Abstract
BACKGROUND
Patent foramen ovale closure represents a potential secondary prevention strategy for cryptogenic stroke, but available trials have varied by size, device studied, and follow-up.
METHODS
We conducted a systematic search of published randomized clinical trials evaluating patent foramen ovale closure versus medical therapy in patients with recent stroke or transient ischemic attack using PubMED, EMBASE, and Cochrane through September 2017. Weighting was by random effects models.
RESULTS
Of 480 studies screened, we included 5 randomized clinical trials in the meta-analysis in which 3440 patients were randomized to patent foramen ovale closure (n = 1829) or medical therapy (n = 1611) and followed for an average of 2.0 to 5.9 years. Index stroke/transient ischemic attack occurred within 6 to 9 months of randomization. The primary end point was composite stroke/transient ischemic attack and death (in 3 trials) or stroke alone (in 2 trials). Patent foramen ovale closure reduced the primary end point (0.70 vs 1.48 events per 100 patient-years; risk ratio [RR], 0.52 [0.29–0.91]; I2 = 55.0%) and stroke/transient ischemic attack (1.04 vs 2.00 events per 100 patient-years; RR, 0.55 [0.37–0.82]; I2 = 42.2%) with modest heterogeneity compared with medical therapy. Procedural bleeding was not different between study arms (1.8% vs 1.8%; RR, 0.94 [0.49–1.83]; I2 = 29.2%), but new-onset atrial fibrillation/flutter was increased with patent foramen ovale closure (6.6% vs 0.7%; RR, 4.69 [2.17–10.12]; I2 = 29.3%).
CONCLUSIONS
In patients with recent cryptogenic stroke, patent foramen ovale closure reduces recurrent stroke/transient ischemic attack compared with medical therapy, but is associated with a higher risk of new-onset atrial fibrillation/flutter.
Keywords: Cryptogenic stroke, Meta-analysis, Patent foramen ovale, Percutaneous
INTRODUCTION
Patent foramen ovale is present in approximately one quarter of the general population. Up to 40% of ischemic strokes do not have a clear identifiable cause despite systematic investigation, and some may be attributed to a patent foramen ovale. Given the promise of a potential reduction in recurrent neurologic events in patients with patent foramen ovale who present with cryptogenic strokes, several percutaneous patent foramen ovale closure devices are now commercially available, including the recently Food and Drug Administration–approved AMPLATZER PFO Occluder (Abbott Vascular, Santa Clara, Calif). However, the overall utility of routine patent foramen ovale closure after cryptogenic stroke remains uncertain and is not currently supported by guidelines.1
In the last several months, new randomized clinical trials (RCTs) evaluating various patent foramen ovale closure devices in more carefully selected patients followed for longer durations have become available, calling for reappraisal of the overall efficacy and safety of this secondary prevention approach. As such, we conducted an updated systematic review and meta-analysis of published RCTs evaluating patent foramen ovale closure versus medical therapy in patients with recent cryptogenic stroke.
METHODS
We queried the PubMED, EMBASE, and Cochrane CENTRAL databases through September 2017, using a predefined search strategy. We retrieved 480 records, and after removing duplicates and screening on the basis of titles and abstracts, 6 full-text articles were examined. Two were short-term and long-term follow-ups of the same RCT; we only included the exploratory analysis with extended follow-up.2 One trial compared patent foramen ovale closure or oral anticoagulation with antiplatelet therapy after cryptogenic stroke.3 We included the prespecified pooled analysis of patients randomized to patent foramen ovale closure plus antiplatelet therapy or antiplatelet therapy alone. We present event rates (%) for procedural complications and incidence rates (expressed per 100 patient-years) for long-term end points, given differential follow-up durations. If unavailable, total observation period was estimated using patient sample size and mean follow-up. We calculated pooled risk ratios (RRs) and 95% confidence intervals (CIs) using a random-effects model by the method of DerSimonian and Laird and assessed heterogeneity with the I2 measure.
RESULTS
We included 5 RCTs2–6 in the meta-analysis, in which 3440 patients were randomized to patent foramen ovale closure (n = 1829) or medical therapy (n = 1611) and followed for an average of 2.0 to 5.9 years. All were multicenter, open-label RCTs, and 2 included blinded end point adjudication.2,6 All trials enrolled patients aged ≤60 years (mean, 42.9–46.3 years in patent foramen ovale closure arms). Index stroke, transient ischemic attack, or peripheral embolism was allowed up to 9 months before randomization. One trial enrolled only patients with patent foramen ovale with an associated atrial septal aneurysm or large interatrial shunt.3 Dual antiplatelet therapy was required after patent foramen ovale closure for ≥3 days to 6 months. The primary end point was the composite of stroke/transient ischemic attack and death2,4,5 or stroke alone.3,6
Patent foramen ovale closure reduced the primary end point (0.70 vs 1.48 events per 100 patient-years; RR, 0.52; 95% CI, 0.29–0.91; I2 = 55.0%) and stroke/transient ischemic attack (1.04 vs 2.00 events per 100 patient-years; RR, 0.55; 95% CI, 0.37–0.82; I2 = 42.2%) with modest heterogeneity compared with medical therapy (Figure). Pooled analysis of 2 trials evaluating the Food and Drug Administration–approved AMPLATZER PFO Occluder device2,5 showed consistent results for both end points. All-cause mortality was low and similar in both patent foramen ovale closure and medical therapy groups (0.17 vs 0.24 deaths per 100 patient-years). Procedural bleeding was not different between study arms (1.8% vs 1.8%; RR, 0.94; 95% CI, 0.49–1.83; I2 = 29.2%). New-onset atrial fibrillation/atrial flutter was increased with patent foramen ovale closure (6.6% vs 0.7%; RR, 4.69; 95% CI, 2.17–10.12; I2 = 29.3%).
Figure.
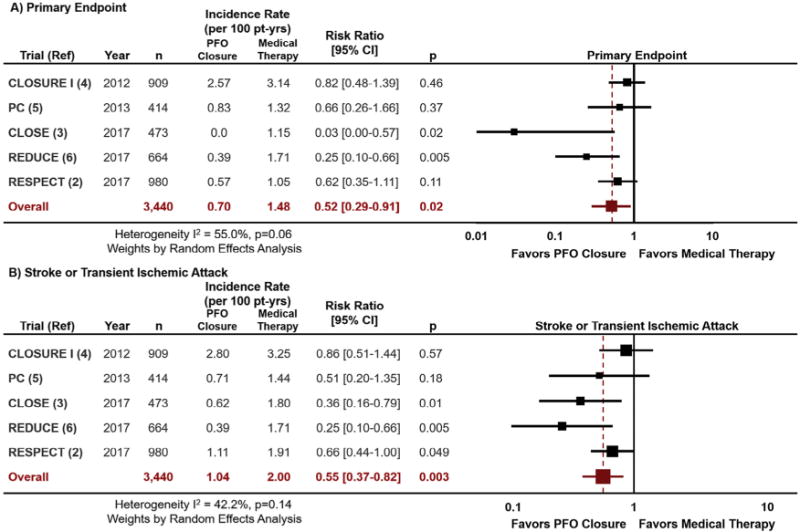
Meta-analysis of 5 published RCTs evaluating percutaneous patent foramen ovale closure versus medical therapy after recent cryptogenic stroke with respect to the primary end point (A) and recurrent stroke or transient ischemic attack (B). I2 represents the degree of heterogeneity. CI = confidence interval; CLOSE = Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence; CLOSURE I = Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale; PC = Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism; PFO = patent foramen ovale; RESPECT = Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment.
DISCUSSION
Our updated meta-analysis supports patent foramen ovale closure as a potential therapeutic strategy to decrease the risk of recurrent neurologic events compared with medical therapy in well-selected patients. Reductions in recurrent stroke/transient ischemic attack were directionally consistent across 5 RCTs and durable with longer-term follow-up.2 With respect to safety, percutaneous device placement was generally well tolerated with low rates of clinically significant bleeding and death. We did identify higher rates of newly detected atrial arrhythmias with patent foramen ovale closure, a finding that requires further investigation.
CONCLUSIONS
Modest heterogeneity in the results was observed across trials likely referable to more than 10 different patent foramen ovale closure devices studied and variation in primary end point selection, mandated antithrombotic therapy in control arms, rigor of evaluation of alternative sources of stroke/transient ischemic attack, and application of certain enrichment criteria. Head-to-head studies are lacking to support one particular patent foramen ovale closure device over another. Despite these remaining questions, at this juncture, select, young patients (aged ≤60 years) presenting with recent cryptogenic stroke may benefit from percutaneous closure of patent foramen ovale at relatively low procedural risk.
CLINICAL SIGNIFICANCE.
In patients with recent cryptogenic stroke, percutaneous closure of patent foramen ovale reduced recurrent stroke/transient ischemic attack compared with medical therapy, but was associated with higher risk of new-onset atrial fibrillation/flutter.
Select, young patients (≤60 years) presenting with recent cryptogenic stroke may benefit from percutaneous closure of patent foramen ovale at relatively low procedural risk.
Acknowledgments
Funding: None.
Conflicts of Interest: MV and AQ are supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training Grant (T32HL007604). AG and NB and are supported by the National Heart, Lung, and Blood Institute T32 postdoctoral training Grant (5T32HL094301-08). DLB discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. All other authors have reported that they have no other relationships relevant to the contents of this paper to disclose.
Footnotes
Authorship: All authors had access to the data and a role in writing the manuscript.
References
- 1.Messe SR, Gronseth G, Kent DM, et al. Practice advisory: recurrent stroke with patent foramen ovale (update of practice parameter): report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87:815–821. doi: 10.1212/WNL.0000000000002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 3.Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 4.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 5.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 6.Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]


