Abstract
Purpose
Depressed coronary endothelial function (CEF) is a marker for atherosclerotic disease, an independent predictor of cardiovascular events, and can be quantified non-invasively with ECG-triggered (ECG-STD) spiral cine MRI combined with isometric handgrip exercise (IHE). However, MRI-CEF measures can be hindered by faulty ECG-triggering, leading to prolonged breath-holds and degraded image quality. Here, a self-gated golden-angle spiral method (SG-GA) is proposed to eliminate the need for ECG during cine MRI.
Methods
SG-GA was tested against retrospectively ECG-gated golden-angle spiral MRI (ECG-GA) and gold-standard ECG-STD in 10 healthy volunteers. CEF data were obtained from cross-sectional images of the proximal right and left coronary arteries in a 3T scanner. Self-gating heart rates were compared to those from simultaneous ECG-gating. Coronary vessel sharpness and cross-sectional area (CSA) change with IHE were compared among the three methods.
Results
Self-gating precision, accuracy, and correlation-coefficient were 7.7±0.5ms, 9.1±0.7ms, and 0.93±0.01, respectively (mean±standard-error). Vessel sharpness by SG-GA was equal or higher than ECG-STD (rest: 63.0±1.7% vs. 61.3±1.3%; exercise: 62.6±1.3% vs. 56.7±1.6%, p<0.05). CSA changes were in agreement among the three methods (ECG-STD=8.7±4.0%, ECG-GA=9.6±3.1%, SG-GA=9.1±3.5%, p=NS).
Conclusion
CEF measures can be obtained with the proposed self-gated high-quality cine MRI method even when ECG is faulty or not available.
Keywords: Self-Gating, Golden Angle, Spiral, Cine MRI, Coronary Endothelial Function, Atherosclerosis
Introduction
Magnetic resonance imaging (MRI) of the cardiovascular system is safe, non-invasive, does not require ionizing radiation, and offers high soft-tissue contrast. In cardiac cine imaging, data collection is conventionally synchronized with an electrocardiogram (ECG) (1) and segmented over multiple cardiac cycles using either prospective ECG-triggering or retrospective ECG-gating (2). Prospective ECG-triggering acquires a fixed number of cardiac phases that is defined before the scan based on an assumed heart rate, and, therefore, is adversely affected by heart rate changes. For example, late diastolic phases may be missed if the heart rate is assumed too high, or triggers may be missed if it is assumed too low. Conversely, ECG-gated data are continuously acquired and retrospectively sorted in cardiac phases, allowing visualization of the entire cardiac cycle (3). Both methods critically rely on accurate detection of the ECG R-wave as a trigger to produce diagnostic-quality images. However, the ECG signal may be distorted by many factors including rapid switching of magnetic field gradients, pulsing of radiofrequency fields (4–6), magnetohydrodynamic effects (7), bulk motion, and arrhythmias, all potentially contributing to decreased image quality.
Recently, ECG-triggered cine MRI was used to measure coronary endothelial function (CEF) (8). Abnormal CEF is a marker for sub-clinical atherosclerotic disease, an independent predictor of cardiovascular events (9), and as such, endothelial function is often considered a “barometer” of vascular health (10). Healthy coronary endothelium responds to specific stressors with nitric oxide (NO) release that results in coronary vasodilation and increased blood flow (11). Conversely, abnormal endothelium responds to the same stressors with decreased NO release, reduced coronary vasodilation (or vasoconstriction) and attenuated blood flow. CEF was conventionally imaged in the cardiac catheterization laboratory where high spatial and temporal resolution could be achieved during the administration of an endothelial-dependent stressor (12). However, the invasive nature of traditional catheterization-based methods often precludes the acquisition of CEF measures in healthy and low-risk populations and thus limits the impact on clinical practice. A non-invasive method that combined ECG-triggered cine MRI and isometric handgrip exercise (IHE) (13), an endothelial-dependent stressor (14–16), was introduced to quantify CEF (8). In healthy subjects, IHE induced significant coronary dilation and increased blood flow as detected by MRI measures of lumen cross-sectional area (CSA) and peak-diastolic coronary flow velocity. Conversely, coronary artery disease (CAD) patients showed unchanged or decreased CSA and flow velocity during IHE. Those MRI-based CEF measures were shown to be primarily NO-dependent, reproducible and impaired in patients with CAD (16,17).
However, cine MRI-CEF methods (8) are critically dependent on ECG triggering and subject to all the aforementioned factors that can degrade ECG signal quality (4–7). Particularly during IHE stress when the heart rate increases, the operator may fail to update the heart rate of the ECG-triggered sequence and muscular activity of the upper body may become an additional source of ECG artifacts. These may lead to missed or erroneous triggers, and hence reduce image quality, prolong scan time, degrade CEF measures and, more rarely, require scan repetitions.
ECG-independent methods for cardiac MRI could solve the above-mentioned issues in MRI-CEF measurements. Early ECG-independent approaches acquired extra echoes or projection images interleaved with image data readouts (18–20). From these additional acquisitions a cardiac signal could be detected directly at the level of the heart and used for self-gating (SG). Self-gated Cartesian acquisitions required modification of the sequence timing to accommodate additional free induction decay (FID) sampling (k-space center) (21–24), resulting in slightly reduced scan efficiency. Larson et al. (25) extracted a cardiac gating signal from the image data themselves by using k-space center samples from a radial acquisition for the first time without the need for additional acquisitions. Those k-space-center-based approaches require careful selection of receive coil elements to obtain SG data, because the signal from each coil has a different sensitivity to cardiac motion. To overcome this limitation, other radial techniques used image-based methods to obtain SG signals by matching regions of interest on high-temporal/low-spatial resolution images extracted from the same image data before retrospective sorting. Such methods were successfully used for cardiac (26) and coronary endothelial (27) function assessment. However, they require additional reconstruction steps (for the low-resolution images) and user interaction to delineate the region of interest. Yet self-gating has not been applied to spiral cine imaging of the coronary arteries for CEF assessment and the current spiral technique predominantly used in the MRI-CEF literature (8,16,17,28,29) still relies on the ECG for cardiac gating.
Here, we propose an SG method that does not need user interaction and automatically extracts a cardiac signal from a multi-coil set of k-space center signals using an independent component analysis (ICA) (30). Instead of a radial trajectory, we use a more efficient spiral readout which also samples the center of k-space at the beginning of every acquired interleave without the need for extra acquisitions. Each spiral arm is continuously rotated by the golden angle (GA) with respect to the previous one and divides the largest azimuthal gap present in the preceding set of acquired arms. This scheme allows continuously acquired data to be sorted into an arbitrary number of cardiac phases while k-space coverage of each cardiac phase approximates uniformity, as samples are very evenly distributed for an arbitrary number of arms (31). This technique eliminates the need for ECG triggering during cine MRI and is here referred to as SG-GA (32). However, when ECG triggers are available, they can be used with the same acquisition for retrospective gating (ECG-GA). The new SG-GA method was tested in healthy volunteers at rest and during IHE in an established MRI-CEF protocol. Heart rate parameters, image quality (vessel sharpness), and CEF measures were all compared among the SG-GA, ECG-GA and the gold-standard ECG-triggered spiral cine (8) (ECG-STD) approach.
Methods
The proposed self-gating approach relies on a continuously acquired golden-angle (137.51°) interleaved spiral sequence to obtain k-space center amplitude values from all receive coils at the beginning of each spiral readout. From these amplitudes, a cardiac signal is extracted and heartbeats are identified to retrospectively sort readouts into cardiac phases before cine image reconstruction.
Cardiac Self-Gating
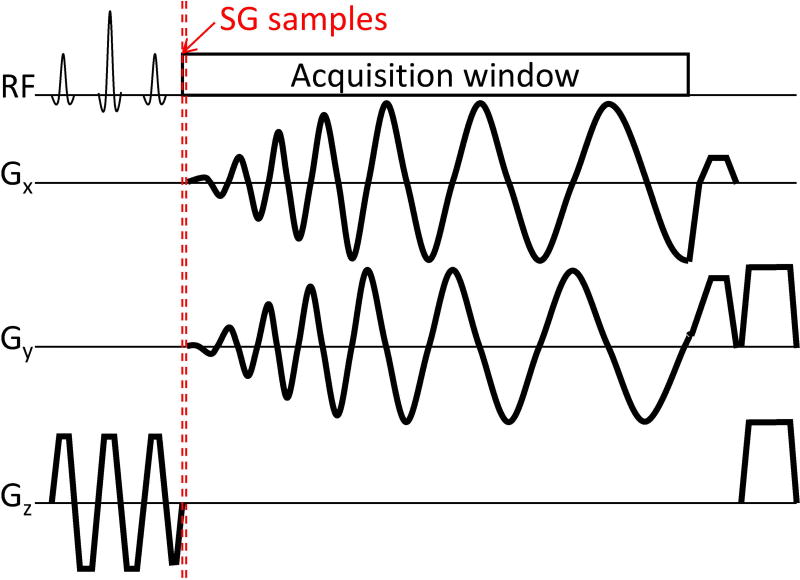
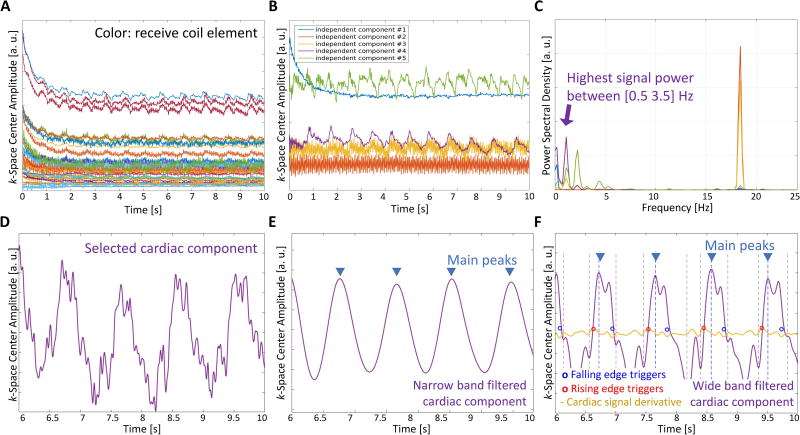
For self-gating, the acquisition window was modified to begin sampling before the spiral readout gradient starts (Figure 1). This enables the acquisition of an FID signal in the approximately 30 µs gap between the end of the slice-selective refocusing gradient and the spiral readout gradient, when no gradient is applied and all excited spins are coherent. This short gap is present in the standard sequence by default to let short-term eddy currents decay before data are acquired. In general, 9 to 15 self-gating samples were acquired before each spiral arm. The number of acquired samples varies between acquisitions as the sampling dwell time depends on the off-center position of the imaging volume. The absolute value of the 9–15 SG samples was averaged to obtain a SG value for each coil. These values were recorded during the entire scan for each interleave, providing a set of signals as shown in Figure 2A. We assume that the amplitude changes are attributed to cardiovascular activity, such as cardiac contraction and fully relaxed blood flowing into the imaging slice (19), and other factors like magnetization variation due to steady state. All these signal components are present and mixed together in the SG signal from each coil. However, the intensity of each component in the SG signal is weighted by its coil sensitivity, and therefore by the coil position with respect to the source of signal fluctuation (e.g. the heart for cardiac activity). Independent component analysis (ICA) (30) is a blind source separation technique that is adopted to separate individual components hidden in a set of mixed signals by exploiting their statistical characteristics. An example usage is the separation of individual voices (sources) from a redundant set of recordings of a group of people that are talking in a room. Similarly, here we test the hypothesis that ICA separates the individual sources related to cardiac activity and other factors (33) by exploiting the redundancy of a multi-coil set of SG recordings. We used the FastICA algorithm (34), a free GPL MATLAB® (Mathworks, Natick, MA) program available at https://research.ics.aalto.fi/ica/fastica/, to reduce the 32-coil signal set to 5 components. The number was chosen empirically to obtain a good estimation of the main sources of variation in the FID signals, including the one related to cardiac motion. The resulting number of independent components was fixed at 5 for all volunteers but the order in which the independent components were extracted varied at each execution (30), and so the cardiac component was selected based on its frequency content. Figure 2 shows an example of 32 SG signals (Figure 2A) that are reduced to 5 independent components (Figure 2B) with fluctuations related to magnetization variation due to steady state (# 1), other sources of variation (# 2, 3), and cardiac activity (# 4, 5). The component with the highest spectral power within the band from 0.5 to 3.5 Hz, as expected for the heart rate, is automatically selected as the cardiac component for self-gating (Figure 2C–D). Next, in a two-step process, the different heartbeats are first identified, and then a trigger is selected for each beat. Before processing, the selected cardiac component was 4-fold interpolated using low-pass filtering and mean-square error minimization (interp function from MATLAB®, (35)) to increase temporal resolution and potentially improve trigger detection, and padded at the extremities with hundred first and last same signal sample copies to minimize filter distortions at the two signal borders (36). To identify heartbeats, the signal was processed with a 6th-order bandpass Butterworth filter with a narrow band (cutoff frequencies: 0.5 and 2 Hz). Main peaks corresponding to cardiac beats were then detected using the findpeaks function from MATLAB® (Figure 2E). To identify trigger points, similar filtering was performed on the cardiac component yet with a wider band to allow higher frequency components in the signal and preserve rapid signal changes for the identification of the trigger (cutoff frequencies: 0.5 and 5 Hz): at each main peak position, two points with highest derivative were selected on the rising and the falling edge (red and blue circle markers, Figure 2F). Either the rising or falling edge was chosen as the final trigger points, depending on which had the higher average absolute derivative. This last wide-band filtered signal is referred to as the cardiac SG signal since it is the one used to detect the final triggers. The SG processing was performed in MATLAB®.
Figure 1.
Sequence diagram of one repetition of the golden angle variable density spiral sequence. Self-gating samples are acquired during a gap at the end of the rephasing lobe of the spatial-spectral excitation pulse and before spiral readout gradients. This ~30 µs gap is currently applied by default to let short-term eddy currents decay before starting data acquisition. Thus, k-space center data are obtained for each spiral interleave and without changes in sequence timing.
Figure 2.
Main steps of the self-gating method. The k-space center amplitude is recorded during the scan for all receive coils (A). Independent component analysis is applied to reduce the set of signals into fewer components (B). The component with highest spectral power in the cardiac frequency range is selected (C, D). The cardiac component is band-pass filtered with a narrow band to detect heartbeats (main peaks, E). The cardiac SG signal is obtained after pass-band filtering the signal in D with a wider band. Here high prominence peaks are found and cardiac triggers are identified on either the rising or falling edges of the detected high prominence peaks where the signal derivative is maximized or minimized–steepest point on the edge (yellow signal, F).
MR experiments
All human studies were approved by the Johns Hopkins School of Medicine Institutional Review Board and written consent was obtained from all study subjects. In vivo experiments were performed in 10 healthy volunteers (7 women, 24±5.5 years, with no history of heart disease, hypertension or diabetes, and non-smokers) on a 3 T clinical scanner (Achieva, Philips Healthcare, Best, The Netherlands) with two-channel multi-transmit excitation and a 32-channel cardiac coil array for signal reception.
CEF assessment of the proximal right and left coronary arteries was performed in the morning after overnight fasting as described previously (8), with the three methods (ECG-STD, ECG-GA, SG-GA). Cine images perpendicular to a proximal, straight segment of left anterior descending (LAD) artery were prescribed perpendicular to an axial plane on a 3D whole-heart MR angiogram. On the same whole-heart scan, the 3-point tool (37) was used to prescribe a targeted scan of the right coronary artery (RCA) where cine images were in turn prescribed perpendicular to a proximal, straight segment of the RCA. Single breath-hold cine images for cross-sectional coronary artery area measurements were performed at rest and during 5–8 minutes of IHE at the same location. Each subject performed sustained isometric handgrip exercise by squeezing an MRI-compatible dynamometer (Stoelting, Wood Dale, Illinois) at 30% of his or her maximum grip strength under direct supervision (13).
For each subject, a total of 8 cine acquisitions were performed: acquisitions in each of two arteries (RCA and LAD), with two acquisition types each (the gold-standard and the proposed golden-angle sequence), and all four of those acquisitions were obtained both at rest and during IHE. Thus for 10 subjects, there were 80 acquisitions (= 10 subjects × 2 arteries × 2 acquisition types × 2 conditions (rest/IHE)). Because the data from the golden-angle sequence were reconstructed two different ways, once using triggers from the proposed SG algorithm, and another using triggers from the simultaneously acquired ECG signal, a total of 120 cine datasets were collected (ECG-STD, ECG-GA, and SG-GA images of the RCA and the LAD at both rest and during IHE in 10 subjects).
The gold-standard ECG-triggered gradient echo 2D spiral sequence (ECG-STD) was acquired as described before (8) with the following protocol parameters: spatial resolution = 0.89 × 0.89 × 8 mm3, field of view = 220 mm, TR/TE = 18/2.1 ms, acquisition window = 13 ms, ~20 s breath-hold duration, 1-2-1 binomial spectral spatial water excitation, 20° radiofrequency excitation angle, 30 – 60 cardiac phases depending on the heart rate. Images were obtained at the scanner using the vendor gridding reconstruction.
For the ECG/SG-GA methods, a golden angle variable density 2D spiral gradient echo sequence was acquired continuously without ECG-synchronization. An Archimedean 2D spiral trajectory with variable density sampling (38) was employed such that the central region of k-space (up to 15% of the normalized radius) is fully sampled in 18 interleaves (arms) with respect to the defined field of view, then the sampling density linearly decreases 1.5-fold till 30% of the normalized radius and stays constant thereafter.
The acquisition was matched with ECG-STD for spatial resolution and acquisition time while other protocol parameters included: field of view = 400 mm, TR/TE = 22/1.87 ms, acquisition window = 16.6 ms, ~20 s breath-hold duration, 875 golden angle rotated spiral interleaves, 1-2-1 binomial spectral spatial water excitation, 15° radiofrequency excitation angle. Data were retrospectively sorted into 40 cardiac phases with 50% sliding window using triggers from the ECG signal for ECG-GA images, and SG-triggers according to the above-described self-gating algorithm for SG-GA images. Beat-to-beat heart rate variability was taken into account using non-linear stretching of the data time stamps for ECG-GA (39) and a linear model for SG-GA. Both ECG- and SG-GA images were reconstructed using non-Cartesian SENSE (40) in the graphical programming interface (GPI) (41). Prior to reconstruction, data were compressed into 10 virtual channels (42) and coil sensitivity maps were generated from a central k-space region, within a 10% normalized radius, using all 875 spiral arms. All cine frames obtained with the ECG-STD and ECG/SG-GA methods were processed using a deblurring algorithm (43) that was centered at the level of the coronary lumen, before data analysis. This deblurring algorithm aims to estimate and remove phase accrual in k-space due to local off-resonance frequency without the use of an additional field map. Specifically, first the cine images were blurred modulating the acquired k-space phase with 21 different frequencies from −50 to 50 Hz. Then, the frequency minimizing the sum of the square root of the imaginary part of the voxels in a 5-mm radius around the vessel was chosen to deblur the image.
Heart rate and blood pressure were measured at rest and during exercise using a noninvasive and MRI-compatible electrocardiogram and calf blood pressure monitor (Invivo, Precess, Orlando, Florida). The rate-pressure product (RPP) was calculated as systolic blood pressure × heart rate and this was determined both at rest and during IHE stress.
Data analysis
To assess the quality of self-gating, the approach of Larson et al. (25) that analyzes trigger times was adapted here to compare heartbeat intervals (time between sequential heart beats) detected with self-gating (x) to those from the ECG (y) (time between two detected R-waves or R-R interval) using metrics for accuracy and precision: mean(|x-y|) and standard deviation(x-y), respectively.
Image quality was assessed with a measure of vessel sharpness quantified at the interface of lumen and surroundings as the maximum image gradient along 32 radial spokes (27,44). Coronary artery CSA measurements were performed using the semi-automatic Cine tool (General Electric, Milwaukee, Wisconsin) based on the full-width-half-maximum algorithm. Percent change of CSA between rest and IHE was quantified as a measure of CEF (8). Vessel sharpness and CSA were determined in three diastolic frames with minimal coronary motion and averaged. Although these frames are selected in diastole from both rest and exercise scans, it should be noted that they may not be acquired precisely at the same moment because rest and exercise images are acquired during different breath-holds, with different heart-rate and quiescent periods. Which may result in subtle anatomical differences between rest and exercise frames.
Statistical analysis
Linear correlation between heartbeat intervals obtained from SG and the ECG was assessed using Pearson’s correlation coefficient (CC) with p<0.05 considered as statistically significant.
Comparisons among the three methods (ECG-STD, ECG-GA, and SG-GA) for vessel sharpness and CSA, separately at rest and during exercise, and CSA change were performed using one-way repeated measures ANOVA and Bonferroni’s multiple comparison tests. These tests performed paired comparisons across the three methods (i.e. only the measurements that could be obtained from all six acquisitions, three methods at both rest and exercise, were included in the analyses). Additionally, CSA measurements and percentage CSA change were also evaluated among the three methods using Bland-Altman plots and linear regression fits. Two-tailed paired Student’s t-test was used to compare RPP between rest and IHE. Statistical analyses were performed in Prism (version 7, GraphPad Software, Inc., La Jolla, CA) with alpha=0.05 as significant threshold and significant difference indicated as (*) p<0.05, (**) p<0.01, (***) p<0.001.
Results
RPP measured at rest was 8055 ± 318 mmHg·beat/s (average ± standard error of the mean (SEM)) and increased 37% during IHE stress (11047 ± 595 mmHg·beat/s, p<0.001).
Out of the total 80 attempted scans, one ECG-STD acquisition and one ECG/SG-GA acquisition for the same RCA segment could not be acquired due to time constraints during exercise. As a result, for self-gating performance assessment, 39 out of 40 ECG/SG-GA acquisitions were available for the comparison of self-gating with the simultaneously acquired ECG signal. For vessel sharpness and CEF assessment, five other scans were discarded: three ECG-STD acquisitions on the LAD and two ECG/SG-GA acquisitions (one on the LAD and one on the RCA) were found to have insufficient image quality during processing. Overall, 7/80 scans were discarded or not available, affecting 6 different coronary segments. As a result, 14/20 imaged coronary segments provided good quality in all 6 datasets (ECG-STD, ECG-GA and SG-GA images at rest and during IHE) and were considered for analysis (84/120 total reconstructed cine datasets).
The processed cardiac SG signal from an exemplary golden angle acquisition shows high correlation with the simultaneously acquired ECG (Figure 3A). Agreement between heartbeat intervals of the two gating signals is confirmed by the linear regression with high cross-correlation as well as small values for accuracy and precision (Figure 3B). Self-gating could successfully be performed in all 39 GA acquisitions. In all subjects studied, self-gated heartbeat intervals showed significant (p<0.0001) and high correlation with those from the ECG. The summary findings were: CC = 0.93 ± 0.01, precision = 7.7 ± 0.5 ms, and accuracy = 9.1 ± 0.7 ms. Scans performed during exercise had slightly worse values (Figure 3C).
Figure 3.
ECG and self-gating signals from one exemplary volunteer showing good visual correlation (A) and in a linear regression model (B). Note that cardiac triggers are detected at the level of the R-wave for the ECG, and on the edge of a main identified peak for self-gating. While the R-wave corresponds to the beginning of systole, the self-gating trigger can correspond to any point in the cardiac cycle. This discrepancy may artificially reduce the correlation between the two triggers. C) Accuracy, precision, and correlation coefficient of self-gated heartbeat intervals compared to R-R intervals from simultaneously acquired ECG. Data averaged from all 39 golden-angle acquisitions and grouped for rest and IHE. Bars indicate standard error of the mean.
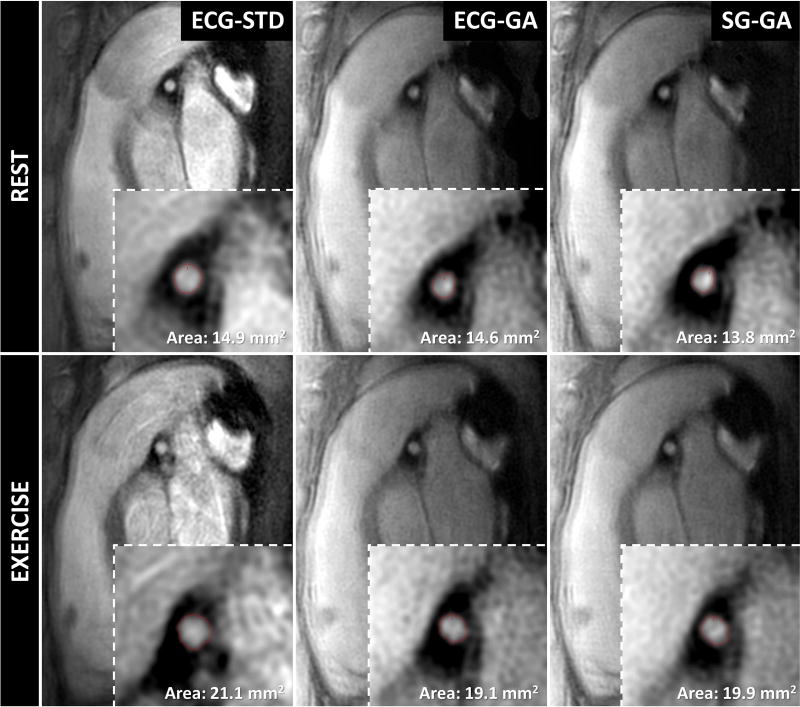
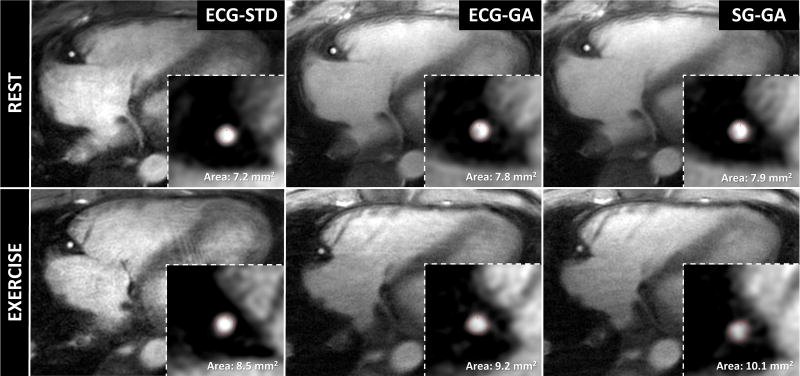
Representative images of the proximal LAD (Figure 4) and RCA (Figure 5) in a diastolic frame depict coronary anatomy with sharp coronary lumen borders (zoomed insets) across all methods and for both rest and exercise conditions.
Figure 4.
Diastolic image frames from one volunteer obtained perpendicular to a proximal segment of the left coronary artery showing good depiction of the lumen (insets show magnified region surrounding coronary artery) with all imaging methods acquired during rest and exercise. Red contours show segmented lumen border with full-width-half-maximum algorithm (Cine tool, GE).
Figure 5.
Diastolic image frames from one volunteer obtained perpendicular to a proximal segment of the right coronary artery showing good depiction of the lumen (insets show magnified region surrounding coronary artery) with all imaging methods acquired during rest and exercise. Red contours show segmented lumen border with full-width-half-maximum algorithm (Cine tool, GE). Subtle anatomical differences in the atrioventricular groove between rest and exercise diastolic frames may be due to different breath-held acquisitions with different heart rates and quiescent periods.
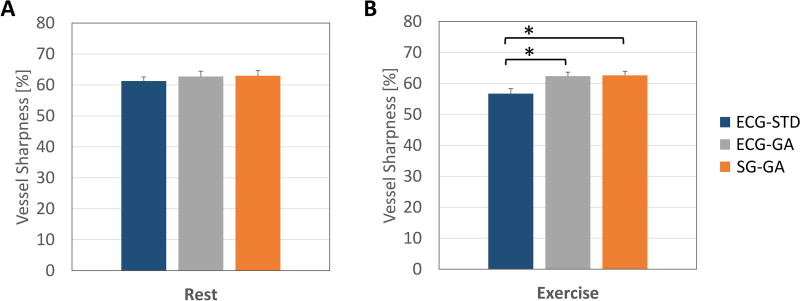
Overall, high image quality and vessel sharpness were observed. Vessel sharpness was similar and not reduced for the new SG-GA method as compared with that of the two ECG-dependent approaches (ECG-STD and ECG-GA) at rest (Figure 6A). Of note, mean vessel sharpness of SG-GA and ECG-GA were significantly higher than those of the reference ECG-STD method during IHE stress (p<0.05, Figure 6B).
Figure 6.
Quantitative image quality results obtained in N=14 left and right coronary artery segments from 10 healthy volunteers. Overall high values of vessel sharpness were observed. The sharpness in the proposed SG-GA method did not decrease without ECG-gating (ECG-GA) at rest (A) and during IHE stress (B) and was significantly higher than the reference ECG-triggered ECG-STD method during IHE stress (B). The sharpness of ECG-GA was also significantly higher than ECG-STD during IHE (B). Bars indicate standard error of the mean; (*) indicates p<0.05, significant difference according to Bonferroni’s multiple comparison test.
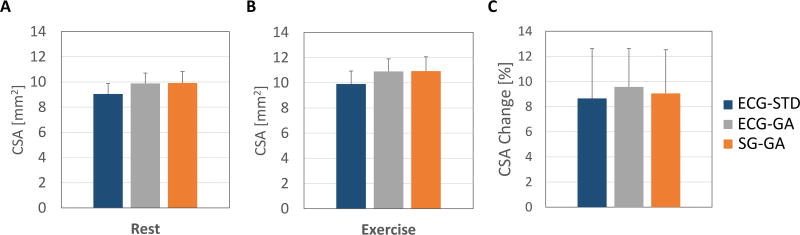
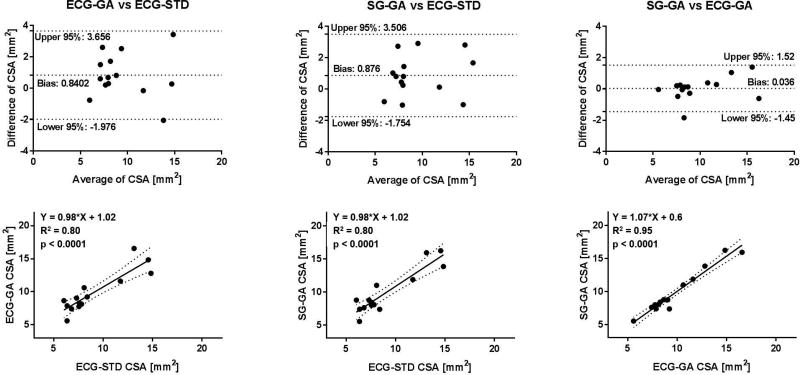
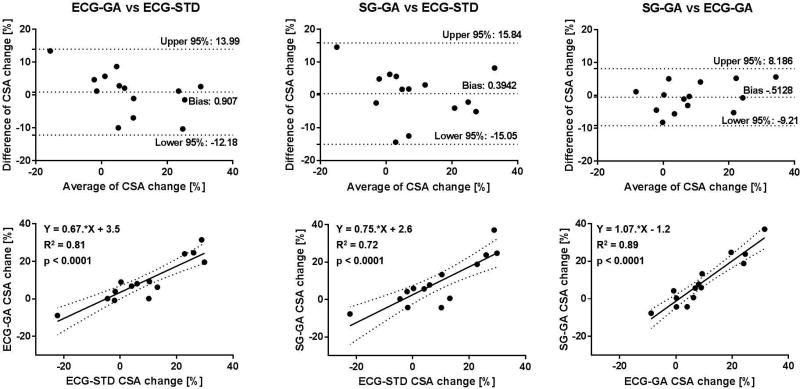
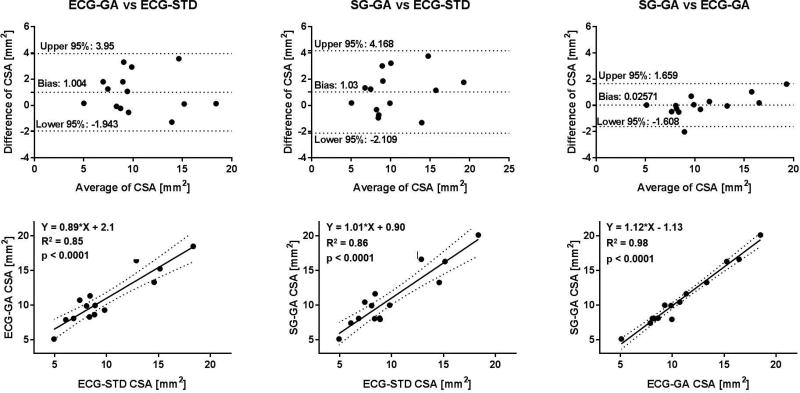
On average, SG-GA images provided coronary CSA values that were not statistically different than those measured in ECG-GA images, and both were in agreement with those obtained in the gold-standard ECG-STD images both at rest and during IHE stress (Figure 7, A and B). Mean CSA derived from GA acquisitions trended higher than those from ECG-STD, but the differences were not significant. Critically for CEF measures, average percentage CSA changes during IHE obtained with the self-gated approach were not significantly different than those with ECG-gating and gold-standard ECG-triggering (Figure 7C). Figures 8–10 show Bland-Altman plots and linear regression analysis between the three methods. CSA measures at rest (Figure 8) and during IHE (Figure 9) showed good agreement of the golden angle methods with ECG-STD in Bland-Altman plots with a non-significant bias of ~1 mm2 and good correlation in linear regression fits with R2 ≥ 0.8 (first and second column, Figures 8 and 9). Agreement between SG-GA and ECG-GA measures was found higher with negligible bias and strong correlation (third column, Figures 8 and 9). For CEF, percentage CSA changes showed good agreement and correlation between golden angle methods and ECG-STD (with less than 1% bias and R2 > 0.7, first and second column, Figure 10), while stronger agreement and correlation was observed between the golden angle methods (third column, Figure 10).
Figure 7.
Quantitative CEF assessment performed in N=14 left and right coronary artery segments from 10 healthy volunteers. The coronary CSA values measured in the proposed SG-GA method were similar to those from ECG-GA at rest (A) and during IHE (B), and both were in agreement with those obtained in the reference ECG-STD method at rest (A) and during IHE (B). Critically for CEF assessment, average percentage CSA change was similar in the SG-GA approach to those in ECG-GA and gold-standard ECG-STD method (C). Bars indicate standard error of the mean.
Figure 8.
Bland-Altman and linear regression plots for coronary CSA measurements at rest. Good agreement and linear correlation in CSA values between the golden angle methods (SG/ECG-GA) and standard ECG-triggered sequence (ECG-STD) can be observed (first and second column). Note bias of ~0.8 mm2 between golden angle and standard methods maybe due to different reconstruction pipelines (see Discussion). Comparison within the proposed golden angle methods (SG-GA vs ECG-GA) shows high agreement and correlation (third column).
Figure 10.
Bland-Altman and linear regression plots for CSA change, a measure of CEF. Good agreement and correlation were observed in a linear fit for the ECG-gated and self-gated golden angle sequences with the standard ECG-triggered sequence (first and second column). High agreement and correlation for the proposed self-gating method were found in comparison to reference ECG-gating golden angle technique (third column).
Figure 9.
Bland-Altman and linear regression plots for coronary CSA measurements during IHE stress. Good agreement and linear correlation in CSA values between the golden angle methods (SG/ECG-GA) and standard ECG-triggered sequence (ECG-STD) can be observed (first and second column). Note bias of ~1 mm2 between golden angle and standard methods maybe due to different reconstruction pipelines (see Discussion). Comparison within the proposed golden angle methods (SG-GA vs ECG-GA) shows high agreement and correlation (third column).
Discussion
ECG-synchronization is used in nearly all current clinical cardiac MRI acquisitions to compensate for cardiac motion, but many factors can degrade the ECG signal and ultimately image quality and thereby increase exam time or reduce the amount of useful information obtained. We report here the first self-gated spiral cine MRI technique and applied it to coronary artery imaging. Specifically, the self-gated spiral approach was developed and tested for coronary endothelial function assessment and was compared with the gold-standard ECG-triggered sequence in healthy subjects. This self-gating approach automatically isolates a cardiac signal from fluctuations of k-space center amplitude by means of independent component analysis. Heart rate parameters determined by self-gating showed high accuracy, precision, and correlation compared to those derived from a simultaneously acquired ECG. Vessel sharpness measured on images obtained with the proposed self-gated technique was as good as those obtained with the standard ECG-dependent sequence and significantly higher during exercise. CEF measurements obtained with the proposed technique were in agreement with those from the standard ECG-triggered sequence.
In this contribution, we combined SG exploiting k-space center sampling with spiral imaging. We adopted spiral imaging, already present in the gold-standard MRI-CEF sequence (8,45), because of its scan efficiency, high SNR, robustness to motion and aliasing, and improved depiction of coronary arteries when combined with spectral spatial pulses (46) for epicardial fat suppression. Glover et al. (47) used the phase of the first few samples of each spiral readout for self-navigated correction of fMRI data. Since the first sample of a spiral readout starts at k-space center, this is a reasonable choice to obtain an FID signal without sequence modification. However, sequence imperfections and gradient delay offsets may alter FID amplitude fluctuations that would otherwise primarily reflect physiological motion. To overcome this limitation and sample a longer and theoretically more robust FID signal, we extended the acquisition window to sampling earlier during the existing, short gap after the slice-select refocusing gradient and before the onset of the readout gradient while no gradients are played out, without prolonging the repetition time. To extract cardiac motion information from the recorded FIDs, the proposed method exploits the redundancy of the multi-coil acquisition by using ICA, under the assumption that the sought hidden components (cardiac activity, radiofrequency field inhomogeneity, steady state magnetization variation, etc. (48)) are statistically independent. In comparison to other methods, ICA avoids the need for tailored filtering methods (21,49) or manual selection of specific coils from which the sought physiological components are expected to be the major contributors to the k-space center signals (i.e. coils closer to the chest/heart) (49,50). A method that extracts a cardiac signal directly from the FID coil signals, without ICA, is another approach but would require more demanding filter parameters due to the larger frequency content of the FID signals and is expected to exhibit lower performance. Alternatively, principal component analysis (PCA) has also been used for self-gating from intensity projections in cine imaging (51). The use of PCA alone was not pursued in this study, because it is part of the FastICA implementation used here (34) and serves as a preprocessing step to reduce the size of the FID signals (32 original to 5 signals). In general, PCA identifies the signals with main variation, then ICA takes such reduced set of signals and, by iteratively maximizing their non-Gaussianity, separates the hidden components (30). However, despite a high performance of the proposed SG method, the cardiac trigger for ECG-gating (R wave) always corresponds to the beginning of systole, while the SG trigger (steepest edge of the main peaks) can be anywhere in the cardiac cycle. Furthermore, Feinstein et al.’s model (39), which improves retrospective sorting by non-linear stretching of heartbeats with different durations, cannot be used since the position of the SG trigger does not necessarily correspond to beginning of systole.
In comparison to image-based SG approaches (25–27,52), there are two main advantages of FID based methods. First, since multiple readouts have to be combined to obtain an image for SG, FID-based SG methods benefit from a higher temporal resolution, i.e. the repetition time. Second, image-based methods usually require manual interaction to identify the region of interest or the profile on the real-time SG images used to extract the gating signal by means of template matching or cross-correlation. Yerly et al. (27) reconstructed images for SG every 10 ms with a sliding acquisition window of 88 ms (15 radial profiles). While their approach requires manual interaction for the above-mentioned reasons, the authors report similar precision to the method proposed here (~10 ms). Nevertheless, an adaptation of previously proposed image-based approaches with radial imaging (26,27) to the spiral trajectory used here would be of interest and would allow for direct comparison of different self-gating methods. This was not performed due to the limited sample size and different focus of this study and remains to be investigated.
Another advantage of the proposed golden-angle spiral cine MRI technique is the ability to use either ECG- or SG-extracted triggers without having to decide beforehand. Compared to the radial golden angle trajectory (27), whose rapidly changing gradients corrupted the ECG in 15 out of 18 investigated subjects, the spiral golden angle trajectory used here allowed the detection of ECG triggers in all 39 acquired ECG/SG-GA scans.
Nevertheless, it is desirable to have an alternative gating method that allows one to obtain useful images even when the ECG signal is not available or gets corrupted during the scan. This is especially important for measures of coronary endothelial function that introduce a stressor like IHE that may cause ECG artifacts.
CSA values measured with the proposed GA methods showed good agreement and high correlation with the gold standard approach. Although not statistically significant, a small bias was observed in the Bland-Altman analysis. This may be caused by differences in the image uniformity scaling in the vendor image reconstruction of the ECG-STD method and the offline reconstruction of the GA methods, leading to different signal intensity levels used in the FWHM algorithm. This is corroborated by a minimal bias between ECG-GA and SG-GA CSA values, when the same reconstruction method was applied. Critically, for CEF measures, the percent CSA change of the GA approaches was not statistically different than those obtained with ECG-STD and high correlation was found among the three techniques.
In terms of robustness of the acquisitions, only 3 out of 39 ECG-STD scans and 2 out of 39 GA acquisitions were discarded and this was because of poor image quality due to a non-perpendicular orientation to the vessel that was appreciated only at the time of analysis.
A potential limitation of the method is the relatively long duration of the breath-holds. Here we performed 20-s breath-holds, the same as the current gold-standard. Although the 20-s breath-holds were well maintained by the participants in this study, it may be too long for certain patients. It is important to emphasize that the breath-hold duration of self-gated acquisitions is not prolonged over standard ECG-gated acquisitions.
However, an advantage of the golden-angle method is that it continuously increases the sampling density in k-space the longer the acquisition. With the same golden-angle approach it is possible to increase patient’s comfort and shorten the breath-hold duration by homogenously undersampling the acquisition and allowing reconstruction with state-of-the-art parallel imaging or compressed sensing approaches (40,53–55).
Conclusions
A novel self-gated golden angle spiral cine MRI technique was developed and tested against a standard ECG-dependent sequence for coronary imaging and CEF assessment in healthy volunteers. The proposed self-gating approach exploits independent component analysis of k-space center information, recorded without prolonging the acquisition time, to automatically extract cardiac triggers. The self-gating derived heart rate parameters were equivalent to those from a simultaneously recorded ECG. The new spiral cine MRI can use triggers from either self-gating or ECG for retrospective gating with improved flexibility in terms of heart rate changes during stress. The obtained GA images showed vessel sharpness at least as good or better than that of the standard ECG-dependent method. CEF measures obtained without ECG were in agreement with those obtained with the published standard sequence. Evaluation of the technique in patients with cardiovascular disease is warranted.
Acknowledgments
Work supported by NIH HL120905, NIH HL125059, NIH HL61912, and AHA 17SDG33671007.
References
- 1.Lanzer P, Botvinick EH, Schiller NB, Crooks LE, Arakawa M, Kaufman L, Davis PL, Herfkens R, Lipton MJ, Higgins CB. Cardiac imaging using gated magnetic resonance. Radiology. 1984;150(1):121–127. doi: 10.1148/radiology.150.1.6227934. [DOI] [PubMed] [Google Scholar]
- 2.van Heeswijk RB, Bonanno G, Coppo S, Coristine A, Kober T, Stuber M. Motion compensation strategies in magnetic resonance imaging. Crit Rev Biomed Eng. 2012;40(2):99–119. doi: 10.1615/critrevbiomedeng.v40.i2.20. [DOI] [PubMed] [Google Scholar]
- 3.Lenz GW, Haacke EM, White RD. Retrospective cardiac gating: a review of technical aspects and future directions. Magn Reson Imaging. 1989;7(5):445–455. doi: 10.1016/0730-725x(89)90399-8. [DOI] [PubMed] [Google Scholar]
- 4.Polson MJ, Barker AT, Gardiner S. The effect of rapid rise-time magnetic fields on the ECG of the rat. Clin Phys Physiol Meas. 1982;3(3):231–234. doi: 10.1088/0143-0815/3/3/008. [DOI] [PubMed] [Google Scholar]
- 5.Shetty AN. Suppression of radiofrequency interference in cardiac gated MRI: a simple design. Magn Reson Med. 1988;8(1):84–88. doi: 10.1002/mrm.1910080110. [DOI] [PubMed] [Google Scholar]
- 6.Damji AA, Snyder RE, Ellinger DC, Witkowski FX, Allen PS. RF interference suppression in a cardiac synchronization system operating in a high magnetic field NMR imaging system. Magn Reson Imaging. 1988;6(6):637–640. doi: 10.1016/0730-725x(88)90086-0. [DOI] [PubMed] [Google Scholar]
- 7.Dimick RN, Hedlund LW, Herfkens RJ, Fram EK, Utz J. Optimizing electrocardiograph electrode placement for cardiac-gated magnetic resonance imaging. Invest Radiol. 1987;22(1):17–22. doi: 10.1097/00004424-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56(20):1657–1665. doi: 10.1016/j.jacc.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 10.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 11.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 12.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 13.Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high-energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323(23):1593. doi: 10.1056/NEJM199012063232304. [DOI] [PubMed] [Google Scholar]
- 14.Brown BG, Josephson MA, Petersen RB, Pierce CD, Wong M, Hecht HS, Bolson E, Dodge HT. Intravenous dipyridamole combined with isometric handgrip for near maximal acute increase in coronary flow in patients with coronary artery disease. Am J Cardiol. 1981;48(6):1077–1085. doi: 10.1016/0002-9149(81)90323-4. [DOI] [PubMed] [Google Scholar]
- 15.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation. 1984;70(1):18–24. doi: 10.1161/01.cir.70.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schär M, Gerstenblith G, Stuber M, Weiss RG. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol. 2015;308(11):H1343–1350. doi: 10.1152/ajpheart.00023.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays AG, Stuber M, Hirsch GA, Yu J, Schär M, Weiss RG, Gerstenblith G, Kelle S. Non-invasive detection of coronary endothelial response to sequential handgrip exercise in coronary artery disease patients and healthy adults. PLoS One. 2013;8(3):e58047. doi: 10.1371/journal.pone.0058047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White RD, Paschal CB, Clampitt ME, Spraggins TA, Lenz GW. Electrocardiograph-independent, "wireless" cardiovascular cine MR imaging. J Magn Reson Imaging. 1991;1(3):347–355. doi: 10.1002/jmri.1880010313. [DOI] [PubMed] [Google Scholar]
- 19.Spraggins TA. Wireless retrospective gating: application to cine cardiac imaging. Magn Reson Imaging. 1990;8(6):675–681. doi: 10.1016/0730-725x(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 20.Kim WS, Mun CW, Kim DJ, Cho ZH. Extraction of cardiac and respiratory motion cycles by use of projection data and its applications to NMR imaging. Magn Reson Med. 1990;13(1):25–37. doi: 10.1002/mrm.1910130105. [DOI] [PubMed] [Google Scholar]
- 21.Buehrer M, Curcic J, Boesiger P, Kozerke S. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med. 2008;60(3):683–690. doi: 10.1002/mrm.21697. [DOI] [PubMed] [Google Scholar]
- 22.Crowe ME, Larson AC, Zhang Q, Carr J, White RD, Li D, Simonetti OP. Automated rectilinear self-gated cardiac cine imaging. Magn Reson Med. 2004;52(4):782–788. doi: 10.1002/mrm.20212. [DOI] [PubMed] [Google Scholar]
- 23.Nijm GM, Sahakian AV, Swiryn S, Carr JC, Sheehan JJ, Larson AC. Comparison of self-gated cine MRI retrospective cardiac synchronization algorithms. J Magn Reson Imaging. 2008;28(3):767–772. doi: 10.1002/jmri.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brau AC, Brittain JH. Generalized self-navigated motion detection technique: Preliminary investigation in abdominal imaging. Magn Reson Med. 2006;55(2):263–270. doi: 10.1002/mrm.20785. [DOI] [PubMed] [Google Scholar]
- 25.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51(1):93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolbitsch C, Prieto C, Schaeffter T. Cardiac functional assessment without electrocardiogram using physiological self-navigation. Magn Reson Med. 2014;71(3):942–954. doi: 10.1002/mrm.24735. [DOI] [PubMed] [Google Scholar]
- 27.Yerly J, Ginami G, Nordio G, Coristine AJ, Coppo S, Monney P, Stuber M. Coronary endothelial function assessment using self-gated cardiac cine MRI and k-t sparse SENSE. Magn Reson Med. 2016;76(5):1443–1454. doi: 10.1002/mrm.26050. [DOI] [PubMed] [Google Scholar]
- 28.Hays AG, Kelle S, Hirsch GA, Soleimanifard S, Yu J, Agarwal HK, Gerstenblith G, Schär M, Stuber M, Weiss RG. Regional coronary endothelial function is closely related to local early coronary atherosclerosis in patients with mild coronary artery disease: pilot study. Circ Cardiovasc Imaging. 2012;5(3):341–348. doi: 10.1161/CIRCIMAGING.111.969691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iantorno M, Schar M, Soleimanifard S, Brown TT, Moore R, Barditch-Crovo P, Stuber M, Lai S, Gerstenblith G, Weiss RG, Hays AG. Coronary artery endothelial dysfunction is present in HIV-positive individuals without significant coronary artery disease. AIDS. 2017;31(9):1281–1289. doi: 10.1097/QAD.0000000000001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13(4–5):411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 31.Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26(1):68–76. doi: 10.1109/TMI.2006.885337. [DOI] [PubMed] [Google Scholar]
- 32.Bonanno G, Hays AG, Weiss RG, Schär M. Proc Intl Soc Magn Reson Med. Vol. 25. Honolulu, HI: 2017. Self-Gated Golden Angle Spiral CINE MRI for Coronary Endothelial Function Assessment; p. 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonanno G, Piccini D, Maréchal B, Zenge MO, Stuber M. Proc Intl Soc Magn Reson Med. Vol. 23. Milan, Italy: 2014. A New Binning Approach for 3D Motion Corrected Self-Navigated Whole-Heart Coronary MRA Using Independent Component Analysis of Individual Coils; p. 936. [Google Scholar]
- 34.Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. 1999;10(3):626–634. doi: 10.1109/72.761722. [DOI] [PubMed] [Google Scholar]
- 35.Digital Signal Processing Committee of the IEEE Acoustics S and Signal Processing Society, editor. Programs for Digital Signal Processing. New York: IEEE Press; 1979. [Google Scholar]
- 36.Oppenheim AV, Schafer RW. Discrete-Time Signal Processing. Upper Saddle River, NJ, USA: Prentice Hall Press; 2009. [Google Scholar]
- 37.Stuber M, Botnar RM, Danias PG, Sodickson DK, Kissinger KV, Van Cauteren M, De Becker J, Manning WJ. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34(2):524–531. doi: 10.1016/s0735-1097(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 38.Pipe JG, Zwart NR. Spiral trajectory design: a flexible numerical algorithm and base analytical equations. Magn Reson Med. 2014;71(1):278–285. doi: 10.1002/mrm.24675. [DOI] [PubMed] [Google Scholar]
- 39.Feinstein JA, Epstein FH, Arai AE, Foo TK, Hartley MR, Balaban RS, Wolff SD. Using cardiac phase to order reconstruction (CAPTOR): a method to improve diastolic images. J Magn Reson Imaging. 1997;7(5):794–798. doi: 10.1002/jmri.1880070505. [DOI] [PubMed] [Google Scholar]
- 40.Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 41.Zwart NR, Pipe JG. Graphical programming interface: A development environment for MRI methods. Magn Reson Med. 2015;74(5):1449–1460. doi: 10.1002/mrm.25528. [DOI] [PubMed] [Google Scholar]
- 42.Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. Magn Reson Med. 2007;57(6):1131–1139. doi: 10.1002/mrm.21237. [DOI] [PubMed] [Google Scholar]
- 43.Noll DC, Pauly JM, Meyer CH, Nishimura DG, Macovski A. Deblurring for non-2D Fourier transform magnetic resonance imaging. Magn Reson Med. 1992;25(2):319–333. doi: 10.1002/mrm.1910250210. [DOI] [PubMed] [Google Scholar]
- 44.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99(24):3139–3148. doi: 10.1161/01.cir.99.24.3139. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch GA, Kelle S, Hays A, Gerstenblith G, Schär M, Weiss RG, Stuber M. Spiral Imaging at 3T for the Measurement of Endothelial-Dependent Coronary Arterial Function. Journal of Cardiovascular Magnetic Resonance. 2008;10(Suppl 1):A139. [Google Scholar]
- 46.Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med. 1990;15(2):287–304. doi: 10.1002/mrm.1910150211. [DOI] [PubMed] [Google Scholar]
- 47.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39(3):361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 48.Rasche V, Holz D, Proksa R. MR fluoroscopy using projection reconstruction multi-gradient-echo (prMGE) MRI. Magn Reson Med. 1999;42(2):324–334. doi: 10.1002/(sici)1522-2594(199908)42:2<324::aid-mrm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Lin W, Guo J, Rosen MA, Song HK. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn Reson Med. 2008;60(5):1135–1146. doi: 10.1002/mrm.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wollny G, Kellman P, Santos A, Ledesma-Carbayo MJ. Automatic motion compensation of free breathing acquired myocardial perfusion data by using independent component analysis. Med Image Anal. 2012;16(5):1015–1028. doi: 10.1016/j.media.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang J, Sharif B, Fan Z, Bi X, Arsanjani R, Berman DS, Li D. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn Reson Med. 2014;72(5):1208–1217. doi: 10.1002/mrm.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul J, Divkovic E, Wundrak S, Bernhardt P, Rottbauer W, Neumann H, Rasche V. High-resolution respiratory self-gated golden angle cardiac MRI: Comparison of self-gating methods in combination with k-t SPARSE SENSE. Magn Reson Med. 2015;73(1):292–298. doi: 10.1002/mrm.25102. [DOI] [PubMed] [Google Scholar]
- 53.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 54.Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014;72(3):707–717. doi: 10.1002/mrm.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schär M, Bonanno G, Hays AG, Wang D, Li Z, Pipe JG, Weiss RG. Golden-Angle Spiral Sparse Parallel-Imaging for Coronary Lumen Area Measurements in Short Breath-Holds. Journal of Cardiovascular Magnetic Resonance. 2017;(Suppl 1):321. [Google Scholar]