Abstract
Women are disproportionately affected by obesity, and obesity increases women’s risk of developing dementia more so than men. Remarkably little is known about how females make decisions about when and how much to eat. Research in animal models with males supports a framework in which previous experiences with external food cues and internal physiological energy states, and the ability to retrieve memories of the consequences of eating, determines subsequent food intake. Additional evidence indicates that consumption of a high-fat, high-sugar diet interferes with hippocampal-dependent mnemonic processes that operate to suppress eating, such as in situations of satiety. Recent findings also indicate that weakening this form of hippocampal-dependent inhibitory control may also extend to other forms of learning and memory, perpetuating a vicious cycle of increased Western diet intake, hippocampal dysfunction, and further impairments in the suppression of appetitive behavior that may ultimately disrupt other types of memorial interference resolution. How these basic learning and memory processes operate in females to guide food intake has received little attention. Ovarian hormones appear to protect females from obesity and metabolic impairments, as well as modulate learning and memory processes, but little is known about how these hormones modulate learned appetitive behavior. Even less is known about how a sex-specific environmental factor – widespread hormonal contraceptive use – affects associative learning and the regulation of food intake. Extending learned models of food intake to females will require considerably investigation at many levels (e.g., reproductive status, hormonal compound, parity). This work could yield critical insights into the etiology of obesity, and its concomitant cognitive impairment, for both sexes.
Keywords: sex differences, learning, energy regulation, obesity, estrogen
1. Introduction
The regulation of food intake and body weight depends critically on the ability of the brain to detect, monitor, and integrate metabolic, hormonal, and neural signals from the periphery that provide information about the body’s energy needs and the status of its energy stores [147,151]. In addition, it is now widely recognized that the decision to eat or refrain from eating also depends on information about the availability of food, the type of food that is available (e.g., is it low fat, gluten-free, Kosher), the effort needed to acquire it, and knowledge about the likely consequences of eating (e.g., will it satisfy me, will it make me fat). The information comes from our past experiences with food and eating, our evaluations of those experiences, and our expectancies about the likely outcomes of food-seeking (i.e., appetitive) and eating behaviors [62,144]. In addition, we can attempt to suppress appetitive and eating behaviors, even when the urge to eat is strong, by actively inhibiting thoughts [33,54,111] or by avoiding or shifting our attention away from cues in the environment that remind us about food and the pleasures of eating [63]. In other words, in addition to metabolic and hormonal mechanisms, energy regulation depends on the operation of cognitive processes involved in remembering and retrieving past experiences with food and eating, with the development of expectations about the likely outcomes eating and appetitive behaviors, and on the ability to control and inhibit those behaviors.
Moreover, disorders of both energy regulation and cognitive functioning appear to be intertwined. Much evidence from human and nonhuman animal models has accumulated indicating that intake of obesity-promoting diets that are high in saturated fats and sugar (i.e., Western diet) can lead to learning and memory impairments and signs of pathophysiology in brain substrates underlying cognition [5,10,45]. Conversely, a number of findings suggest that excess energy intake and weight gain may be a consequence of interference with the cognitive controls of eating (for review see Yeomans [148]). This pattern of findings is consistent with what has been termed a vicious-cycle of obesity and cognitive decline [33, 58]. According to this hypothesis based on rat models, eating a Western diet high in saturated fats and sugars gives rise to disturbances in learning and memory processes that contribute to the inhibitory control of eating. A consequence of this reduced inhibitory control is increasing intake (i.e., overeating) of Western diet and further deterioration of inhibitory cognitive functioning. The hippocampus, a brain structure long implicated as a crucial substrate for learning and memory (e.g., Squire [128]), has received increasing research attention for its role in the control of eating and appetitive behavior [73,130,132]. However, this work has largely been conducted with male rodents. The role of the hippocampus and learning and memory processes in the control of energy intake and body weight in females has received little attention.
There are many reasons why it is important to fill this gap in knowledge. Women have a greater incidence of obesity [103], and are at greater risk for developing Alzheimer’s disease and other forms of dementia, two disorders that are known to harm the hippocampus. Recent reviews have detailed links among sex, the developmentof Alzheimer’s disease [84], and obesity [101]. Furthermore, estrogens are potent regulators of food intake, metabolic homeostasis, and adipose tissue distribution ([105]; also see Clegg et al., this issue). Animal models are clear that estrogens have anorexigenic and anti-obesogenic actions. Yet, despite the protection that estradiol should be affording, premenopausal women are as susceptible to obesity as men [103]. In addition, the cluster of risk factors that define the metabolic syndrome (i.e., abdominal obesity, hypertension, elevated fasting plasma glucose, high serum triglycerides, low high-density lipoprotein (HDL) levels) has reportedly increased most in young women in the 20–39 age range (NHANES from 1988–1994 to 1999–2006; [161]). Another sex-specific variable that has largely been neglected are the short-and longer-term effects of hormonal contraceptives on female energy regulation and cognitive functioning. It is possible that these contraceptives, which the majority of women in the United States use or have used [21], may diminish the protections normally afforded to the brain by estrogens.
The purpose of this paper is to consider the influence of sex on the cognitive controls of energy regulation. This review will be guided by our previously developed model of the learned controls of intake. We will begin by summarizing the associative relationships described by the model. We then examine what is known about sex differences in the learning and memory processes relevant to the model and in the brain substrates of those processes with emphasis on the hippocampus. The paper will conclude with a discussion of how differences in sex hormones may impact both body weight regulation and cognitive functioning. As part of this discussion, we will present a case that research on energy regulation, cognitive functioning, and their interrelationships should consider the effects of widely-used hormonal contraceptives to increase the generality of their findings with respect to human females.
2. An integrative model of the physiological and cognitive controls of energy intake
Food-related environmental cues gain the power to evoke appetitive behaviors that anticipate the occurrence of rewarding postingestive outcomes [70]. This anticipatory response evocation can be accomplished to the extent that such environmental stimuli excite or retrieve the memories of their associated rewarding postingestive outcomes [16]. The stronger is the excitement of those memories, the greater the strength of the appetitive response. However, it has been increasingly recognized that the strength of memory retrieval is also subject to inhibitory learning processes that antagonize or weaken the ability of environmental cues to excite reward memories. This type of inhibitory learning occurs when the memory of a reward is retrieved but the actual reward does not occur [139]. For example, with respect to eating and appetitive behavior, environmental food cues are typically followed by rewarding postingestive stimulation at the outset of a meal, whereas those same cues may be followed by nonrewarding or even aversive postingestive consequences if eating continues after the need for food has been met. Based on longstanding principles of Pavlovian conditioning, inhibitory associations are formed when environmental cues retrieve the memory of postingestive rewards under conditions in which those rewarding postingestive outcomes are not forthcoming [113]. As a result the ability of an environmental cue to excite the memory of rewarding postingestive stimulation will be countered to the extent that those cues are embedded concurrently in inhibitory associations that antagonize the excitement of reward memories (Bouton [14]).
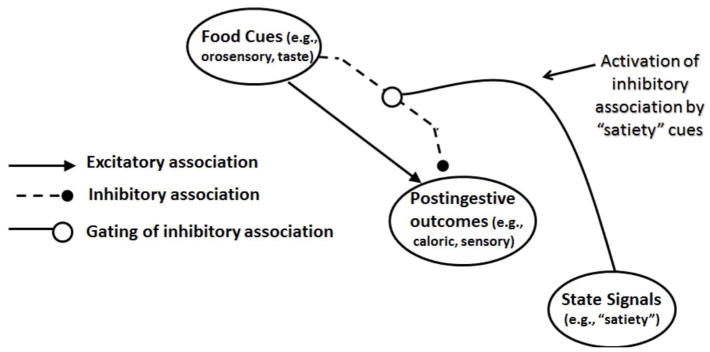
Within this framework, the decision to eat or refrain from eating is determined by the degree to which the inhibitory association can block or weaken the retrieval of reward memories. When the inhibitory association is strongly activated, the ability of food cues to excite reward memories will be reduced and feeding behavior will be suppressed. When the inhibitory association is weak, food cues will more strongly excite the memories of rewarding postingestive stimulation which will, in turn, evoke appetitive and eating behavior more strongly. From this perspective, a key question is what determines the degree to which the inhibitory association is activated. We have proposed previously that interoceptive physiological satiety states suppress appetitive and eating behavior by signaling that food cues will not be followed postingestive reward. In other words, this energy state information activates the inhibitory association to suppress feeding [35] (see Fig. 1).
Figure 1.
Mechanisms underlying the learned control of intake by external food cues, memories of postingestive outcomes, and energy state signals. Adapted from Davidson, Sample, & Swithers, 2014; Davidson, Tracy, Schier, & Swithers, 2014.
According to this model, three fundamental learning and memory processes are involved with the regulation of energy intake: (1) the formation of excitatory associations between environmental food cues and rewarding postingestive outcomes; (2) the formation of inhibitory associations between environmental food cues and rewarding postingestive outcomes; (3) the modulation of the strength of the inhibitory associations by satiety signals. The next section of this paper will briefly review relevant findings about sex differences in each of these processes as a means of understanding differences in energy and body weight regulation.
3. Sex differences in learning and memory
3.1. Simple cue-reward excitatory learning
In simple learning situations, an excitatory association is formed between two events when one event (a conditioned stimulus (CS) or a response) predicts the occurrence of another event (an unconditioned stimulus (US)). A typical demonstration of simple Pavlovian learning in the laboratory might involve training rodents with a brief auditory or visual CS which signals the subsequent availability of a biologically relevant US such as food or drugs. As a consequence of exposure to this predictive relationship, the CS comes to elicit a behavior change or conditioned response (e.g., salivation, approaching the place where food is delivered) in anticipation of the presentation of the US. This type of behavior change is one example of simple cue-reward learning (see [113]). Evidence from rodent models indicates that the excitatory CS→US associations formed during simple-cue-reward learning may be stronger and more persistent in females than males. This phenomenon is most established with research on drugs of abuse, in which female rodents learn to self-administer psychostimulants more rapidly than males [88]. While less work has been conducted with food cues, female rats have been reported to learn that a CS signals an appetitive US more rapidly than males [55,109]. Sex differences in performance have also been observed in another simple form of learning, termed extinction. In extinction, the presentation of the CS is no longer followed by the US. The result appears to be learning of a new, inhibitory association, which reduces the ability of the CS to elicit the previously learned response (e.g., [153]). Females have been reported to extinguish conditioned responding to cues for shock (i.e., conditioned fear; [159]) and drugs (e.g., [157]) more slowly than males. It is unclear whether this slower “extinction” of responding occurs due to weaker inhibition, differences in the strength of the original excitatory learning, or performance factors related to the particular unconditioned stimulus (e.g., greater psychomotor sensitization to amphetamines by females [8]. In addition to differences in the speed and magnitude of discrimination, the way in which females associate cues with reward (i.e., associative structure) may differ from males. One response tendency is “sign-tracking”, in which the animal engages with (e.g., manipulates, licks) the signal for reward (CS) more than the location of the reward (US). A reward-processing framework proposes that this sign-tracking behavior results from rats ascribing the CS with “incentive salience”, or assigns the CS value beyond that of predicting the occurrence of the US [115].
Characterizing these response profiles, and how they differ by sex, may help in predicting later behavioral outcomes and targeting more effective behavioral intervention therapies. The propensity to sign-track when associations are formed during training has been found to predict poorer extinction outcomes and resistance to some forms of outcome devaluation [99]. Outcome devaluation typically diminishes responding to the CS by pairing the US with an aversive (e.g., illness-inducing) stimulus or through unlimited access to reach US-specific satiety. Examining the influence of sex on updating predictions about reward, Hammerslag and Gulley [55] found that female rats were less sensitive to reward devaluation through reinforcer-specific satiety of liquid sucrose compared to males. They suggest that females exhibit greater stimulus-directed behavior to exogenous environmental cues, as opposed to “goal-directed” behavior based on endogenous information, compared to males. According to work with male rats, this impaired ability to use interoceptive information (e.g., satiety signals; illness) to update predictions about CS associated with reward is considered a component of what has been termed an addiction-like behavioral profile, in which the animal persists in responding despite the loss of reinforcement [43,116]. Collectively, these findings suggest the possibility that a female bias towards food-and drug-related behavioral excess might involve differences in the ways in which they associate cues with rewarding postingestive outcomes.
3.2. Sex differences in inhibiting conditioned responses and role of context modulation of retrieval
The recall of previous experiences to determine that reward is not forthcoming is important for the regulation of food intake. This ability to form and retrieve inhibitory associations, like those that antagonize conditioned appetitive responding to food cues in our model, is another component process by which females’ learned control of food intake might differ from males. When a previously conditioned food cue (CS +) is presented in the absence of reward (CS−), this second-learned inhibitory association is particularly sensitive to contextual changes [15]. In other words, alterations in the temporal, environmental, or interoceptive stimulus context in which the inhibitory association was formed weakens its capacity to oppose excitatory conditioned responding. This contextual dependence of inhibitory learning corresponds to the associative structure outlined in our model of energy regulation, in which satiety states contextualize the absence of reward to previously rewarded food cues.
The crucial role of context in guiding behavior is demonstrated by the renewal phenomenon [17], in which conditioned responding is reinstated when the extinction context (B) is replaced by return to the original conditioning context (A; in ABA designs) or with a new context (C; in ABC designs). Previous studies have reported sex differences mediated by gonadal hormones in the ability to use contexts to retrieve inhibitory fear memories [26, 36]. However, barely any work has examined the contextual dependence of retrieving inhibitory associations with food, which signal the absence of forthcoming reward to a previously reinforced cue or response. In one such study, Todd et al. [134] trained sated female rats to lever press for food in a distinctive exteroceptive context (A), and extinguished responding in a different context (B). When returned to the original conditioning context (A), females showed renewal of food-seeking behavior compared to the extinction context (i.e., ABA renewal). These findings indicate that the inhibition of previously learned appetitive responses in females is context-specific. In a Pavlovian preparation directly examining sex as a variable, Anderson and Petrovich [2] found sex effects in the contextual renewal of conditioned responding to a discrete food cue under food deprivation conditions. Estradiol-replaced females showed renewal of food cue responding, similarly to males, while intact (estrous cycle stage unspecified) and OVX females did not show renewal to the original learning context. Differences across these studies with respect to inclusion of males, deprivation conditions, and Pavlovian versus instrumental conditioning make interpretation difficult, but the proposition that estradiol is involved in the contextual recall of food-related memories and withholding responding for food warrants further study. More research is needed to understand the influence of sex and sex hormones on the ability to use contexts to disambiguate the predicted outcomes of food-related cues in the environment.
3.3. Deprivation state contexts
Interoceptive energy states produced by food deprivation or satiation are widely recognized as contexts (e.g., [66,137]). In a similar manner to exteroceptive contexts, interoceptive hunger or satiety states can serve as contexts that determine the outcome of the competition between excitatory and inhibitory associations of food cues with their postingestive consequences. Work from our lab established that varying intensities of food deprivation could enter into associations like any other conditioned stimulus or context (e.g., [155]). According to this view, the ability to withhold responding to previously rewarded food cues depends on the utilization of contextual cues produced by food satiety [35,58]. In other words, satiety states function as contexts to facilitate the retrieval of the inhibitory food cue →reward memory association to counter excitatory response evocation by these food cues.
However, this work has predominantly used only male rodents. Differences in the capacity of deprivation state contextual cues to facilitate memory inhibition and antagonize excitatory responding to food cues might be expected to contribute to a sex bias in overeating. To begin an investigation of these associative processes in females, we [118] assessed sex differences in the ability to use varying levels of food deprivation alone or in conjunction with external food cues to predict appetitive outcomes. Male and free-cycling female rats received training on a compound deprivation discrimination paradigm, in which both 24 h food deprivation (i.e., rats had no access to food for the preceding 24 h to produce a low metabolic fuel state) and an external visual or auditory cue inside the conditioning chamber (e.g., tone) preceded the delivery of sucrose into the food cup, whereas 0 h deprivation (i.e., 24+ hours of ad lib food to produce satiety) and an alternate external cue (e.g., white noise) did not signal reward (see [118] for detailed methods). As we have shown previously with males, females approached the food cup, presumably in anticipation of sucrose reward delivery, according to their previous deprivation state training experience. This discriminative appetitive responding to the level of food deprivation occurred both alongside competing external food cues and following the removal of external cues from the conditioning apparatus. An additional group for which external cues predicted sucrose reward but deprivation states varied noncontingently (i.e., random) with reward established that the pattern of appetitive responding (i.e., food cup approach) was based on associative learning rather than motivational properties associated with food deprivation.
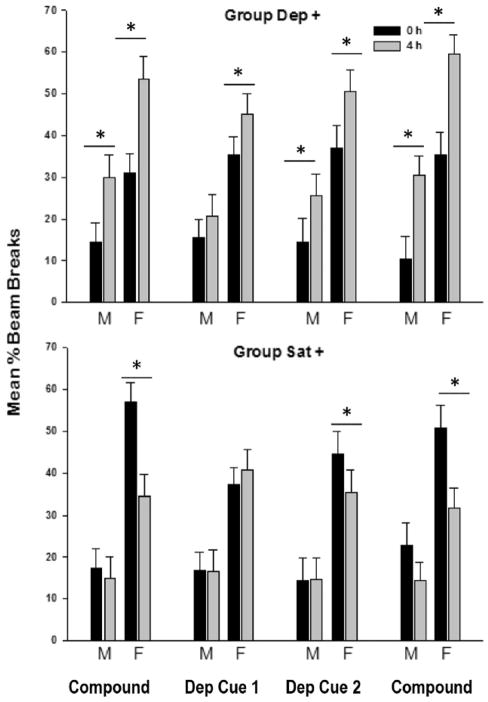
To extend the ecological validity of these findings and further parallel previous work in male rats, an additional experiment trained lower level 4 h food deprivation states that approximate those encountered under free-feeding conditions in compound with external cues. In addition to the contingency rewarded under food deprivation (Group Dep+), another group of rats of each sex was trained to associate satiation (i.e., 24 h free access to food) with reward delivery and 4 h food deprivation with no reward (Group Sat+). Following training with the compound deprivation state and external cues (e.g., sucrose dispensed to 4 h deprived rat after a tone plays in the conditioning chamber), subsequent removal of the external cues from the conditioning chamber revealed better performance by females in discriminating between 0 and 4 h deprivation states (see Dep Cue Test 1 and 2 in Fig. 2). Females in the deprivation rewarded contingency (Group Dep+) learned about deprivation states more readily than males, but this difference did not persist with additional training (Dep Cue 2). In the opposite contingency, in which rats received sucrose under the satiated deprivation level (Group Sat+), females outperformed male rats, who never significantly discriminated between 4 h deprivation and satiated states in order to predict the occurrence of sucrose reward. Corresponding to previous work in males [119,120], these findings provided the first evidence in females that interoceptive deprivation states can gain stimulus control over appetitive responding, in the presence or absence of external cues. In other words, this work suggests females form and use associations between their energy states and appetitive reinforcement. The female rats used for this set of experiments were free cycling, and we did not attempt to synchronize estrous cycle stage with multi-week behavioral training and testing. The modest female benefit revealed with the lower intensity deprivation discrimination introduces the possibility that intact females may be better at forming associative relationships with interoceptive energy contexts, particularly satiety states, than males. While extending the validity of our model of food intake regulation to free cycling females, this work raises questions about potential sex specific vulnerabilities of these associative mechanisms to environmental perturbations.
Fig. 2.
Performance on the low-level deprivation discrimination as measured by the mean ± S.E.M. percent magazine entries for each learning period for Dep+ (top panel) and Sat + (lower panel) males (M) and females (F). Experimental learning periods are as follows: terminal compound cue acquisition, deprivation cues alone Test 1 and 2 (Dep Cue 1 and Dep Cue 2), and reinstatement of the deprivation state/external cue compound; *indicates p < 0.05 for post hoc pairwise comparisons. This figure was adapted from [118].
4. Hippocampal contributions to learned control of energy intake
4.1. Studies with male rats
As referenced earlier, the hippocampus is receiving increasing attention as a critical substrate in the mnemonic processes involved in regulating energy intake. For example, as illustrated in the model above, the control of appetitive behavior depends on the ability to predict the likely consequences of intake. This requires animals to resolve the conflict produced by the existence of conflicting inhibitory and excitatory associations between food cues and the rewarding and nonrewarding postingestive outcomes that compete to control behavior. Recent research shows that the ability to inhibit one of two or more competing or conflicting response tendencies depends on the hippocampus. For example, male rats with hippocampal lesions are less able to refrain from making a previously rewarded approach response in the presence of stimuli that signal aversive or nonrewarded outcomes [67,124]. Additional evidence indicates that the ability of rats to learn not to make a previously rewarded response is impaired following hippocampal lesions [141]. Consistent with our model, White and Naeem interpreted this finding by suggesting that the association underlying learning not to respond competes with excitatory associations that promote conditioned appetitive responding. Evidence from rat models of learning are clear that hippocampal damage increases conditioned responding by interfering with the utilization of the inhibitory association to suppress responding.
There is strong evidence that the hippocampus is an important substrate for the inhibitory control of feeding behavior. Male rats with selective neurotoxic hippocampal lesions initiate a greater number of eating episodes [25], make more frequent contacts with food in the home cage, and show increased instrumental responding for sucrose rewards [29,79,123]. Direct evidence for hippocampal involvement in the inhibition of feeding behavior was also provided by Parent and colleagues, who reported that temporary inactivation of either dorsal [60] or ventral hippocampus [56] reduced the duration of time between meals for male rats. There are similar findings from human amnesic patients with hippocampal damage such as Henry G. Moliason (better known as HM), who underwent a bilateral medial temporal lobectomy to treat his epileptic seizures. Not only could HM not recall previous eating episodes, but he also failed to rate his internal state as satiated even after consuming multiple consecutive meals in a single session [59]. This case study is consistent with our associative model, as satiety signals would no longer modulate the inhibitory association between food cues and their postingestive consequences. In other words, the memory of the rewarding postingestive outcomes of food would no longer be suppressed in situations where those outcomes were not forthcoming.
Other studies with male rats showed that lesions confined to the hippocampus impaired the ability to use cues produced by different levels of food deprivation as discriminative signals for shock [31, 65] or sucrose rewards [32]. As noted above, different deprivation states can be seen as contexts that serve to retrieve or inhibit retrieval of associations between external cues and the US. More direct evidence for such a hippocampal-dependent contextual retrieval function was provided by [80], who showed that without the hippocampus rats were unable to use hunger and thirst cues to retrieve the memory of the locations of food and water (also see Hirsh et al. [64]). Furthermore, following recovery from surgery, rats with hippocampal lesions gain more weight than sham-lesioned controls [29,119].
4.2. Sex differences in the hippocampal-dependent learned control of intake
Although a number of studies have investigated the effects of sex differences in hippocampal-dependent learning tasks (for review, see [82]), few have focused explicitly on the control of eating and appetitive behavior. For example, males typically outperform females in hippocampal-dependent spatial tasks, though findings are transient and inconsistent depending on task parameters. Males tend to exhibit shorter latencies to learn the location of the hidden platform in the Morris water maze [107]; however, females show greater thigmotaxis, swimming along the walls of the pool, and pre-training to the apparatus attenuates or abolishes females’ performance decrement [71, 107]. There is also evidence for sex differences in hippocampal-dependent nonspatial learning and memory problems. For example, in studies of fear conditioning, the ability of a discrete stimulus that has been associated with a shock to elicit fear responses depends on the context in which that cue is presented. This type of contextual control of the cue → shock association is hippocampal-dependent [68, 91]. Previous studies have reported sex differences in contextual fear conditioning with males exhibiting an advantage relative to females. Corresponding to these sex differences in the expression of contextual fear, researchers have identified differences in molecular mechanisms critical for learning and memory formation, including long-term potentiation [90] and activation of the extracellular signal-related kinase (ERK) pathway [51].
Other investigations have found differences in learning strategy as a function of circulating ovarian hormones. Indeed, the degree to which females attend to various environmental cues to guide responding can depend on circulating levels of estradiol over the 4-day estrous cycle, with greater estradiol being associated with attention to a wider range of stimuli and contexts (e.g., Sava and Markus [122]). As discussed in detail by Korol (this issue), females in the high estrogen phase (proestrus) favor a spatial, allocentric “place” strategy in learning the Y-maze, while those in the low estrogen (estrous) phase tend to use an egocentric “response” strategy. This body of work demonstrates that circulating estradiol dynamically modulates stimulus salience and learning strategy in hippocampal-sensitive tasks.
Unfortunately, the relevance of findings from studies of spatial learning and fear conditioning to the role of sex in the learned control of appetitive behavior is unclear. Little research has examined if hippocampal functioning influences the learned control of energy and body weight regulation differently for males and females. One exception is a recent study by Anderson & Petrovich [3] following up work cited earlier in this review [2] reporting sex differences in context dependent renewal of extinguished Pavlovian conditioned responding to food cues. Like their earlier report, [3] found that male rats showed renewal of responding when returned to a previously rewarded context, whereas female rats did not. In addition, these researchers showed that these behavioral differences varied with the differential recruitment of a medial prefrontal cortex-hippocampal-thalamic circuit, as indicated by Fos induction, during context dependent appetitive renewal. These results suggest that this brain circuit may be an important site for sex differences in appetitive behavior. One implication of these findings for understanding the hippocampal-dependent control of eating and appetitive behavior is that while the suppression of feeding behavior in male rats may involve the hippocampal-dependent activation of inhibitory associations by contextual satiety cues, suppression of intake for females could involve another, perhaps hippocampal-independent, mechanism. Additional research is needed to further evaluate this possibility.
5. Western diet interferes with learned control of intake regulation and hippocampal functioning
Much recent evidence shows that impaired hippocampal-dependent learning and memory functioning is also a consequence of consuming a high-fat, high-sugar Western diet. Rats that are maintained on Western diet show impaired performance relative to standard chow-fed controls, in the water maze [12, 98], Y-maze [57], radial-arm maze, [72], and conditional discrimination problems [34]. With the exception of Molteni et al. [98], who used only female rats in the water maze, these studies were conducted with male rats. Additionally, the rats fed Western diet in each of these studies were unimpaired on hippocampal-independent tasks. The finding of performance deficits in hippocampal-dependent, but not hippocampal-independent, problems makes it unlikely that the disruptive effects of Western diet were produced by changes in reward, motivation, arousal, general behavioral competency, or other global deficits. These types of changes would also be expected to disrupt performance on hippocampal-independent problems. Rather, the results indicate that Western diet intake resulted in a selective impairment in hippocampal function. Moreover, just as hippocampal damage has been shown to increase food intake and body weight, promote appetitive responding to external food cues, and impair discrimination of internal states, we have also found these effects to be consequences of Western diet consumption in rodents [30,120]. Converging evidence from humans who consume diets high in fat and sugar [5, 19,44, 102] support this animal model linking Western diet, hippocampal function, and food intake regulation.
Interference with cognition and energy intake regulation may be related to the emergence of Western diet- or obesity-induced hippocampal pathophysiology. For example, Beilharz et al. [9] reported that hippocampal inflammation occurred after male rats were maintained for one month on a high-fat cafeteria or standard chow diet when each were supplemented with a 10% sucrose solution. Degree of inflammation was associated with spatial memory deficit in a hippocampal-dependent place task. Western diet intake also interferes with the hippocampal expression of brain-derived neurotrophic factor (BDNF) which has an important role in the survival, maintenance, and growth of many types of neurons [6, 98]. Interference with hippocampal BDNF has been shown to disrupt several processes, such as synaptic plasticity and neurogenesis, that are thought to contribute to hippocampal-dependent memory processes [83]. A number of neuroendocrine signals that contribute to the regulation of energy intake and body weight, including insulin (e.g., [27,106], leptin (e.g., [46,125], and ghrelin (e.g., [38, 74] also act on specific receptors in the hippocampus. Recent research in male rats shows that hippocampal -dependent learning and memory functions are influenced by these signals, that Western diet intake can interfere with each of these signaling systems, and that this interference is correlated with impairments in hippocampal-dependent cognitive functions (see [75,76,129].
There is a dearth of studies investigating the impact of dietary challenges on cognitive function in female rodents. Underwood and Thompson [136] reported that, while intact female rats were protected from diet-induced metabolic alterations relative to males, rats of both sexes showed impaired spatial memory following 12 weeks of high-fat diet exposure. Barron et al. [7] reported a similar pattern of results in a transgenic mouse model of Alzheimer’s disease, in which high-fat diet fed females were spared from peripheral metabolic impairments, yet showed behavioral deficits and increased amyloid β accumulation in hippocampus like males. In an investigation of another type of dietary exposure, Abbott et al. [1] asserted that estradiol can counteract sucrose solution-induced deficits in hippocampal functioning. They found that females in the high estrogen phase (proestrus) were spared from deficits observed in females in the low estrogen phase (metestrus) and males on a hippocampal-dependent spatial task. More work is needed in females to understand the effects of western-style diet intake and potential interactions with sex steroids on indices of learning and memory.
5.1. The effects of Western diet on the blood-brain barrier
The mechanisms by which Western diet disrupts hippocampus functioning are poorly understood, but some or all of these effects may be secondary to disturbances in the blood-brain barrier (BBB). The BBB is a semipermeable network of cerebral endothelial cells that maintains the internal chemical milieu of the brain by regulating the transport of nutrients and other required substances and denying access to potentially toxic substances carried in the blood (Zheng et al. [152].). The structural integrity of the BBB depends on the maintenance of tight junctions between adjacent endothelial cells. The efficacy of these tight junctions depends on the expression of a number of proteins (viz. tight junction proteins) that are linked to the cytoskeleton to form a seal that can be rapidly modulated to maintain homeostasis in the brain microenvironment [150]. One form of BBB dysfunction is increased BBB permeability, which allows leakage of circulating neurotoxic substances into the brain and with disruption of transporter functions which result in reduced nutrient supply (Zheng et al. [152]). These pathologies are often most pronounced in the hippocampal formation [57], which is believed to be especially vulnerable to insult as a result of its high nutrient demands and pronounced cellular plasticity [145].
The integrity of the hippocampal BBB is compromised for obese rats maintained on Western diet [45]. Using animal models, our lab has demonstrated links between Western diet intake, hippocampal BBB permeability, and performance on hippocampal-dependent tasks, including modulating the inhibition of learned responding in males (i.e., serial feature negative tasks) [30,77]). Maintenance on Western diet is also accompanied by reductions in the expression of tight junction proteins in BBB [77]. Similar results have also been reported in rat models of Type 2 diabetes [149]. Moreover, increased BBB permeability was not observed in the striatum and prefrontal cortex of rats fed Western diet, indicating hippocampal-specific vulnerability to BBB disruption produced by dietary challenges. Recent work manipulating BBB permeability in obese male mice supports a causal role for BBB leakiness and resultant macrophage infiltration in hippocampal dysfunction [131].
In addition to increased permeability, there is evidence that consuming a Western-style diet disrupts glucose transport across the BBB into the brain. Glucose is the primary energy source used by the brain. Glucose uptake in the brain occurs primarily via a family of specific glucose transporters. Glucose transporter 1 (GLUT1) is the predominant glucose transporter across the BBB [133]. Reductions in the expression of GLUT1 in the BBB can therefore reduce glucose uptake by the hippocampus and other brain structures. Jais et al. [69] observed significant, although transient, reductions in GLUT-1 expression at the BBB and significant reductions in glucose uptake by the brain in mice that were fed a high-fat diet for just 3–7 days. GLUT-1 expression at the hippocampal BBB was also observed by Hargrave et al. [58] in male rats that had been maintained on Western diet for 10 days. In this study, reductions in GLUT-1 occurred in conjunction with impairments on a hippocampal spatial memory task. While the reduction in GLUT-1 expression appeared to greatest at the 10-day time point, smaller but significant reductions, compared to chow controls, were also observed at 40 and 90 days after initiation of Western diet. In sum, there is ample evidence that Western diet maintenance compromises the integrity of the hippocampal BBB and selectively impairs hippocampal-dependent cognitive functioning.
While the impact of dietary challenges on female BBB has not been investigated, previous work indicates sex differences in BBB changes in response to various stressors [39,89, 104]. It has been suggested that endogenous estradiol and estrogen replacement therapy protect against neurodegenerative diseases by preserving the blood-brain barrier [127]. Indeed, BBB endothelial cells express all three major estrogen receptor forms - ERα, ER β, and GPER. Estrogen does appear to protect BBB following ischemic and immune challenges. In response to a peripheral lipolysaccharide (LPS), estradiol treatment prevents inflammation-induced damage to BBB [89]. Notably, this BBB protection appears to be mediated by estrogenic regulation of Annexin A1 (ANXA1), an anti-inflammatory protein that is implicated in amyloid β clearance [94]. An investigation into the impact of Western diet exposure and hormonal status on female BBB permeability would be expected to yield important insights.
6. Sex steroids in energy regulation and cognition
Sexual dimorphisms in energy homeostasis and consummatory behavior underscore the need to consider sex effects in investigations of learned appetitive responding. Prior to the transition to menopause or estropause, ovarian hormones exert antiobesogenic effects. Estradiol tonically inhibits food intake from puberty to reproductive senescence, with ovariectomy increasing basal levels of food intake. Fluctuations in estradiol across the estrous cycle phasically suppress meal size (e.g., female rats consume 25% less following the pre-ovulatory estradiol surge) ([11]; see [4, 41, 47] for reviews). Likewise, exogenous estradiol administration potently inhibits meal size. Estradiol both enhances the potency of anorectic satiety signals (e.g., leptin, [23]) and weakens the potency of orexigenic feeding signals (e.g., ghrelin, [24]) (see [4, 92] for reviews). For instance, estradiol augments the intake suppressing effects of CCK, a short-term satiety signal released when food enters the gut [40,48]. In addition to influencing food intake, research has demonstrated that estrogen protects against body weight gain and obesity by influencing energy expenditure (e.g., [117]). While this review focuses on food intake behavior, metabolic fuel pathways and physical activity such as nonexercise activity thermogenesis (i.e., NEAT) are additional important regulators of body weight and adiposity [158] that differ by sex (see [85] for review).
Estrogen confers additional protection against the negative metabolic consequences of obesity. Estrogens regulate adipose tissue distribution, a determining factor in dementia risk for women [142]. Premenopausal women exhibit a gluteal-femoral distribution of adipose tissue, while men and post-menopausal women accrue fat in the visceral depot. Adipose tissue distribution is important in regulating metabolic homeostasis, wherein abdominal fat is associated with the buildup of free fatty acids and eventually increased inflammation [105], precursors to the metabolic syndrome. Peripheral and central estrogenic action, particularly by ERα, has been shown to regulate these phenotypic differences (see Hevener et al. [61] for review). For example, estrogen increases the brain’s sensitivity to short- (e.g., CCK, insulin) and long-term (e.g., leptin) satiety signals [23]. Further, novel pharmacotherapies that target estradiol delivery to tissues expressing the short-term satiety signal GLP-1 have been shown to reverse the metabolic syndrome in mice [42].
When challenged with a diet high in saturated fat, intact females show improved metabolic outcomes. Underwood and Thompson [136] found sex-dependent effects of long-term high-fat diet maintenance on metabolic markers of Type 2 diabetes. Unlike males, female rats did not show impaired glucose homeostasis or obesity following 12 weeks of high-fat diet maintenance. Some evidence suggests females are protected from the Western diet-induced inflammatory response. For example, Morselli et al. [100] found that ERα protected female mice from high-fat diet-induced increases fatty acid levels and associated inflammation in hypothalamus exhibited by males. These findings indicate that estrogenic action, particularly through ERα, confers sex-specific protection against obesity, inflammation, and the metabolic syndrome.
Later in life, these metabolic benefits are lost with the rapid onset of estrogen deprivation in menopause [22,28, 93]. In addition to abdominal adiposity, nonhuman animal models and epidemiological studies indicate that estrogen deprivation is further associated with inflammation and increased risk for Alzheimer’s disease (for review, [101]). While beyond the scope of this review, evidence from rodent models [28,81,154] and observational studies in women (e.g., 143)indicate that certain hormone replacement therapies may restore some of this protection, leading to better metabolic and cognitive outcomes when intervention is made prior to estrogen deprivation.
Despite estrogen’s protection against obesity and against its deleterious metabolic consequences, premenopausal women have a higher prevalence of obesity than men [103]. We do not know of any epidemiological surveys on the prevalence of obesity or the metabolic syndrome that segregate data by hormonal contraceptive use. A closer consideration of hormonal status may help to reconcile this seeming paradox between estrogen’s metabolic benefits and the current obesity and diabetes trends as well as to expand the external validity of food intake models for women. The next section discusses how another environmental factor, the widespread use of hormonal contraceptives, could potentially influence associative learning and memory processes like those involved in regulating food intake.
6.1. Hormonal contraceptives
The CDC estimates that 80% of women of reproductive age in the United States have ever used hormonal contraceptives [21], which have been widely available for the past 50 years. Age of use increasingly begins in adolescence, when ovarian estrogens are still exerting organizational changes on the brain. Despite ubiquitous use of hormonal contraceptives, their immediate and potential later life effects on the brain and cognition have received remarkably little attention.
The synthetic steroids that constitute hormonal contraceptives, as well as hormone replacement therapy regimens, differ from endogenous forms in their pharmacokinetic profiles and endocrine influence. The most common form of oral hormonal contraception is the combination of synthetic estrogens and progestins, though some formulations use progestins only. Compared to endogenous 17-β estradiol, the synthetic estrogen ethinyl estradiol has a five times greater binding affinity for the ER and cannot be converted to weaker estrogens (e.g., estrone). While the estrogenic component of hormonal contraceptives is overwhelmingly ethinyl estradiol, the wide variability in the progestin compound presents methodological challenges in comparing across formulations. For example, the effects of hormonal contraceptive use on indices of visuospatial learning and verbal memory in women have been found to vary depending on the androgenicity of the progestin component. More androgenic, second-generation progestins have been associated with improved mental-rotation [140] as well as poorer verbal memory performance [52] compared to non-users. In contrast, newer fourth-generation progestins (e.g., drospirenone), which bind more specifically to the progesterone receptor, seem to promote “feminizing” effects [110] have been associated with worse performance on mental rotation task compared to nonusers [140].
Variability in the dose, route of administration, and rate of steroid release further complicate research on hormonal contraception. Transdermal patches and intrauterine devices produce tonic hormone delivery, while the oral contraceptive “pill” is characterized by phasic hormone release [37]. In rodent models of these hormonal contraceptive regimens, cyclic versus tonic hormone administration has been found to produce disparate effects on learning and memory processes [96], as noted in more detail below. The labyrinthine nature of endogenous sex steroid synthesis pathways makes discerning the effects of these hormonal regimens on brain and behavior difficult (see McCarthy [95] for review). Progesterone is a precursor to androgens and estrogens, and estradiol can be locally synthesized from androgens in the brain via aromatase. The mechanism of action of synthetic sex steroids and how they interact with endogenous steroid action has yet to be elucidated. Generally, combined oral contraceptives inhibit GnRH pulsatility, which in turn prevents LH and FSH pulsatile secretion, suppressing follicular development and thus reducing estradiol levels, preventing ovulation [114].
Rodent studies of hormonal contraceptive regimens indicate that synthetic estrogens and progestins could alter hippocampal-dependent mnemonic processes. Mennenga et al. [96] found that higher dose of ethinyl estradiol impaired spatial memory performance in ovariectomized female rats with cyclic or tonic hormone delivery. Santoru et al. [121] orally administered combination ethinyl estradiol/levonorgestrel (EE/LNG) did not affect Morris water maze performance in gonadally-intact females. Simone et al. [126] reported biphasic dose-dependent differences in novel object recognition performance in intact female rats receiving chronic ethinyl estradiol, levonorgestrel, and combination ethinyl estradiol/levonorgestrel regimens. Across these few studies, methodological diversity in hormone combination, dose and administration, and ovarian status make interpretation difficult.
In a cross-species investigation, Graham and Milad [50] reported that levonorgestrel (LNG; an androgenic progestin)-treated intact female rats and women using hormonal contraceptives showed impaired fear extinction recall compared to their free cycling counterparts. This deficit was rescued in rats with ER agonists or in women by a single administration of estradiol prior to extinction. Consistent with the critical role of estrogen in memory consolidation and retrieval, this work suggests that a prolonged reduction in circulating estradiol, as occurs with hormonal contraceptive regimens, could interfere with the contextual consolidation and/or retrieval of inhibitory memories. How this extends to appetitive paradigms remains unknown. To our knowledge, no studies have investigated the effects of hormonal contraceptive regimens on appetitive learning and memory paradigms. The cognitive impact of sustained reductions in ovarian hormones, which often enhance cognitive performance, coupled with the introduction of synthetic estrogen and progesterone, remains understudied.
The effects of hormonal contraceptives on current and later life cognitive function are likewise unclear in humans. Many studies have reported differences in affective mnemonic processing between OC users and non-users [97,108] suggest that, through their reduction in endogenous sex steroid levels, oral contraceptives modulate the amygdalar regulation of emotionally-valenced stimuli. Indeed, affective state is considered an interoceptive context that can modulate learned associations [18], including conditioned appetitive food cue responding [13]. To our knowledge, the impact of hormonal contraceptive use on appetitive associative learning and memory inhibition has not been investigated. Considering the history of hormonal contraceptive experience (e.g., specific formulation, length of use) within the dynamic nature of brain structural changes in development and adulthood poses difficulty.
7. Limitations
A complete understanding of the role of sex differences in the control of food intake and body weight will ultimately require an integrative analysis of the complex interplay of many processes that can be investigated at many levels of analysis (e.g., molecular, genetic, structural, physiological, cognitive, behavioral). However, gaps in current knowledge make the integration of learning and memory processes with other types of regulatory control mechanisms difficult. Accordingly, this review is limited to identifying those gaps, describing what is known about the influence of sex on learning and memory processes involved appetitive and eating behavior, and on bringing attention to the effects of dietary factors on those processes and the neural substrates that support them.
This review also summarizes a theoretical perspective which relies primarily on principles derived from studies of associative learning and memory to address the broad questions of how humans and nonhuman animals regulate their energy intake and body weight and how disorders of intake and weight regulation might occur. There are many other theoretical approaches to these questions, most of which focus on processes other than learning and memory. While an enumeration and critical analysis of these other perspectives is beyond the scope of the present review, we will note here what have been termed as the “prevailing” and “alternative” models of obesity in a recent paper by Ludwig and Friedman [86]. The prevailing model links energy dysregulation leading to obesity to an environment where increased energy intake results from an abundance of highly palatable, energy-dense food and where reduced requirements for physical activity decrease energy expenditure. Ludwig and Friedman’s alternative model proposes that poor diet quality, combined with genetic and lifestyle factors, such as stress and inadequate sleep, promote increased fat storage which causes excess caloric intake and decreased energy expenditure, in part, by reducing access to circulating metabolic fuels.
Our model advances the “prevailing” view by describing how interference with basic learning and memory mechanisms could enable environmental cues associated with highly palatable, energy-dense, food can evoke energy intake in excess of energy needs. Ludwig and Friedman’s alternative model is distinguished from the prevailing view by the counter-intuitive suggestion that adiposity may cause overeating, rather than overeating causes increased adiposity. In a similar twist, our model suggests impairment in hippocampal-dependent cognitive functioning may cause overeating, in contrast to views which suggest that overeating causes cognitive dysfunction. In both Ludwig and Friedman’s alternative view and our learning and memory account, eating a poor-quality diet produces the pathophysiological changes that are responsible for overeating. A limitation of our current model is that mechanisms that enable intake of a Western diet to interfere with hippocampal functioning remain to be specified. An interesting, although speculative, possibility that is derived from Ludwig and Friedman’s alternative view is that Western diet-induced increases in fat storage is part of the mechanism that produces the type of hippocampal-dependent learning and memory impairments that promote excess energy intake and further increases in fat storage.
Our theoretical model draws support from findings, derived primarily from studies of rodents, which show that increases in peripheral estrogenic activity are strongly and negatively correlated with increased energy intake and body weight gain (e.g., [20,41]) and with improved performance on tasks that rely on hippocampal-dependent learning and memory processes (e.g., [82,87]). However, this support is tempered by reports that weight loss associated with administration of estradiol and weight gain associated with ovariectomy sometimes do not require changes in energy intake (e.g., [11,160] but see [49,53]). It appears that decreased body weight and adiposity when estrogen levels are elevated, and weight and adiposity gain in the aftermath ovariectomy can also occur a result of increased and decreased energy expenditure, respectively (e.g., [138,146]). The learning and memory model we outline in this review accounts for changes in body weight via alterations in the ability refrain from eating and appetitive behavior, but requires modification to account for weight changes are the result of only differences in energy expenditure.
8. Conclusions
In this review, we have interrogated the interactive mnemonic and physiological processes of food intake regulation in females. Using our previously defined model for the learned control of food intake regulation as a framework, we have reported what is known about sex effects in simple excitatory learning, inhibiting learned responses, and the utilization of exteroceptive contexts and internal deprivation states to guide appetitive responding. Rodent models indicate accelerated learning about food cues and more exteroceptive stimulus control over appetitive responding, but the mechanisms underlying this remain unclear. Sex effects in the contextual retrieval of inhibitory associations have barely been investigated in appetitive paradigms. Limited evidence indicates that this contextual retrieval and related associative processes that depend on hippocampus (e.g., spatial learning) are modulated by estradiol Counterintuitive to the higher prevalence of obesity in women, rodent models indicate that females are protected from diet-induced obesity and its deleterious metabolic consequences. Both sexual dimorphisms in the physiology of eating and sex effects on hippocampal-dependent learning and memory appear to be mediated by estradiol. We have called attention to the omission of hormonal contraceptives, which reduce estradiol levels for prolonged periods in women of reproductive age, from investigations into basic learning and memory processes and food intake control. Filling in these gaps of how these basic cognitive processes operate in females, and their susceptibility to environmental challenges, may yield critical insight into the female bias in obesity prevalence and increased susceptibility to associated cognitive dementia.
Acknowledgments
Work on this paper was supported in part by Grant R01DK110412 from the National Institutes of Diabetes and Digestive and Kidney Diseases. Some of the work reported in this manuscript was performed as part of completing Ph.D. requirements by Camille Sample at American University, Washington D.C.
References
- 1.Abbott KN, Morris MJ, Westbrook RF, Reichelt AC. Sex-specific effects of daily exposure to sucrose on spatial memory performance in male and female rats, and implications for estrous cycle stage. Physiol Behav. 2016;162:52–60. doi: 10.1016/j.physbeh.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LC, Petrovich GD. Renewal of conditioned responding to food cues in rats: sex differences and relevance of estradiol. Physiol Behav. 2015;151:338–344. doi: 10.1016/j.physbeh.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LC, Petrovich GD. Sex specific recruitment of a medial prefrontal cortex -hippocampal-thalamic system during context-dependent renewal of responding to food cues in rats. Neurobiol Learn Mem. 2017;139:11–21. doi: 10.1016/j.nlm.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond Ser B Biol Sci. 2006;361(1471):1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attuquayefio T, Stevenson RJ, Boakes RA, Oaten MJ, Yeomans MR, Mahmut M, Francis HM. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J Exp Psychol Anim Learn Cogn. 2016;42(4):415–428. doi: 10.1037/xan0000118. [DOI] [PubMed] [Google Scholar]
- 6.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron AM, Rosario ER, Elteriefi R, Pike CJ. Sex-specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: implications for Alzheimer’s disease. PLoS One. 2013;8(10):e78554. doi: 10.1371/journal.pone.0078554. http://dx.doi.org/10.1371/journal.pone.0078554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker JB, Taylor JR. Sex differences in motivation. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young E, editors. Sex Differences in the Brain, From Genes to Behavior. Oxford University Press; New York, NY: 2008. [Google Scholar]
- 9.Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. http://dx.doi.org/10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7(8):6719–6738. doi: 10.3390/nu7085307. http://dx.doi.org/10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17(2):201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 12.Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Laye S, Ferreira G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun. 2014;40:9–17. doi: 10.1016/j.bbi.2014.03.005. http://dx.doi.org/10.1016/j.bbi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Bongers P, Jansen A. Emotional eating and Pavlovian learning: evidence for conditioned appetitive responding to negative emotional states. Cogn Emot. 2015:1–14. doi: 10.1080/02699931.2015.1108903. http://dx.doi.org/10.1080/02699931.2015.1108903. [DOI] [PubMed]
- 14.Bouton ME. Context, ambiguity, and classical conditioning. Curr Dir Psychol Sci. 1994;3(2):49–53. http://dx.doi.org/10.1111/1467-8721.ep10769943. [Google Scholar]
- 15.Bouton ME. Learning and the persistence of appetite: extinction and the motivation to eat and overeat. Physiol Behav. 2011;103(1):51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28(7):663–674. doi: 10.1016/j.neubiorev.2004.09.001. http://dx.doi.org/10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Bower GH. Mood and memory. Am Psychol. 1981;36(2):129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- 19.Brannigan M, Stevenson RJ, Francis H. Thirst interoception and its relationship to a Western-style diet. Physiol Behav. 2015;139:423–429. doi: 10.1016/j.physbeh.2014.11.050. http://dx.doi.org/10.1016/j.physbeh.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99(2):175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23(25):1–160. [PubMed] [Google Scholar]
- 22.Christensen A, Pike CJ. Menopause, obesity and inflammation: interactive risk factors for Alzheimer’s disease. Front Aging Neurosci. 2015;7:130. doi: 10.3389/fnagi.2015.00130. http://dx.doi.org/10.3389/fnagi.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 24.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, … Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–1058. doi: 10.2337/db06-0015. http://dx.doi.org/10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 25.Clifton PG, Vickers SP, Somerville EM. Little and often: ingestive behavior patterns following hippocampal lesions in rats. Behav Neurosci. 1998;112(3):502–511. doi: 10.1037//0735-7044.112.3.502. [DOI] [PubMed] [Google Scholar]
- 26.Cossio R, Carreira MB, Vasquez CE, Britton GB. Sex differences and estrous cycle effects on foreground contextual fear conditioning. Physiol Behav. 2016;163:305–311. doi: 10.1016/j.physbeh.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, … Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 28.Daniel JM, Witty CF, Rodgers SP. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015;74:77–85. doi: 10.1016/j.yhbeh.2015.04.012. http://dx.doi.org/10.1016/j.yhbeh.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. http://dx.doi.org/10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson TL, Hargrave SL, Swithers SE, Sample CH, Fu X, Kinzig KP, Zheng W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience. 2013;253:110–122. doi: 10.1016/j.neuroscience.2013.08.044. http://dx.doi.org/10.1016/j.neuroscience.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59(2):167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 32.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124(1):97–105. doi: 10.1037/a0018402. http://dx.doi.org/10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86(5):731–746. doi: 10.1016/j.physbeh.2005.09.004. http://dx.doi.org/10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and dietresistant rats. Physiol Behav. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. ttp://dx,doi.org/10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davidson TL, Sample CH, Swithers SE. An application of Pavlovian principles to the problems of obesity and cognitive decline. Neurobiol Learn Mem. 2014;108:172–184. doi: 10.1016/j.nlm.2013.07.014. http://dx.doi.org/10.1016/j.nlm.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daviu N, Andero R, Armario A, Nadal R. Sex differences in the behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Horm Behav. 2014;66(5):713–723. doi: 10.1016/j.yhbeh.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Devineni D, Skee D, Vaccaro N, Massarella J, Janssens L, LaGuardia KD, Leung AT. Pharmacokinetics and pharmacodynamics of a transdermal contraceptive patch and an oral contraceptive. J Clin Pharmacol. 2007;47(4):497–509. doi: 10.1177/0091270006297919. http://dx.doi.org/10.1177/0091270006297919. [DOI] [PubMed] [Google Scholar]
- 38.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, … Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9(3):381–388. doi: 10.1038/nn1656. http://dx.doi.org/10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 39.Diler AS, Uzum G, Akgun Dar K, Aksu U, Atukeren P, Ziylan YZ. Sex differences in modulating blood brain barrier permeability by NO in pentylenetetrazol-induced epileptic seizures. Life Sci. 2007;80(14):1274–1281. doi: 10.1016/j.lfs.2006.12.039. http://dx.doi.org/10.1016/j.lfs.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. http://dx.doi.org/10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104(4):517–524. doi: 10.1016/j.physbeh.2011.04.014. http://dx.doi.org/10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX, … Tschop MH. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18(12):1847–1856. doi: 10.1038/nm.3009. http://dx.doi.org/10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. http://dx.doi.org/10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis HM, Stevenson RJ. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav Neurosci. 2011;125(6):943–955. doi: 10.1037/a0025998. http://dx.doi.org/10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- 45.Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. 2014;17(6):241–251. doi: 10.1179/1476830513Y.0000000092. http://dx.doi.org/10.1179/1476830513y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283(26):18238–18247. doi: 10.1074/jbc.M800053200. http://dx.doi.org/10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geary N. Lovejoy, Sex differences in energy metabolism, obesity, & eating behavior. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain, From Genes to Behavior. Oxford University Press; New York, NY: 2008. pp. 253–274. [Google Scholar]
- 48.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56(2):281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 49.Giles ED, Jackman MR, Johnson GC, Schedin PJ, Houser JL, MacLean PS. Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1634–1642. doi: 10.1152/ajpregu.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73(4):371–378. doi: 10.1016/j.biopsych.2012.09.018. http://dx.doi.org/10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gresack JE, Schafe GE, Orr PT, Frick KM. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience. 2009;159(2):451–467. doi: 10.1016/j.neuroscience.2009.01.009. http://dx.doi.org/10.1016/j.neuroscience.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Griksiene R, Ruksenas O. Effects of hormonal contraceptives on mental rotation and verbal fluency. Psychoneuroendocrinology. 2011;36(8):1239–1248. doi: 10.1016/j.psyneuen.2011.03.001. http://dx.doi.org/10.1016/j.psyneuen.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Guyard B, Fricker J, Brigant L, Betoulle D, Apfelbaum M. Effects of ovarian steroids on energy balance in rats fed a highly palatable diet. Metabolism. 1991;40(5):529–533. doi: 10.1016/0026-0495(91)90236-p. [DOI] [PubMed] [Google Scholar]
- 54.Hall PA. Executive control resources and frequency of fatty food consumption: findings from an age-stratified community sample. Health Psychol. 2012;31(2):235–241. doi: 10.1037/a0025407. http://dx.doi.org/10.1037/a0025407. [DOI] [PubMed] [Google Scholar]
- 55.Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol. 2014;56(4):611–621. doi: 10.1002/dev.21127. http://dx.doi.org/10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hannapel RC, Henderson YH, Nalloor R, Vazdarjanova A, Parent MB. Ventral hippocampal neurons inhibit postprandial energy intake. Hippocampus. 2017;27(3):274–284. doi: 10.1002/hipo.22692. http://dx.doi.org/10.1002/hipo.22692. [DOI] [PubMed] [Google Scholar]
- 57.Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behav Neurosci. 2016;130(1):123–135. doi: 10.1037/bne0000110. http://dx.doi.org/10.1037/bne0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hargrave SL, Jones S, Davidson TL. The outward spiral: a vicious cycle model of obesity and cognitive dysfunction. Curr Opin Behav Sci. 2016;9:40–46. doi: 10.1016/j.cobeha.2015.12.001. http://dx.doi.org/10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebben N, Corkin S, Eichenbaum H, Shedlack K. Diminished ability to interpret and report internal states after bilateral medial temporal resection: case H.M. Behav Neurosci. 1985;99(6):1031–1039. doi: 10.1037//0735-7044.99.6.1031. [DOI] [PubMed] [Google Scholar]
- 60.Henderson YO, Smith GP, Parent MB. Hippocampal neurons inhibit meal onset. Hippocampus. 2013;23(1):100–107. doi: 10.1002/hipo.22062. http://dx.doi.org/10.1002/hipo.22062. [DOI] [PubMed] [Google Scholar]
- 61.Hevener AL, Clegg DJ, Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol Cell Endocrinol. 2015;418(Pt 3):306–321. doi: 10.1016/j.mce.2015.05.020. http://dx.doi.org/10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgs S. Cognitive processing of food rewards. Appetite. 2016;104:10–17. doi: 10.1016/j.appet.2015.10.003. http://dx.doi.org/10.1016/j.appet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Higgs S, Dolmans D, Humphreys GW, Rutters F. Dietary self-control influences top-down guidance of attention to food cues. Front Psychol. 2015;6:427. doi: 10.3389/fpsyg.2015.00427. http://dx.doi.org/10.3389/fpsyg.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirsh R, Leber B, Gillman K. Fornix fibers and motivational states as controllers of behavior: a study stimulated by the contextual retrieval theory. Behav Biol. 1978;22(4):463–478. doi: 10.1016/s0091-6773(78)92583-x. [DOI] [PubMed] [Google Scholar]
- 65.Hock BJ, Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18(17):7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9(2):195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 67.Ito R, Lee AC. The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav Brain Res. 2016;313:345–357. doi: 10.1016/j.bbr.2016.07.039. http://dx.doi.org/10.1016/j.bbr.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 68.Izquierdo I, Furini CR, Myskiw JC. Fear memory. Physiol Rev. 2016;96(2):695–750. doi: 10.1152/physrev.00018.2015. http://dx.doi.org/10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 69.Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, … Bruning JC. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;166(5):1338–1340. doi: 10.1016/j.cell.2016.08.010. http://dx.doi.org/10.1016/j.cell.2016.08.010. [DOI] [PubMed] [Google Scholar]; memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. http://dx.doi.org/10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Johnson AW. Eating beyond metabolic need: how environmental cues influence feeding behavior. Trends Neurosci. 2013;36(2):101–109. doi: 10.1016/j.tins.2013.01.002. http://dx.doi.org/10.1016/j.tins.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. http://dx.doi.org/10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process. 2010;36(2):313–319. doi: 10.1037/a0017228. http://dx.doi.org/10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- 73.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103(1):59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73(9):915–923. doi: 10.1016/j.biopsych.2012.07.002. http://dx.doi.org/10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanoski SE, Grill HJ. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatry. 2017;81(9):748–756. doi: 10.1016/j.biopsych.2015.09.011. http://dx.doi.org/10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, Grill HJ. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36(9):1859–1870. doi: 10.1038/npp.2011.70. http://dx.doi.org/10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21(1):207–219. doi: 10.3233/JAD-2010-091414. http://dx.doi.org/10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kelley SP, Mittleman G. Effects of hippocampal damage on reward threshold and response rate during self-stimulation of the ventral tegmental area in the rat. Behav Brain Res. 1999;99(2):133–141. doi: 10.1016/s0166-4328(98)00097-7. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24(31):6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. http://dx.doi.org/10.1523/jneurosci.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koebele SV, Bimonte-Nelson HA. Trajectories and phenotypes with estrogen exposures across the lifespan: what does goldilocks have to do with it? Horm Behav. 2015;74:86–104. doi: 10.1016/j.yhbeh.2015.06.009. http://dx.doi.org/10.1016/j.yhbeh.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koss WA, Frick KM. Sex differences in hippocampal function. J Neurosci Res. 2017;95(1–2):539–562. doi: 10.1002/jnr.23864. [DOI] [PubMed] [Google Scholar]
- 83.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16(3):216–224. doi: 10.1002/hipo.20153. http://dx.doi.org/10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 84.Li R, Singh M. Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinol. 2014;35(3):385–403. doi: 10.1016/j.yfrne.2014.01.002. http://dx.doi.org/10.1016/j.yfrne.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;28(8):411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 86.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167–2168. doi: 10.1001/jama.2014.4133. http://dx.doi.org/10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- 87.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. doi: 10.1016/j.yhbeh.2014.08.011. http://dx.doi.org/10.1016/j.yhbeh.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lynch W, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 89.Maggioli E, McArthur S, Mauro C, Kieswich J, Kusters DH, Reutelingsperger CP, … Solito E. Estrogen protects the blood-brain barrier from inflammation-induced disruption and increased lymphocyte trafficking. Brain Behav Immun. 2016;51:212–222. doi: 10.1016/j.bbi.2015.08.020. ttp://dx.doi.org/10.1016/j.bbi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 90.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661(1–2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 91.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417–428. doi: 10.1038/nrn3492. http://dx.doi.org/10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. http://dx.doi.org/10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. doi: 10.1210/er.2016-1146. http://dx.doi.org/10.1210/er2016-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McArthur S, Loiola RA, Maggioli E, Errede M, Virgintino D, Solito E. The restorative role of annexin A1 at the blood-brain barrier. Fluids Barriers CNS. 2016;13(1):17. doi: 10.1186/s12987-016-0043-0. http://dx.doi.org/10.1186/s12987-016-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88(1):91–124. doi: 10.1152/physrev.00010.2007. http://dx.doi.org/10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CW, Engler-Chiurazzi EB, … Bimonte-Nelson HA. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology. 2015;54:1–13. doi: 10.1016/j.psyneuen.2015.01.002. ttp://dx.doi.org/10.1016/j.psyneuen.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merz CJ. Contribution of stress and sex hormones to memory encoding. Psychoneuroendocrinology. 2017;82:51–58. doi: 10.1016/j.psyneuen.2017.05.002. http://dx.doi.org/10.1016/j.psyneuen.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 99.Morrison SE, Bamkole MA, Nicola SM. Sign tracking, but not goal tracking, is resistant to outcome devaluation. Front Neurosci. 2015;9:468. doi: 10.3389/fnins.2015.00468. http://dx.doi.org/10.3389/fnins.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, … Clegg DJ. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep. 2014;9(2):633–645. doi: 10.1016/j.celrep.2014.09.025. http://dx.doi.org/10.1016/j.celrep.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moser VA, Pike CJ. Obesity and sex interact in the regulation of Alzheimer’s disease. Neurosci Biobehav Rev. 2016;67:102–118. doi: 10.1016/j.neubiorev.2015.08.021. http://dx.doi.org/10.1016/j.neubiorev.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newby PK, Muller D, Hallfrisch J, Qiao N, Andres R, Tucker KL. Dietary patterns and changes in body mass index and waist circumference in adults. Am J Clin Nutr. 2003;77(6):1417–1425. doi: 10.1093/ajcn/77.6.1417. [DOI] [PubMed] [Google Scholar]
- 103.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 104.Oztas B, Camurcu S, Kaya M. Influence of sex on the blood brain barrier permeability during bicuculline-induced seizures. Int J Neurosci. 1992;65(1–4):131–139. doi: 10.3109/00207459209003284. [DOI] [PubMed] [Google Scholar]
- 105.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. http://dx.doi.org/10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68(4):509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 107.Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110(6):1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- 108.Petersen N, Cahill L. Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc Cogn Affect Neurosci. 2015;10(9):1266–1272. doi: 10.1093/scan/nsv010. http://dx.doi.org/10.1093/scan/nsv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pitchers KK, Flagel SB, O’Donnell EG, Woods LC, Sarter M, Robinson TE. Individual variation in the propensity to attribute incentive salience to a food cue: influence of sex. Behav Brain Res. 2015;278:462–469. doi: 10.1016/j.bbr.2014.10.036. http://dx.doi.org/10.1016/j.bbr.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception-time to find out, what it does to our brain. Front Neurosci. 2014;8:256. doi: 10.3389/fnins.2014.00256. http://dx.doi.org/10.3389/fnins.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Price M, Higgs S, Lee M. Self-reported eating traits: underlying components of food responsivity and dietary restriction are positively related to BMI. Appetite. 2015;95:203–210. doi: 10.1016/j.appet.2015.07.006. http://dx.doi.org/10.1016/j.appet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 113.Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. http://dx.doi.org/10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- 114.Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 Pt 1):1263–1269. doi: 10.1016/s0002-9378(99)70120-1. [DOI] [PubMed] [Google Scholar]
- 115.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. http://dx.doi.org/10.1080/09652140020016996. [DOI] [PubMed] [Google Scholar]
- 116.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. http://dx.doi.org/10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saito K, He K, Yang Y, Zhu L, Wang C, Xu P, … Xu Y. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016;6:23459. doi: 10.1038/srep23459. http://dx.doi.org/10.1038/srep23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sample CH, Jones S, Dwider F, Davidson TL. Discriminative control by deprivation states and external cues in male and female rats. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.08.019. http://dx.doi.org/10.1016/j.physbeh.2017.08.019. [DOI] [PMC free article] [PubMed]
- 119.Sample CH, Jones S, Hargrave SL, Jarrard LE, Davidson TL. Western diet and the weakening of the interoceptive stimulus control of appetitive behavior. Behav Brain Res. 2016;312:219–230. doi: 10.1016/j.bbr.2016.06.020. http://dx.doi.org/10.1016/j.bbr.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sample CH, Martin AA, Jones S, Hargrave SL, Davidson TL. Western-style diet impairs stimulus control by food deprivation state cues: implications forobesogenic environments. Appetite. 2015;93:13–23. doi: 10.1016/j.appet.2015.05.018. http://dx.doi.org/10.1016/j.appet.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santoru F, Berretti R, Locci A, Porcu P, Concas A. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology. 2014;231(17):3351–3364. doi: 10.1007/s00213-014-3539-9. http://dx.doi.org/10.1007/s00213-014-3539-9. [DOI] [PubMed] [Google Scholar]
- 122.Sava S, Markus EJ. Intramaze cue utilization in the water maze: effects of sex and estrous cycle in rats. Horm Behav. 2005;48(1):23–33. doi: 10.1016/j.yhbeh.2005.01.011. http://dx.doi.org/101016/j.yhbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 123.Schmelzeis MC, Mittleman G. The hippocampus and reward: effects of hippocampal lesions on progressive-ratio responding. Behav Neurosci. 1996;110(5):1049–1066. doi: 10.1037//0735-7044.110.5.1049. [DOI] [PubMed] [Google Scholar]
- 124.Schumacher A, Vlassov E, Ito R. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 2016;26(4):530–542. doi: 10.1002/hipo.22542. http://dx.doi.org/10.1002/hipo.22542. [DOI] [PubMed] [Google Scholar]
- 125.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21(24):Rc186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, Holmes PV. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain -derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265–278. doi: 10.1016/j.psyneuen.2015.08.015. http://dx.doi.org/10.1016/j.psyneuen.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 127.Sohrabji F. Guarding the blood-brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr. 2007;13(6):311–319. doi: 10.3727/000000006781510723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 129.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, … de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 130.Stevenson RJ, Francis HM. The hippocampus and the regulation of human food intake. Psychol Bull. 2017 doi: 10.1037/bul0000109. http://dx.doi.org/10.1037/bul0000109. [DOI] [PubMed]
- 131.Stranahan AM, Hao S, Dey A, Yu X, Baban B. Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16642233. http://dx.doi.org/10.1177/0271678X16642233. [DOI] [PMC free article] [PubMed]
- 132.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 2015;6:10188. doi: 10.1038/ncomms10188. http://dx.doi.org/10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H. Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem Biophys Res Commun. 1990;173(1):67–73. doi: 10.1016/s0006-291x(05)81022-8. [DOI] [PubMed] [Google Scholar]
- 134.Todd TP, Winterbauer NE, Bouton ME. Contextual control of appetite. Renewal of inhibited food-seeking behavior in sated rats after extinction. Appetite. 2012;58(2):484–489. doi: 10.1016/j.appet.2011.12.006. http://dx.doi.org/10.1016/j.appet.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Underwood EL, Thompson LT. A high-fat diet causes impairment in hippocampal memory and sex-dependent alterations in peripheral metabolism. Neural Plast. 2016;2016:7385314. doi: 10.1155/2016/7385314. http://dx.doi.org/10.1155/2016/7385314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Urcelay GP, Miller RR. The functions of contexts in associative learning. Behav Process. 2014;104:2–12. doi: 10.1016/j.beproc.2014.02.008. http://dx.doi.org/10.1016/j.beproc.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, … Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308(6):R530–542. doi: 10.1152/ajpregu.00401.2014. http://dx.doi.org/10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wasserman EA, Castro L. Surprise and change: variations in the strength of present and absent cues in causal learning. Learn Behav. 2005;33(2):131–146. doi: 10.3758/bf03196058. [DOI] [PubMed] [Google Scholar]
- 140.Wharton W, Hirshman E, Merritt P, Doyle L, Paris S, Gleason C. Oral contraceptives and androgenicity: influences on visuospatial task performance in younger individuals. Exp Clin Psychopharmacol. 2008;16(2):156–164. doi: 10.1037/1064-1297.16.2.156. http://dx.doi.org/10.1037/1064-1297.16.2.156. [DOI] [PubMed] [Google Scholar]
- 141.White NM, Naeem M. Learning not to respond: role of the hippocampus in withholding responses during omission training. Behav Brain Res. 2017;318:61–70. doi: 10.1016/j.bbr.2016.11.011. http://dx.doi.org/10.1016/j.bbr.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 142.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 143.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69:163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilkinson LL, Hinton EC, Fay SH, Ferriday D, Rogers PJ, Brunstrom JM. Computer-based assessments of expected satiety predict behavioural measures of portion-size selection and food intake. Appetite. 2012;59(3):933–938. doi: 10.1016/j.appet.2012.09.007. http://dx.doi.org/10.1016/j.appet.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 145.Williamson LL, Bilbo SD. Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. http://dx.doi.org/10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 146.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166(3):520–528. doi: 10.1016/j.ygcen.2010.01.006. http://dx.doi.org/10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Woods SC, Begg DP. Regulation of the motivation to eat. Curr Top Behav Neurosci. 2016;27:15–34. doi: 10.1007/7854_2015_381. http://dx.doi.org/10.1007/7854_2015_381. [DOI] [PubMed] [Google Scholar]
- 148.Yeomans MR. Adverse effects of consuming high fat-sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc. 2017:1–11. doi: 10.1017/S0029665117000805. http://dx.doi.org/10.1017/s0029665117000805. [DOI] [PubMed]
- 149.Yoo DY, Yim HS, Jung HY, Nam SM, Kim JW, Choi JH, … Hwang IK. Chronic type 2 diabetes reduces the integrity of the blood-brain barrier by reducing tight junction proteins in the hippocampus. J Vet Med Sci. 2016;78(6):957–962. doi: 10.1292/jvms.15-0589. http://dx.doi.org/10.1292/jvms.15-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–1078. doi: 10.1016/j.cell.2015.10.067. http://dx.doi.org/10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. http://dx.doi.org/10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192(1):1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. http://dx.doi.org/10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 154.Christensen A, Pike CJ. Age-dependent regulation of obesity and Alzheimer-related outcomes by hormone therapy in female 3xTg-AD mice. PLoS One. 2017;12:e0178490. doi: 10.1371/journal.pone.0178490. http://dx.doi.org/10.1371/journal.pone.0178490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Davidson TL. Learning about deprivation intensity stimuli. Behav Neurosci. 1987;101(2):198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- 156.Davidson TL, Tracy AL, Schier LA, Swithers SE. A view of obesity as a learning and memory disorder. J Exp Psychol Anim Learn Cogn. 2014;40(3):261–279. doi: 10.1037/xan0000029. http://dx.doi.org/10.1037/xan0000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- 158.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Phys Regul Integr Comp Phys. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 159.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem. 2014;60:44–52. doi: 10.1373/clinchem.2013.202549. http://dx.doi.org/10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]