Abstract
Recombinant adeno-associated viruses (rAAVs) are the leading in vivo gene delivery platform, and have been extensively studied in gene therapy targeting various tissues, including the central nervous system (CNS). A single-bolus rAAV injection to the cerebrospinal fluid (CSF) space has been widely used to target the CNS, but it suffers from several drawbacks, such as leakage to peripheral tissues. Here, a protocol is described using an osmotic pump to infuse rAAV slowly into the mouse CSF space. Compared to the single-bolus injection technique, pump infusion can lead to higher CNS transduction and lower transduction in the peripheral tissues.
Keywords: : adeno-associated virus, central nervous system, gene therapy, intrathecal delivery
Introduction
Numerous studies have demonstrated that recombinant adeno-associated viruses (rAAVs) can deliver their DNA payload in various tissues in vivo, and mediate safe and efficient transgene expression. In many cases, a simple systemic delivery of rAAV suffices to target multiple peripheral tissues, such as the liver, heart, and skeletal muscle. However, rAAV delivery to the central nervous system (CNS) encounters the blood–brain barrier (BBB) that restrains most drugs, including rAAV-based therapeutics, from entering the CNS from blood circulation. A major breakthrough in CNS gene therapy came from several laboratories, demonstrating that rAAV packaged in several serotypes such as AAV9 and AAV.rh10 can cross the BBB and transduce neurons and glia in rodents and nonhuman primates (NHPs).1–4 Based on the preclinical success, a clinical trial using systemic rAAV9 to treat spinal muscular atrophy (SMA) in infants was initiated, and the remarkable safety and therapeutic efficacy was recently reported.5 Nevertheless, using systemic rAAV to target CNS as a therapeutic strategy faces several obstacles. First, it requires a high vector dosage, especially for adult patients, and therefore poses a significant manufacturing burden. Second, the gene delivery efficacy can be greatly reduced by the presence of pre-existing neutralizing antibodies (NAbs) that are prevalent in adult human populations. Third, it carries the risk of off-target gene delivery to peripheral tissues and inducing potential immunotoxicities. To address these considerations, direct rAAV delivery to the CNS remains an attractive option for therapeutic development.
Intraparenchymal injection of rAAVs can result in localized gene delivery6 to treat CNS disease that afflicts a defined brain region. To treat more diffused CNS pathology, multiple injections would be required for a human brain, and therefore may not be practically feasible. Because the CNS is bathed in cerebrospinal fluid (CSF), injecting rAAVs directly to the CSF has been tested for CNS gene delivery.7–9 Later studies have mainly focused on targeting the dorsal root ganglion (DRG) containing the cell bodies of sensory neurons in the spinal cord, aiming to treat neuropathic pain, whereas targeting the cells residing deep in the parenchymal has been limited or not studied at all.10–15 More recently, several BBB-crossing AAV serotypes such as AAV9 and AAV.rh10 were tested for more widespread CNS gene delivery following CSF injection. Overall, these studies have demonstrated that direct injection of rAAV9 or rAAV.rh10 into the CSF space can lead to robust transduction of neurons and glia in multiple species, including the mouse,6,16–18 rat,19 cat,20,21 dog,22,23 pig,24,25 and NHPs.23,25–30 Peripheral tissue transduction can be reduced compared to systemic delivery of the same dose, although not completely diminished in most cases.16,19,24,26,27 In NHP studies, CSF rAAV delivery has been shown to resist peripheral NAbs to some extent.26–28 Toward translation to clinic use, several studies have demonstrated the therapeutic efficacy of CSF rAAV to treat different CNS diseases in animal models, including SMA,25 amyotrophic lateral sclerosis (ALS),31,32 Rett syndrome,33 and lysosomal diseases with CNS involvement.22,34–37 A clinical trial is underway to treat giant axon neuropathy (GAN) by intrathecal injection of a rAAV9 vector (ClinicalTrials.gov Identifier: NCT02362438).
CSF delivery is usually performed by intracerebral ventricular (ICV) injection, intra-cisterna magna (CM) injection, or intrathecal (IT) injection. ICV and CM injections require specialized equipment, whereas IT injection is a relatively simple procedure and can be performed in an outpatient setting. Currently, a single-bolus rAAV injection is used in CSF delivery, although it was shown that three boluses to the cervical, thoracic, and lumbar regions are required for a widespread spinal cord gene delivery in pigs.24 For both rodents and larger model species, further refinement and evaluation of CSF rAAV delivery are underway to improve CNS targeting.38 For IT injection in mice, it has been previously reported that the injection speed has an impact on the gene delivery pattern along the neuroaxis, with slow injection favoring the spinal cord.39 Recently, an osmotic pump was used to infuse rAAV9 or an engineered vector slowly to the mouse subarachnoid space over a period of about 1 day, and widespread spinal cord transduction was found.40 In this protocol, a step-by-step procedure is described involving the IT placement of a catheter in a mouse model for the slow infusion of rAAV9 by an osmotic pump. In addition, a similar procedure for ICV infusion is also described. Compared to a single-bolus injection, both infusion routes can mediate higher CNS targeting and reduced peripheral tissue transduction.
Materials
1. Reagents are listed in Table 1
Table 1.
List of reagents
| Reagents | Supplier | Specific handling | Storage conditions |
|---|---|---|---|
| 0.9% sodium chloride | Hospira | — | RT |
| rAAV solution | Variable | — | <4°C; avoid repeated freezing and thawing |
| Isoflurane | Piramal | Subjected to regulatory policy | RT |
| Ketoprofen | Zoetis | Subjected to regulatory policy | RT |
| Ketamine | Henry Schein Animal Health | Subjected to regulatory policy | RT |
| Xylazine | Santa Cruz Animal Health | Subjected to regulatory policy | RT |
rAAV, recombinant adeno-associated virus; RT, room temperature.
1.1. Preparation of ketamine/xylazine
Mix 2 mL of ketamine stock (100 mg/mL), 0.2 mL of xylazine stock (100 mg/mL), and 7.8 mL of 0.9% saline to a total volume of 10 mL. Store at 4°C for a maximum of 2 weeks. For intraperitoneal (i.p.) injection, use 100 μL/20 g of body weight.
1.2. Preparation of ketoprofen
Dilute 100 μL of stock (100 mg/mL) in 9.9 mL of 0.9% saline. For i.p. injection, use 100 μL/20 g of body weight.
2. Supplies are listed in Table 2
Table 2.
List of supplies
| Item | Supplier | Note |
|---|---|---|
| Alzet mini-osmotic pump, model 2001D | Alzet, order # 0000292 | — |
| Tissue culture plate, 48-well | Variable | — |
| Tubes, 2 mL | Variable | Sterilize by autoclaving |
| Mouse intrathecal catheter | Alzet, order # 0007743 | — |
| Small bead (∼1 mm ID; ∼3 mm OD) | handcraft store | Sterilize by autoclaving |
| Krazy Glue | Variable | — |
| Tissue culture plate, six-well | Variable | — |
| Conical tube, 50 mL | Variable | — |
| Filling needle for 200 μL pumps | Alzet, order # 0007987 | — |
| Syringe, 1 mL | BD, ref # 309659 | — |
| Gauze | Dukal, order # 1212 | — |
| Insulin syringe, 28G1/2 | Becton Dickinson, order # 329461 | — |
| Puralube® ophthalmic ointment | Dechra | — |
| Alcohol pads | Dukal, ref # 852 | — |
| Povidone-iodine prep pads | Dynarex, order # 1108 | — |
| Scalpel | Miltex, ref # 4-410 | — |
| Cotton-tip applicator | Puritan, ref # 25-806 1WC | — |
| Suture | Ethicon, 661G | — |
| Razor blade | Variable | — |
| Brain infusion kit 3 | Alzet, order # 0008851 | — |
| Loctite 454 adhesive | Alzet, order # 0008670 | — |
| Marker pen, fine-tip | ASTM, D-4236 | — |
3. Tools and equipment are listed in Table 3
Table 3.
List of tools and equipment
| Equipment | Brand and model | Note |
|---|---|---|
| Hair clipper | Oster | — |
| Laminar flow hood | Variable | Providing an aseptic environment |
| Forceps, general use | Variable | Sterilize by autoclaving |
| Hemostatic forceps | Variable | Sterilize by autoclaving |
| Incubator | Variable | Set to 37°C |
| Forceps, fine-tip | Variable | Sterilize by autoclaving |
| Surgical scissors, general use | Variable | Sterilize by autoclaving |
| Anesthesia machine | VetEquip | — |
| Spatula | Fisherbrand, cat. # 21-401-10 | Sterilize by autoclaving |
| Stereotaxic instrument | Stoelting | — |
| Micro-drill with a fine bit | Ideal, part # 67-1000 | Sterilize the bit by autoclaving |
| Cannula holder | Stoelting, item # 51636 | — |
| Heat therapy pump | Stryker, model # TP700 | — |
| Heating pad | Adroit, AP-R 60 | — |
| Cautery | World Precision Instrument, item # 500392 | — |
Procedure
Note: Wherever possible, work under a laminar flow hood, and use aseptic techniques with sterilized materials, as indicated in the Materials section. All animal procedures require approval from regulatory agencies such as the Institutional Animal Care and Use Committee.
1. Intrathecal delivery
1.1. Priming osmotic pumps
The osmotic pumps have to be primed before implantation, so that infusion starts immediately after subcutaneous implantation. The 2001D (200 μL; 1 day infusion time) pumps are primed in 0.9% saline for 4 h, according to the manufacturer's instruction.
1. Open the number of pumps and flow moderators needed.
2. Obtain the same number of 2 mL tubes, and add 0.5 mL of 0.9% saline to each tube.
3. Insert the metal tube of a flow moderator into a pump.
4. Place one pump and flow moderator assembly into a 2 mL tube, so that the pump body is submerged in 0.9% saline but the flow moderator is not. Close the tube.
5. Incubate at 37°C for 4 h.
1.2. Preparation of intrathecal catheters
6. Open the number of catheters needed, and obtain enough beads.
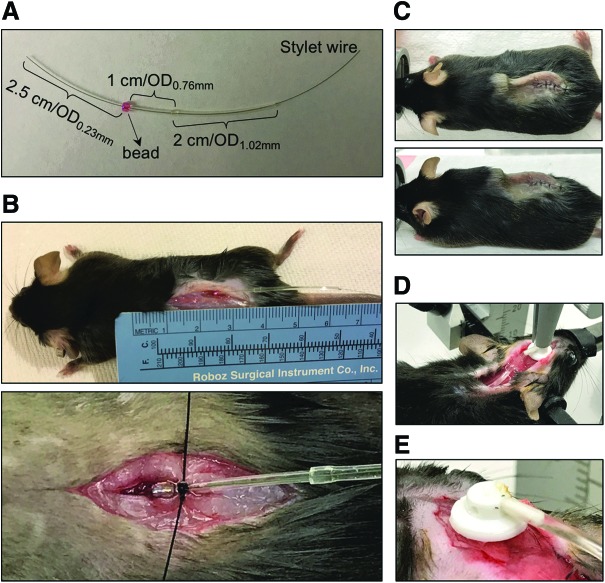
7. Thread the OD0.23mm portion of a catheter through a small bead (to be used later as a suture anchor), and place the bead at the junction of the OD0.23mm portion and the OD0.76mm portion (Fig. 1A).
8. Add a small drop of Krazy Glue onto the bead. The glue will fill in the space between the catheter and the bead.
9. Place the catheter and bead assembly on a six-well tissue culture plate. Do not let the glue touch the plasticware.
10. Air dry for 30 min, and verify that the bead is secured onto the catheter. Collect all catheters into a 50 mL conical tube for temporary storage.
Figure 1.
Procedure demonstration. (A) An intrathecal (IT) catheter with a bead secured on it. (B) A catheter is placed into the subarachnoid space, with a bead sutured onto the muscle to prevent displacement of the catheter. (C) A catheter and pump assembly is covered under the skin after surgery. Top view (top panel) and side view (bottom panel) are shown. (D) The placement of a cannula, catheter tube, and pump assembly during surgery. (E) The removable tab on the cannula is removed by heating using a cautery.
1.3. Filling the primed osmotic pumps with rAAV preparation
11. After the pumps have been primed for 4 h, place them at room temperature.
12. Attach a blunt filling needle to a 1 mL syringe, and withdraw enough rAAV preparation (∼200 μL for each pump).
13. With the needle pointing up, gently tap the syringe to remove air bubbles. Push the plunger to dispense air in the syringe. Place aside.
14. Take one pump and flow moderator assembly out of 0.9% saline, and place in an upright position in a 48-well tissue culture plate. Remove excess saline near the opening of the pump with gauze.
15. Pull the flow moderator out of the empty pump using a pair of forceps and place aside.
16. Insert the filling needle attached to the syringe to the bottom of the pump. Slowly push the plunger to dispense the rAAV preparation. While filling, gradually lift the syringe and needle assembly for complete filling. Once the pump is full, a small drop of rAAV preparation appears near the opening of the pump. Pull the filling needle out of the pump.
17. Hold the metal tube of the flow moderator tightly with a pair of hemostatic forceps, and remove the rubber part and plastic part with a pair of forceps.
18. Insert the metal tube partially into the filled pump until a small drop of rAAV preparation appears at the end of the flow moderator. Discard the other parts of the flow moderator.
19. Return the filled pump and flow moderator assembly to the 2 mL tube containing 0.9% saline. Close the tube and label with proper information, such as rAAV name and experimental group.
20. Repeat steps 14–19 until all pumps are filled. Use a new filling needle and syringe for each rAAV preparation to prevent cross-contamination.
1.4. Filling the catheters with rAAV preparation
21. Pick up one catheter using a pair of forceps. Pull the stylet from the OD0.23mm portion of the catheter, so that the other end of stylet stays inside the catheter near the junction of the OD0.76mm portion and OD1.02mm portion.
22. Withdraw enough rAAV preparation (∼10 μL for each catheter) using an insulin syringe. Remove air in the syringe as described in step 13.
23. Insert the syringe needle into the OD1.02mm portion of the catheter. Use a pair of forceps to hold the catheter onto the syringe tightly, and slowly push the plunger to fill the catheter with the rAAV preparation until a small drop appears at the OD0.23mm tip. Slowly remove the syringe needle out of the catheter while continuing to fill the entire catheter. Avoid trapping air inside the catheter.
24. Reposition the stylet, so that one end is flush with the OD0.23mm tip.
Note: Filling each catheter immediately prior to use is recommended.
1.5. Preparation of mice for surgery
25. Shave the fur on the lower back using a hair clipper. The skin region subjected to surgery should be exposed. Return the shaved mice to their housing cages.
26. Turn on oxygen flow and isoflurane vaporizer.
27. Turn valves to control the oxygen/isoflurane flow to the induction chamber, and place one mouse in the induction chamber until fully anesthetized.
28. Administer 100 μL of diluted ketoprofen per 20 g of body weight by i.p. injection.
29. Turn valves to control the oxygen/isoflurane flow to the nose cone, and place the anesthetized mouse under the nose cone in a prone position for continuous anesthesia. Verify successful anesthesia by a toe pinch resulting in no reflection.
30. Cover both eyes with eye ointment.
31. Clean the shaved skin with a povidone-iodine pad followed by an alcohol pad three times.
Note: Using mice that are older than 6 weeks or that weigh at least 20 g is recommended. Using the C57BL/6J strain of either sex has been successful.
1.6. Intrathecal placement of a catheter (Supplementary Video S1; Supplementary Data are available online at www.liebertpub.com/hgtb)
32. Hold the mouse by pinching the hip joints, and make a 1.5 cm incision along the midline on the skin of the lower back with a scalpel. Make another small incision in the muscle underneath the skin incision. The muscle incision should be ∼1 mm to the left or right of the midline. Use a cotton applicator to remove blood in case of bleeding.
33. Use a blunt pump filling needle to probe the spine gently under the muscle incision to identify a catheter entry point. A tail flick may be observed when the needle is placed correctly.
34. Place the probing needle aside, and pick up the filled catheter with a pair of fine forceps. Make sure the stylet is flush with the OD0.23mm tip.
35. Insert the OD0.23mm tip through the entry point previously identified by using the probing needle, and gently advance the catheter until the bead reaches the muscle surface. Successful positioning of the catheter should result in mouse tail flick reflex during catheter advancement.
36. Suture the muscle, and make a knot around the bead to secure the catheter onto the muscle (Fig. 1B).
1.7. Implantation of the filled osmotic pump
37. Gently pull the stylet out of the catheter.
38. Make an open space at the upper back region using a small spatula to gently separate the skin from muscle.
39. Attach the filled pump and flow moderator assembly to the OD1.02mm end of the catheter. The connection region between the flow moderator and catheter should be ∼2 mm. Insert the flow moderator into the pump as deep as possible.
40. Place the pump in the open space at the upper back. It is necessary to bend the catheter.
41. Suture the skin so that the pump and the entire catheter are covered under the skin (Fig. 1C).
1.8. Postoperative recovery
42. Administer 200 μL of 0.9% saline by i.p. injection, and place the mouse to a housing cage with bedding. Mice should be housed individually after surgery.
43. The mouse is expected to wake up from anesthesia within a few minutes. Successful operation should result in no motor defect.
Note: Paralysis shortly after surgery may indicate spinal cord damage during catheter placement. However, it is not common (<10% of mice) after establishing the method by extensive practice.
1.9. Removal of an implanted osmotic pump
44. Anesthetize a mouse as described in step 1.5. Cut open the suture on the skin, and cut the suture on the bead.
45. Slowly pull the catheter attached to the pump out of the spinal space, and put aside.
46. Suture the muscle and skin, and return the mouse to the housing cage for recovery.
47. To verify successful operation of osmotic pressure, remove the flow moderator and quickly cut open the pump with a sharp razor blade. The reservoir inside of the pump should appear to be squeezed.
Note: Typically, the 2001D pumps are removed 40–42 h after implantation. Leaving a pump in an animal for longer period of time may result in pump damage and leakage of salt from the pump.
2. ICV delivery
2.1. Priming osmotic pumps and filling with rAAV preparation
1. Refer to steps 1.1 and 1.3.
2.2. Preparation of stereotaxic instrument and mice for surgery
2. Place stereotaxic instrument on a flat surface, and connect all cables and accessories.
3. Administer 100 μL of diluted ketamine/xylazine per 20 g of body weight to a mouse by i.p. injection. Verify successful anesthesia by a toe pinch.
4. Administer 100 μL of diluted ketoprofen by i.p. injection, and cover both eyes with ointment.
5. Shave the fur on the head and neck region using a hair clipper.
6. Secure the mouse in a prone position using ear bars attached to a stereotaxic instrument. The head surface should be horizontal by visual examination.
7. Clean the shaved region using a povidone-iodine pad followed by an alcohol pad three times.
2.3. ICV placement of a cannula for brain infusion
8. Cut open the skin on the head and neck region (∼2.5 cm) with a pair of small scissors, and pull the skin apart to the sides to expose the skull.
9. Dry the skull surface with a cotton-tip applicator to visualize the bregma better.
10. Open a set of brain infusion cannula, including a cannula with a removable tab, a catheter, and four depth-adjustment spacers. Slide one depth-adjustment spacer onto the cannula tube and glue it to the cannula base using Loctite adhesive. This modification shortens the cannula length to 2.5 mm and places the cannula tip into lateral ventricles when the cannula is properly placed.
11. After the adhesive dries, secure the cannula to a cannula holder by tightly clamping the removable tab, and attach the cannula holder to the stereotaxic frame. The cannula tube should be straight and upright by visual examination. The connector to catheter tube should be in parallel with the mouse body, pointing backward.
12. Adjust the X, Y, and Z arms of the stereotaxic frame to place the cannula tip right on top of the bregma. Set the frame position as (0, 0, 0).
13. Slightly lift the cannula by adjusting the Z arm, and adjust the X and Y arms to (−0.9, −0.2) for the left ventricle, or (+0.9, −0.2) for the right ventricle.
14. Lower the Z arm to bring the cannula tip close to the skull surface, and mark a small dot on the skull surface right below the cannula tip with a fine-tip marker pen.
15. Lift the cannula by adjusting the Z arm, and drill a burr hole at the marked position with a fine-bit electric drill. Gently drill through the skull without damaging brain tissue.
16. Cut the catheter tube to 2 cm long, and attach the catheter tube to the cannula. Fill the cannula and catheter tube assembly with rAAV preparation using an insulin syringe until a small drop appears at the cannula tip.
17. Lower the cannula by adjusting Z arm to bring the cannula tip close to the burr hole.
18. Make an open space at the neck and upper back region using a small spatula to separate the skin gently from the muscle.
19. Attach the flow moderator of a filled osmotic pump (from step 2.1) to the catheter tube. The connection region between the flow moderator and catheter should be ∼2 mm. Insert the flow moderator into the pump as deep as possible.
20. Place the pump in the open space at the upper back region.
21. Apply several drops of Loctite adhesive to the cannula base.
22. Slowly lower the cannula to insert the cannula tube into the brain until the cannula base is flush with the skull surface (Fig. 1D).
23. After 5 min, release the cannula from the cannula holder, and lift the cannula holder by adjusting the Z arm.
24. Break the junction between the cannula and the removable tab by heating with a cautery (Fig. 1E).
25. Suture the skin, so that the cannula and the catheter tube are covered under the skin.
2.4. Postoperative recovery from ICV placement of a cannula
26. Release the mouse from the ear bars, and administer 500 μL of 0.9% saline by i.p. injection.
27. Place the mouse into a housing cage with bedding. Keep the cage on a heating pad helps recovery. After surgery, mice should be housed individually.
28. The mouse is expected to wake up from anesthesia within 1 h. Successful operation should result in no motor defect.
2.5. Removal of an implanted osmotic pump for brain infusion
29. Anesthetize a mouse as described in step 1.5. Cut open the suture.
30. Cut the catheter tube in the middle, and remove the pump and the attached catheter.
31. Seal the end of the catheter attached to the cannula using a cautery (Supplementary Video S2).
32. Place the remaining catheter attached to the cannula under the skin, and suture the skin.
33. Return the mouse to the housing cage.
34. Verify successful operation of osmotic pressure, as described in step 1.9.
Note: Typically, the 2001D pumps are removed 40–42 h after implantation. Leaving a pump in an animal for longer period of time may result in pump damage and leakage of salt from the pump.
3. Expected results
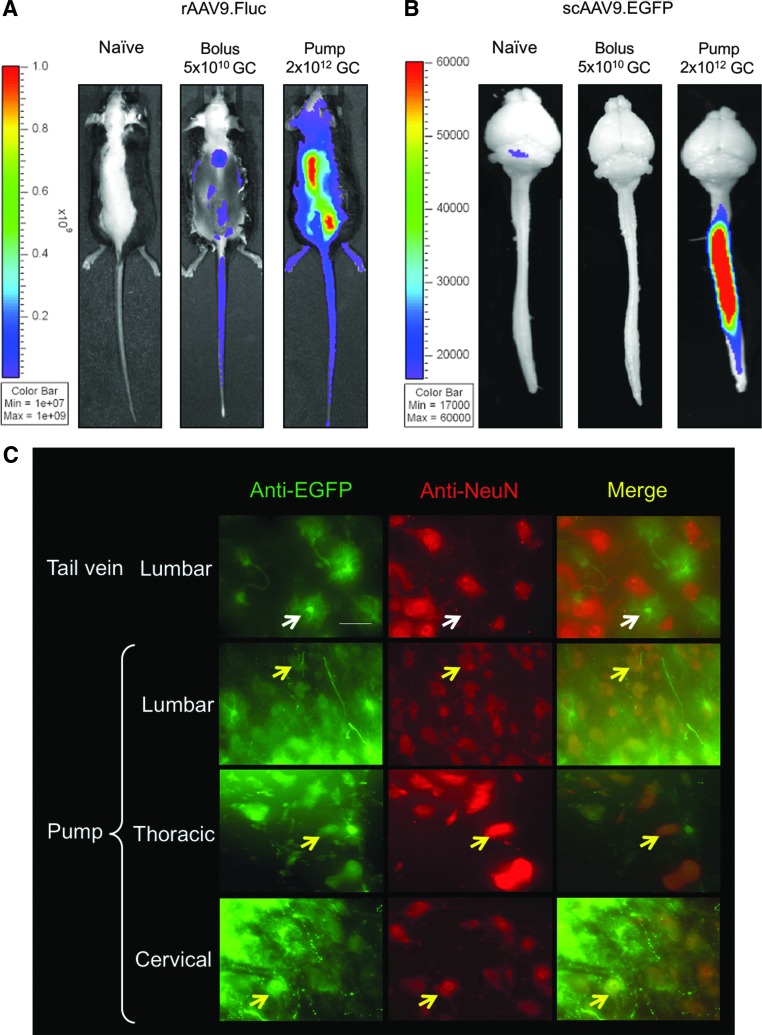
3.1. With rAAV9 packaging either a single-stranded genome or a self-complementary genome, it was found that IT pump infusion resulted in more widespread and higher transduction of the spinal cord than a bolus IT injection (Fig. 2A and B). This is in part due to a higher delivery volume (200 μL) and dose enabled by the pump infusion, whereas bolus injection is typically limited to <10 μL. Neurons in the ventral horns can be transduced following IT pump infusion (Fig. 2C).
Figure 2.
IT pump infusion of recombinant adeno-associated virus serotype 9 (rAAV9) in mouse transduced spinal cord. (A) In vivo imaging showing the firefly luciferase expression from a single-stranded rAAV9.CB6-Fluc vector delivered by either a single-bolus IT injection of 5 × 1010 GC in 5 μL (bolus) or IT pump infusion of 2 × 1012 GC in 200 μL (pump). (B) Epi-fluorescence imaging of native EGFP signal from a self-complementary (sc) AAV9.CB6-EGFP vector. Brain and spinal cord were dissected from mice receiving either a single-bolus IT injection of 5 × 1010 GC in 5 μL (bolus) or IT pump infusion of 2 × 1012 GC in 200 μL (pump). (C) Immunofluorescence staining of mouse spinal cord tissue cryosections. Tail vein: injection of 2 × 1012 GC of scAAV9.CB6-EGFP through tail vein in an adult mouse. Pump: IT pump infusion of the same vector of the same dose. Yellow arrows: co-localization of EGFP and NeuN (a neuronal cell marker) staining signals indicates transduction of neurons in the ventral horns. White arrows: lack of co-localization. Scale bar: 50 μm.
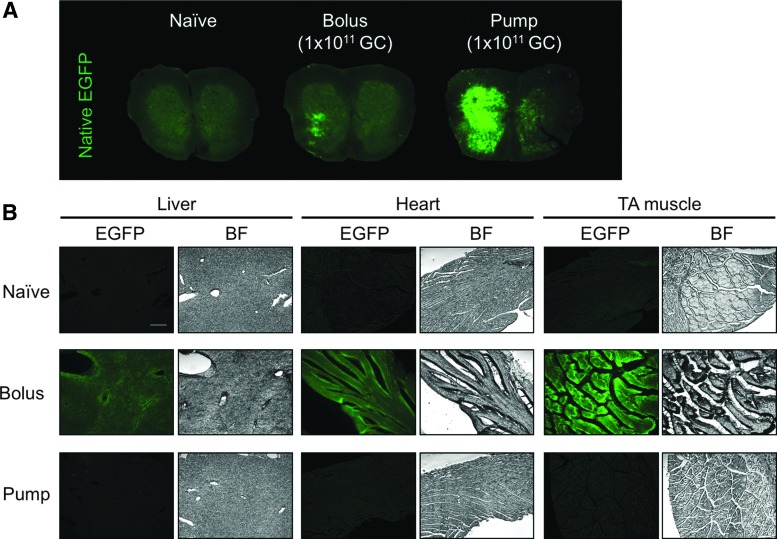
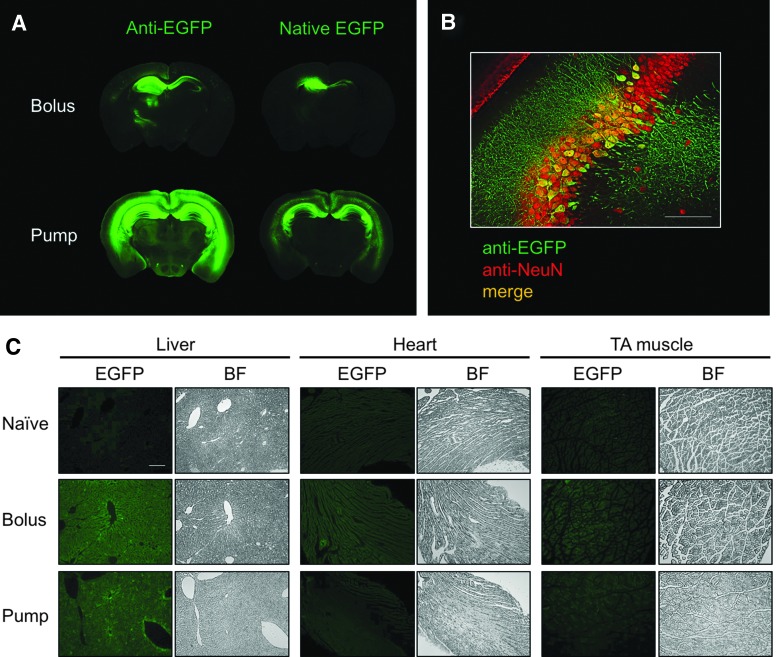
3.2. When the same dose of rAAV9 (1 × 1011 GC) was delivered by either a bolus IT injection or pump infusion, it was observed that pump infusion resulted in higher spinal cord transduction than the bolus injection (Fig. 3A). Notably, transduction of peripheral organs, including liver, heart, and skeletal muscle, was reduced compared to a bolus injection (Fig. 3B).
Figure 3.
Comparing IT bolus injection and pump infusion of the same dose (1 × 1011 GC) of scAAV9.CB6-EGFP. (A) Native EGFP signal from lumbar spinal cord tissue sections. (B) Native EGFP signal and bright field (BF) images of cryosections of the liver, heart, and tibialis anterior (TA) muscle. Scale bar: 200 μm.
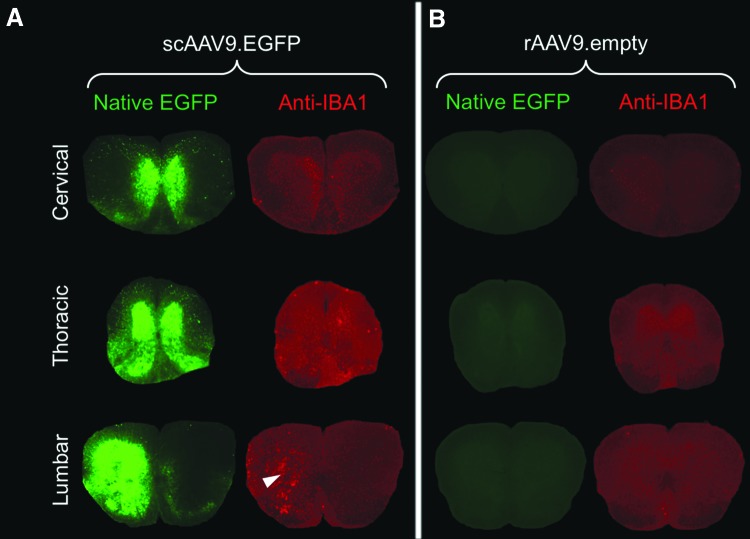
3.3. We observed an increase in immunostaining against IBA1 after IT pump infusion of scAAV9-EGFP, indicating microgliosis (Fig. 4A). This is likely caused by the overexpression of the foreign protein EGFP, because strong IBA1 immunostaining overlaps with the region of high EGFP expression (Fig. 4A). It is not caused by the AAV9 capsid or the surgical procedure because infusion of empty rAAV9 particles did not lead to microgliosis (Fig. 4B).
Figure 4.
The high EGFP expression in spinal cord resulting from IT pump infusion caused microgliosis. Native EGFP and anti-IBA1 immunostaining signals from spinal cord tissue sections. Mice were treated with either (A) 1 × 1011 GC of scAAV9.CB6-EGFP (scAAV9.EGFP) or (B) 1 × 1011 viral particles of empty rAAV9 vectors without a packaged genome (rAAV9.empty). Arrowhead indicates the increased IBA1 immunostaining signal that corresponds to the region with strong EGFP expression in the lumbar region.
3.4. Following ICV pump infusion of 1 × 1011 GC of scAAV9-EGFP, it was observed that pump infusion led to more widespread and higher EGFP expression in the brain than a single-bolus injection of 2 × 1010 GC of the same vector (Fig. 5A). Neurons can be transduced following ICV pump infusion (Fig. 5B). Reduced transduction in the heart with ICV pump infusion, and similar transduction in the liver and skeletal muscle was observed compared to a bolus injection (Fig. 5C), although more vectors were delivered with pump infusion.
Figure 5.
Comparing intracerebral ventricular (ICV) bolus injection and pump infusion of scAAV9.CB6-EGFP. (A) The immunostaining or native EGFP signals of mouse brain sections following either an ICV bolus injection of 2 × 1010 GC (bolus), or ICV pump infusion of 1 × 1011 GC (pump). (B) Immunostaining of EGFP and NeuN of brain sections following ICV pump infusion. Co-localization of the signals indicates transduction of neurons in the hippocampus. Scale bar: 100 μm. (C) Native EGFP signal and BF images of cryosections of the liver, heart, and TA muscle. Scale bar: 50 μm.
Troubleshooting
1. No tail flicker reflex is observed during IT catheter placement, indicating that the catheter is not placed correctly. During initial practice of this surgical procedure, euthanized animals were taken and the muscle tissue covering the lumbar region was removed to expose the vertebrae. This allowed the catheter entry point to be visualized, therefore allowing better control over catheter placement. In experiments using anesthetized mice, using a blunt-end probing needle helps to identify the proper catheter entry point, but the procedure still needs extensive practice. To verify correct placement of an IT catheter, a syringe can be attached to the catheter tubing (instead of a pump) to inject some tracer dye, followed by examining the stained spinal cord.
2. Hind-limb paralysis immediately after IT catheter placement. During catheter insertion into the spinal region, keep the stylet wire flush with the opening of the catheter tubing. An exposed stylet tip will likely cause damage of the spinal cord tissue, resulting in paralysis. If the insertion end of the catheter is bent after several failed trials, pull the stylet out of the catheter, cut off the bent part, and reposition the stylet back to the catheter. Do not shorten the catheter tubing.
3. Brain infusion cannula is not secured on skull. Dry the skull surface with a cotton-tip applicator. Keep the skull surface horizontal. A tilted skull surface will not align with the cannula base well, and therefore the cannula will not be glued firmly onto the skull. Apply the adhesive right before lowering the cannula tube into the brain to prevent drying of the adhesive.
Supplementary Material
Acknowledgments
We would like to thank Jake Metterville for his technical guidance on intrathecal catheterization. This work was supported by grants from the National Institutes of Health to G.G. (1P01AI100263-05, 1R01NS076991-05, R01HL097088, and 4P01HL131471-01).
Author Disclosure
G.G. is a scientific co-founder of Voyager Therapeutics and holds equity in the company. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceuticals. For all other authors, no competing financial interests exist.
References
- 1.Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 2009;27:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duque S, Joussemet B, Riviere C, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 2009;17:1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Yang B, Mu X, et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther 2011;19:1440–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 2011;19:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722 [DOI] [PubMed] [Google Scholar]
- 6.Snyder BR, Gray SJ, Quach ET, et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther 2011;22:1129–1135 [DOI] [PubMed] [Google Scholar]
- 7.Elliger SS, Elliger CA, Aguilar CP, et al. Elimination of lysosomal storage in brains of MPS VII mice treated by intrathecal administration of an adeno-associated virus vector. Gene Ther 1999;6:1175–1178 [DOI] [PubMed] [Google Scholar]
- 8.Peel AL, Zolotukhin S, Schrimsher GW, et al. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell type-specific promoters. Gene Ther 1997;4:16–24 [DOI] [PubMed] [Google Scholar]
- 9.Watson G, Bastacky J, Belichenko P, et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther 2006;13:917–925 [DOI] [PubMed] [Google Scholar]
- 10.Storek B, Harder NM, Banck MS, et al. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain 2006;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storek B, Reinhardt M, Wang C, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A 2008;105:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto N, Watanabe A, Yamamoto M, et al. Global diffuse distribution in the brain and efficient gene delivery to the dorsal root ganglia by intrathecal injection of adeno-associated viral vector serotype 1. J Gene Med 2009;11:498–505 [DOI] [PubMed] [Google Scholar]
- 13.Towne C, Pertin M, Beggah AT, et al. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain 2009;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason MR, Ehlert EM, Eggers R, et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther 2010;18:715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vulchanova L, Schuster DJ, Belur LR, et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain 2010;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster DJ, Dykstra JA, Riedl MS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 2014;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Wang D, Qiao T, et al. A single injection of recombinant adeno-associated virus into the lumbar cistern delivers transgene expression throughout the whole spinal cord. Mol Neurobiol 2016;53:3235–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bey K, Ciron C, Dubreil L, et al. Efficient CNS targeting in adult mice by intrathecal infusion of single-stranded AAV9-GFP for gene therapy of neurological disorders. Gene Ther 2017;24:325–332 [DOI] [PubMed] [Google Scholar]
- 19.Hordeaux J, Dubreil L, Deniaud J, et al. Efficient central nervous system AAVrh10-mediated intrathecal gene transfer in adult and neonate rats. Gene Ther 2015;22:316–324 [DOI] [PubMed] [Google Scholar]
- 20.Bucher T, Colle MA, Wakeling E, et al. scAAV9 intracisternal delivery results in efficient gene transfer to the central nervous system of a feline model of motor neuron disease. Hum Gene Ther 2013;24:670–682 [DOI] [PubMed] [Google Scholar]
- 21.Bucher T, Dubreil L, Colle MA, et al. Intracisternal delivery of AAV9 results in oligodendrocyte and motor neuron transduction in the whole central nervous system of cats. Gene Ther 2014;21:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurda BL, De Guilhem De Lataillade A, Bell P, et al. Evaluation of AAV-mediated gene therapy for central nervous system disease in canine mucopolysaccharidosis VII. Mol Ther 2016;24:206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinderer C, Bell P, Katz N, et al. Evaluation of intrathecal routes of administration for adeno-associated viral vectors in large animals. Hum Gene Ther 2018;29:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federici T, Taub JS, Baum GR, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther 2012;19:852–859 [DOI] [PubMed] [Google Scholar]
- 25.Passini MA, Bu J, Richards AM, et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther 2014;25:619–630 [DOI] [PubMed] [Google Scholar]
- 26.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 2012;23:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray SJ, Nagabhushan Kalburgi S, McCown TJ, et al. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 2013;20:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samaranch L, Salegio EA, San Sebastian W, et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum Gene Ther 2013;24:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinderer C, Bell P, Vite CH, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev 2014;1:14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samaranch L, San Sebastian W, Kells AP, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Mol Ther 2014;22:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Yang B, Qiu L, et al. Widespread spinal cord transduction by intrathecal injection of rAAV delivers efficacious RNAi therapy for amyotrophic lateral sclerosis. Hum Mol Genet 2014;23:668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dirren E, Aebischer J, Rochat C, et al. SOD1 silencing in motoneurons or glia rescues neuromuscular function in ALS mice. Ann Clin Transl Neurol 2015;2:167–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinnett SE, Hector RD, Gadalla KKE, et al. Improved MECP2 gene therapy extends the survival of MeCP2-null mice without apparent toxicity after intracisternal delivery. Mol Ther Methods Clin Dev 2017;5:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haurigot V, Marco S, Ribera A, et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 2013. July 1 [Epub ahead of print]; DOI: 10.1172/JCI66778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinderer C, Bell P, Gurda BL, et al. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther 2014;22:2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinderer C, Katz N, Louboutin JP, et al. Delivery of an adeno-associated virus vector into cerebrospinal fluid attenuates central nervous system disease in mucopolysaccharidosis type II mice. Hum Gene Ther 2016;27:906–915 [DOI] [PubMed] [Google Scholar]
- 37.Hordeaux J, Dubreil L, Robveille C, et al. Long-term neurologic and cardiac correction by intrathecal gene therapy in Pompe disease. Acta Neuropathol Commun 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer K, Ferraiuolo L, Schmelzer L, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Mol Ther 2015;23:477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Liu C, Yang C, et al. Slow intrathecal injection of rAAVrh10 enhances its transduction of spinal cord and therapeutic efficacy in a mutant SOD1 model of ALS. Neuroscience 2017;365:192–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murlidharan G, Sakamoto K, Rao L, et al. CNS-restricted transduction and CRISPR/Cas9-mediated gene deletion with an engineered AAV vector. Mol Ther Nucleic Acids 2016;5:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.