Abstract
Violaxanthin de-epoxidation, chlorophyll fluorescence quenching, and photosynthetic O2 evolution in the presence of paraquat (Pq) were studied in intact attached leaves of Pq-susceptible, and Pq-resistant (PqR) biotypes of Erigeron canadensis under different light conditions. Initially, similar changes were induced in the two biotypes, but the effects relaxed only in the PqR plants, indicating a Pq elimination process. The penetration of Pq into the chloroplasts of PqR plants proved to be somewhat restricted and highly light-dependent, as revealed by both the light response curves of violaxanthin de-epoxidation and fluorescence quenching and the short-term high-light pre-illumination experiments. An irregular down-regulation of the non-photochemical fluorescence quenching processes was observed, reflected by lower steady-state zeaxanthin and non-photochemical fluorescence quenching levels as compared with the corresponding non-treated high-light controls. It is concluded that light is essential not only for the initiation of the mechanism of resistance to Pq, but also for the penetration of Pq into the chloroplasts in the PqR E. canadensis. Also, the Pq elimination process may cause a modification to the regulation of the non-radiative energy dissipation in PqR plants in the presence of Pq.
Paraquat (methylviologen, 1,1′-dimethyl-4,4′-bipyridinium, the active ingredient of the non-selective herbicide Gramoxone; Pq) has been widely used in weed control. Pq is a strong autooxidable electron acceptor in PS I, and it is generally accepted that in light-exposed plants the presence of Pq in the chloroplasts has several important consequences. (a) Pq will accept electrons from the iron-sulfur cluster Fe-SA/Fe-SB of PS I (Fujii et al., 1990) resulting in a depletion of NADPH and the inhibition of CO2 fixation (Dodge, 1971; Preston, 1994); (b) the Pq radical thus formed will react directly with O2 to produce superoxide. With the rapid regeneration of oxidized Pq by O2, Pq is effective in small amounts inside the chloroplast. The increased efficiency of electron capture will enhance the linear photosynthetic electron transport rate and ΔpH formation across the thylakoid membranes, providing favorable conditions for xanthophyll cycle de-epoxidation (Büch et al., 1994; Pfündel and Bilger, 1994; Thiele and Krause, 1994); and (c) the toxic oxygen species (such as superoxide anion, hydrogen peroxide, and hydroxyl radical) produced as a result of primary Pq action will rapidly destroy the chloroplast membranes (Dodge, 1994). In this way, Pq treatment can cause damaging effects even at low-light (LL) intensities.
As a consequence of the repeated use of Pq, resistance to Pq has emerged in several weed species (Shaaltiel and Gressel, 1986; Pölös et al., 1988; Fuerst and Vaughn, 1990; Preston et al., 1992). The site of resistance may be situated along the route of penetration into the plant cell or the chloroplasts. A number of possible mechanisms of Pq resistance have been suggested (Fuerst and Vaughn, 1990; Preston, 1994): (a) There may be a restricted movement of Pq into the leaves and chloroplasts (Fuerst et al., 1985; Vaughn and Fuerst, 1985; Tanaka et al., 1986); (b) there is an enhanced activity of the Halliwell-Asada pathway, an antioxidative enzyme cascade in chloroplasts that eliminates active oxygen species (Shaaltiel and Gressel, 1986; Jansen et al., 1989); and (c) Pq may be readily sequestered and not allowed to act as an electron acceptor in PS I (Fuerst et al., 1985; Powles and Cornic, 1987; Vaughn et al., 1989; Lasat et al., 1997).
The mode of action of Pq in leaves of Pq-atrazine (Atr) coresistant (PqAtrR) Erigeron canadensis originating from Hungary has been investigated in detail (Lehoczki et al., 1992). A most important and characteristic phenomenon was the transitory inhibition of the photosynthetic activity measured as CO2 fixation, O2 evolution, and variable fluorescence. All of these parameters reach their minimum by the 1st or 2nd h of Pq treatment. This indirectly proved that Pq can reach the site of action in the chloroplasts even in the PqAtrR biotype. Atr acts at the QB site of PS II and it is well known that modification at this and related sites in PS II can have a marked effect on the susceptibility to photoinhibition (Holt et al., 1981; Sundby et al., 1993; Váradi et al., 1994; Darkó et al., 1996, 2000). It is presumed that resistance to Pq and Atr emerged simultaneously, but independently when both Pq and Atr were used repeatedly for many years in weed control. It cannot be excluded, however, that the existence of Atr resistance in a Pq-resistant plant (PqAtrR biotype) modifies the light response in the absence or presence of Pq as compared with PqR plants.
The elevated levels of antioxidative enzymes in PqR Conyza bonariensis have led to the assumption that the mechanisms of Pq resistance may protect against high-light (HL) stress (Jansen et al., 1989). Despite the extremely high level of Pq resistance (resistance factors of 160 and 650 to Pq for the PqR and PqAtrR biotypes, respectively; defined as the ratio of the Pq concentrations that cause 50% inhibition of Fv/Fm [optimum quantum yield of PSII]), no photosynthetic advantage, e.g. a higher photosynthetic activity or a higher tolerance to HL stress, has been detected in our PqR and PqAtrR biotypes (Váradi et al., 1994; Darkó et al., 1996) as compared with the control biotypes (PqS and Atr-resistant). We also recently investigated whether antioxidant enzymes might be involved in Pq resistance (Turcsányi et al., 1994, 1998) and found that the activities of oxyradical-detoxifying enzymes (superoxide dismutases, ascorbate peroxidase, glutathione reductase, and catalase) did not correlate with the Pq resistance in PqAtrR and PqR biotypes of E. canadensis (Turcsányi et al., 1998).
Results of Pq treatment under different light conditions and dark-plus-light combinations (Váradi et al., 1990; Lehoczki et al., 1992) suggested that light plays a basic role in the mechanism of resistance to Pq. It was found that PqAtrR E. canadensis plants recovered from the inhibitory effect of Pq only in the light, and an increase in light intensity proved to have a pronounced enhancing effect on the recovery of variable fluorescence. It was suggested that light is essential not only for the photosynthetic process and Pq action, but also for the Pq resistance mechanism in photosynthesizing plant tissues.
Excessive light as well as Pq generates highly reactive oxygen species that can cause oxidative damage to the photosynthetic apparatus. Oxygenic photosynthetic organisms have evolved multiple photoprotective mechanisms to cope with the potentially damaging effects of light and oxidative stress. The xanthophyll cycle is known to be one of the main photoprotective mechanisms in photosynthesizing higher plant cells (Demmig-Adams and Adams, 1990; Owens, 1994; Pfündel and Bilger 1994). As yet, there is no agreement on the mechanism of the energy dissipation processes, but it is presumed that the xanthophyll cycle may directly or indirectly play an important role. Additionally, Lichtenthaler and Schindler (1992) and Schubert et al. (1994) proposed that the enzymatic and nonenzymatic epoxidation reactions of zeaxanthin may consume potentially damaging oxygen species. It was reported recently that the xanthophyll epoxidation cycle my protect the photosynthetic apparatus by several mechanisms (Havaux and Niyogi, 1999).
This widely accepted key role of the xanthophyll cycle in photoprotection and oxidative stresses, and the evidence for the essential role of light in the Pq action and Pq resistance mechanism (Váradi et al., 1990; Lehoczki et al., 1992) led to the proposal that the xanthophyll cycle might be a useful indicator in studies of the elementary processes of the Pq resistance mechanism. Preliminary experiments revealed that there is an altered response of the xanthophyll cycle and non-photochemical fluorescence quenching (NPQ) processes in the PqR biotype of E. canadensis during the first hours of Pq treatment as compared with the PqS (wild) biotype (Váradi et al., 1998). It was found that after 1 to 4 h of Pq treatment, violaxanthin was less de-epoxidized in the PqR plants than that in the PqS plants, and NPQ correlated well with the xanthophyll cycle function in Pq-treated PqR plants, but not in the Pq-treated PqS biotype.
This paper presents results of analyses of the xanthophyll cycle and fluorescence quenching on Pq-treated PqS and PqR biotypes of E. canadensis. Plants were treated under dim-light conditions, and the transient effects of illumination in the presence of Pq were then followed after a 10-min dark period. This experimental approach using dark-adapted Pq-treated plants allowed us to investigate light-induced transient processes that would be difficult to observe under natural conditions, to reveal some light-dependent elements of the Pq resistance mechanism.
RESULTS
Transient Inhibition of Photosynthetic Functions in the PqR Biotype
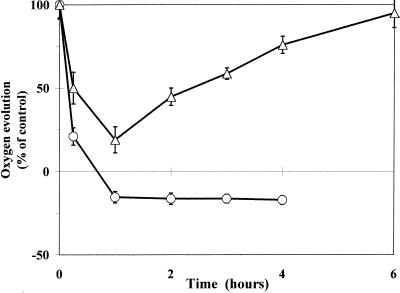
The effects of Pq on photosynthetic functions were demonstrated by O2 evolution measurements on leaf discs (Fig. 1) and chlorophyll fluorescence measurements on intact attached leaves (Fig. 2) under HL. The time course of the net O2 evolution of leaf discs in the presence of 0.5 mm Pq (the concentration usually applied in the field) in PqS and PqR biotypes of E. canadensis was measured at a photosynthetically active photon flux density (PPFD) of 850 μmol m−2 s−1 PPFD. Pq at a concentration usually applied in the field for weed control rapidly inhibited the net O2 evolution in both biotypes (Fig. 1). Leaf discs from the PqS biotype exhibited a more pronounced decrease in net O2 evolution, and about 1 h after Pq treatment they exhibited only a light-dependent O2 consumption due to the superoxide generation process. The net O2 evolution activity of the PqR biotype was less inhibited similarly to that of the PqAtrR biotype. The Pq-induced decrease of net O2 evolution was transient in the PqR leaves and they started to recover 1 h after Pq treatment.
Figure 1.
Time course of the net photosynthetic O2 evolution of leaf discs of PqR (▵) and PqS (○) biotypes of E. canadensis (in percentage of untreated control) in the presence of 0.5 mm Pq at a PPFD of 850 μmol m−2 s−1 (untreated leaves of the PqS and PqR biotypes evolved 53 ± 6 and 55 ± 5 μmol O2 mg−1 chlorophyll h−1, respectively). Pq was applied under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before any illumination. The measurement of O2 evolution was carried out in a leaf disc electrode and the linear rate of O2 evolution corrected for dark respiration was used to calculate the light-dependent O2 evolution or O2 consumption. The data are means of six replicates and se are shown when larger than the symbols.
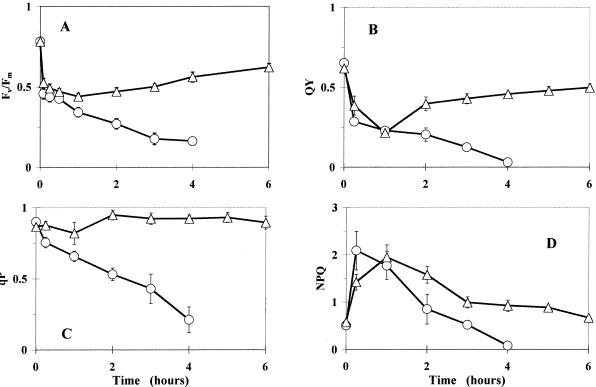
Figure 2.
Time courses of chlorophyll fluorescence parameters in intact attached leaves of PqR (▵) and PqS (○) biotypes of E. canadensis in the presence of 0.5 mm Pq under HL (1,400 μmol m−2 s−1). Pq was applied under dim-light (15 μmol m−2 s−1) conditions and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before HL illumination. Fluorescence induction kinetics were recorded after 10-min dark adaptation to obtain the Fv/Fm of PS II (A), and subsequent saturating flashes (3,000 μmol m−2 s−1) were applied to determine the QY of PS II electron transport (B), qP (C), and the non-photochemical quenching levels (D) at the end of 10 min at a PPFD of 600 μmol m−2 s−1. The data are means of five replicates and se are shown when larger than the symbols.
There was also a transient reduction of Fv/Fm in the PqR biotype in the first 2 h of Pq treatment, as has been shown for the PqAtrR E. canadensis biotype (Lehoczki et al., 1992). Fv/Fm in the PqS biotype was approaching zero after 4 h under similar conditions (Fig. 2A). The relative quantum yield of PS II photochemistry (QY) exhibited the same tendency (Fig. 2B), but it was more pronounced than in the case of the photochemical quenching coefficient (qP). The latter displayed only a slight transitory decline in the PqR plant, but decreased markedly in the PqS (wild) biotype (Fig. 2C). NPQ demonstrated a marked increase in the 1st h of Pq treatment, and there was then an expressed decline in both biotypes (Fig. 2D). After 4 h, however, NPQ in the PqR biotype approached the initial level (NPQ ≅ 0.9, corresponding to LL conditions in the absence of Pq, in contrast with the appropriate HL control, where NPQ was approximately 2.5), whereas it was negligible by the end of the 4th h of Pq treatment in the PqS plant.
The light response of Fv/Fm of the PqR biotype in the presence of 0.1 mm Pq was studied in attached intact leaves at low PPFDs (100, 200, and 500 μmol m−2 s−1) and in the dark to observe the light intensity dependence of the effect of Pq (Fig. 3). Pq caused only a slight modification of Fv/Fm in PqR plants in the dark, similar to that in PqAtrR E. canadensis (Lehoczki et al., 1992). Both the magnitude of the transient inhibition and the delay in the start of the recovery process demonstrated a clear light intensity dependence. It is striking that at PPFDs of 200 or 500 μmol m−2 s−1 Fv/Fm reached its minimum very quickly, indicating that Pq reached the site of action within a few minutes in the PqR plants. Another important observation is that the higher the PPFD applied to the PqR plant in the presence of Pq, the longer the maximum inhibition lasted, whereas plants exposed to all irradiances approached the same recovery level after 12 h (data not shown). Since light appeared to play a key role in the Pq effect and the mechanism of resistance to Pq, further experiments were focused on light-driven mechanisms (the violaxanthin epoxidation cycle and fluorescence quenching processes) of the photosynthetic apparatus.
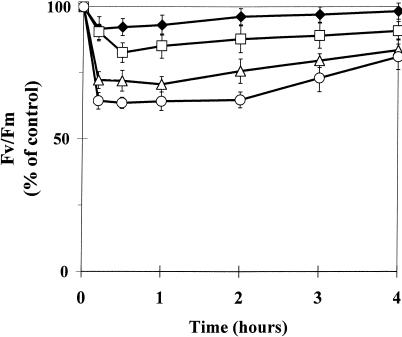
Figure 3.
Light-intensity dependence of the transient inhibition of the optimum quantum yield of PS II photochemistry (Fv/Fm) measured after 10-min dark adaptation in intact attached leaves of the PqR biotype of E. canadensis in the presence of 0.1 mm Pq. Pq was applied under dim-light (15 μmol m−2 s−1) conditions and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before the illumination at different PPFDs (dark control, ♦; 100 μmol m−2 s−1, □; 200 μmol m−2 s−1, ▵; and 500 μmol m−2 s−1, ○). Fluorescence induction kinetics were recorded after another 10-min dark adaptation. The data are means of eight replicates and se are shown when larger than the symbols.
Light Intensity Response of Violaxanthin De-Epoxidation in the Presence of Pq
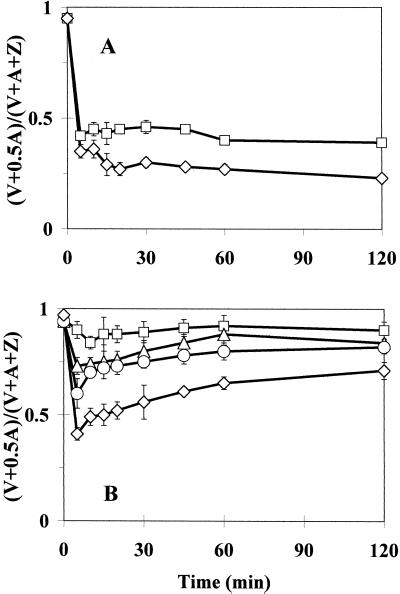
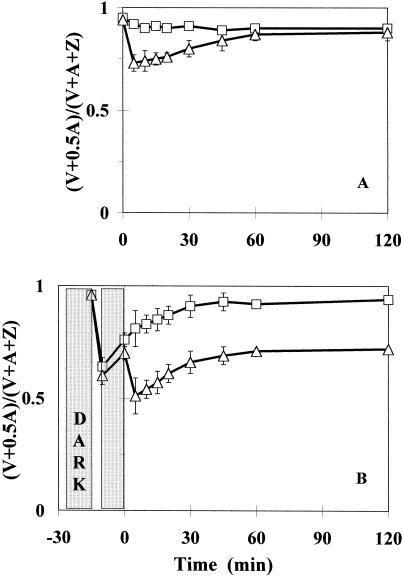
The light-driven de-epoxidation of violaxanthin (V), resulting in an accumulation of zeaxanthin (Z) via antheraxanthin (A) in the thylakoids, was characterized using the epoxidation index, Ei (epoxidation index) [where Ei = (V + 0.5 × A)/(V + A + i)]. The xanthophyll cycle de-epoxidation in 0.1 mm Pq-treated leaves of the E. canadensis biotypes was determined as a function of light intensity. Characteristic kinetics are shown at PPFDs of 100 and 1,400 μmol m−2 s−1 in the PqS biotype, whereas light-intensity-dependence in the PqR plants is demonstrated at PPFDs of 100, 200, 300, and 1,400 μmol m−2 s−1 (Fig. 4). There was a decrease in Ei (mainly due to zeaxanthin accumulation) within the first 5 min of illumination of Pq-treated leaves of both biotypes, similar to that observed in non-treated dark-adapted control leaves when illuminated (data not shown). In the PqS biotype, however, the magnitude of this Pq-plus-light-induced drop in Ei appeared to be independent of the light intensity, whereas a clear light intensity dependence was observed in the PqR biotype. It was not obvious whether this light intensity dependence of the Pq effect in the PqR biotype came from the light dependence of the electron transport rate in the presence of Pq or was a consequence of a light intensity-dependent Pq uptake into the chloroplasts of the PqR biotype. The Ei values in the Pq-treated leaves of the PqS biotype remained unchanged, keeping the relatively small initial difference in the steady-state levels of the plants illuminated at PPFDs of 100 and 1,400 μmol m−2 s−1. The slight dependence of the Pq effect on the light intensity in the PqS biotype suggested that the light intensity-dependence of the electron transport rate was not the main factor determining the magnitude of the transient de-epoxidation of violaxanthin in the presence of Pq in the PqR biotype. Moreover, after this light intensity-dependent first drop in Ei in the leaves of the PqR biotype, a recovery process started immediately and the Ei values approached their asymptotic levels after 1 or 2 h of Pq treatment. These steady-state levels also showed a characteristic light intensity-dependence in the PqR biotype, but the most interesting feature was the recovery of Ei to levels (0.7 in the case of 1,400 μmol m−2 s−1) much higher than the steady-state control in the PqR plants (0.38 for the untreated control exposed to 1,400 μmol m−2 s−1).
Figure 4.
Time course of changes in the Ei of the xanthophyll cycle at different light intensities in intact attached leaves of PqS (A) or PqR (B) biotypes of E. canadensis in the presence of 0.1 mm Pq. The Ei is expressed as (V + 0.5 × A)/(V + A + Z). Pq was applied under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before the illumination at different PPFDs of 100 (□), 200 (▵), 300 (○), and 1,400 (⋄) μmol m−2 s−1. The data are means of five replicates and se are shown when larger than the symbols.
These light intensity-dependent responses of the xanthophyll cycle in PqR plants in the presence of Pq suggested a light-mediated uptake of Pq in the case of PqR biotype.
Effect of HL Pre-Illumination on Violaxanthin De-Epoxidation in Pq-Treated PqR Leaves
De-epoxidation of the xanthophyll cycle pigments was used as an intrinsic probe to follow the effects of Pq at a thylakoidal level. Samples were collected from a separate experiment conducted under LL (200 μmol m−2 s−1) conditions to investigate the in vivo role of light in primary Pq action and the resistance mechanism in intact leaves of PqR E. canadensis plants (Fig. 5). The non-treated LL control showed only slight de-epoxidation, whereas the Pq plus LL treatment caused a marked, but transient decline in Ei (Fig. 5A).
Figure 5.
Time course of changes in the Ei of the xanthophyll cycle, expressed as (V + 0.5 × A)/(V + A + Z) in intact attached leaves of PqR E. canadensis (A) under LL (200 μmol m−2 s−1) in the absence (□, LL control) or In the presence (▵, Pq + LL) of 0.1 mm Pq. Effect of a short-term (5-min) HL (1,100 μmol m−2 s−1 PPFD) pre-illumination (B) on the time course of Ei in intact attached leaves of PqR E. canadensis under LL (200 μmol m−2 s−1) in the absence (□, 5 min HL + LL) or in the presence (▵, Pq + 5 min HL + LL) of 0.1 mm Pq. Pq was applied under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before any illumination. The short-term pre-illumination was followed by another 10-min dark period before the LL measurement to synchronize the fluorescence (Figs. 6 and 7) and xanthophyll cycle measurements. The data are means of six replicates and ses are shown when larger than the symbols.
This LL experiment was repeated by using a 5-min HL (1,100 μmol m−2 s−1) pre-illumination before the usual 10-min dark adaptation (Fig. 5B). The 5-min HL plus LL treatment led to a sudden drop in Ei within the first 5 min due to the HL illumination, and there was a noteworthy relaxation in the de-epoxidation during the 10-min dark period, which continued under LL conditions, reaching the LL control level (Fig. 5A) after 45 min. The effect of the 5-min HL pre-illumination on the time course of Ei under LL conditions was also investigated in the presence of Pq (see Fig. 5B; Pq plus 5-min HL plus LL treatment). In the HL pre-illumination period, Pq induced a marked reduction of Ei close to that of the 5-min HL effect without Pq (5-min HL plus LL) and Ei relaxed at a similar rate under the 10-min dark period. There was, however, a second and larger transient decline in Ei when the LL was switched on and the asymptotic level of that transient was near Ei = 0.7 (similar to the 1,400 μmol m−2 s−1 treatment in Fig. 4). These results suggested that in the case of the PqR biotype the light-mediated uptake of Pq may be the factor determining the Pq effect, but not the light intensity dependence of the electron transport rate in the presence of Pq.
In Vivo Chlorophyll a Fluorescence Quenching in Pq-Treated Leaves
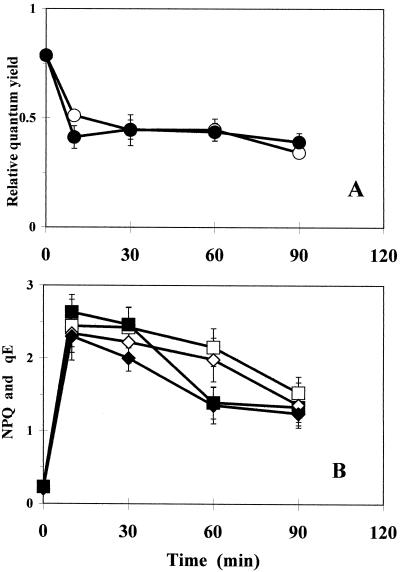
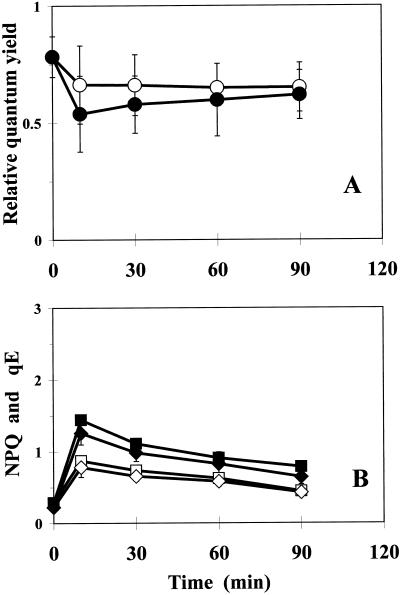
As a consequence of the observed transient kinetics of violaxanthin de-epoxidation in the PqR plants (see above), fluorescence quenching studies were focused on the events of the initial phase (the first 1–2 h) of Pq action in PqS and PqR plants. Chlorophyll fluorescence quenching processes were characterized by calculating the QY, NPQ, and high energy-dependent fluorescence quenching (qE) in Pq-treated PqS and PqR E. canadensis plants (Figs. 6 and 7, respectively) using a low PPFD of 200 μmol m−2 s−1. After a marked initial drop, QY declined in Pq-treated leaves of the PqS biotype (Fig. 6A), whereas both NPQ and qE (Fig. 6B) increased markedly initially, but then gradually declined. The QY (Fig. 7A), NPQ, and qE (Fig. 7B) were less affected in PqR plants under low PPFD (200 μmol m−2 s−1) as compared with the PqS biotype.
Figure 6.
Time course of QY (○) expressed as 1 − Fs/F'm (A), NPQ (□), and its qE, (⋄; B) in intact attached leaves of PqS E. canadensis in the presence of 0.1 mm Pq and under low PPFD (200 μmol m−2 s−1; open symbols). Closed symbols represent the effect of a short-term (5-min) HL (1,100 μmol m−2 s−1) pre-illumination (HL + LL). Pq was applied under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before any illumination. The quenching analysis was carried out after another 10-min dark adaptation and under low actinic PPFD (200 μmol m−2 s−1) to prevent additional effects caused by photoinhibition and related processes. The qE component of NPQ was calculated from the relaxation of the maximum fluorescence yield after the AL was switched off. The data are means of 10 replicates and ses are shown when larger than the symbols.
Figure 7.
Time course of QY (○) given by 1 − Fs/F'm (A), NPQ (□), and its qE, (⋄; B) in intact attached leaves of PqR E. canadensis in the presence of 0.1 mm Pq and at a low PPFD of 200 μmol m−2 s−1 (open symbols). Closed symbols represent the effect of a short-term HL (5-min, 1,100 μmol m−2 s−1) pre-illumination (HL + LL). Pq was applied under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before any illumination. The quenching analysis was carried out after another 10-min dark adaptation and under low actinic PPFD (200 μmol m−2 s−1) to prevent additional effects caused by photoinhibition and related processes. The qE component of NPQ was calculated from the relaxation of the maximum fluorescence yield after the AL was switched off. The data are means of 10 replicates and ses are shown when larger than the symbols.
When this quenching analysis at a PPFD of 200 μmol m−2 s−1 was repeated after a short, but intensive pre-illumination (5 min, 1,100 μmol m−2 s−1 PPFD) before the 10-min dark adaptation (Figs. 6 and 7), there were different responses in the two biotypes. The QY was only slightly affected in the initial phase in the PqS biotype (Fig. 6A), whereas there was a more pronounced longer-lasting effect in the PqR plant (Fig. 7A).
Effect of Pq Concentration
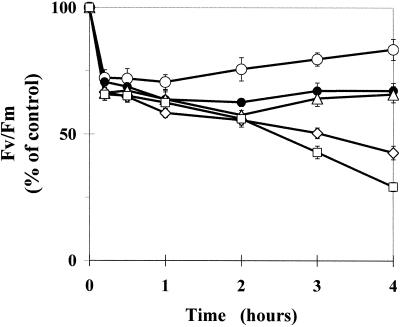
The concentration dependence of Pq action in the PqR biotype was characterized using Fv/Fm measurements. The effect of the concentration of Pq applied to attached intact leaves of PqR E. canadensis on the transient inhibition of Fv/Fm at a PPFD of 200 μmol m−2 s−1 is shown in Figure 8, where the Pq concentration ranged from 0.1 to 5.0 mm. A concentration of 0.5 mm Pq corresponds to the concentration usually applied in agricultural practice. Below this concentration (i.e. 0.1 mm), a similar prompt effect with a more rapid recovery is observed than at higher concentrations (0.5–5.0 mm). The data in Figure 8 demonstrates that the effect of Pq on Fv/Fm in the PqR biotype is not simply a function of the applied Pq concentration.
Figure 8.
Time course of the maximum quantum yield of PS II photochemistry (Fv/Fm) in intact attached leaves of the PqR biotype of E. canadensis in the presence of different Pq concentrations under a low PPFD of 200 μmol m−2 s−1, expressed as a percentage of the untreated control value. Pq was applied to the leaf surface in different concentrations (○, 0.1 mm; ●, 0.5 mm; ▵, 1.0 mm; ⋄, 2.5 mm; and □, 5.0 mm) under dim-light (15 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min to allow the surface of the leaves to become dry before any illumination. Fluorescence induction kinetics were recorded after a further 10-min dark adaptation. The data are means of six replicates and ses are shown when larger than the symbols.
DISCUSSION
In light-exposed plants, Pq exerts its primary effect as a strong electron acceptor in PS I, with the rapid regeneration of oxidized Pq by O2. Pq is therefore available to transfer electrons to molecular O2 and it is effective for a long time inside the chloroplasts of PqS plants. The light-driven electron flow from PS II is diverted on the acceptor side of PS I to Pq instead of ferredoxin-NADP reductase, resulting in the depletion of NADPH and a consequent inhibition of CO2 fixation. An unbalanced photosynthetic system with a low photochemical efficiency, together with a decreasing NPQ, and a highly de-epoxidized state of the xanthophyll cycle pigments, appear to be the first signs of the phytotoxic process of Pq action in PqS plants (Figs. 2, 4, and 6). The kinetics of NPQ were similar for the PqS and PqR biotypes during the first few hours of Pq treatment, whereas qP markedly decreased in the PqS biotype, but remained almost unchanged in the PqR plants (Fig. 2). The QY, however, declined in the PqS plants, whereas in the PqR plants, after an initial decline in the 1st hour, it gradually recovered. This suggests an uncontrolled destructive process in the PqS plants, but some Pq elimination/inactivation process in the PqR plant leaves. Furthermore, the highly de-epoxidized state of the xanthophyll cycle, observed in the PqS biotype during several hours of Pq action (Fig. 4), does not seem to support the hypothesis that zeaxanthin may be epoxidized by harmful oxygen species in a nonenzymatic process (Lichtenthaler and Schindler, 1992; Schubert et al., 1994). Furthermore, the enzymatic epoxidation of zeaxanthin to antheraxanthin and violaxanthin might be prevented in the PqS, but not in the PqR biotype, in consequence of the presumed depletion of NADPH during constant Pq action at PSI, diverting electrons from NADP reduction in PqS plants.
In accordance with other authors (Gilmore and Yamamoto, 1991; Büch et al., 1994; Günther et al., 1994; Thiele and Krause, 1994), we found that Pq is able to induce in vivo NPQ and zeaxanthin formation in photosynthesizing leaves of PqS plants. Rapid effects of Pq in the leaves of both the PqS and PqR biotypes confirmed our earlier hypothesis that Pq can reach the site of action in the chloroplasts immediately, not only in the PqS, but also in the PqR and PqAtrR biotypes of E. canadensis (Váradi et al., 1990; Lehoczki et al., 1992). Studies on the effects of the Pq concentration applied to the PqR plants on the time course of Fv/Fm at low PPFD (Fig. 8) revealed that within the first 2 h of treatment with relatively high Pq concentrations (ranging from the normal agricultural concentration up to 10 times), there was almost no concentration dependence in the transient inhibition of Fv/Fm. At the highest Pq concentrations applied (2.5 and 5.0 mm), however, there was a concentration-dependent and irreversible breakdown of PqR plants, starting after 2 h. This may indicate that (a) The penetration of Pq into the chloroplasts of the PqR biotype is not simply a function of the Pq concentration gradient, and that (b) there is a limited capacity of the eliminating/protecting processes and that this was overloaded by the highest Pq concentration after 2 h.
Ei revealed a characteristic transient in the PqR biotype at all light intensities (Fig. 4), indicating that Pq entered the chloroplast and that some elimination of Pq may have been quickly induced when the leaves were illuminated. This seems to be the earliest biochemical response relating to the primary action of Pq in photosynthesizing plant tissues, and also the most rapidly relaxing of the well known biophysically and biochemically detected transients in Pq-treated PqR E. canadensis. The magnitude of the initial drop in Ei in the PqR biotype appeared to be dependent on the light intensity, but only slightly so in the case of the PqS biotype, indicating some light-driven mechanism in the PqR biotype. Another noteworthy difference in time course in the PqR biotype was the light-dependent asymptotic level of Ei, which was approached after some hours of Pq treatment. This Ei was much higher in Pq-treated PqR plants than in the untreated control at a PPFD of 1,400 μmol m−2 s−1 (0.70 and 0.38, respectively). The rapid elimination of Pq in the PqR biotype may not be sufficient itself to explain the unusually low de-epoxidation state of the xanthophyll cycle in the Pq-treated PqR plants at high PPFDs. Furthermore, the similarity of the light responses of the xanthophyll cycle (Váradi et al., 1994) in the untreated PqS and PqR biotypes (Ei values of 0.42 and 0.38, respectively) does not explain these differences in light response in the presence of Pq.
After Pq treatment NPQ and qE initially correlated well with the zeaxanthin accumulation in both biotypes. Later, however, NPQ declined gradually in both biotypes, but did not further correlate with zeaxanthin in the PqS biotype (Figs. 2D and 4A). This implies that the quenching process was functionally impaired in the PqS biotype, but not in the PqR plants (Figs. 2D and 4B). An unusual down-regulation of the xanthophyll cycle de-epoxidation and NPQ was observed in PqR E. canadensis treated with Pq, resulting in low steady-state levels of zeaxanthin and NPQ (Figs. 2D and 4B) under HL conditions (Ei ≅ 0.7 and NPQ ≅ 0.9 as compared with the corresponding non-treated light control values of Ei ≅ 0.4 and NPQ ≅ 2.5). It is difficult to explain this misadjustment of non-radiative energy dissipation in the PqR plant in the presence of Pq at the present. Furthermore, it is difficult to determine whether this phenomenon is involved in the resistance mechanism or is only an indicator of other processes. We can hypothesize that, in parallel with the restriction of the Pq uptake and the suggested elimination of Pq in PqR plants, some unknown process takes place in Pq-treated PqR plants, which results in this irregular light response of the xanthophyll cycle and xanthophyll cycle-mediated NPQ formation in Pq-treated PqR E. canadensis. We presume that (in parallel with the elimination of Pq in the PqR plant) some overcompensation (against the increased electron transport and the large ΔpH) takes place in the regulatory processes. Further experiments are necessary to clarify this point.
A short but intensive (5-min, 1,100 μmol m−2 s−1) pre-illumination of the Pq-treated leaves of the PqR biotype before the 10-min dark adaptation preceding the LL (200 μmol m−2 s−1) monitoring of fluorescence quenching (Fig. 7) and xanthophyll cycle (Fig. 5B) resulted in the same time course as when the experiment had been conducted under continuous HL irradiation (Fig. 4). This implies that the amount of Pq reaching the target site was determined by the initial light intensity, whereas the activity of the elimination process did not demonstrate such a light intensity-dependence, though a minimum flux of light quanta was essential to induce it.
A difference in overall linear electron transport rate between the two biotypes has recently been implied to exist in the background of resistance (Chase et al., 1998), and electron transfer rate differences have been reported between PqS and PqR biotypes of Solanum americanum (Chase et al., 1998) and E. canadensis (Lehoczki et al., 1992). The data for E. canadensis cited from Lehoczki et al. (1992), however, were related to the PqAtrR biotype, which has been shown to have a lowered photosynthetic electron transport rate due to its strong Atr resistance (Váradi et al., 1994; Darkó et al., 1996). Furthermore, however, it has also been reported that PqR E. canadensis does not differ significantly from the PqS biotype in the functioning of the xanthophyll cycle under different light regimes or in its susceptibility to HL treatment (Váradi et al., 1994; Darkó et al., 1996), and it can be assumed that the light responses of electron transport in the two biotypes are also similar in the presence of Pq.
In summary, we can conclude that (a) Light is essential to initiate the mechanisms of resistance to Pq in PqR E. canadensis; (b) light is essential for the effective initial uptake of Pq by the chloroplasts of PqR plants; (c) the Pq uptake seems to be limited in the early phase of Pq action in PqR plants, possibly in consequence of light plus Pq-induced barrier formation; (d) the amount of Pq entering the chloroplasts of Pq-treated PqR plants is apparently determined by the initial PPFD; (e) the unusual violaxanthin de-epoxidation and NPQ formation in PqR plants during Pq treatment may indicate a Pq-induced down-regulation of photosynthetic electron transport, overcompensating the electron-capturing effect of Pq and presumably decreasing the harmful effects of the action of Pq until it is sequestered/eliminated; and (f), as the penalty for Pq resistance, the Pq-treated PqR plants may suffer from a photoinhibition of photosynthesis, due to the lack of effective non-radiative energy dissipation.
MATERIALS AND METHODS
Plant Material
Seeds of PqR and PqS biotypes of Erigeron canadensis (L.) were collected at various locations in Hungary (Lehoczki et al., 1984; Pölös et al., 1988). They were germinated and grown in soil containers in the greenhouse for 2 to 3 months and then transferred to a natural environment (natural light conditions with a daily maximum PPFD of about 1,600 μmol m−2 s−1). Rosette-stage plants aged 14 to 16 weeks with fully-developed leaves were used for experiments.
Pq and Light Treatments
All treatments and measurements were carried out under laboratory conditions. Formulated Pq (Gramoxone, 25% active ingredient) was applied in diluted form to the leaf surface. The concentrations of the Pq solutions applied to the leaf surface were as indicated in “Results” and in the figure legends. Pq solutions were sprayed onto the selected leaves or the leaves were wrapped in tissue paper wetted with Pq solution and then covered with aluminum foil (both methods gave similar results). Pq was applied under dim-light conditions (15–20 μmol m−2 s−1) and the treated leaves were then kept in the dark for 10 min before any following treatment or measurement to allow the surface of the treated leaves to become dry for better reproducibility. After this uniform Pq treatment, the treated leaves were exposed to different light regimes according to the aim of the experiment in question. The exact light conditions of the treatments and measurements are indicated in the “Results” sections relating to the fluorescence and the xanthophyll cycle measurements and in the figure legends.
Fluorescence Induction Measurements
All fluorescence measurements were started after an additional 10-min dark adaptation (different from that applied after the Pq treatment) within the measuring head of the instrument. Fluorescence quenching analysis was carried out with a dual channel modulated fluorimeter (Hansatech, King's Lynn, UK) in the region of 730 nm emission, with 1-s saturating pulses of 3,000 μmol m−2 s−1 (sufficient to close all of the PS II reaction centers even in the presence of Pq), and the terminology suggested by van Kooten and Snel (1990) was used. After the 10-min dark adaptation, the leaves were initially exposed to a weak (0.5 μmol m−2 s−1) yellow-modulated measuring beam for measurement of the initial fluorescence yield (Fo). The maximum fluorescence yield (Fm) was obtained by exposing the leaf sample to the saturating pulse, and the quenched levels of maximum fluorescence (Fm′) were then determined under low actinic light (AL; 200 μmol m−2 s−1) conditions at the end of the 10-min AL illumination. After the AL had been switched off, far-red light was applied for the determination of the minimal level of fluorescence at steady-state (Fo′). The qP was calculated according to Schreiber et al. (1986) and NPQ was calculated according to the equation: NPQ = (Fm − Fm′)/Fm′ (Bilger and Björkman, 1990). The QY = ΔF/Fm′ (or 1 − Fs/F′m), was determined according to Genty et al. (1989) where Fs is the steady-state fluorescence level and ΔF = Fm′ − Fs. For estimation of the qE of NPQ, the relaxation kinetics of NPQ after AL off were followed by using saturating pulses delivered at 90-s intervals. The fast-relaxing (within the first 10 min of dark relaxation after light treatment) component of fluorescence quenching was assigned to the qE. qE was calculated according to Thiele et al. (1997): qE = Fm/Fm′ − Fm/Fm", where Fm" is the maximum fluorescence yield after 10 min of dark relaxation of the samples subsequent to illumination with AL.
Xanthophyll Cycle Analysis
Xanthophyll cycle pigments were determined by HPLC. Samples for HPLC analysis were fixed and stored in liquid nitrogen until the sample preparation. Samples were ground in liquid nitrogen in Eppendorf tubes, extracted overnight with acetone:water (85:15, v/v) at 0°C and then centrifuged for 30 min at 8,000g. The pellet was re-extracted twice with pure acetone under similar conditions. Combined extracts were homogenized, recentrifuged, and injected directly onto a reversed phase HPLC column (5 μm, 4 × 150 mm, C18, Nucleosil 120, BST, Budapest). For separation of zeaxanthin from lutein, the gradient program of A-eluent: acetonitrile:water (9:1, v/v) plus 0.1% (v/v) triethylamine and B-eluent: ethyl acetate was used. Linear gradient from 0% to 32% (v/v) was used in the first 18 min, followed by 32% to 100% for 6 min, then a plateau (100% B) for the next 6 min was followed by a drop to 0% B at the end (30 min). The column was re-equilibrated with A-eluent for 10 min (flow rate of 1 mL min−1). Xanthophyll peaks were detected at 450 nm and quantified by using zeaxanthin from Roche (Basel) and violaxanthin and antheraxanthin prepared by thin-layer chromatography.
O2 Evolution Measurements
A leaf disc electrode unit (LD 2, Hansatech) with a high-intensity light source (LS 2, Hansatech) and a heat filter (Melles Griot, Irvine, CA) was used to measure the O2 evolution by leaf discs at 25°C according to Delieu and Walker (1983). In each experiment 6 cm2 leaf pieces were cut from fully expanded young leaves and placed into the leaf chamber unit, which contained 21% (w/v) O2 and 1% (w/v) CO2 (from 1 m carbonate/bicarbonate buffer solution at pH 9). The leaf discs were illuminated with white light of 850 μmol m−2 s−1. The linear rate of O2 evolution corrected for dark respiration was used to calculate the light-dependent O2 evolution or O2 consumption in Pq-treated leaves, expressed as micromole O2 per milligram chlorophyll per hour. The chlorophyll content per unit leaf area was determined spectrophotometrically in 80% (w/v) acetone according to Lichtenthaler (1987).
Footnotes
This work was supported by the Hungarian Research Fund (OTKA T–16445).
LITERATURE CITED
- Bilger W, Björkman O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Büch K, Stransky H, Bigus HJ, Hager A. Enhancement by artificial electron acceptors of thylakoid lumen acidification and zeaxanthin formation. J Plant Physiol. 1994;144:641–648. [Google Scholar]
- Chase CC, Bewick TA, Shilling DG. Differential photosynthetic electron transport and oxidative stress in paraquat-resistant and sensitive biotypes of Solanum americanum. Pestic Biochem Physiol. 1998;60:83–90. [Google Scholar]
- Darkó É, Váradi G, Dulai S, Lehoczki E. Atrazine-resistant biotypes of Conyza canadensis have altered fluorescence quenching and xanthophyll cycle pattern. Plant Physiol Biochem. 1996;34:843–852. [Google Scholar]
- Darkó É, Váradi G, Lemoine Y, Lehoczki E. Defensive strategies against high-light stress in wild and D1 protein mutant biotypes of Erigeron canadensis. Aust J Plant Physiol. 2000;27(4):325–333. [Google Scholar]
- Delieu TD, Walker DA. Simultaneous measurement of oxygen evolution and chlorophyll fluorescence from leaf pieces. Plant Physiol. 1983;73:542–549. doi: 10.1104/pp.73.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW. The carotenoid zeaxanthin and “high-energy-state quenching” of chlorophyll fluoresence. Photosynth Res. 1990;25:187–197. doi: 10.1007/BF00033160. [DOI] [PubMed] [Google Scholar]
- Dodge AD. The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavor. 1971;30:130–135. doi: 10.1016/0160-9327(71)90039-1. [DOI] [PubMed] [Google Scholar]
- Dodge AD. Herbicide action and effects on detoxification processes. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 219–236. [Google Scholar]
- Fuerst EP, Nakatani HY, Dodge AD, Penner D, Arntzen CJ. Paraquat resistance in Conyza. Plant Physiol. 1985;77:984–989. doi: 10.1104/pp.77.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst EP, Vaughn KC. Mechanism of paraquat resistance. Weed Technol. 1990;4:150–156. [Google Scholar]
- Fujii T, Yokoyama E, Inoue K, Sakurai H. The sites of electron donation of photosystem I to methyl viologen. Biochim Biophys Acta. 1990;1015:41–48. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Biochim Biophys Acta. 1989;990:87–92. doi: 10.1007/BF00033166. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamamoto HY. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 1991;96:197–209. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther G, Thiele A, Laasch H. A new method for the determination of the transthylakoid pH gradient in isolated chloroplasts: the pH-dependent activity of violaxanthin de-epoxidase. Plant Sci. 1994;102:19–30. [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JS, Stemler AJ, Radosevich SR. Differential light responses of photosynthesis by triazine-resistant and triazine-susceptible Senecio vulgaris biotypes. Plant Physiol. 1981;67:744–748. doi: 10.1104/pp.67.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Shaaltiel Y, Kazzes D, Canaani O, Malkin S, Gressel J. Increased tolerance to photoinhibitory light in paraquat-resistant Conyza bonariensis measured by photoacoustic spectroscopy and 14CO2-fixation. Plant Physiol. 1989;91:1174–1178. doi: 10.1104/pp.91.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasat MM, DiTomaso JM, Hart JJ, Kochian LV. Evidence for vacuolar sequestration of paraquat in roots of a paraquat-resistant Hordeum glaucum biotype. Physiol Plant. 1997;99:255–262. [Google Scholar]
- Lehoczki E, Laskay G, Gaál I, Szigeti Z. Mode of action of paraquat in leaves of paraquat-resistant Conyza canadensis (L.) Cronq. Plant Cell Environ. 1992;15:531–539. [Google Scholar]
- Lehoczki E, Laskay G, Pölös E, Mikulás J. Resistance to triazine herbicides in horseweed (Conyza canadensis) Weed Sci. 1984;32:669–674. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Lichtenthaler HK, Schindler C. Studies on the photoprotective function of zeaxanthin at high-light conditions. In: Murata N, editor. Research in Photosynthesis. IV. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 517–520. [Google Scholar]
- Owens TG. Excitation energy transfer between chlorophylls and carotenoids: a proposed molecular mechanism for non-photochemical quenching. In: Baker NR, Bowyered JR, editors. Photoinhibition of Photosynthesis. Oxford: Bios Scientific; 1994. pp. 95–107. [Google Scholar]
- Pfündel EE, Bilger W. Regulation and possible function of the violaxanthin cycle. Photosynth Res. 1994;42:89–109. doi: 10.1007/BF02187121. [DOI] [PubMed] [Google Scholar]
- Pölös E, Mikulás J, Szigeti Z, Matkovics B, Hai DQ, Párducz Á, Lehoczki E. Paraquat and atrazine co-resistance in Conyza canadensis (L.) Cronq. Pestic Biochem Physiol. 1988;30:142–154. [Google Scholar]
- Powles SB, Cornic G. Mechanism of paraquat resistance in Hordeum glaucum: I. Studies with isolated organelles and enzymes. Aust J Plant Physiol. 1987;14:81–89. [Google Scholar]
- Preston C. Resistance to photosystem I disrupting herbicides. In: Powles SB, Holtum JAM, editors. Herbicide Resistance in Plants: Biology and Biochemistry. Boca Raton, FL: Lewis Publishers; 1994. pp. 61–82. [Google Scholar]
- Preston C, Holtum JAM, Powles SB. On the mechanism of resistance to paraquat in Hordeum glaucum and H. leporinum: delayed inhibition of photosynthetic O2 evolution after paraquat application. Plant Physiol. 1992;100:630–636. doi: 10.1104/pp.100.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical quenching of chlorophyll fluorescence with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Schubert H, Kroon BMA, Matthijs HCP. In vivo manipulation of the xanthophyll cycle and the role of zeaxanthin in the protection against photodamage in the green alga Chlorella pyrenoidosa. J Biol Chem. 1994;269:7267–7272. [PubMed] [Google Scholar]
- Shaaltiel Y, Gressel J. Multienzyme oxygen radical detoxifying system correlated with paraquat resistance in Conyza bonariensis. Pestic Biochem Physiol. 1986;26:22–28. [Google Scholar]
- Sundby C, Chow WS, Anderson JM. Effects on photosystem II function, photoinhibition, and plant performance of the spontaneous mutation of serine-264 in the photosystem II reaction center D1 protein in triazine-resisant Brassica napus L. Plant Physiol. 1993;103:105–113. doi: 10.1104/pp.103.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Chisaka H, Saka H. Movement of paraquat in resistant and susceptible biotypes of Erigeron philadelphicus and E. canadensis. Physiol Plant. 1986;66:605–608. [Google Scholar]
- Thiele A, Krause GH. Xanthophyll cycle and thermal energy dissipation in photosystem II: relationship between zeaxanthin formation, energy-dependent fluorescence quenching and photoinhibition. J Plant Physiol. 1994;144:324–332. [Google Scholar]
- Thiele A, Winter K, Krause GH. Low inactivation of D1 protein of photosystem II in young canopy leaves of Anacardium excelsum under high-light. J Plant Physiol. 1997;151:286–292. [Google Scholar]
- Turcsányi E, Darkó É, Borbély G, Lehoczki E. The activity of oxyradical-detoxifying enzymes is not correlated with paraquat resistance in Conyza canadensis (L.) Cronq. Pestic Biochem Physiol. 1998;60:1–11. [Google Scholar]
- Turcsányi E, Surányi GY, Lehoczki E, Borbély G. Superoxide dismutase activity in response to paraquat resistance in Conyza canadensis (L.) Cronq. J Plant Physiol. 1994;144:599–606. [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Váradi G, Darkó É, Lehoczki E. Altered light response of xanthophyll cycle in herbicide-resistant Erigeron canadensis biotypes in the presence of paraquat. In: Garab G, editor. Photosynthesis: Mechanisms and Effects. V. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 3901–3904. [Google Scholar]
- Váradi G, Darkó É, Pölös E, Szigeti Z, Lehoczki E. Xanthophyll cycle patterns and in vivo photoinhibition in herbicide-resistant biotypes of Conyza canadensis. J Plant Physiol. 1994;144:669–674. [Google Scholar]
- Váradi G, Lehoczki E, Szigeti Z, Pölös E. Short-term paraquat effects on paraquat-resistant horseweed Conyza canadensis Cronq. Proceedings of the 9th Australian Weeds Conference, August 6–10, 1990, Adelaide, South Australia. 1990. pp. 257–259. [Google Scholar]
- Vaughn KC, Fuerst E. Structural and physiological studies of paraquat-resistant Conyza. Pestic Biochem Physiol. 1985;24:86–94. [Google Scholar]
- Vaughn KC, Vaughan MA, Camilleri P. Lack of cross-tolerance of paraquat-resistant hairy fleabane (Conyza bonariensis) to other toxic oxygen generators indicates enzymatic protection is not the resistance mechanism. Weed Sci. 1989;37:5–11. [Google Scholar]