Abstract
Background
Due to waning levels of maternal antibodies (measles; enterovirus 71, EV71; and coxsackievirus A16, CoxA16), some infants may lose protection against infection prior to vaccination. Using a longitudinal design, we examine how maternal antibody levels evolve over time in infants prior to vaccination.
Methods
In 2013–2014, we collected sera at ages 0, 3 and 6 months from infants. We assayed for levels of measles IgG antibody (717, 233 and 75 sample sera tested at months 0, 3 and 6, respectively), and neutralizing antibodies for EV71 and CoxA16 (225, 217, and 72). Demographic and health information were collected, and a linear mixed model (LMM) was used to describe antibody levels over time.
Results
Pre-vaccination monotonic antibody decreases were observed for measles (1410, 195 and 22 mIU/ml, p < 0.001), EV71 (1:19.9, 6.3 and 4.5, p < 0.001) and CoxA16 (1:16.3, 5.9, and 4.5, p < 0.001). At 6 months of age, only 2.7% (95%CI, 0.6–8.3), 6.8% (95%CI, 2.7–14.4) and 5.6% (95%CI, 1.9–12.7) of infants were antibody positive for measles, EV71 and CoxA16, respectively. LMM findings indicated that infants with higher antibody titers at birth experienced a greater loss of antibody level. An infection rate of 1.3% (95%CI, 0.1–6.1) was reported for both EV71 and CoxA16.
Conclusions
Further modifications of vaccination strategies for measles, earlier vaccination for EV71 infection, and deployment of a CoxA16 vaccine need to be considered to limit infection among the very young.
Keywords: Maternal antibodies, Infants, Measles, Enterovirus 71, Coxsackievirus 16
1. Introduction
Specific maternal antibodies provide vital, passive immunity against infectious pathogens for infants during the first months of life [1]. However, depending on an infant’s immunization schedule, this maternal protection may wane prior to vaccination [2–4]. Over the past decade, measles and hand-foot-mouth disease (HFMD) have remained public health challenges among infants in some countries, including China [5,6]. This phenomenon may, in part, be due to the timing of immunization.
Measles vaccination is free and mandatory for children 8 months to 14 years of age, and since vaccination began in China, reported measles cases have decreased substantially. Indeed, mean annual measles incidence was 572.0 per 100,000 between 1960 and 1969, 355.3 per 100,000 between 1970 and 1979, 52.9 per 100,000 between 1980 and 1989, and 7.6 per 100,000 between 1990 and 1999 [7]. Since 1986, the control of measles incidence in China has been supported by the Expanded Program on Immunization (EPI), which provides measles vaccination using a two-dose successive vaccination schedule. In the 30 years since EPI implementation, the epidemiology of measles has changed greatly. For example, over the past 10 years, data show that for children younger than 8 months, who are ineligible for vaccination, the relative burden of measles has increased. Specifically, although the number of annual measles cases in China generally decreased during 2005 to 2012 (123,136, 99,602, 109,023, 131,441, 52,461, 38,159, 9943, and 6183 respectively), the percentage of annual cases that occurred in Chinese infants aged <8 months increased from 11.3% to 24.5% [8]. In the initial EPI schedule, the first dose, a monovalent attenuated vaccine, was given at 8 months, and the second dose, either a monovalent vaccine, measles-rubella/MR vaccine, or measles-mumps-rubella/MMR vaccine, was administered at 7 years. In 2006, the schedule was revised so that the second dose is administered at 18–24 months.
Since 2000, annual measles incidence has remained below 10 per 100,000. Between 2005 and October 2013, a total of 596,391 measles cases and 368 measles-related deaths were reported in China, and annual incidence, in cases per 100,000 population, decreased from 9.95 in 2008 to 0.46 in 2012 but then rose to more than 1.96 in 2013 [6]. The reason for this increase under the EPI may be explained by waning maternal antibody protection, which for most infants is likely derived from maternal vaccination rather than natural infection [5,9–12].
Hand foot and mouth disease (HFMD) is a common infectious disorder caused by enterovirus. In the western Pacific region, two enteroviruses, human enterovirus 71 (EV71) and coxsackievirus A16 (CoxA16), co-circulate and are the principal agents of HFMD. Some patients with EV71 infection rapidly develop neurological and systemic complications that can be fatal [13,14]. In China 7,200,092 probable cases of HFMD were reported to the national surveillance system during 2008–2012, of which 2457 (0.03%) were fatal [6]. Two alum-adjuvant inactivated EV71 vaccines developed in mainland China, which showed high efficacy (94.8% against disease, 100% against EV71-associated hospitalization or neurologic complications), good immunogenicity persistence and acceptable safety profiles in clinical trials, have been approved for HFMD prevention among Chinese infants [15,16]. The vaccine, which is initially administered at 6 months, has been licensed in China since December 2015 [16,17]. The current vaccination schedules for both measles and EV71, as well as the absence of a vaccine for CoxA16, permit assessment of how waning maternal antibody protection is affecting rates of infant infection in China.
The effects of waning maternal measles antibodies and very early susceptibility to measles among infants have been well studied in countries where measles has been eliminated. For example, in Belgium, geometric mean titres (GMT) of IgG against measles (enzyme linked immunosorbent assay; ELISA) and proportions of positive samples decreased monotonically from 1593 mIU/ml (181/214) for women at week 36 of pregnancy, to 1369 mIU/ml (152/189) for cord blood, 928 mIU/ml (122/160) for infants at 1 month, 304 mIU/ml (72/158) at 3 months, 79 mIU/ml (11/72) 6 months, and 11 mIU/ml (0/156) at 12 months [5]. Similarly, in Taiwan, a cohort study was conducted to understand the dynamics of maternal EV71 antibodies in infants at 0 and 6 months [18]; however, no study has been specifically designed to reveal the antibody dynamics in infants for CoxA16, which typically produce less severe complications than EV71.
In China, prior studies examining measles, EV71 and CoxA16 antibodies among different infant age-groups mostly employed cross-sectional designs [9,17,19–21], which cannot reveal the ‘true’ evolution of antibody levels. Using a longitudinal study design, we here aim to report how maternally-derived antibodies in infants for measles, EV71 and CoxA16 evolve over time in infants during the first 6 months post-partum prior to vaccination.
2. Methods
Guangzhou is the economic, educational and cultural hub of southern China. The city has a sub-tropical climate, a permanent population of 7.94 million residents, a transient population of 4.67 million, and approximately 60 000 births each year in 2010. With 45 beds and nearly 2500 children delivered per year, the Liwan District Maternal and Child Health Hospital provides medical services for pregnant women living in the area, as well as some women from nearby Southsea county. During July 2013 to April 2014, infants born at the Liwan District Maternal and Child Health Hospital in Guangzhou, southern China were enrolled for this study by convenience sampling. We included healthy pregnant women and their healthy offspring. Cord blood from newborns, and venous blood at 3 months and 6 months of age were collected. Inclusion criteria were age 16–45 years and residence in Guangzhou or the Southsea county in Guangdong province, southern China for the duration of the study. Exclusion criteria were the presence of an immunodepressive condition or an acute infection, or administration of immunoglobulins or blood products during the study period. Maternal demographic and health information including age, education, hypertension, diabetes, anemia, and gestation week before delivery, as well as the corresponding newborn gender, birth weight and Apgar Score after birth, were collected by questionnaire by the study nurses. In China, no specific infectious disease reporting or vaccination records system existed two decades ago. In lieu of these data, we attempted to collect information regarding prior infection and vaccination from study participants; however, most of the participants found it difficult to recall their infection or vaccination history. As a consequence, we did not include this information in our analysis.
Several tests were employed to assess antibody levels in the collected serum samples: (1) the ELISA (Anti-Measles IgG and IgM, Virion/Serion, Germany) [20] was used to quantify measles antibody concentration; and (2) a modified cytopathogenic effect assay [22] was performed to evaluate neutralizing antibody titers against coxA16 (CoxA16; G10 strain, 7.0 lgCCID50/ml) and EV71 (H07 strain, 7.3 lgCCID50/ml [22]. Equivocal assay results were retested once.
Protective antibody cut-off values of 200 mIU/ml for measles were used in this analysis, per prior work [20,23]. WHO Western Pacific Region Regional Reference Measles Laboratory tested a panel of sera and found that the enzyme immunoassay (Vrion/Serion kit) with a cut-off of 200 mIU/ml, when compared to the Plaque Reduction Neutralization Test (PRNT), showed a sensitivity of 94.9% and a specificity of 100% [24]. Consequently, the ELISA provides a conservative measure for elevated measles antibody levels.
Positive cut-off values of 1:8 for CoxA16 and EV71 were also employed [22]. GMT and 95% confidence intervals (CI) were calculated to compare the neutralization antibody levels for EV71 and CoxA16. The occurrence of disease symptoms for measles or HFMD, e.g. rash or fever, were recorded by the study nurse during the 3-month and 6-month collections. If rash or fever were observed at 3 or 6 months of age by the guardians, the infants were immediately referred for clinical management and venous blood was collected and tested for measles-IgM and IgG antibodies and neutralizing antibodies titers against EV71 and CoxA16. Infection for EV71 or CoxA16 virus was defined as a 4-fold increase of neutralization antibody level [25].
We used a linear mixed model (LMM) to describe antibody levels (measles, EV71 and CoxA16) in infants over time while accounting for heterogeneity among and homogeneity within infants [26]. To facilitate analysis of antibody waning rates as a function of initial titre, antibody levels at birth were categorized as high (≥3200 mIU/ml), medium (800–3200 mIU/ml) or low (<800 mIU/ml) for measles [20]; and high (≥1:48) or low (<1:48) for EV71 and CoxA16 antibodies [22]. The possibility that different subjects might have different intercepts defining their antibody waning trajectories, in addition to different slopes (representing the relationship of antibody level with month of age) was considered [26]. Fixed effects associated with antibody level, month of age, and the interaction between antibody level and age were also included.
Data analysis was conducted using SPSS statistical software (Version 23.0, SPSS, Inc.). A repeated measure test (GLM) was employed to assess the trend of antibody levels for measles, EV71 and CoxA16 (GMT) at 0, 3 and 6 months. For all analyses, P values less than 0.05 were regarded as significant. The study protocol was reviewed and approved by the Guangzhou Center for Disease Control and Prevention Ethics Board and written consent was obtained from all guardians (ClinicalTrials.gov Identifier: NCT02219061).
3. Results
Seven hundred and seventeen newborns were enrolled for this study. Of 715 pregnant women, three had twins and 36.4% (260) gave birth by caesarean. 71.2% (509) had not attended college; 64.8% (463) lived in a family with 2 or 3 persons, and 35.2% (252) with ≥4 persons. Fifty-three percent of the newborns (380/717) were male. General characteristics of the enrolled subjects are provided in Table 1, which shows an overlap of the 95% CIs for the baseline characteristics and antibodies levels for all subjects at 0, 3 and 6 months.
Table 1.
Baseline characteristics and antibody levels (mean value) for all subjects of 0, 3, 6 months of age. Changes in maternal age, gestational age, birth weight, and Apgar Score reflect differing sample sizes.
| 0 mo (N = 717) | 3 mo (N = 233) | 6 mo (N = 75) | |
|---|---|---|---|
| Mean (min, max) | |||
| Mother age (years) | 27 (16,45) | 28 (18,42) | 28 (20,42) |
| Gestational age (weeks) | 39 (32,43) | 39 (35,41) | 39 (36,41) |
| Birth weight (kg) | 3.19 (1.38, 4.82) | 3.24(2.10,4.44) | 3.22 (2.35, 4.04) |
| Apgar score 1-min | 9 (6,10) | 9 (7,10) | 9 (8,10) |
| Apgar score 5-min | 10 (8,19) | 9 (8,10) | 10 (10,10) |
| Means with 95% CIs | |||
| Measles (mIU/ml) | 1409.9 (1305.8,1514.5) | 1192.4 (1029.5,1363.9) | 1631.6 (1284.3,1995.1) |
| Enterovirus 71 (1:) | 19.9 (16.6, 24.0) | 19.6 (16.1, 23.9) | 20.5 (14.4, 29.0) |
| Coxsackievirus A16 (1:) | 16.3 (13.9, 19, 1) | 16.6 (14.0, 19.7) | 16.2 (12.3, 21.4) |
Differences in maternal age, gestational age, birth weight, and Apgar score (1 and 5 min) between infants at 0 month and 3 months of age, or between infants at 0 month and 6 months of age were non-significant.
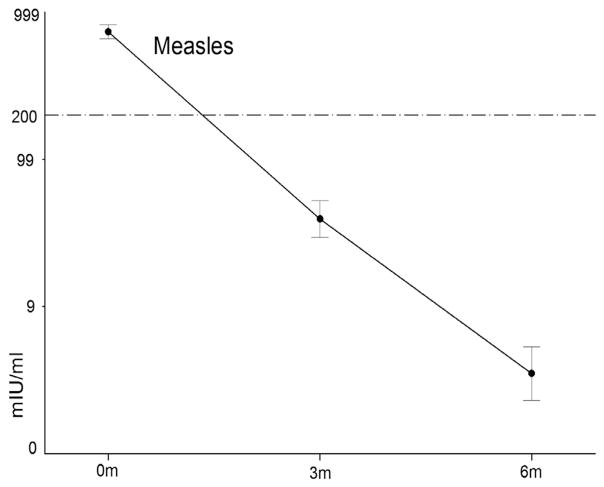
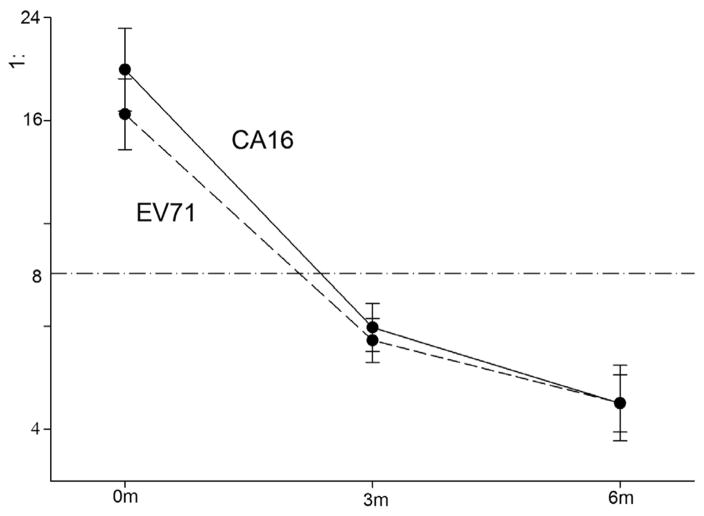
We assessed measles IgG antibody concentration from 717, 233 and 75 sera in infants of 0 month, 3 months and 6 months and 225, 217, and 72 sera for EV71 and CoxA16, respectively. Waning of maternal antibodies for measles, EV71 and CoxA16 was observed during the first 6 months of age. For measles, there was a monotonic decrease of antibody concentration from month 0 (1410 mIU/ml), to month 3 (195 mIU/ml), and month 6 (22 mIU/ml) (Test of Sphericity, p < 0.001; F = 60.759, p < 0.001) (Fig. 1). A similar significant trend was observed for EV71 (1:19.9, 6.3 and 4.5) (F = 54.765, p < 0.001) and CoxA16 (1:16.3, 5.9, and 4.5) (F = 65.578, p < 0.001) (Fig. 2). Based on these assays, 25.3% (95% CI, 20.1–31.2) infants 3 months old and 2.7% (95%CI, 0.6–8.3) 6 months old were measles antibody positive. The percentage of infants antibody seropositive for EV71 and CoxA16, respectively, was 72.2% (95%CI, 66.2–77.8) and 72.7% (95%CI, 66.6–78.2) at 0 months, 33.2% (95%CI, 27.2–39.6) and 30.4% (95%CI, 24.6–36.8) at 3 months, and 6.8% (95%CI, 2.7–14.4) and 5.6% (95%CI, 1.9–12.7) at 6 months. When we restricted the analysis to only those sera obtained from infants at all three time points, similar trends for the three antibodies were observed (Table 2).
Fig. 1.
Profile for the decay in log measles antibodies based all sera at 0, 3 and 6 months. Log (concentration + 1) was computed for measles antibody. The mean and 95% confidence intervals are shown. All infants tested measles-IgM negative and no cases of measles infection were identified. No. of sera: 717, 233, 75 at 0, 3 and 6 months of age.
Fig. 2.
Profiles for the decay in log antibodies based all sera at 0, 3 and 6 months for coxsackievirus 16 and enterovirus 71. Note. The number of sera was 225, 217 and 72 at 0, 3 and 6 months.
Table 2.
Measles, Enterovirus 71 and coxsackievirus 16 antibody GMT at 0, 3 and 6 months of age, based on the paired sera.
| N | Mo 0 | Mo 3 | Mo 6 | |
|---|---|---|---|---|
| Measles | 56 | 1482.6 (1103.8, 1861.3) | 253.4 (152.9, 353.9) | 20.0 (4.7, 35.3) |
| CoxA16 | 51 | 17.4 (12.5, 24.3) | 6.1 (4.8, 7.7) | 4.2 (4.0, 4.4) |
| EV71 | 52 | 19.7 (13.0, 29.7) | 6.4 (5.2, 7.9) | 4.2 (4.0, 4.4) |
LMM findings indicated that infants with higher antibody titers at birth experienced a greater depletion of antibody levels. Specifically, infants with high measles antibody levels (≥3200 mIU/ml) at birth (mean, 4512 mIU/ml) waned by 2552 mIU/ml every 3 months versus 1028 mIU/ml every 3 months for those with medium levels (800–3200 mIU/ml) at birth (mean, 1716 mIU/ml), and 246 mIU/ml for those with low levels (<800 mIU/ml) (mean, 366 mIU/ml at birth) (F = 356.6, p < 0.001). Similarly, for EV71 and CoxA16, infants born with titers ≥ 1:48 waned by 5.7 and 4.8 every 3 months compared to 1.6 and 1.7 for titers < 1:48 at birth (F = 268.2 and 175.5, p < 0.001). Despite these different antibody depletion rates, infants born with higher measles antibody levels maintained greater maternal protection. The average measles antibody loss as a percentage of original concentration was 56.6% (2552/4512), 59.9% (1028/1716), and 67.2% (246/366) for the categories ≥3200, 800–3200 and <800 mIU/ml at birth, respectively. However, the waning of EV71 or CoxA 16 antibody titers were equivalent, regardless of antibody levels at birth (see Tables 3 and 4 ).
Table 3.
Mean measles IgG antibody concentrations (mIU/ml) at 0, 3 and 6 months of age for the 3 initial antibody level categories. 95% confidence intervals are given in parentheses and sample size is in brackets.
| Initial antibody category | Mo 0 | Mo 3 | Mo 6 |
|---|---|---|---|
| ≥3200 (N) | 4512.1 (4190.6, 4833.7) [78] | 905.3 (408.9, 1401.6) [21] | 91.6 (13.7, 169.6) [10] |
| Proportion | 100% | 20.0% | 2.0% |
| 800–3200(N) | 1716.0 (1642.7, 1789.3) [315] | 240.7 (190.2, 291.2) [92] | 15.5 (6.3, 24.7) [39] |
| Proportion | 100% | 14.0% | 0.9% |
| <800(N) | 365.5 (340.6, 390.4) [324] | 35.0 (20.9,49.2) [119] | 4.0 (−4.0,12.2) [26] |
| Proportion | 100% | 9.6% | 1.1% |
| Total | 1409.9 (1305.8,1514.5) | 194.6 (138.5,250.6) | 21.7 (9.5, 33.8) |
The initial antibody level (Mo 0) was taken as 100% and the proportion for measles antibody were shown for Mo 3 and Mo 6.
Table 4.
Enterovirus 71 and coxsackievirus 16 antibody GMT at 0, 3 and 6 months of age for the 2 initial antibody level categories. 95% confidence intervals are given in parentheses and sample size is in brackets.
| Initial antibody category | Mo 0 | Mo 3 | Mo 6 | |
|---|---|---|---|---|
| EV71(N) | ≥1:48 | 115.2 (96.1, 138.1) [71] | 13.3 (10.5, 16.9) [63] | 4.7 (3.9, 5.8) [22] |
| Proportion | 100% | 11.5% | 4.1% | |
| <1:48 | 8.9 (7.9, 10.1) [156] | 4.4 (4.2,4.6) [138] | 4.4 (3.7, 5.3) [47] | |
| Proportion | 100% | 49.4% | 49.4% | |
| CoxA16(N) | ≥1:48 | 83.9 (67.2,104.9) [55] | 14.3 (10.9,18.9) [50] | 4.5 (3.8, 5.4) [15] |
| Proportion | 100% | 17.0% | 5.4% | |
| <1:48 | 9.7 (8.6, 10.8) [172] | 4.5 (4.3,4.8) [151] | 4.5 (3.6,5.7) [53] | |
| Proportion | 100% | 46.4% | 46.4% |
The initial antibody level (Mo 0) was taken as 100% and the proportion for EV71 and CoxA16 antibodies were shown for Mo 3 and Mo 6.
No relevant symptoms (such as rash or fever) for measles or HFMD were identified among study subjects, and all infants were measles-IgM negative. However, one infant’s CoxA16 neutralizing antibody titer increased from 1:4 at month 0 to >1:1024 at month 6; and one infant’s EV71 antibody increased from 1:24 at month 0 and month 3 to 1:256 at month 6, indicating an infection rates of 1.33% (95%CI, 0.1–6.1) for both EV71 and CoxA16.
4. Discussion
This study is among the first to present the evolution of measles and EV71 antibody levels among newborns in China, and is also the first to report the specific dynamics of CoxA16 antibodies. Using pre-vaccination longitudinal data at 0, 3 and 6 months, we find considerable waning of maternal measles IgG antibody (1410, 195, and 22 mIU/ml), and neutralizing antibodies against EV71 (1:19.9, 6.3 and 4.5) and CoxA16 (1:16.3, 5.9, and 4.5). For subjects at 6 months of age, 2.7% (95%CI, 0.6–8.3), 6.8% (95%CI, 2.7–14.4) and 5.6% (95%CI, 1.9–12.7) were antibody positive for measles, EV71 and CoxA16, respectively. Similar trends are observed when the analysis is restricted to infants contributing sera samples at all three times points. LMM findings indicate that infants with higher antibody levels at birth experienced a more substantial depletion of those antibodies.
This study provides solid evidence of the rapid waning of measles antibodies in infants prior to vaccination under the EPI schedule in China. It also confirms the rapid decrease of measles antibody levels suggested by prior cross-sectional studies in China. Compared with our findings of 87.4%, 25.3%, and 2.7% sero-positivity among infants at 0, 3 and 6 months, Xu et al. reported overall sero-positivity of 89.3% (month 0), 22.3% (month 3), and 6.9% (month 6) in three cities in eastern China using the same assay kit; the study also documented sero-positivity at 6 months of 14.9%, 4.1% and 1.6% in Jinan, Ningbo, and Harbin City, respectively [21]. Employing a different ELISA kit, manufactured by the China Center for Disease Control and Prevention, Zhang et al. found a decreased sero-positivity rate in Qinghai, the poorest province in China, of 98.0% (95%CI, 87.4–99.7) at month 0, 91.8% (80.2–96.9) at month 3 and 68% (54.0–79.4) at month 6 in 2009 [21]. In Qinghai province, measles has remained endemic since the establishment of EPI with quadrennial epidemic cycles, and the average incidence of measles during the 1990s was 33 cases per 100,000 [7]. These circumstances may be indicative of a higher prior infection rate, low vaccination coverage, and population groups unvaccinated or partially vaccinated, all of which could contribute to continual measles outbreaks. As a result, more maternal antibodies derived from natural infection and higher infant antibody levels may be induced. Unlike the Qinghai province, most areas in China have experienced low measles incidence records in recent years [8].
The present study found a sero-positivity rate of 2.7% at 6 months of age in Guangzhou, China; however, in a previous cross-sectional study, we found a sero-positivity rate for measles antibody of 48.5% (95%CI, 41.7–55.4) among infants 5–7 months of age in Guangzhou city [9]. The difference between the former and present study is unclear, but may stem from discrepancies in sampling approach, the ELISA kit employed, or the study year.
By using longitudinal data, we were also able to report depletion rates among groups with different measles antibody levels at birth. Infants born with higher titers tend to have more antibody loss than those born with lower titers. The mechanism for this difference is unclear and further study may be needed [4]. On the other hand, although newborns with higher measles antibody levels usually experienced more antibody loss, their titer levels remained higher than infants born with lower antibody levels.
Due to waning maternally-derived measles antibody levels, young infants are at greater risk for measles. Indeed, in a city in southern China, from 2005 to 2014, the proportion of total measles cases among children <1 year increased from 24.3% to 47.9% [27]. In Tianjin, northern China during 2014, 558 measles cases (20.58%) were among infants aged <1 year [28].
As a consequence, strategies for measles vaccination among young infants or women of childbearing age may need to be reconsidered in China. For young infants, it is critical that the optimal age for measles vaccination be identified that balances the risk from maternal antibody loss with the risk of primary vaccine failure due to the presence of maternal antibodies [29,30]. Indeed, it has been shown that measles vaccination at an early age may not be effective, as maternal antibodies in young infants may impede vaccine protection by disrupting vaccine-induced immune responses [31]. Such interference has generally motivated against early measles vaccination. Alternatively, if more infants are born with higher antibody levels, the risk of infection between 0 and 8 months might be reduced. High maternal antibody levels in pregnant women are vital for efficiently transferring these antibodies to the newborn [32].
For this study, we did not have the opportunity to measure measles antibody levels at 8 months of age and determine explicitly the possible benefit of vaccination 2 months earlier at 6 months of age. We obtained the sera from infants ≤6 months of age in the study hospital where parents take their infants for regular medical examination; however, children >6 months age seldom go to the hospital, which thus precluded explicit assessment of the additional antibody level loss from 6 months to 8 months. Future measurement of this difference would more definitively determine the optimal age for initial measles vaccination.
This longitudinal study reveals how EV71 antibody levels wane in infants during the first half year of life. In Taiwan, Luo et al. reported that maternal EV71 neutralizing antibodies declined from 50% in neonates to undetectable levels in 99% of children at 6-months [18]. A retrospective study conducted in Jiangsu Province in eastern China showed that antibody seropositive rates against EV71 among infants aged 2 and 7 months were 57.6% (95%CI, 54.5–60.8) and 39.5% (95%CI, 36.4–42.6), respectively [22]. The rates from this latter study are higher than what we report here. Further, in the first 6 months post-partum, the HFMD incidence rate of 0.72% (7/975) from EV71 in Jiangsu study was lower than our findings (1.33%), albeit with a smaller sample size. These differences could stem from different infection rates among the populations in southern and eastern China [33].
The immunogenicity of the EV71 vaccine (strain H07, subgenotype C4; 400 U of EV71 antigen with alum adjuvant) administered at 6 months has been noted in randomized trials in China [16,34]. Although the incidence for EV71 infection was very low in infants ≤6 months of age, the case-fatality risk, case-severity risk, and severity-fatality risk have been reported much higher than for other age groups [6]. Given the decline of maternal neutralizing antibodies, this date of immunization appears to be too late, at least for the Guangzhou population, to provide protection to infants ≤6 months who more commonly incur severe outcomes from EV71 infection [6].
Our findings show that 72.7% of newborns, 30.4% of infants at 3 months, and 5.6% of infants at 6 months are protected from CoxA16 infection. These levels are lower than reported previously in the Jiangsu retrospective study (41.3% and 26.4% at months 2 and 7, respectively) [22]. Although CoxA16 infection usually causes mild symptoms, severe and fatal HFMD cases due to CoxA16 infection have been reported in the United States, France, and Japan [35–37]. In Shenyang, northeast China, 20.7% (19/92) of HFMD cases presenting with neurological symptoms were due to CoxA16 infection, with 2 patients presenting with brainstem encephalitis and one with acute flaccid paralysis[38]. Additionally, studies indicate that co-infection with CoxA16 and EV71 viruses might increase the possibility of genetic recombination between the two viruses [39–41]. Given the dramatic waning of maternal immunity and possible severe clinical outcomes from CoxA16 infection[42], it is advised that development of human CoxA16 vaccine (monovalent, or bivalent EV71 and CoxA16) should be a priority.
There are several limitations in this study. The first is the high drop out rate – i.e, 85% of subjects did not provide a sample at the third point. However, the general demography and initial antibody levels among subjects providing 3 samples are comparable to the overall cohort at month 0. The second possible limitation is that the subjects from the one hospital we used for sampling may not be representative of the overall population. However, some of subject characteristics, such as women’s education level (71.2% who had not attended college) or gender ratio of newborns (53% were male) in the sample are similar to the general demographics in Guangzhou (76.3% and 51.2% respectively) [43]. Finally, we failed to collect information on measles infection or vaccination from the pregnant mothers, and thus we cannot compare the antibody levels and wane rates between these two groups. These limitations suggest that additional investigations are needed before applying these findings broadly in China to inform vaccination policy.
In sum, our findings demonstrate a waning of maternally-derived antibodies in infants prior to vaccination for measles, EV71 and CoxA16. Further modifications of vaccination strategies for measles, earlier vaccination for EV71 infection, and development and provision of a CoxA16 vaccine should be investigated and considered in the future.
Acknowledgments
We appreciate the participation of the children in this study. Study nurses are also appreciated for their work in collecting the serums.
This publication was made possible by the research grants from the Guangzhou Science Technology and Innovation Commission (201707010204), and the Project for Key Medicine Discipline Construction of Guangzhou Municipality (2017-2019-07).
Footnotes
Author contributions
C.F. and M.W. designed the study; L.L. collected the serum samples; Y.L. and Y.C. did the experiments; Q.G., S.P. and Z.Y. built the dataset; C.F. and J.S. analyzed the data and C.F., Q.G. and J.S. wrote the manuscript text.
Competing interests
JS discloses partial ownership of SK Analytics.
References
- 1.Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–5. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
- 2.Mollema L, Smits GP, Berbers GA, Van Der Klis FR, Van Binnendijk RS, De Melker HE, et al. High risk of a large measles outbreak despite 30 years of measles vaccination in The Netherlands. Epidemiol Infect. 2014;142:1100–8. doi: 10.1017/S0950268813001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Dagan R, Slater PE, Duvdevani P, Golubev N, Mendelson E. Decay of maternally derived measles antibody in a highly vaccinated population in southern Israel. Pediatr Infect Dis J. 1995;14:965–9. doi: 10.1097/00006454-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 6.Xing WJ, Liao QH, Viboud C, Zhang J, Sun JL, Wu JT, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14:308–18. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lixia W, Guang Z, Lee LA, Zhiwei Y, Jingjin Y, Jun Z, et al. Progress in accelerated measles control in the People’s Republic of China, 1991–2000. J Infect Dis. 2003;187(Suppl 1):S252–7. doi: 10.1086/368045. [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Hao L, Zhang Y, Su Q, Rodewald L, An Z, et al. Monitoring progress towards the elimination of measles in China: an analysis of measles surveillance data. Bull World Health Organ. 2014;92:340–7. doi: 10.2471/BLT.13.130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu CX, Xu JX, Liu WB, Zhang W, Wang M, Nie J, et al. Low measles seropositivity rate among children and young adults: A sero-epidemiological study in southern China in 2008. Vaccine. 2010;28:8219–23. doi: 10.1016/j.vaccine.2010.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Lennon JL, Black FL. Maternally derived measles immunity in era of vaccine-protected mothers. J Pediatr. 1986;108:671–6. doi: 10.1016/s0022-3476(86)81039-3. [DOI] [PubMed] [Google Scholar]
- 11.Chui LW, Marusyk RG, Pabst HF. Measles virus specific antibody in infants in a highly vaccinated society. J Med Virol. 1991;33:199–204. doi: 10.1002/jmv.1890330311. [DOI] [PubMed] [Google Scholar]
- 12.Pabst HF, Spady DW, Marusyk RG, Carson MM, Chui LW, Joffres MR, et al. Reduced measles immunity in infants in a well-vaccinated population. Pediatr Infect Dis J. 1992;11:525–9. doi: 10.1097/00006454-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Chen KT, Chang HL, Wang ST, Cheng YT, Yang JY. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998–2005. Pediatrics. 2007;120:e244–52. doi: 10.1542/peds.2006-3331. [DOI] [PubMed] [Google Scholar]
- 14.Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, Lin KH, et al. Reemergence of enterovirus 71 in 2008 in taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol. 2009;47:3653–62. doi: 10.1128/JCM.00630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Li JX, Jin PF, Wang YX, Zhu FC. Enterovirus 71: a whole virion inactivated enterovirus 71 vaccine. Exp Rev Vaccines. 2016;15:803–13. doi: 10.1080/14760584.2016.1191357. [DOI] [PubMed] [Google Scholar]
- 16.Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–28. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 17.Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, et al. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Exp Rev Vaccines. 2016;15:129–37. doi: 10.1586/14760584.2016.1096782. [DOI] [PubMed] [Google Scholar]
- 18.Luo ST, Chiang PS, Chao AS, Liou GY, Lin RY, Lin TY, et al. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerg Infect Dis. 2009;15:581–4. doi: 10.3201/1504.081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Lu L, Chen M, Huang F, Zeng Y, Li XM, et al. Analysis of measles immunity level in persistent populations in Beijing, 2012. Zhonghua Yu Fang Yi Xue Za Zhi. 2013;47:916–9. [PubMed] [Google Scholar]
- 20.Xu GZ, Ma R, Xu HJ, Ma YH, Dong HJ, Li Y, et al. Levels of transition on maternal transferred measles antibody in infants in 3 cities in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:1074–7. [PubMed] [Google Scholar]
- 21.Zhang X, Shirayama Y, Zhang Y, Ba W, Ikeda N, Mori R, et al. Duration of maternally derived antibody against measles: a seroepidemiological study of infants aged under 8 months in Qinghai, China. Vaccine. 2012;30:752–7. doi: 10.1016/j.vaccine.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 22.Zhu FC, Liang ZL, Meng FY, Zeng Y, Mao QY, Chu K, et al. Retrospective Study of the Incidence of HFMD and Seroepidemiology of Antibodies against EV71 and CoxA16 in Prenatal Women and Their Infants. Plos One. 2012:7. doi: 10.1371/journal.pone.0037206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, Li F, Zheng X, Zhang H, Duan M, Yang Y, et al. Measles vaccine coverage estimates in an outbreak three years after the nation-wide campaign in China: implications for measles elimination, 2013. BMC Infect Dis. 2015;15:23. doi: 10.1186/s12879-015-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao NY, Zhu Z, Jiang XH. Comparison and evaluation of enzyme-linked immunization assay kits with plague reduction neutralization test for detection of measles IgG antibody. Zhongguo Yi Miao He Mian Yi. 2009;15:215–8. [PubMed] [Google Scholar]
- 25.China Minister of Health. National guideline for Hand-foot-mouth disease diagnose and treatment. 2010. [Google Scholar]
- 26.West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval Health Prof. 2009;32:207–28. doi: 10.1177/0163278709338554. [DOI] [PubMed] [Google Scholar]
- 27.Han K, Chen S, Tang C, Wen J, Li J, Ni J, et al. The epidemiological and serological characteristics of measles in Dongguan, China, 2005–2014. Hum Vaccin Immunother. 2016:1–7. doi: 10.1080/21645515.2016.1159364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Boulton ML, Montgomery JP, Carlson B, Zhang Y, Gillespie B, et al. The epidemiology of measles in Tianjin, China, 2005–2014. Vaccine. 2015;33:6186–91. doi: 10.1016/j.vaccine.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gagneur A, Pinquier D, Aubert M, Balu L, Brissaud O, De Pontual L, et al. Kinetics of decline of maternal measles virus-neutralizing antibodies in sera of infants in France in 2006. Clin Vaccine Immunol. 2008;15:1845–50. doi: 10.1128/CVI.00229-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19:635–41. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Kumar ML, Johnson CE, Chui LW, Whitwell JK, Staehle B, Nalin D. Immune response to measles vaccine in 6-month-old infants of measles seronegative mothers. Vaccine. 1998;16:2047–51. doi: 10.1016/s0264-410x(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 32.Caceres VM, Strebel PM, Sutter RW. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin Infect Dis. 2000;31:110–9. doi: 10.1086/313926. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S, Liao Q, Van Boeckel TP, Xing W, Sun J, Hsiao VY, et al. Hand, foot, and mouth disease in China: modeling epidemic dynamics of enterovirus serotypes and implications for vaccination. PLoS Med. 2016;13:e1001958. doi: 10.1371/journal.pmed.1001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381:2024–32. doi: 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 35.Wright HT, Jr, Landing BH, Lennette EH, Mc AR. Fatal infection in an infant associated with Coxsackie virus group A, type 16. N Engl J Med. 1963;268:1041–4. doi: 10.1056/NEJM196305092681904. [DOI] [PubMed] [Google Scholar]
- 36.Legay F, Leveque N, Gacouin A, Tattevin P, Bouet J, Thomas R, et al. Fatal coxsackievirus A-16 pneumonitis in adult. Emerg Infect Dis. 2007;13:1084–6. doi: 10.3201/eid1307.070295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto K, Sanefuji M, Kusuhara K, Nishimura Y, Shimizu H, Kira R, et al. Rhombencephalitis and coxsackievirus A16. Emerg Infect Dis. 2009;15:1689–91. doi: 10.3201/eid1510.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W, Liu CF, Yan L, Li JJ, Wang LJ, Qi Y, et al. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol J. 2012;9:8. doi: 10.1186/1743-422X-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan H, Zhu YF, Qi X, Zhang YJ, Li L, Deng F, et al. Analysis on the epidemiological and genetic characteristics of enterovirus type 71 and Coxsackie A16 virus infection in Jiangsu, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:339–43. [PubMed] [Google Scholar]
- 40.Yip CC, Lau SK, Zhou B, Zhang MX, Tsoi HW, Chan KH, et al. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71. Arch Virol. 2010;155:1413–24. doi: 10.1007/s00705-010-0722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao Q, Wang Y, Yao X, Bian L, Wu X, Xu M, et al. Coxsackievirus A16: epidemiology, diagnosis, and vaccine. Hum Vaccin Immunother. 2014;10:360–7. doi: 10.4161/hv.27087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guangzhou Beurea of Statistics. 2016 http://www.gzstats.gov.cn/tjdt/201607/t20160704_24446.html.