates in biological samples and in vivo have been thoroughly investigated (49). Intracellular reductions proceed faster, and for cell-permeable nitroxides, it makes major contribution to overall nitroxide decay. In relation to hydroxylamine spin probes, this means that intracellular ROS measurement is more difficult. It is hard to compare extracellular and intracellular ROS production even if we use specifically targeted probes. Nitroxides differ much in their ability to reduce. The main structural features determining the reduction rate include ring size (44), charge and electronic effects of the substituents (63), and steric hindrance (39, 47). Sterically shielded nitroxides have never been used for hydroxylamine spin probe preparation yet. Five-membered pyrrolidine nitroxides demonstrate the highest stability to reduction compared with other structural types of nitroxides. For example, 4-isobutyramido-TEMPO (TMT nitroxide) is reduced by components of PETC inside the thylakoid membrane, whereas 3-methoxycarbonyl-proxyl (CM nitroxide) is not (51).
Permeability and targeting
Majority of ROS have limited lifetime and limited ability to diffuse. Therefore, to make the hydroxylamine oxidation efficient, it is important to ensure that spin probe can reach the source of ROS, or even accumulate in tissue or cellular compartment where ROS are generated. Ability of organic substances to diffuse through cellular membranes is mainly determined by partition coefficients (Kp). Kp octanol/water of some hydroxylamines is listed in Table 1. Remarkably, CMH usually gives the most intensive EPR spectra of nitroxide in cellular cultures and in tissues, because it is cell permeable, shows the highest reactivity among pyrrolidine derivatives, and gives highly stable nitroxide of pyrrolidine series.
It is always reasonable to test more than one hydroxylamine for measurements, choosing the probe with optimal redox properties, minimal artifacts, and maximal cellular responses. Specifically targeted hydroxylamines are of special interest as they can provide information about site of ROS origin (22).
Superoxide Measurement
Superoxide radical anion is a product of one-electron reduction of oxygen molecule, which is constantly produced in biochemical redox chains and may be generated by various enzymatic systems. It should be noted that some popular superoxide-producing enzymatic systems, such as xanthine–xanthine oxidase system, produce large amount of H2O2, which can cause secondary processes accompanied with production of free radicals. Therefore, supplementation with catalase is highly desirable. Enzymatic systems usually produce ROS with constant rate; therefore, at high hydroxylamine concentration we observe a linear growth of nitroxide EPR signal, allowing for quantitative measurement of superoxide production rate. Biological function of superoxide is broad. On one hand, it is an important mediator in signaling system regulating cell growth, differentiation, migration, and proliferation (94). On the other hand, it is implicated in cell death (8). Superoxide is often considered an initial source of all biogenic ROS, including highly reactive oxygen-centered radicals (alkoxy, alkylperoxy) and highly reactive molecules (H2O2, peroxynitrite). Unlike majority of oxygen-centered radicals demonstrating close-to-diffusion rates of reaction with various organic compounds, superoxide is a moderately reactive radical. Owing to high biological importance of superoxide, many methods have been suggested for its measurement, including specially designed spin traps and fluorescent dyes.
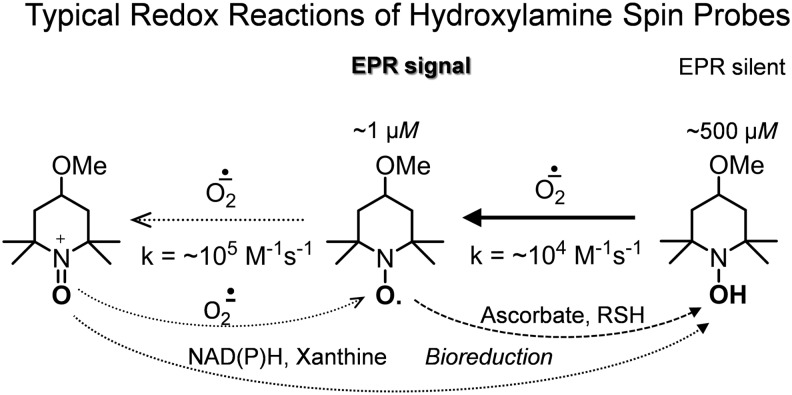
Superoxide measurement is the main field of application of hydroxylamine spin probes. After invention by Rosen et al. (75), it was successfully used by numerous researchers. Reaction of hydroxylamine spin probes with superoxide is relatively fast (Table 1), two orders of magnitude faster than spin trapping, and the nitroxides formed are more stable than spin adducts. As a result, strong EPR signals are obtained in cells with small amount of cyclic hydroxylamine spin probes (25–500 μM). High reactivity of cyclic hydroxylamine spin probes toward superoxide and high resistance of nitroxides to reduction allows for detection of intracellular superoxide. Sensitivity of this method is high enough for measurements of basal levels of superoxide production by cells.
The oxidation of hydroxylamine spin probes with superoxide can be completely suppressed by SOD. Application of SOD allows for validation of superoxide detection and separation from nonspecific oxidation, making the data on superoxide measurement unambiguous. Indeed, the rate of nitroxide accumulation usually has inverse dependence on SOD supplementation, but it is not affected by scavengers of other ROS, such as urate, catalase, or dimethylsulfoxide (54). Cu, Zn-SOD, and Mn-SOD are cell impermeable; therefore, for validation of intracellular superoxide detection, one can use cell-permeable polyethylene glycol-conjugated SOD (50 U/mL) or use cells overexpressing SOD1 or SOD2 (22).
Superoxide was shown to oxidize nitroxides to oxoammonium cations in the cell-free samples and the rate of this reaction is much higher than that of reaction of hydroxylamine and superoxide (95). Oxoammonium cations are highly reactive strong oxidants, they can oxidize superoxide to form oxygen and nitroxides. In biological media, they usually undergo fast two-electron reduction with various reductants (nicotinamide adenine dinucleotide, reduced form [NADH], thiols, alcohols, amines, etc.) to hydroxylamines. Thus, a redox cycle of interconversions occurs between hydroxylamine, nitroxide, and oxoammonium cation mediated by superoxide and cellular reductants, which accounts for catalytic “SOD-mimetic” activity of nitroxides (hydroxylamines) (49, 53, 95) (Fig. 3). The rate of ROS formation can be underestimated if the ratio nitroxide/hydroxylamine exceeds 1% (19). To perform quantitative measurements, one should have low degree of conversion (dilute cells or increase spin probe concentration) to allow linear time-course accumulation of nitroxide. Direct reduction of nitroxides with cellular reductants can also contribute to the equilibrium position. If the hydroxylamine concentration is not high enough, accumulation of the nitroxide can deviate from expected kinetics (68). In cell-free enzymatic systems and in cultured cells, there are usually no significant deviations from linear kinetics of nitroxide accumulation at conversions <1% (22).
FIG. 3.
Interconversion of cyclic hydroxylamines and nitroxides in superoxide-generating systems.
The influence of hydroxylamine structure on the kinetics of nitroxide accumulation strongly depends on the system where the measurement is performed. For instance, piperidine-based hydroxylamine probe CAT1H reacts with superoxide with higher rate than pyrrolidine-based probes, and this leads to faster accumulation of CAT1 in cell-free systems; however, in cell cultures and in tissues, piperidine nitroxides are more rapidly reduced to EPR-silent hydroxylamines compared with pyrrolidine nitroxides, and, therefore, pyrrolidine hydroxylamines provide stronger EPR signals of corresponding nitroxides.
Measurement of ROS in Cell-Free Systems
The method was successfully used for measurement of activities of oxidative enzymes, producing superoxide: xanthine/xanthine oxidase (17), 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase (84), NADPH-cytochrome P-450 reductase (25, 75), and flavinadenindinucleotide (FAD)-containing monooxygenase (75), for testing antioxidant capacity of preservation solutions (77), etc. It was found that in the absence of a competing scavenger, such as SOD, 3-carboxy-1-hydroxy-2,2,5,5-tetramethylpyrrolidine (CPH) reacts with 90% of superoxide from the membrane fraction (18). Thus, O2•− detection by CPH should be used in cell-free systems or cellular fractions wherein detection of superoxide can be confirmed by inhibition of the EPR signal with SOD.
Production of superoxide in the presence of nitric oxide leads to formation of peroxynitrite. Hydroxylamines have been successfully used for quantification ofperoxynitrite in various systems, and urate is the efficient reagent for validation of these measurements (17).
It was already mentioned that H2O2 does not react with hydroxylamines with significant rate. However, in presence of H2O2, horse radish peroxidase can oxidize acetamidophenol to corresponding phenoxyl radical, and the latter readily oxidizes hydroxylamines to corresponding nitroxides (3). EPR measurement of the nitroxide formed provides quantitative measurement of H2O2 with detection limit of 3 pmol/test (4). The validation of the method can be performed using catalase. Owing to high rate of reaction of hydroxylamines with acetamidophenoxyl radical, the results of H2O2 measurement are not affected by the presence of NADPH (25–500 μM) or xanthine (25–500 μM) (18). The method can be used for measurement of H2O2 production by cells or mitochondria (18, 26). It should be noted that supplementation of superoxide-generating systems with SOD leads to H2O2 formation and quantification of H2O2 is an alternative to direct superoxide measurements (25, 34). Measurement of H2O2 along with superoxide production in subcellular fractions may give more information about enzymatic activity and function (18).
Since H2O2 is formed in various enzymatic reactions, measurement of H2O2 is a useful approach for measurement of enzymatic activity or substrate concentration. For instance, Nox4 NADPH oxidase activity was measured in membrane fractions of smooth muscle cells (18, 46). H2O2 formation in the enzymatic oxidation of glucose can be used for quantification of glucose. Highly sensitive immunoassays have been developed for measurement of thyroid-stimulating hormone (detection limit 0.0025 μU/mL) (2) and for clinical detection of hepatitis B virus (2, 61).
Measurements of ROS in Cellular and Subcellular Systems
Basal production of superoxide by intact nonstimulated cells and subcellular compartments (e.g., mitochondria) is usually very low; therefore, reliable measurements in cellular systems require good X-band EPR spectrometer with high-sensitivity resonator. Specific stimulation can strongly increase production of ROS, which can be measured by hydroxylamine spin probes. The nitroxide accumulation rate under these conditions depends on cellular location of ROS source and on permeability of the hydroxylamine used.
Cell permeability has been estimated for a set of hydroxylamines, showing the following order: CMH > PPH >> mTH > TMH ≈ TMTH >> CAT1H (22). This row does not show complete correlation with Kp values because of specific accumulation of some hydroxylamines. For example, 1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine (PPH) can be accumulated in cells via active transport, similar to that of phosphates and was shown to be mitochondria dependent (22); however, PPH does not permeate isolated mitochondria (69). Intracellular and intramitochondrial accumulation of mitoTEMPO-H, 1-hydroxy-4-[2-triphenylphosphonio)-acetamido]-2,2,6,6-tetramethylpiperidine (mTH) is driven by electrical transmembrane potential. Low lipophilicity of CPH most likely results from ionization of the carboxylic group (pKa is ca. 5) at physiological pH values. However, ionization of the carboxylic group only attenuates the cell permeability but does not abolish it completely (22). Difference in cell permeability of various hydroxylamines opens unique possibilities for identification of sources of cellular ROS. For example, site-specific superoxide formation was studied in human lymphoblasts with NADPH oxidase activator phorbol-12-myristate-13-acetate (PMA) or with stimulator of mitochondrial superoxide production antimycin A in the presence of cell-impermeable CAT1H probe and mitochondria-targeted mTH. PMA stimulation leads to nitroxide accumulation, which was five times higher in the presence of CAT1H compared with the presence of mTH. In contrast, stimulation of mitochondrial superoxide production by antimycin A strongly increased oxidation of mTH, whereas nitroxide accumulation with CAT1H probe was not affected by antimycin A (23).
CMH is the most efficient spin probe for measurement of overall ROS production. It allows for ROS detection in various cellular compartments and in different types of cells, usually giving the highest nitroxide accumulation rate (35, 40, 55, 67, 85, 89, 90). However, it also shows high background oxidation rate. Comparison of efficacy of various hydroxylamines in cellular systems and the use of inhibitors to identify ROS can be found in Dikalov et al. (22).
Measurement of ROS in Tissues and In Vivo
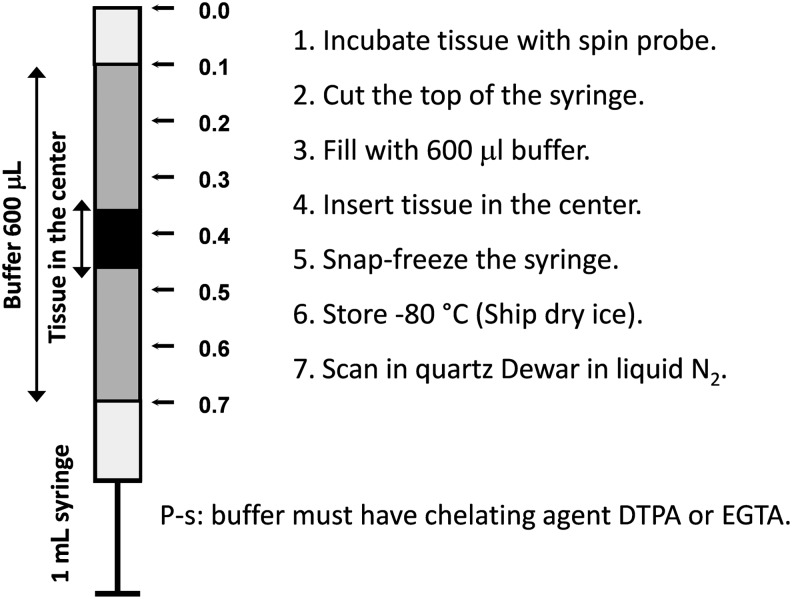
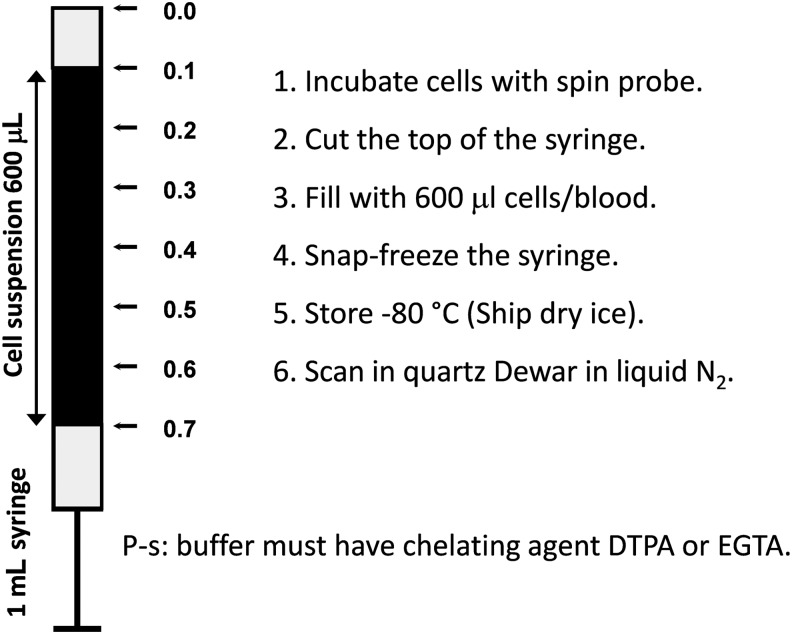
Hydroxylamines have found broad application in investigation of various oxidative stress-related phenomena in living tissues. Superoxide production can be measured by intraperitoneal (i.p.) infusion of cyclic hydroxylamines followed by ex vivo analysis of the blood or tissue samples using X-band (9 GHz) EPR spectroscopy (13, 29, 50). The typical protocols for ROS measurements are given in Dikalov et al. (14) and Fabbri et al. (28). Similar procedures were used in numerous publications (30, 31, 55, 56, 58, 64, 71, 73, 74, 78). Typically, the samples are recorded immediately after incubation with hydroxylamine spin probe (usually CMH or CPH). If the immediate measurements after incubation are not possible, the samples can be frozen. Freezing stops the enzymatic reactions leading to ROS formation; however, after thawing the background oxidation rate may increase (28). To avoid this effect, a special procedure has been developed with registration of immobilized nitroxide spectrum in the frozen sample (1, 7, 6, 12, 14, 57, 81, 86). Specifically, animals can be injected with hydroxylamine probe (20 mg/kg) for 60 min, sacrificed, and isolated tissue can be placed in the center of 1 mL plastic syringe (Fig. 4), snap frozen and stored at −80°C. Then frozen block can be removed by gentle push from the warmed up syringe and analyzed in the quartz Dewar with liquid nitrogen. As a reference one can freeze standard solutions of stable nitroxide product and use the EPR intensity of immobilized nitroxide to calculate the amount of detected ROS in vivo.
FIG. 4.
Preparation of the frozen tissue for ROS measurements.
It should be noted that these measurements cannot be attributed to certain types of ROS without validation using specific inhibitors or scavengers. It has been shown that supplementation of wild-type mice myocardium samples with polyethylene glycol-conjugated superoxide dismutase (PEG-SOD) decreased amount of nitroxide after incubation with CMH (42). Transgenic animals such as overexpressing SOD2 mice or SOD2-depleted mice (SOD2−/+) can also be used for validation. In cell culture experiments, one can confirm specific site of O2•− detection by testing the effect of supplementation with cell-impermeable Mn-SOD, cell-permeable PEG-SOD, or overexpression of SOD2 (22).
To provide a cell and tissue-specific measurements of superoxide, we recommend treating samples with hydroxylamine probe ex vivo. In these experiments, blood, isolated cells, or dissected tissue is incubated with the probe (50–500 μM) for 30 min. Tissue samples can be placed at the center of the syringe (Fig. 4). Blood or cell suspension can be placed into the 1 mL precut syringe and snap frozen in liquid nitrogen as shown in Figure 5. After incubation with the probe, cultured cells attached to the bottom of the dish can be quickly resuspended in 600 μL of fresh buffer and placed into the syringe.
FIG. 5.
Preparation of the frozen samples with the blood or cell suspension.
Combination of cell-impermeable spin probe (e.g., CAT1H) and a spin probe capable of intracellular/intramitochondrial accumulation may provide information about mechanisms of cellular response development. Recently we have found that simultaneous use of CAT1H-15N, D12, and mTH can reveal peculiarities of various agents known to stimulate phagocytic NADPH oxidase and mitochondrial O2•− production (20).
ROS Imaging in Animals
Numerous efforts have been made to visualize ROS production in tissues of living animals; however, ROS imaging in vivo still remains a challenge. There are following problems:
Technical aspect
Standard commercial EPR spectrometers operate at 9.5 GHz (0.34 T). This frequency is efficiently absorbed by liquid water in biological systems; therefore, the EPR spectra can only be recorded in ampoules with rather low cross section. Measurements in living animals have to be performed at much lower frequencies: L-band, around 1 GHz, 30–40 mT for objects smaller than 20 mm (mice or isolated organs), or radio frequencies below 300 GHz, 10 mT for larger objects (70 mm, rat or rabbit) (5, 45, 52, 59). Recording of a spectrum is usually not enough for in vivo studies, because nitroxide content in tissues is wide ranging. EPR imaging (EPRI) is a time-consuming procedure, because it requires a set of spectra recorded at different directions of the magnetic field gradient. Thus, there is always a tradeoff between temporal and spatial resolution. Moreover, EPRI does not give any references on anatomy; therefore, to use EPR images one should overlay them with magnetic resonance imaging (5). Mixed technologies, such as overhauser-enhanced magnetic resonance imaging (PEDRI), are currently considered more promising for radicals imaging (5, 62). Most of the advances in this field were made using self-made equipment.
Chemical aspect
Dynamics of nitroxide concentration in tissues upon injection of hydroxylamine reflects pharmacokinetics of both hydroxylamine and nitroxide, their metabolic (redox) transformations, and clearance by excretion. It has been shown that i.p. injection of TEMPOL or TEMPOL-H in mice leads to essentially the same concentration of TEMPOL in blood in <20 min (80). From this point of view, injection of nitroxide or hydroxylamine should lead to the same result. Most of the researchers prefer to follow kinetics nitroxide decay, because this allows to observe signal decay at higher nitroxide concentration and, therefore, requires lower device sensitivity. Another question is whether these measurements actually reflect ROS production in tissues. Peculiarities of pharmacokinetics of hydroxylamine and of corresponding nitroxide can play a major role. For example, higher level of 3-carboxy-2,2,5,5-tetramethylpyrroline-1-oxyl in brain tumors obviously results from blood-brain barrier damage (60). Nitroxide reduction rate was found oxygen dependent, or even determined by redox state of oxygen-consuming enzymatic complexes (10, 49, 83). However, in vivo the nitroxide–hydroxylamine equilibrium is different for different types of cells and tissues and depends on the pathophysiological condition of the living animal (11).
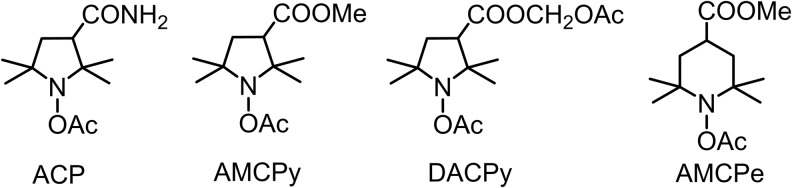
Acyl-protected hydroxylamines (acetoxyamines) represent an alternative to hydroxylamine or nitroxide spin probes (Fig. 6). These compounds themselves do not react with ROS, but they can be converted into reactive hydroxylamine spin probes by intracellular esterases.
FIG. 6.
Acyl-protected hydroxylamine probes.
Investigation of the 1-acetoxy-3-carbamoyl-2,2,5,5-tetramethylpyrrolidine (ACP) in cellular system indicated that SOD can suppress >50% of nitroxide accumulation in PMA-treated neutrophils. This means that after deprotection with esterases, hydroxylamine can diffuse out of the cell and this hydroxylamine (and corresponding nitroxide) can redistribute in the system (38). It has been shown that in mice after i.p. or intravenous. injection, ACP quickly reaches equilibrium with the other tissues. Deacylation proceeds very fast within liver and, probably, kidney; the rates of hydrolysis in other tissues were much lower, and resulting hydroxylamine was redistributed in organs. The measurement of hydroxylamine concentration in blood and majority of organs showed almost homogeneous distribution with minor changes in a period 10–30 min after injection, whereas accumulation of the compound in brain and muscle proceeded with slower rate. In 30 min almost all ACP was converted into hydroxylamine, and the nitroxide concentration never exceeded 10–20% of that of hydroxylamine (76).
1-acetoxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine and 1-acetoxy-3-acetoxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidine in mice showed somewhat different pattern of distribution of corresponding hydroxylamine and nitroxide, with the highest concentration of both compounds 10 and 60 min after i.p. injection observed in kidney. AMCP hydroxylamine reached higher concentration in all tissues than DACP, whereas 1-acetoxy-4-methoxycarbonyl-2,2,6,6-tetramethylpiperidine hydroxylamine did not show significant accumulation (92).
ACP was used for rat brain EPRI after kainic acid (KA)-induced epileptic seizures. Difference in accumulation of the nitroxide in different areas of brain in control and KA-treated rats was used to reveal brain structures subjected to stronger oxidative stress (92).
In general, the acyl-protected hydroxylamines do not show apparent advantage over free hydroxylamines because the molecules are not retained by cells, and the products of their hydrolysis (deacylation) and oxidation are redistributed in tissues in agreement with their pharmacokinetics. In this respect, design of acyl-protected hydroxylamine probes with specific targeting groups might be a fruitful approach.
Conclusions
Cyclic hydroxylamine probes are the effective scavengers of superoxide radical. Cyclic hydroxylamines provide quantitative measurements of superoxide radical with high sensitivity both in vitro and ex vivo in cells and tissue samples. SOD-inhibited nitroxide formation can confirm the amount of detected superoxide. Cyclic hydroxylamines can also be used to detect peroxynitrite. Inhibition by peroxynitrite scavengers such as urate shows the amount of trapped peroxynitrite. Production of H2O2 can be measured by co-oxidation of CPH in peroxidase–acetamidophenol reaction. Cyclic hydroxylamines have been successfully used to assay the production of superoxide radical and H2O2 by NADPH oxidases, mitochondria, intact neutrophils, vascular cells, and tissue. To minimize nonspecific oxidation, buffers must be treated with Chelex resin and supplemented with iron and copper chelators (Desferal, DTPA, EGTA). New cell-permeable and acyl-protected cyclic hydroxylamines have a great potential in the study of intracellular ROS production. These new EPR techniques demonstrated great potential to study ROS in biological samples.
Abbreviations Used
- ACP
1-acetoxy-3-carbamoyl-2,2,5,5-tetramethylpyrrolidine
- CAT1H
1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium
- CM
3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl
- CMH
1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine
- CPH
3-carboxy-1-hydroxy-2,2,5,5-tetramethylpyrrolidine
- DCF
2,7-dichlorodihydrofluorescein
- DCFH-DA
dichlorodihydrofluorescein diacetate
- DCPH
3,4-dicarboxy-1-hydroxy-2,2,5,5-tetramethylpyrrolidine
- DTPA
diethylenetriaminepentaacetic acid
- EGTA
ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- EPR
electron paramagnetic resonance (synonym for ESR)
- EPRI
EPR imaging
- FAD
flavinadenindinucleotide
- H2O2
hydrogen peroxide
- i.p.
intraperitoneal
- KA
kainic acid
- mTH
mitoTEMPO-H, 1-hydroxy-4-[2-triphenylphosphonio)-acetamido]-2,2,6,6-tetramethylpiperidine
- NADH
nicotinamide adenine dinucleotide, reduced form
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
- O2•−
superoxide
- ONOO−
peroxynitrite
- OXANOH
2-ethyl-1-hydroxy-2,5,5-trimethyl-3-oxazolidine
- PEG-SOD
polyethylene glycol-conjugated superoxide dismutase
- PETC
photosynthetic electron transport chain
- PMA
phorbol-12-myristate-13-acetate
- PPH
1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TMH
1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine
- TMT
4-isobutyramido-TEMPO
- TMTH
1-hydroxy-4-isobutyramido-2,2,6,6-tetramethylpiperidine
Acknowledgments
This work was supported by funding from National Institutes of Health (R01HL124116) and Russian Foundation for Basic Research (17-03-01132-a).
References
- 1.Agouni A, Lagrue-Lak-Hal A, Mostefai HA, Tesse A, Mulder P, Rouet P, Desmoulin F, Heymes C, Maria Martínez C, and Andriantsitohaina R. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa). PLoS One 4: e5557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki M, Saito T, Watanabe H, Matsuo T, Saito K, Togashi H, Kawata S, Ishikawa K, Aoyama M, Kamada H, and Shinzawa H. Clinical significance of a highly sensitive enzyme immunoassay of hepatitis B surface antigen using a novel electron spin resonance technique. J Med Virol 66: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aoyama M. and Shiga M. Method and reagent for detecting peroxidase or hydrogen peroxide. US Grant EP 754760B1, CA2181500 A1, US5780257 A (Priority 20.07.1995)
- 4.Aoyama M, Shiga M, Ohya H, and Kamada H. A novel ESR method for horseradish peroxidase activity using a combination of p-acetamidophenol and hydroxylamine, and its application to enzyme immunoassays. Anal Sci 14: 1107–1113, 1998 [Google Scholar]
- 5.Bačić G, Pavićević A, and Peyrot F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol 8: 226–242, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A, Weidinger A, Hofer M, Steinborn R, Lindenmair A, Hennerbichler-Lugscheider S, Eibl J, Redl H, Kozlov AV, and Wolbank S. Different metabolic activity in placental and reflected regions of the human amniotic membrane. Placenta 36: 1329–1332, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Berg K, Ericsson M, Lindgren M, and Gustafsson H. A high precision method for quantitative measurements of reactive oxygen species in frozen biopsies. PLoS One 9: e90964, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brookes PS, Levonen AL, Shiva S, Sarti P, and Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med 33: 755–764, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cadenas E. and Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222–230, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chen K, Glockner JF, Morse PD, and Swartz HM. Effects of oxygen on the metabolism of nitroxide spin labels in cells. Biochemistry 28: 2496–2501, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Colacicchi S, Ferrari M, and Sotgiu A. In vivo electron paramagnetic resonance spectroscopy/imaging: first experiences, problems, and perspectives. Int J Biochem 24: 205–214, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, and Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dikalov S, Fink B, Skatchkov M, and Bassenge E. Comparison of glyceryl trinitrate-induced with pentaerythrityl tetranitrate-induced in vivo formation of superoxide radicals: effect of vitamin C. Free Radic Biol Med 27: 170–176, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Dikalov S, Griendling KK, and Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalov S, Grigor'ev IA, Voinov M, and Bassenge E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: quantification of extracellular superoxide radicals formation. Biochem Biophys Res Commun 248: 211–215, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Dikalov S, Skatchkov M, and Bassenge E. Quantification of peroxynitrite, superoxide, and peroxyl radicals by a new spin trap hydroxylamine 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine. Biochem Biophys Res Commun 230: 54–57, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Dikalov S, Skatchkov M, and Bassenge E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem Biophys Res Commun 231: 701–704, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HHHW, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalov SI, Dikalova AE, and Mason RP. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch Biochem Biophys 402: 218–226, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Dikalov SI, Dikalova AE, Uzhachenko R, Morozov DA, and Kirilyuk IA. Studies of cellular accumulation and antioxidant activity of pyrrolidine and piperidine nitroxides. In: Book of Abstracts of International Conference on Electron Paramagnetic Resonance and Imaging of Biological Systems EPR 2017 2017: Oral Presentation 32. Morgantown, WV [Google Scholar]
- 21.Dikalov SI. and Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal 20: 372–382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dikalov SI, Kirilyuk IA, Voinov M, and Grigor'ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 45: 417–430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalov SI, Li W, Doughan AK, Blanco RR, and Zafari AM. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am J Physiol Regul Integr Comp Physiol 302: R1134–R1142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikalov SI. and Nazarewicz RR. Measurements of reactive oxygen species in cardiovascular studies. In: Systems Biology of Free Radicals and Antioxidants, edited by Laher I. Berlin Heidelberg: Springer-Verlag, 2014, pp. 1435–1450 [Google Scholar]
- 25.Doughan AK. and Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxid Redox Signal 9: 1825–1836, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Dudley SC, Hoch NE, McCann LA, Honeycutt C, Diamadopoulos L, Fukai T, Harrison DG, Dikalov SI, and Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage. Role of the NADPH and xanthine oxidases. Circulation 112: 1266–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Fabbri R, Sapone A, Paolini M, Vivarelli F, Franchi P, Lucarini M, Pasquinelli G, Vicenti R, Macciocca M, Venturoli S, and Canistro D. Effects of N-acetylcysteine on human ovarian tissue preservation undergoing cryopreservation procedure. Histol Histopathol 30: 725–735, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Fink B, Dikalov S, and Bassenge E. A new approach for extracellular spin trapping of nitroglycerin-induced superoxide radicals both in vitro and in vivo. Free Radic Biol Med 28: 121–128, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, and Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, and Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 529: 2549–2561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griendling KK, Touyz RM, Zweier JL, Dikalov S, Chilian W, Chen Y-R, Harrison DG, and Bhatnagar A. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res 119: 39–75, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigorev IA, Shchukin GI, and Volodarskii LB. Reaction of 1-hydroxy-4-dihalomethyl-2,2,5,5-tetramethyl-Δ-3-imidazoline-3-oxides and their radicals with nucleophylic reagents. Russ Chem Bull 11: 1332–1337, 1975 [Google Scholar]
- 34.Guzik TJ, Chen W MD, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, and Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity via increased mitochondrial uncoupling and biogenesis. Diabetes 65: 2639–2651, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins CL. and Davies MJ. Detection and characterization of radicals in biological materials using EPR methodology. Biochim Biophys Acta 1840: 708–721, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Israeli A, Patt M, Orona M, Samuni A, Kohena R, and Goldstein S. Kinetics and mechanism of the comproportionation reaction between oxoammonium cation and hydroxylamine derived from cyclic nitroxides. Free Radic Biol Med 38: 317–324, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Itoh O, Aoyama M, Yokohama H, Obara H, Ohya H, and Kamada H. Sensitive ESR determination of intracellular oxidative stress by using acyl-protected hydroxylamines as new spin reagents. Chem Lett 29: 304–305, 2000 [Google Scholar]
- 39.Jagtap AP, Krstic I, Kunjir NC, Hänsel R, Prisner TF, and Sigurdson ST. Sterically shielded spin labels for in-cell EPR spectroscopy: analysis of stability in reducing environment. Free Radic Res 49: 78–85, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Jaishy B, Zhang Q, Chung HS, Riehle C, Soto J, Jenkins S, Abel P, Cowart LA, Van Eyk JE, and Abel ED. Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. Lipid Res 56: 546–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, and Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med 52: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang PT, Chen C-L, Ohanyan V, Luther DJ, Meszaros JG, Chilian WM, and Chen Y-R. Overexpressing superoxide dismutase 2 induces a supernormal cardiac function by enhancing redox-dependent mitochondrial function and metabolic dilation. J Mol Cell Cardiol 88: 14–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato Y, Shimizu Y, Lin YJ, Unoura K, Utsumi H, and Ogata T. Reversible half-wave potentials of reduction processes on nitroxide radicals. Electrochim Acta 40: 2799–2802, 1995 [Google Scholar]
- 44.Keana JFW. and Van Nice FL. Influence of structure on the reduction of nitroxide MRI contrast-enhancing agents by ascorbate. Physiol Chem Phys Med NMR 16: 477–480, 1984 [PubMed] [Google Scholar]
- 45.Khan N, Mupparaju SP, Mintzopoulos D, Kesarwani M, Righi V, Rahme LG, Swartz HM, and Tzika AA. Burn trauma in skeletal muscle results in oxidative stress as assessed by in vivo electron paramagnetic resonance. Mol Med Rep 1: 813–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, and Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Kirilyuk IA, Bobko AA, Semenov SV, Komarov DA, Irtegova IG, Grigor'ev IA, and Bagryanskaya E. Effect of sterical shielding on the redox properties of imidazoline and imidazolidine nitroxides. J Org Chem 80: 9118–9125, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Kirilyuk IA, Grigor'ev IA, Fadeeva VP, Nikulicheva ON, and Dikalov SI. Hydroxylamine spin probes for superoxide detection, reactive oxygen species, nitric oxide, antioxidants and human health. In: Proceedings of 8th National Scientific Practical Conference with International Participation 2014, pp. 90–93. Moscow, Russia [Google Scholar]
- 49.Kocherginsky N. and Swartz HM. Nitroxide Spin Labels: Reactions in Biology and Chemistry. Boca Raton, FL: CRC Press, 1996. [Google Scholar]
- 50.Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nohl H, Redl H, and Bahrami S. EPR analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic Biol Med 34: 1555–1562, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Kozuleva M, Klenina I, Mysin I, Kirilyuk I, Opanasenko V, Proskuryakov I, and Ivanov B. Quantification of superoxide radical production in thylakoid membrane using cyclic hydroxylamines. Free Radic Biol Med 89: 1014–1023, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Krishna MC, Devasahayam N, Cook JA, Subramanian S, Kuppusamy P, and Mitchell JB. Electron paramagnetic resonance for small animal imaging applications. ILAR J 42: 209–218, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Krishna MC, Grahame DA, Samuni A, Mitchell JB, and Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A 89: 5537–5541, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70: 343–354, 2005 [DOI] [PubMed] [Google Scholar]
- 55.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce G, and Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lejay A, Choquet P, Thaveau F, Singh F, Schlagowski A, Charles A-L, Laverny G, Metzger D, Zoll J, Chakfe N, and Geny B. A new murine model of sustainable and durable chronic critical limb ischemia fairly mimicking human pathology. Eur J Vasc Endovasc Surg 49: 205–212, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Leonetti D, Soleti R, Clere N, Vergori L, Jacques C, Duluc L, Dourguia C, Martínez MC, and Andriantsitohaina R. Estrogen receptor a participates to the beneficial effect of red wine polyphenols in a mouse model of obesity-related disorders. Front Pharmacol 7: 529, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorthioir A, Joannidès R, Rémy-Jouet I, Frguin-Bouilland C, Iacob M, Roche C, Monteil C, Lucas D, Renet S, Audrézet M-P, Godin M, Richard V, Thuillez C, Guerrot D, and Bellien J. Polycystin deficiency induces dopamine-reversible alterations in flow-mediated dilatation and vascular nitric oxide release in humans. Kidney Int 87: 465–472, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Lurie DJ. Commentary: electron spin resonance imaging studies of biological systems. Br J Radiol 69: 983–984, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Massota P, Parzya E, Pourtaua L, Melleta P, Madelin G, Marque S, Franconia J-M, and Thiaudiere E. In vivo high-resolution 3D Overhauser-enhanced MRI in mice at 0.2T. Contrast Media Mol Imaging 7: 45–50, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Matsuo T, Shinzawa H, Togashi H, Aoki M, Sugahara K, Saito K, Takahashi T, Yamaguchi I, Aoyama M, and Kamada H. Highly sensitive hepatitis B surface antigen detection by measuring stable nitroxide radical formation with ESR spectroscopy. Free Radic Biol Med 25: 929–935, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Maulucci G, Bačić G, Bridal L, Schmidt HH, Tavitian B, Viel T, Utsumi H, Yalçin AS, and De Spirito M. Imaging reactive oxygen species-induced modifications in living systems. Antioxid Redox Signal 24: 939–958, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris S, Sosnovsky G, Hui B, Huber CO, Rao NUM, and Swartz HM. Chemical and electrochemical reduction rates of cyclic nitroxides (nitroxyls). J Pharm Sci 80: 149–152, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Mrakic-Sposta S, Gussoni M, Montorsi M, Porcelli S, and Vezzoli A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid Med Cell Longev 2012: 973927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nilsson UA, Olsson LI, Thor H, Moldeus P, and Bylund-Fellenius AC. Detection of oxygen radicals during reperfusion of intestinal cells in vitro. Free Radic Biol Med 6: 251–259, 1989 [DOI] [PubMed] [Google Scholar]
- 66.Ouari O, Hardy M, Karoui H, and Tordo P. Recent developments and applications of the coupled EPR/Spin trapping technique (EPR/ST). Electron Paramagnetic Res 22: 1–40, 2011 [Google Scholar]
- 67.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, Sydykov A, Haag D, Esfandiary A, Kojonazarov B, Veit F, Fuchs B, Weisel FC, Hecker M, Schermuly RT, Grimminger F, Ghofrani HA, Seeger W, and Weissmann N. Mitochondrial hyperpolarization in pulmonary vascular remodeling mitochondrial uncoupling protein deficiency as disease model. Am J Respir Cell Mol Biol 49: 358–367, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Pignitter M, Mayer B, Klyanaraman B, and Vasques-Vivar J. Limitations of the cyclic hydroxylamine spin probe CPH in quantitative measurements of superoxide: 262. Free Radic Biol Med 41: S103, 2006 [Google Scholar]
- 69.Piskernik C, Haindl S, Behling T, Gerald Z, Kehrer I, Redl H, and Kozlov AV. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim Biophys Acta 1782: 280–285, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Polienko JF, Schanding T, Voinov MA, and Grigor'ev IA. Improved synthesis of 1-hydroxy-2,2,5,5-tetramethyl-3-imidazoline 3-oxide (HTIO). Synth Commun 36: 2763–2768, 2006 [Google Scholar]
- 71.Ponnuswamy P, Ostermeier E, Schrüttle A, Chen J, Huang PL, Ertl G, Nieswandt B, and Kuhlencordt PJ. Oxidative stress and compartment of gene expression determine proatherosclerotic effects of inducible nitric oxide synthase. Am J Pathol 174: 2400–2410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ribou AC. Synthetic sensors for reactive oxygen species detection and quantification: a critical review of current methods. Antioxid Redox Signal 25: 520–533, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, and Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivera-Ingraham GA, Barri K, Boël M, Farcy E, Charles A-L, Geny B, and Lignot J-H. Osmoregulation and salinity-induced oxidative stress: is oxidative adaptation determined by gill function? J Exp Biol 219: 80–89, 2016 [DOI] [PubMed] [Google Scholar]
- 75.Rosen GM, Finkelstein E, and Rauckman EJ. A method for the detection of superoxide in biological systems. Arch Biochem Biophys 215: 367–378, 1982 [DOI] [PubMed] [Google Scholar]
- 76.Saito K, Takeshita K, Anzai K, and Ozawa T. Pharmacokinetic study of acyl-protected hydroxylamine probe, 1-acetoxy-3-carbamoyl-2,2,5,5-tetramethylpyrrolidine, for in vivo measurement of reactive oxygen species. Free Radic Biol Med 36: 517–525, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Semmelmann A, Neeff H, Sommer O, Thomusch O, Hopt UT, and von Dobschuetz E. Evaluation of preservation solutions by ESR-spectroscopy: superior effects of University of Wisconsin over histidine–tryptophan–ketoglutarate in reducing renal reactive oxygen species. Kidney Int 71: 875–881, 2007 [DOI] [PubMed] [Google Scholar]
- 78.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, and Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sosnovsky G. and Cai ZW. A study of the Favorskii rearrangement with 3-bromo-4-oxo-2,2,6,6-tetramethylpiperidin-1-oxyl. J Org Chem 60: 3414–3418, 1995 [Google Scholar]
- 80.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, and Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radic Biol Med 42: 1632–1650, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spescha RD, Shi Y, Wegener S, Keller S, Weber B, Wyss MM, Lauinger N, Tabatabai G, Paneni F, Cosentino F, Hock C, Weller M, Nitsch RM, Lüscher TF, and Camici GG. Deletion of the ageing gene p66Shc reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J 34: 96–103, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Steffen-Heinz A. and Steffens B. EPR spectroscopy and its use in planta—a promising technique to disentangle the origin of specific ROS. Front Environ Sci 3: 15, 2015 [Google Scholar]
- 83.Swartz HM. Principles of the metabolism of nitroxides and their Implications for spin trapping. Free Radic Res Commun 9: 399–405, 1990 [DOI] [PubMed] [Google Scholar]
- 84.Thierbach S, Bui N, Zapp J, Chhabra SR, Kappl R, and Fetzner S. Substrate-assisted O2 activation in a cofactor-independent dioxygenase. Chem Biol 21: 217–225, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Thomas VC, Sadykov MR, Chaudhari SS, Jones J, Endres JL, Widhelm TJ, Ahn J-S, Jawa RS, Zimmerman MC, and Bayles KW. A central role of carbon-overflow pathways in the modulation of bacterial cell death. PLoS Pathog 10: e1004205, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Usselman RJ, Hill I, Singel DJ, and Martino CF. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS One 9: e93065, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valgimigli L, Pedulli GF, and Paolini M. Measurement of oxidative stress by EPR radical-probe technique. Free Radic Biol Med 31: 708–716, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Weissmann N, Kuzkaya N, Fuchs B, Tiyerili V, Schäfer RU, Schütte H, Ghofrani HA, Schermuly RT, Schudt C, Sydykov A, Egemnazarow B, Seeger W, and Grimminger F. Detection of reactive oxygen species in isolated, perfused lungs by electron spin resonance spectroscopy. Respir Res 6: 86, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia M, Zhang C, Boini KM, Thaker AM, and Li P-L. Membrane raft–lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin. Cardiovasc Res 89: 401–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu M, Xia M, Li X-X, Han W-Q, Boini KM, Zhang F, Zhang Y, Ritter JK, and Li P-L. Requirement of translocated lysosomal V1 H+-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells. Mol Biol Cell 23: 1546–1557, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan'shole VV, Kirilyuk IA, Grigor'ev IA, Morozov SV, and Tsentalovich YP. Antioxidative properties of nitroxyl radicals and hydroxyamines in reactions with triplet and deaminated kynurenine. Russ Chem Bull 59: 66–74, 2010 [Google Scholar]
- 92.Yokoyama H, Itoh O, Aoyama M, Obara H, Ohya H, and Kamada H. In vivo EPR imaging by using an acyl-protected hydroxylamine to analyze intracerebral oxidative stress in rats after epileptic seizures. Magn Reson Imaging 18: 875–879, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Yordanov AT, Yamada K, Krishna MC, Russo A, Yoo J, English S, Mitchell JB, and Brechbiel MW. Acyl-protected hydroxylamines as spin label generators for EPR brain imaging. J Med Chem 45: 2283–2288, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Zhang DX. and Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Zhang R, Goldstein S, and Samuni A. Kinetics of superoxide-induced exchange among nitroxide antioxidants and their oxidized and reduced forms. Free Radic Biol Med 26: 1245–1252, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, and Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A 102: 5727–5732, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zielonka J. and Kalyanaraman B. Hydroethidine- and mitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med 48: 983–1001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zielonka J, Vasquez-Vivar J, and Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Zwicker K, Dikalov S, Matuschka S, Mainka L, Hofmann M, Khramtsov V, and Zimmer G. Oxygen radical generation and enzymatic properties of mitochondria in hypoxia/reoxygenation. Arzneimittelforschung 48: 629–636, 1998 [PubMed] [Google Scholar]