Abstract
Proton functional magnetic resonance spectroscopy (1H fMRS) is a noninvasive neuroimaging technique capable of detecting dynamic changes in glutamate related to task-related demands at a temporal resolution under 1 min. Several recent 1H fMRS studies demonstrated elevated steady-state levels of glutamate of 2% or greater during different ‘task-active’ conditions, relative to a ‘non-task-active’ control condition. However, the ‘control’ condition from these studies does vary with respect to the degree of constraining behavior, which may lead to different glutamate levels or variability between ‘control’ conditions. The purpose of this 1H fMRS study was to compare the steady-state levels and variability of glutamate in the left dorsolateral prefrontal cortex (dlPFC) of 16 healthy adults across four different putative ‘non-task-active’ conditions: relaxed with eyes closed, passive visual fixation crosshair, visual flashing checkerboard, and finger tapping. Results showed significantly lower glutamate levels during the passive visual fixation crosshair than the visual flashing checkerboard and the finger tapping conditions. Moreover, glutamate was significantly less variable during the passive visual fixation crosshair and the visual flashing checkerboard than the relaxed eyes closed condition. Of the four conditions, the passive visual fixation crosshair condition demonstrated the lowest and least variable glutamate levels potentially reflecting the least dlPFC engagement, but greatest behavioral constraint. These results emphasize the importance of selecting a proper ‘control’ condition to reflect accurately a ‘non-task-active’ steady-state level of glutamate with minimal variability during 1H MRS investigations.
Keywords: Functional proton magnetic resonance spectroscopy, 1H fMRS, Dorsolateral prefrontal cortex, glutamate, Resting state
Introduction
Proton magnetic resonance spectroscopy (1H MRS) facilitates non-invasive quantification of brain metabolite levels. Improved hardware, acquisition approaches, and higher B0 field-strength magnets have spurred interest in investigating the temporal dynamics of task-modulated neurochemistry (Duncan et al., 2014a; Stanley and Raz, 2018 - pending revision). Specifically, glutamate, a key neurochemical of interest in 1H fMRS, is the prominent excitatory neurotransmitter in the brain; ~80% of cortical neurons are glutamatergic (Shepherd and Oxford University Press. 2004). Additionally, glutamate acts as a metabolic substrate in the tricarboxylic acid (TCA) cycle, as a precursor for other metabolites (GABA and glutamine), and is important for ammonia detoxification (Cooper and Jeitner, 2016; Suarez et al., 2002). Recently, several 1H fMRS studies demonstrated increased steady-state levels of glutamate in response to task-based stimulation related to the hippocampus and in sensory, motor, and prefrontal cortices (Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2013; Schaller et al., 2014; Stanley et al., 2017; Woodcock et al., 2018 - under review). Table 1 provides a summary of 1H fMRS studies reporting changes in glutamate with task. Task-related increase in glutamate levels relative to a ‘non-task-active’ comparison condition likely reflects increased glucose extraction and metabolism in brain regions associated with stimulation driven by task-related demands (Mangia et al., 2012; Sonnay et al., 2016). The compelling evidence demonstrates high sensitivity in the ability of 1H fMRS to detect task engagement via glutamate modulation related to the functional specialization of the associated brain area under investigation. However, little attention is given to the selection of the ‘non-task-active’ comparison conditions, which differ greatly between 1H fMRS studies (Table 1).
Table 1.
Summary of 1H fMRS studies reporting task-related changes in glutamate.
| Study | Sample Size | Acquisition Protocol | Task | Non-Task-Active Control Condition | Results |
|---|---|---|---|---|---|
| Mullins et al. (2005) |
|
|
|
|
|
| Mangia et al. (2007) |
|
|
|
|
|
| Gussew et al. (2010) |
|
|
|
|
|
| Lin et al. (2012) |
|
|
|
|
|
| Schaller et al. (2013) |
|
|
|
|
|
| Schaller et al. (2014) |
|
|
|
|
|
| Bednařík et al. (2015) |
|
|
|
|
|
| Taylor et al. (2015a) |
|
|
|
|
|
| Taylor et al. (2015b) |
|
|
|
|
|
| Huang et al, 2015 |
|
|
|
|
|
| Apsvalka et al. (2015) |
|
|
|
|
|
| Jahng et al. (2016) |
|
|
|
|
|
| Woodcock et al. (2017) |
|
|
|
|
|
| Stanley et al. (2017) |
|
|
|
|
|
| Lindner et al. (2017) |
|
|
|
|
|
active’ comparison condition is expected to be inert with respect to the functional specialization of that region leading to relatively lower
The intent of the ‘task-active’ condition is to drive metabolic demand, and thus, modulate glutamate levels in the brain region of interest, based on the region's putative functional specialization. In contrast, the ‘non-task-steady-state and less variable glutamate levels. In sensory and motor cortices, the ‘non-task-active’ condition may appear obvious. For example, an optimal comparison condition for the investigation of visual stimulation in the occipital cortex is the absence of visual stimuli, such as a blank screen or blank screen with a crosshair (Table 1). However, the complexity of conducting 1H fMRS in executive and associative cortices, such as the dorsolateral prefrontal cortex (dlPFC), is magnified. The dlPFC is responsible for numerous cognitive functions and is susceptible to modulation by intrinsic neural activity, such as intrusive thoughts or environmental distractions, that encompass the broader umbrella of executive functions (Miller and Cohen, 2001). Thus, the dlPFC is a particularly challenging region to establish a proper ‘non-task-active’ comparison condition for task-based 1H fMRS studies. In a 1H fMRS study, Woodcock et al. (2018 - under review) used a visual crosshair condition as the ‘non-task-active’ condition which preceded the N-back working memory task, because the interspersed rest epochs between the N-back blocks demonstrated greater variability in the dlPFC glutamate compared to the glutamate during the N-back blocks. Similarly, Stanley et al. (2017) used a finger tapping condition to contrast a hippocampal learning and memory task because the variability of glutamate in the hippocampus during rest epochs using a crosshair was much greater than the task epochs (unpublished results). Additionally, several 1H fMRS studies have noted greater variability in glutamate during the ‘rest’ comparison condition than the ‘task-active’ condition (Ip et al., 2017; Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2013; Schaller et al., 2014). Collectively, these observations suggest that the level of constraint on behavior imposed during these different ’non-task-active’ control conditions (e.g., relaxed eyes closed vs fixation on a crosshair) may impact the steady-state level of glutamate and its variability during acquisition. Thus, the focus of the present 1H fMRS study is to assess the degree to which different control conditions truly reflect a ‘non-task-active’ state.
To address this issue, we conducted a series of 1H fMRS experiments in the left dlPFC of healthy young adults using four different putative ‘non-task-active’ conditions: relaxed eyes closed, passive visual fixation crosshair, visual flashing checkerboard, and a finger tapping task. Steady-state glutamate levels and their variability during these conditions were quantified and compared. The dlPFC is a region of broad interest across psychiatric disorders and cognitive neuroscience. Additionally, these ‘non-task-active’ conditions were selected due to their lack of primary neural engagement associated with the functional specialization of the dlPFC. However, because of the variability in the level of behavioral engagement between conditions, we are hypothesizing significant differences in the steady-state level of glutamate and variability between conditions. We hypothesize the visual flashing checkerboard will exhibit lower and less variable steady-state levels of glutamate.
Materials and methods
Participants
The Wayne State University Institutional Review Board approved all study procedures. Sixteen right hand dominant adult volunteers narrowly selected for age (9 males; mean age ± SD: 24.3 ± 3.5 years old) were recruited. Participants reported no psychiatric or neurological disorders, MRI contraindications, or current use of psychoactive medications. Participants provided written informed consent and were compensated for their time.
Experimental conditions
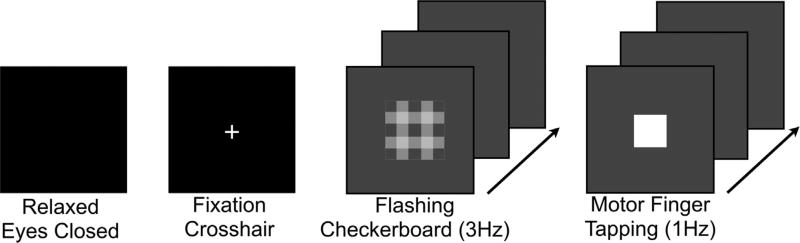
All scans were completed in the morning (between 9:30 a.m. and 11:30 a.m.). The 1H fMRS data were acquired during each of the four ‘non-task-active’ conditions. Subjects were given an explicit verbal description of each one of the four conditions prior to the scans. The four conditions were as follows: 1) relaxed eyes closed – subjects were instructed to relax and keep eyes closed; 2) passive visual fixation crosshair - subjects focused on a static, white crosshair projected in the center of the screen; 3) visual flashing checkerboard - subjects focused on a flashing grayscale checkerboard pattern (3 Hz) projected in the center of the screen; and 4) finger tapping - subjects pressed a button with their right index finger each time a white box (1 Hz) was projected in the center of the screen. The relaxed eyes closed and visual flashing checkerboard conditions were always the first and last to be administered, respectively; passive visual fixation crosshair and finger tapping were administered randomly (Fig. 1).
Fig. 1.
The four 1H fMRS ‘control’ conditions acquired as separate scans from left to right include: relaxed with eyes closed, passive visual fixation crosshair, visual flashing checkerboard and motor finger tapping.
The acquisition order of the imaging protocol included acquiring T1-weighted MRI images followed by setting up the 1H fMRS (voxel placement and shimming) followed by acquiring the 1H fMRS of the four conditions. The rationale for always starting with the “relaxed eyes closed condition”, which was the least constrained condition, was to ensure subjects were indeed relaxed without being confounded by thoughts from previous conditions as may be the case if the order was random. The visual checkerboard condition was always presented last because it was the first part of a longer two-part N-back working memory task (Woodcock et al., 2018 - under review). Brief breaks were given, and verbal instructions provided, before the start of the next condition.
1H fMRS acquisition and processing
All scans were completed on a 3T Siemens Verio system with a 32-channel volume head-coil. T1-weighted images were collected using the Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (TR = 2.2s, TE = 3 ms, TI = 799 ms, flip angle= 13°, field-of-view (FOV) = 256 × 256 × 160 mm3, matrix= 256 × 256 × 160, 1 mm3 pixel resolution). Shimming of the B0-field was initially conducted using FASTESTMAP (Tkac and Gruetter, 2005) and by specifying a slightly larger voxel covering the left dlPFC (25 × 25 × 25 mm3) because FASTESTMAP is ineffective when prescribing a voxel that is rotated or angulated. Finally, the T1-weighted images were used to guide voxel placement (15 × 20 × 15 mm3; 45 mm3) in the left dlPFC (Brodmann Areas 45 and 46) using the automated voxel placement (AVP) approach (Woodcock et al., 2018 - pending revision) in 15 of 16 subjects (the one exception due to experimenter error).
1H fMRS spectra were continuously acquired every 16s during each condition (PRESS with OVS and VAPOR, TE = 23 ms, TR =4.0s, 4 averages per measurement, 13 measurements per condition, bandwidth =2 kHz, 2048 complex data points; scan duration per condition= 3:28min). The scan duration for each condition was chosen to mimic the typical block length of control condition measurements observed in 1H fMRS studies (Table 1) without being too lengthy to avoid fatigue and/or loss of task engagement. Prior to quantification, raw spectra, without water suppression, were phase-, shift-, and eddy current-corrected. After removing the first 1H fMRS measurement for each condition to avoid data not meeting the steady-state condition, consecutive spectra were averaged to increase the signal-noise ratio, resulting in a 32s temporal resolution or 6 quantitated measurements per condition (representative spectrum shown in Fig. 2). LC Model (version 6.3) with a simulated basis set for a priori knowledge was used to quantify metabolite levels (Provencher, 1993). Additional details on the fully automated post-processing and quantification are provided by Stanley et al. (2017). Briefly, B1-field correction and voxel tissue segmentation were done on the T1-weighted structural images via FreeSurfer and FSL tools (Dale et al., 1999; Smith et al., 2004). Voxel tissue composition and unsuppressed water levels were used to calculate absolute glutamate concentration (Gasparovic et al., 2006).
Fig. 2.
Depiction of a typical 1H MRS spectrum acquired with eight averages yielding a 32s temporal resolution. The acquired spectrum in black is superimposed on the LC Model fitted spectrum in red. Below includes the glutamate signal (Glu) in blue and the residual of the fitting in grey. The chemical shift axis in ppm is depicted at the bottom.
Statistical analyses
The two primary questions were to test whether glutamate levels and/or glutamate variability differed across conditions. The outcome variables of interest were glutamate levels and the percent coefficient of variation (% CV) from the 6 measurements for each condition. Other metabolites, N-acetyl-aspartate (NAA), phosphocreatine plus creatine (PCr + Cr), trimethylamines [glycerophosphocholine plus phosphocholine (GPC + PC)], and myo-inositol, were assessed as well. The main effect of condition was examined using repeated measures generalized estimating equations and post-hoc differences were evaluated using least square means (SAS GENMOD; SAS Institute Inc.). Given the exploratory nature of this pilot study, post-hoc pairwise comparisons were carried out even if the main condition effect was non-significant. Descriptive statistics are presented mean ± one standard deviation, unless otherwise noted. The threshold for significance was: p ≤ .05.
Results
Voxel overlap
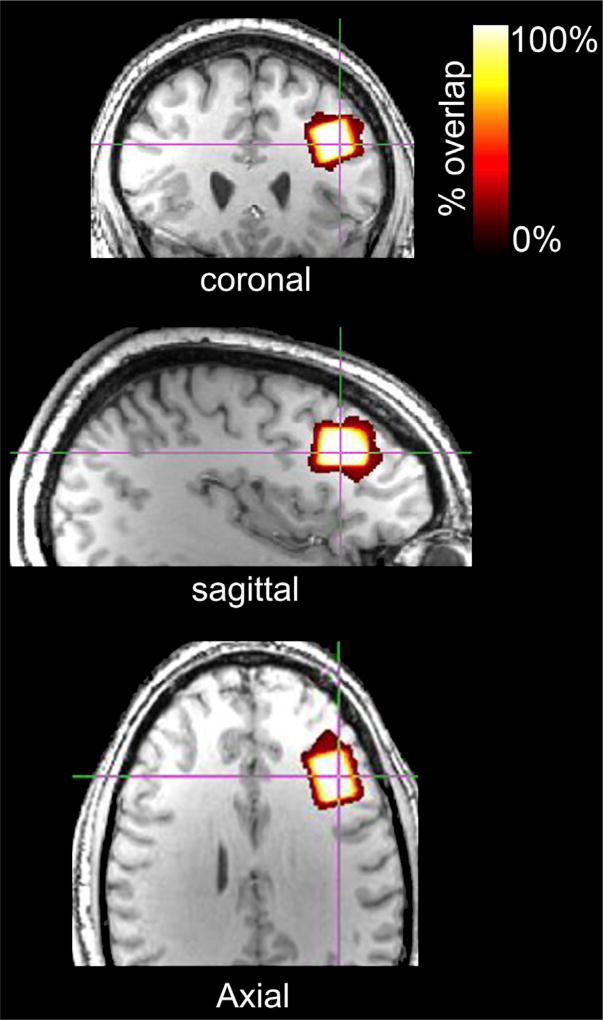
Geometric voxel overlap indicated highly accurate (mean % overlap of each subjects’ voxel and the template voxel in template space = 92.3 ± 4.7%) and reliable (voxel overlap across all subjects = 88.6%) voxel placement across subjects (Fig. 3). Mean (±1 SD) voxel tissue composition was 36.8 ± 3.8% gray matter and 60.8 ± 4.5% white matter.
Fig. 3.
Voxel overlap across all subjects depicted in the template brain. Red-to-white color gradient represents percentage of voxel overlap across subjects (white = 100% voxel overlap; red = 0% voxel overlap).
LC model fit characteristics
The overall quality of the 1H fMRS data representing a 32s temporal resolution was reasonable and consistent across conditions, with an overall mean S/N of 11.7 ± 2.0, FWHM of 4.8 ± 1.0 Hz, and CRLB for glutamate of 7 ± 1%, which did not significantly differ between conditions (Table 2).
Table 2.
LCModel fitting characteristics (values ± SEM).
| Non-Task-Active ‘Control’ Conditions | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Relaxed Eyes Closed | Fixation Crosshair | Flashing Checkerboard | Motor Finger Tapping | X2 | p-value | |
| FWHM in Hz | 4.7 (0.8) | 4.9 (1.1) | 4.9 (0.9) | 4.9 (1.0) | 0.02 | 0.10 |
| S/N | 11.9 (1.9) | 11.8 (2.0) | 11.6 (1.9) | 11.4 (2.1) | 0.15 | 0.98 |
| % CRLB of Glutamate | 7 (1) | 7 (1) | 7 (1) | 7 (1) | 0.06 | 0.10 |
Glutamate levels across conditions
The overall mean (±1 SD) glutamate level for the four conditions; relaxed with eyes closed, passive visual fixation, finger tapping, and visual checkerboard, were 11.9 ± 1.1, 11.6 ± 0.9, 12.0 ± 0.9, and 12.2 ± 0.9 (mmol/kg wet weight), respectively. When analyzed for mean concentration differences across the four conditions, the condition term for glutamate failed to reach significance (X2 = 5.09; p = 0.17). However, significance was revealed by the post-hoc comparisons; the glutamate level was significantly lower during the passive visual fixation crosshair (11.6 ± 0.9 mmol/kg wet weight) compared to both the visual flashing checkerboard (12.2 ± 0.9 mmol/kg wet weight; p = 0.0080) and the finger tapping (12.0 ± 0.9 mmol/kg wet weight; p = 0.023) conditions (Fig. 4). The condition term was not significantly different for the other metabolites, NAA, PCr + Cr, GPC + PC and myo-inositol.
Fig. 4.
Mean (±SEM) levels of glutamate based on the six 32s acquisitions per subjects for each condition and expressed in institutional units (IU). Significant differences from the post-hoc analyses between conditions are noted with ‘*’ (p = 0.023) and ‘**’ (p = 0.0080).
Glutamate variability across conditions
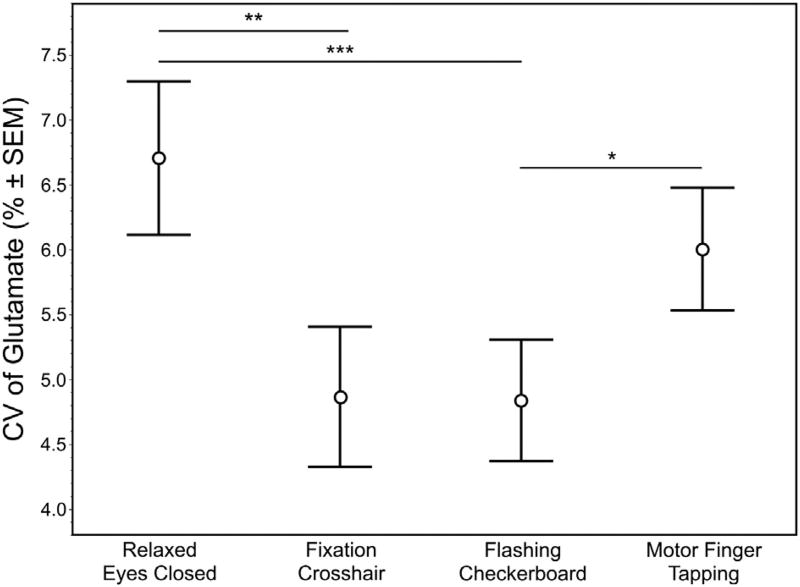
As with the analysis of the mean glutamate levels, fluctuation of the glutamate signal at a temporal resolution of 32s and expressed as a % CV failed to reach significance across conditions (X2 = 6.60, p = 0.086) (Fig. 5). However, post-hoc analysis demonstrated significantly lower % CV for glutamate during the visual flashing checkerboard compared to both the relaxed eyes closed (p = 0.0037) and finger tapping (p = 0.035) conditions. In addition, glutamate levels during the passive visual fixation crosshair were significantly less variable than the relaxed eyes closed (p = 0.0087) (Fig. 6) condition.
Fig. 5.
Variability of glutamate across the six 32s acquisitions for the four ‘control’ conditions: relaxed eyes closed (A), passive visual fixation crosshair (b), visual flashing checkerboard (C) and motor finger tapping (D). Values are expressed as the mean % change (±SEM) relative to the mean glutamate from the six 32s acquisitions of each subjects.
Fig. 6.
The mean (±SEM) variability of glutamate expressed a % CV based on the six 32s acquisitions per subject for each condition. Significant differences from the post-hoc analyses between conditions are noted with ‘*’ (p = 0.035), ‘**’ (p = 0.0037) and ‘***’ (p = 0.0087).
Passive visual fixation crosshair and visual flashing checkerboard exhibited the least glutamate signal variability (lowest % CV) among the four conditions examined. Glutamate was the only metabolite to show significant differences in the % CV across conditions.
Discussion
The motivation of this study was to investigate whether differences in glutamate can be detected across different putative ‘non-task-active’ conditions, knowing that 1H fMRS is sensitive to detecting glutamate modulation induced by neural engagement. Specifically, the steady-state level and variability of glutamate in the left dlPFC were assessed during different conditions of varying degree of behavioral/cognitive constraints (e.g., visual vs. no visual, motor vs. visual, task engaged vs. rest). These conditions were chosen specifically because the left dlPFC is not the dominant brain region engaged. Unexpectedly, steady-state glutamate level was the lowest during the passive visual fixation crosshair condition, while the visual flashing checkerboard and motor finger tapping conditions had significantly higher glutamate (by 4.7% and 3.2%, respectively) (Fig. 4). Moreover, the passive visual fixation crosshair and visual flashing checkerboard conditions produced the least variability in glutamate with CV values under 5%, which were both significantly lower than the eyes closed condition (mean CV = 6.7%; Figs. 5 and 6).
Increased glutamate modulation related to task-demand is consistent with new metabolic steady-states from the neural output that has been postulated to be driven by shifting the net glutamatergic excitatory and GABAergic inhibitory (E/I) balance towards greater excitability (Duncan et al., 2014a; Isaacson and Scanziani, 2011; Lauritzen et al., 2012; Maffei, 2017; Stanley and Raz, 2018 - pending revision; Tatti et al., 2017). Likewise, a relative decrease in the steady-state glutamate level is consistent with a net neural output reflecting a shift in the E/I balance towards a state of less excitability (or a greater inhibitory drive). This implies that the four ‘control’ conditions differed in their level of engagement of the left dlPFC by shifting the level of excitability (i.e., E/I drive). One may speculate that the level of directed attention or executive control exercised during these different conditions, which would modulate dlPFC engagement, was greatest for the visual flashing checkerboard, and to a lesser degree the finger tapping condition, relative to the passive visual fixation crosshair condition. Interestingly, the relatively most engaged condition, visual flashing checkerboard, produced the least glutamate variability, which is consistent within the framework. Counterintuitive to our hypothesis was the passive visual fixation crosshair condition, which revealed the lowest level of steady-state glutamate, as well as the second to least variable glutamate levels. These findings indicated the passive visual fixation condition was the most robust ‘non-task-active’ condition of the four tested herein. Lastly, the relaxed eyes closed condition, which is viewed as the least behaviorally constrained task (i.e., with the most ambiguous set of instructions), produced the largest variability.
This series of experiments exhibits the criticality of developing an appropriate ‘non-task-active’ comparison condition for task-based 1H fMRS and provides evidence supporting a dependency on the level of constraint of behavior for the brain area of interest. For some brain regions, the ‘non-task-active’ state can be presumed to be the absence of any neural engagement. In sensory and motor cortices, this is the absence of task-related stimulation (e.g., blank screen for the visual cortex) (Lin et al., 2012; Mangia et al., 2007c; Schaller et al., 2013; Schaller et al., 2014). However, executive and associative brain regions, such as the dlPFC, which is a central node of the executive network, receiving, and processing a variety of sensory inputs for multiple cognitive functions, pose a greater challenge (Miller and Cohen, 2001).
The observation that the passive visual fixation crosshair condition induced the lowest steady-state glutamate level, in addition to low glutamate level variability, has important implications for both functional and non-functional 1H MRS studies. Though, our findings are limited to the dlPFC, the conceptual framework of constraining behavior is generalizable across brain areas accordingly to their functional specialization. As noted above, several 1H fMRS studies investigating different brain areas have reported greater variability in glutamate during the control condition relative to the ‘task-active’ condition, which illustrates the effect observed herein is not limited to the dlPFC (Ip et al., 2017; Lin et al., 2012; Mangia et al., 2007; Schaller et al., 2013; Schaller et al., 2014). Intuitively, minimizing glutamate variability, especially for 1H fMRS experiments using block designs, will lead to greater statistical power in detecting significant differences between alternating periods of task-active and non-task-active. However, the majority of non-functional 1H MRS studies are acquired without any specific instructions or behavioral constraints, aside from asking the participants to relax and keep their head still during acquisition. This is potentially problematic knowing that the steady-state glutamate levels may differ depending on whether behavior is being constrained (or not) during data collection, including the level of vigilance, anxiety or agitation, which can vary between participants or patient groups being studied. Also, the impact of participants sleeping during scans has shown differences in glutamine levels (Bartha et al., 1999). Collectively, this new knowledge illustrates the importance of constraining behavior during 1H MRS to ensure that the steady-state level of glutamate best reflects the ‘non-task-active’ condition with minimal variability. Ultimately, further studies are warranted to confirm its impact on research investigating different disorders or conditions.
Not surprising were the negative results in the other tested metabolites: NAA, PCr + Cr, GPC + PC, and myo-inositol, which displayed no significant differences in steady-state levels nor % CV between the four conditions. This is consistent with the growing evidence from 1H fMRS studies primarily showing task-related modulation is specifically limited to glutamate, GABA, lactate, aspartate and glucose (Duncan et al., 2014b; Stanley and Raz, 2018 - pending revision).
We do acknowledge that our explanation of different steady-state glutamate levels and variability between conditions reflecting differences in the level of engagement in the left dlPFC is broadly defined and lacks empirical support. Other limitations include the small sample size, which limited statistical power.
Conclusions
In all, this study demonstrated that putative ‘non-task-active’ control conditions require careful consideration for 1H fMRS investigations. We found that steady-state glutamate levels, and the variability therein, differed across ‘control’ conditions. Our findings indicated that the passive visual fixation crosshair condition was the most optimal ‘non-task-active’ condition, of those tested herein, for investigations of glutamate levels in the dlPFC. This finding provides compelling evidence that glutamate levels are highly susceptible to the behavioral constraints of ‘non-task-active’ conditions.
Acknowledgments
Role of funding source
Research reported in this publication was supported, in part, by the National Institute of Mental Health under Award Number R01 MH111177 (JAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding also provided by the State of Michigan (Joe Young Sr./Helene Lycaki funds) and the Detroit Wayne Mental Health Authority. Funding sources were not involved in the design, execution, analysis or interpretation of data described in this manuscript.
The authors thank Caroline Zajac-Benitez and Muzamil Arshad for their assistance.
Footnotes
Contributors
JL, EAW and JAS authored the manuscript and developed the figures. EAW and JAS developed the experimental task. JL and CA analyzed the data and assisted with data analyses. CA assisted in the editing of the manuscript. DK operated the MRI scanner and assisted with data collection. All authors have read and approved of this manuscript.
Conflict of interest
All authors declare no conflict of interest with respect to the conduct or content of this work.
References
- Bartha R, Williamson PC, Drost DJ, Malla AK, Neufeld RW. Medial prefrontal glutamine and dreaming. Br. J. Psychiatr. J. Ment. Sci. 1999;175:288–289. doi: 10.1192/bjp.175.3.288b. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules. 2016;6 doi: 10.3390/biom6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci. Biobehav. Rev. 2014a;47:36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—a review of multimodal imaging studies. Neurosci. Biobehav. Rev. 2014b;47:36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Huang Z, Davis HI, Yue Q, Wiebking C, Duncan NW, Zhang J, Wagner NF, Wolff A, Northoff G. Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: a combined functional mrs and fMRI study. Hum. Brain Mapp. 2015;36:3204–3212. doi: 10.1002/hbm.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip IB, Berrington A, Hess AT, Parker AJ, Emir UE, Bridge H. Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain. Neuroimage. 2017;155:113–119. doi: 10.1016/j.neuroimage.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY, Shin W, Paik JW, Lee KM, Park S, Choe BY, Ryu CW. Glutamine and glutamate complex, as measured by functional magnetic resonance spectroscopy, alters during face-name association task in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2016;52:145–159. doi: 10.3233/JAD-150877. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Mathiesen C, Schaefer K, Thomsen KJ. Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses. Neuroimage. 2012;62:1040–1050. doi: 10.1016/j.neuroimage.2012.01.040. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J. Cerebr. Blood Flow Metabol. 2012;32:1484–1495. doi: 10.1038/jcbfm.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A. Fifty shades of inhibition. Curr. Opin. Neurobiol. 2017;43:43–47. doi: 10.1016/j.conb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Giove F, Dinuzzo M. Metabolic pathways and activity-dependent modulation of glutamate concentration in the human brain. Neurochem. Res. 2012;37:2554–2561. doi: 10.1007/s11064-012-0848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J. Cerebr. Blood Flow Metabol. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Schaller B, Mekle R, Xin L, Kunz N, Gruetter R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J. Neurosci. Res. 2013;91:1076–1083. doi: 10.1002/jnr.23194. [DOI] [PubMed] [Google Scholar]
- Schaller B, Xin L, O'Brien K, Magill AW, Gruetter R. Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7Tesla. Neuroimage. 2014;93(Pt 1):138–145. doi: 10.1016/j.neuroimage.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Shepherd GM Oxford University Press. The Synaptic Organization of the Brain. Oxford University Press; Oxford: 2004. p. xiv.p. 719. 1 online resource. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sonnay S, Duarte JM, Just N, Gruetter R. Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: a 13C MRS study in vivo at 14.1 T. J. Cerebr. Blood Flow Metabol. 2016;36:928–940. doi: 10.1177/0271678X16629482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Burgess A, Khatib D, Ramaseshan K, Arshad M, Wu H, Diwadkar VA. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo (1)H functional magnetic resonance spectroscopy. Neuroimage. 2017;153:189–197. doi: 10.1016/j.neuroimage.2017.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Raz N. Functional magnetic resonance spectroscopy: the “New” MRS for cognitive neuroscience and psychiatry research. Frontiers in Psychiatry, Neuroimaging and Stimulation. 2018 doi: 10.3389/fpsyt.2018.00076. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem. Int. 2002;41:123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Tatti R, Haley MS, Swanson OK, Tselha T, Maffei A. Neurophysiology and regulation of the balance between excitation and inhibition in neocortical circuits. Biol. Psychiatr. 2017;81:821–831. doi: 10.1016/j.biopsych.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkac I, Gruetter R. Methodology of H NMR spectroscopy of the human brain at very high magnetic fields. Appl. Magn. Reson. 2005;29:139–157. doi: 10.1007/BF03166960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Anand C, Khatib D, Diwadkar VA, Stanley JA. Working memory modulates glutamate levels in the prefrontal cortex during 1H fMRS. Frontiers in Psychiatry, Neuroimaging and Stimulation. 2018a doi: 10.3389/fpsyt.2018.00066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Arshad M, Khatib D, Stanley JA. Automated Voxel Placement: A Linux-based Suite of Tools for Accurate and Reliable Single Voxel Coregistration. Journal of Neuroimaging in Psychiatry & Neurology. 2018b doi: 10.17756/jnpn.2018-020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]