Abstract
The ultimate goal of Fontan surgical planning is to provide additional insights into the clinical decision making process. In its current state, surgical planning offers an accurate hemodynamic assessment of the preoperative condition, provides anatomical constraints for potential surgical options, and produces decent postoperative predictions if boundary conditions are similar enough between the preoperative and postoperative states. Moving forward, validation with postoperative data is a necessary step in order to assess the accuracy of surgical planning and determine which methodological improvements are needed. Future efforts to automate the surgical planning process will reduce the individual expertise needed and encourage use in the clinic by clinicians. As postoperative physiologic predictions improve, Fontan surgical planning will become an even more effective tool to accurately model patient specific hemodynamics.
Keywords: Fontan, patient specific, surgical planning, pre-operative planning, hepatic flow distribution
Introduction
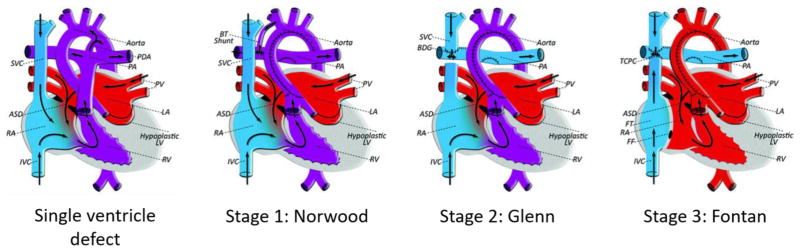
For the 1% of children born with congenital heart defects (CHD), complex surgeries are often inevitable. Single ventricle abnormalities are among the most severe, requiring several, staged operations for palliation. These operations include a stage I procedure such as the Norwood, Blalock-Taussig shunt or PA-band, the Glenn procedure (directly connecting the superior vena cava to the pulmonary artery) and the Fontan procedure (Fig. 1). Short of transplantation, the Fontan procedure is the only long-term option to palliate most single ventricle congenital heart defects. In the Fontan procedure, a baffle or conduit is used to redirect blood from the inferior vena cava (IVC) to the pulmonary circulation, bypassing the subpulmonary ventricle [1]. First performed in 1971, the Fontan operation has since progressed through several iterations including the atriopulmonary connection, intra-atrial baffle, and extracardiac conduit with several more subtle variations along the way. The resulting configuration prevents the mixing of oxygenated and deoxygenated blood by putting the systemic and pulmonary circulation in series, and allows the body to function with only one working ventricle.
Fig. 1.
Staged palliation for single ventricle congenital heart defects. Blood volume is colored by blue (deoxygenated) and red (oxygenated). ASD: atrial septal defect, BDG: bidirectional Glenn anastomosis, BT: Blalock-Taussig, FF: Fontan fenestration, FT: Fontan tunnel, IVC/SVC: inferior/superior vena cava, LA: left atrium, LV: left ventricle, PA: pulmonary artery, PDA: patent ductus arteriosus, PV: pulmonary vein, RA: right atrium, RV: right ventricle, TCPC: total cavopulmonary connection
Though the Fontan procedure offers promising short term outcomes, serious complications such as pulmonary arteriovenous malformations (PAVMs), liver disease, protein-losing enteropathy and exercise intolerance can occur as patients age [2–7]. It is hypothesized that the altered hemodynamics inherent to Fontan physiology play a role in the development of these complications, which has motivated a plethora of computational and experimental studies [8–12]. Various groups have investigated new connection designs (Optiflo and Y-graft), mechanical circulatory assistance, and hemodynamic correlations with outcomes among others [13–19].
Because of advances in medical imaging and the planned nature of the Fontan surgery, the use of “surgical planning” to preoperatively optimize relevant hemodynamic metrics is hypothesized to improve patient outcomes. Fontan surgical planning is a collaborative effort between clinicians and engineers has been used over the past 15 years. Generally, surgical planning has been reserved for the most complex Fontan cases, including those with interrupted IVC with azygous (AZ) continuation, multiple superior vena cavae (SVC) and other types of complex anatomies [11, 20, 21].
Fontan Surgical Planning: Current methodology and impact
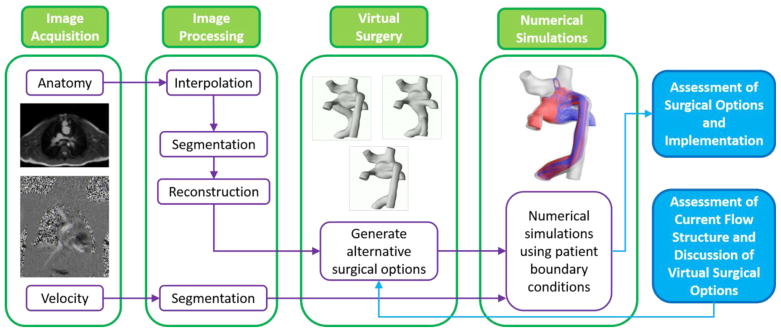
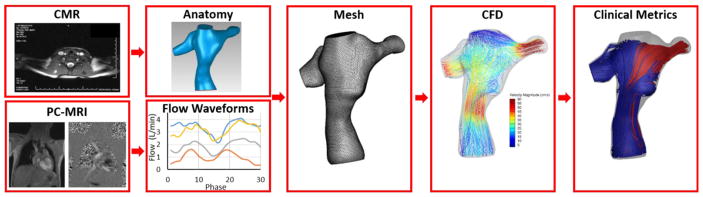
The current surgical planning paradigm is a multi-step process that involves collaboration between clinicians and engineers. The major steps include preoperative image acquisition, image processing, creation of virtual surgical options, and numerical simulations of those proposed options (Fig. 2) [22–24]. Communication between clinicians and engineers is essential to verify segmented images, discuss the viability of surgical options and review simulation results. Overall, surgical planning is meant to analyze the performance of potential surgical options and therefore help inform the decision of graft type and positioning.
Fig. 2.
Surgical planning paradigm
Potential surgical options are evaluated and ranked according to various clinically important metrics. To date, the major goals of Fontan surgical planning have included minimizing energy loss in the total cavopulmonary connection (TCPC), providing a balanced hepatic flow distribution (HFD) to the left and right lungs, and avoiding extreme wall shear stress. Energy loss has been related to exercise intolerance and is hypothesized to affect overall patient outcome, HFD is a known factor in PAVM progression, and extreme wall shear stress (both low and high) may be prone to clot or blood damage respectively [25–29]. In situations where one specific surgical option is not optimal for all metrics, clinicians must evaluate which complication is of most concern for a given patient. For example, the progression of PAVMs is a very common motivation for surgical planning and therefore would focus on achieving balanced HFD.
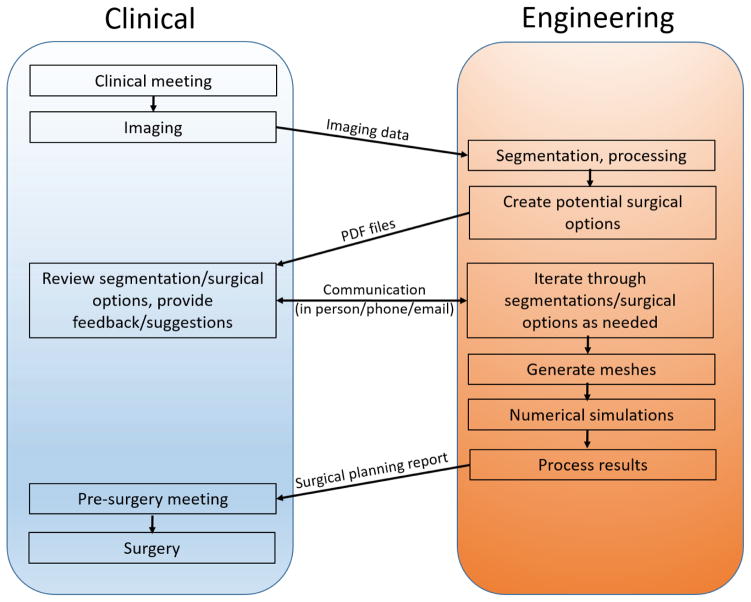
The data flow, order of events, and forms of data transfer between the clinical and engineering setting are shown in Fig. 3. The only physical transfer of data from the clinical setting to engineering setting is the imaging data which begins the surgical planning process. Analyses including 3D reconstructions of the preoperative anatomy and proposed surgical options, as well as a final surgical planning report, are ultimately sent back to the clinic at the respective steps in the surgical planning process. Frequent communication between the two settings is important during the entire process, including segmentation, creation of realistic surgical options (requires the most clinical input), and review of comprehensive results.
Fig. 3.
Interaction and types of data transfer between clinical and academic settings. Events are positioned chronologically from top to bottom
Methodology Overview
Image acquisition
The foundation for surgical and interventional planning is accurate imaging data. In general, cross sectional imaging provides a superior substrate for anatomic reconstructions when compared to two or even three dimensional echocardiography due to the need for high spatial resolution and isotropic voxels (medical imaging cube with equal length sides in each dimension, analogous to pixels in a two dimensional image). Our lab typically utilizes cardiovascular magnetic resonance imaging (CMR) studies on children and young adults with congenital heart disease. Anatomic reconstructions can be created from stacks of contiguous, high-resolution, isotropic, static datasets. Most often we utilize pre-contrast, respiratory navigated, T2 prepared, steady state free precession (SSFP) readout images or post-contrast, respiratory navigated, inversion recovery prepared gradient echo (IR GRE) readout images. Ideally, the datasets should be isotropic with <1.5 mm resolution.
Flow boundary conditions for the simulations are typically provided via phase contrast magnetic resonance imaging (PCMRI). Through plane, retrospectively gated, PCMRI is used to assess flows in the cavae, branch pulmonary arteries, pulmonary veins and across the aortic valve. Velocity encoding (VENC) is generally 150 cm/sec for the aorta and 60 cm/sec for the other vessels. Slice thickness is generally 4–5 mm with inplane resolution of 1.25×1.25 mm. The number of phases acquired is a function of the heart rate and generally ranges from 20–30.
Image processing
Image segmentation is used to reconstruct both a patient’s anatomy and blood flow waveforms. Most often, axial CMR images are the sequence of choice for anatomical reconstruction. Image segmentation has advanced steadily over the last 20 years, and now a number of both commercial and open-source software packages exist that can interpolate, convert between axial/coronal/sagittal orientations, and segment patient anatomies with varying levels of automation [30–32]. Thus far, the high variability between Fontan anatomies has delayed fully automatic segmentation in this space.
Blood flow waveforms for all vessels of interest are reconstructed from PC-MRI images to provide boundary conditions for modeling purposes. These vessels include the IVC, SVC, left and right pulmonary arteries (LPA and RPA), left superior vena cava (LSVC), and AZ as appropriate. Similar to anatomic segmentation, a handful of software packages are available for velocity segmentation. To highlight one, Segment (http://segment.heiberg.se) is an open source software that provides a very straightforward and user friendly workflow for segmentation [33–34]. This software (similar to others) offers a wide variety of functionality including automatic region of interest refining and propagation, vessel tracking throughout the cardiac cycle, automatic parameter calculation and 3D velocity profile segmentation among others.
Generating surgical options
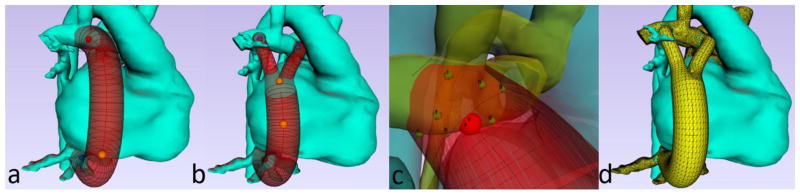
The preoperative anatomical segmentation is the basis for generating potential surgical options, which is performed following detailed discussion with the clinicians. The Glenn anatomy (for pre-Fontan planning) or original Fontan anatomy (for Fontan revisions), as well as structures that could create anatomical constraints for the proposed options (heart, aorta etc.) are included in the modeling. While standard CAD software can be used to generate surgical options, the use of modeling software specifically designed for Fontan surgical planning drastically reduces the time and effort required, and reduces the need for any prior modeling expertise. For example, SURGEM III is a software which has been developed over the last decade specifically for Fontan surgical planning [11,23, 35]. SURGEM III is an interactive solid modeling tool whose name stems from “surgery modeler.” It is designed to simplify and accelerate the surgical planning process, specifically the steps involved in creating and evaluating potential surgical options. With this software, a user can easily choose a graft type (traditional conduit or Y-graft) and graft size (in 2 mm increments) and position the insertion locations as desired (Fig. 4). The user can modify graft centerline placement, insertion angle and offset options as needed (Fig. 4c). SURGEM III can also automatically generate a large number of combinations of graft size and insertion angles/offsets in order to evaluate the robustness of given surgical option [36]. Finally, this software can preview and export a surface mesh of the proposed surgical option for the next step in the surgical planning process (Fig. 4d).
Fig. 4.
Creation of surgical options. (a) Fontan extracardiac baffle option. (b) Fontan bifurcated Y-graft option. (c) Automatic creation of baffle insertion angle/offset variations. (d) Preview of unioned (pre-op anatomy with proposed graft placement) mesh
Numerical simulations
Computational fluid dynamics (CFD) has been used for more than a decade to analyze blood flow through the Fontan connection [11, 37–40]. Using the patient-specific anatomy and blood flow waveforms as the domain and boundary conditions respectively, CFD techniques first discretize the patient’s anatomy into many computational nodes/cells (on the order of 105–106).
The CFD solver then solves the Navier-Stokes and continuity equations (momentum and mass conservation) at every node/cell within the domain. The pressure and velocity values at each node/cell are then updated, and this process repeats (iterates) until it converges on a solution. This occurs for every time step throughout the cardiac cycle. The output of a CFD simulation is the pressure and velocity components that satisfies the Navier-Stokes and continuity equations, as well as the specified boundary conditions at every node/cell in the domain. The resulting pressure and velocity fields can be used to calculate a number of potentially important clinical metrics including energy loss, hepatic flow distribution and wall shear stress among others. The general process for patient-specific CFD simulations in shown in Fig. 5.
Fig. 5.
General process for patient-specific CFD simulations
Sophisticated lumped parameter networks (LPN) are often used to model the full body circulation in an attempt to produce more accurate flow boundary conditions for the postoperative state and assess global hemodynamics [39, 41–45]. In this process, a 0-D LPN is coupled to a 3-D patient specific model (stage 1, 2 or 3). The LPN is then tuned to match patient specific conditions by carefully adjusting parameters (resistances, compliances and impedances) throughout the LPN. When available, pressure measurements via catheterization allow for more patient-specific modeling of the full circulation (pulmonary and systemic vascular resistance etc.) and can be integral in making broader surgical decisions [46]. This is usually accomplished using an optimization algorithm with some final manual adjustments to best match patient flow waveforms [39]. Once the model has been successfully tuned to match the pre-operative conditions, the virtual “surgery” is implemented by either updating the 3-D patient specific model, reconfiguring the LPN, or both.
In addition, there is a wide range of fidelity in surgical planning simulations. Multiple variations including steady vs pulsatile flow waveforms as boundary conditions, Newtonian vs non-Newtonian fluid properties, rigid vs compliant vessel walls, free-breathing vs breath-held flow effects, patient-specific vs idealized anatomies and others must be considered. In general, current surgical planning employs pulsatile, non-Newtonian, rigid, patient-specific and breath-held conditions. Though some of these conditions are obviously not physiologically true, there is an important trade-off between physiological accuracy and computational time. Overall, the inclusion of more representative conditions (pulsatile, free-breathing, compliant etc.) leads to longer computational times which may not fit the relatively short clinical timeline for most surgical planning cases. Several studies have investigated the effects of these various options, but more work is needed to determine how they may affect the ranking of surgical options [24, 47–48].
Personnel Involvement
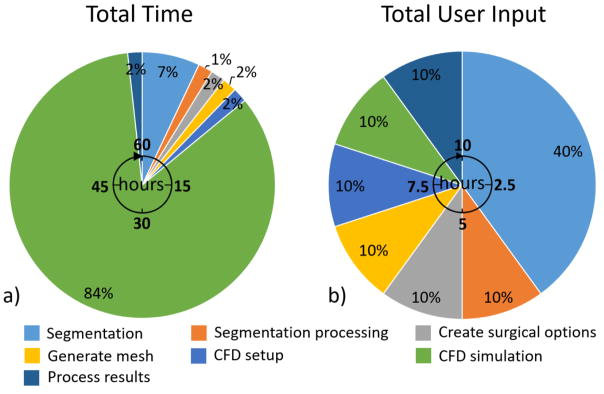
Fig. 6a shows the total time (human effort and computational time) required for each step in the surgical planning process. This timeline is representative of a standard case with high quality imaging data, and gives estimates based on our experience for modeling only one potential surgical option at one physiologic condition. Image segmentation and CFD simulation require over 90% of the total time needed for surgical planning. Furthermore, the estimation of 48 hours to complete a CFD simulation assumes a significant amount of computational resources (64 AMD 2.4 GHz Opteron 6378 processors, 2 MB cache). If multiple surgical options are modeled as well as multiple physiologic conditions (usually the case), the time required for mesh generation, simulation setup, simulation run time and processing results will be multiplied accordingly. While the steps in the surgical planning process are sequential, various surgical options could be distributed and evaluated in parallel by multiple people if sufficient computational resources exist.
Fig. 6.
Total time and user input required for surgical planning. These estimates are repreentative of modeling one surgical option at one physiologic condition
Fig. 6b shows the total user input time (human effort only). In terms of user input, image segmentation requires around 40% of total user input (Fig. 6b). This percentage can increase for patients with poor image quality or imaging artifacts. For the remaining steps in the surgical planning process, relatively minimal user input is required. Multiple surgical options with specific graft sizes can be generated within an hour using the software mentioned above. Standard protocols can be followed to generate meshes for CFD simulations. Even with more advanced meshes including inflation layers and variable element sizing this process can still be completed in less than one hour per surgical option. Simulation setup is a somewhat routine process and can also be accomplished in less than one hour per surgical option. Once the simulation is complete, processing results to calculate various metrics can be finished within one hour. Overall, around 10 hours of user input is currently required to model one surgical option, with an additional 4 hours for each added surgical option or physiologic condition.
Current clinical impact
At present, prospective Fontan surgical planning patients are identified by their clinician, who then contacts either the clinical or basic science members of our team to discuss feasibility of modeling for the patient-specific case. Specific imaging needs for accurate analysis are communicated to the referring center, and after the medical imaging is performed, the imaging data along with a clinical summary are de-identified and sent to our center. As mentioned above, we have received a mix of both pre-Fontan patients (where adverse streaming and difficult hemodynamics are predicted due to complex anatomy, so a traditional lateral tunnel or extra-cardiac Fontan is anticipated to have suboptimal results) as well as failing Fontan patients (with either PAVMs or poor hemodynamics).
After the surgical planning options are created and the hemodynamics such as energy loss and flow splits to each lung are calculated, the results are recorded in a report that is discussed with the cardiologist and the surgeon who are involved in the patient care; ideally, the cardiologist and surgeon are the same as the ones who were in collaboration prior to creation of the surgical options, however, in practice, this is not necessarily the case. Nevertheless, all involved discuss the options to determine which is best to implement in the operating room, both from a hemodynamic standpoint and a practical surgical standpoint, the latter being less of an issue as most of this is taken into account earlier. The ventricles, atria and other anatomic details are included in the 3-dimensional reconstruction to ensure the operation is able to be performed. In general, surgical planning data, both CFD and anatomy, are presented at surgical conference.
For the vast majority of surgery in congenital heart disease, surgical planning is not performed. However, for complex lesions, surgical planning plays an important role by providing unique information. Specifically, this planning process has found utility in patients with bilateral superior vena cavae or in patients with heterotaxy syndrome (e.g. those with interrupted inferior vena cava with azygous continuation to a superior vena cava). In addition, surgical planning has been utilized in double outlet right ventricle to guide repair of the outflow tracts. Normally, pre-operative imaging allows for cardiologists and surgeons to view the “current state” of the patient’s anatomy and hemodynamics and the healthcare provider needs to imagine how the repair will be performed. Surgical planning is the only methodology for healthcare providers to actually visualize and quantify the multiple “future states” and to decide between them.
Since timing for most of these procedures is relatively elective, the analyses are completed prior to formulation of a clinical surgical plan. The detailed engineering and clinical summaries are composed and sent back to the original center. The referring clinicians then review all analyses, and a post-analysis conference call is scheduled to ensure clear communication of all ideas and findings. In each case we have completed to date, the clinical center has voiced that the results were helpful in formulating a treatment plan for the patient. In most cases, the most hemodynamically ideal virtual surgical option is implemented, but in select cases there have been additional factors that precluded this option (surgeon’s preference, etc.). Regardless of which virtual option is implemented, we request in all cases that any follow up data, including future cross-sectional imaging or catheterization data, is also sent to our center, so the virtual surgical decision making process can continue to be refined.
Example case
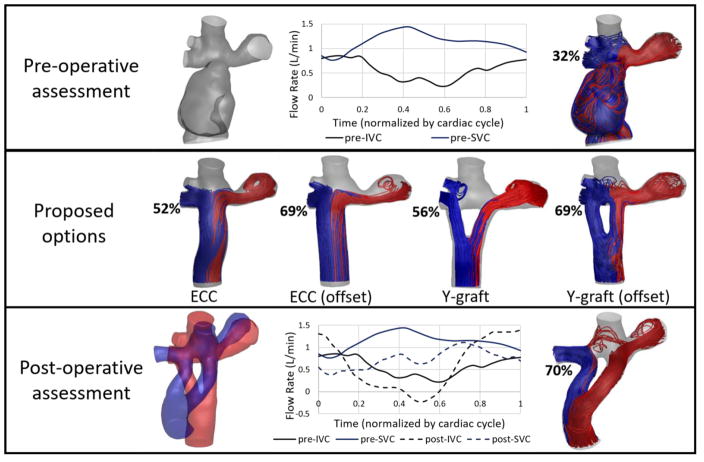
The following example case is a 12 year old female with an existing lateral tunnel Fontan connection. Arterial oxygen saturations were in the upper 80s, with diagnosis of right lung PAVMs on the basis of systemic venous bubble injections. This patient was enrolled in the surgical planning protocol in order to evaluate surgical options for a Fontan revision with the goal of providing increased hepatic flow to the right lung.
The patient’s anatomy and blood flow waveforms were reconstructed from preoperative imaging (Fig. 7, pre-operative). CFD simulations revealed an unbalanced hepatic flow favoring the left lung, confirming a lack of hepatic factor to the RPA are therefore PAVM progression in the right lung.
Fig. 7.
Representative surgical planning case. Only inlet waveforms are shown for clarity. Streamlines colored by outlet vessel (LPA=red, RPA=blue) HFD to the right lung is indicated by percentage. The Y-graft (offset) option was implemented in the patient. Post-operative assessment was conducted at a 2 month follow up visit. To compare anatomies, the proposed option (red) is overlaid with the actual post-operative anatomy (blue). (ECC: extracardiac conduit)
Several potential surgical options were modeled including multiple variations of extracardiac conduit (ECC) and Y-graft connections. Each option was evaluated in terms of hepatic flow distribution (Fig. 7, proposed options). Both the ECC (offset) and Y-graft (offset) options directed the majority of hepatic flow (69%) to the right lung, suggesting either option could provide sufficient hepatic factor to combat PAVM progression.
The offset Y-graft option was chosen and implemented in the patient. The post-operative assessment based on a two month follow up visit is shown in Fig. 7. The proposed (red) and post-operative (blue) anatomies are overlaid for comparison. The post-operative Y-graft connection had very similar insertion points as the proposed option, however the general shape of the Y-graft was much more tortuous than proposed. A comparison of the pre- and postoperative inlet flow waveforms are also shown (outlet waveforms not shown for clarity).
Finally, the most important comparison is the predicted vs post-operative HFD. In this case, the HFD prediction of 69% to the RPA was very similar to the post-operative 70%. In general, we have seen HFD predictions to be within 10 percentage points of the post-operative result for Fontan revision cases closely resembling the predicted anatomy. However, this value is subject to change over time with physiologic growth and adaptation.
Future vision - Methodology
The current capabilities of surgical planning offer accurate modeling of the preoperative condition, anatomical constraints for proposed surgical options, and decent predictions of the postoperative state. Building upon these early successes, future improvements on both the engineering and clinical side are needed to progress the effectiveness and applicability of Fontan surgical planning. Important future steps include validation with post-operative data, improved boundary condition prediction, and automation.
Validation - The necessity of follow-up data
The only true measure for the effectiveness/usefulness of surgical planning is post-operative patient outcome. Surgical planning validation is this single most important task for the future of surgical planning. However, despite ongoing efforts, a lack of follow-up data continues to be a major barrier limiting the progression of surgical planning.
Because of this current shortcoming, a number of relatively basic and necessary questions remain: How accurate are surgical planning predictions? Are patient outcomes improved as a result of surgical planning? Does surgical planning reduce operating times? Does surgical planning reduce clinical costs or the need for re-operations? Can surgical planning provide useful insights even with patient growth and adaptation over time? To address these questions as well as evaluate current surgical planning methods, validation must be a focus in the coming years. A substantial, multi-center clinical trial randomizing the use of Fontan surgical planning and funding post-op data collection may be necessary to acquire the data required to answer these questions.
Additionally, in order to refine the process of surgical planning, validation is needed in order to determine which, if any, metrics are causal or correlated to long-term Fontan complications. Future studies are needed to identify specific metrics that may be associated with liver disease, protein losing enteropathy and thromboembolic events among others. Furthermore, the various simulation fidelities mentioned earlier can be assessed for their impact on these metrics. Through this process, surgical planning can be improved and more substantially related to patient outcomes.
Until this validation data exists, Fontan surgical planning can continue to provide insights into the pre-operative state and provide estimates of post-operative hemodynamics. Nonetheless, clinicians should view surgical planning results as a “second opinion” and not a divine revelation. Years of clinical and surgical experience are not to be over-ruled by the surgical planning process, nor are they meant to be. However, the complex hemodynamics resulting from multiple colliding inlet jets (all with different flow rates varying in time) inside a complicated Fontan anatomy cannot always be visualized and do not always follow intuition, even with years of experience.
Boundary conditions – Physiologic prediction
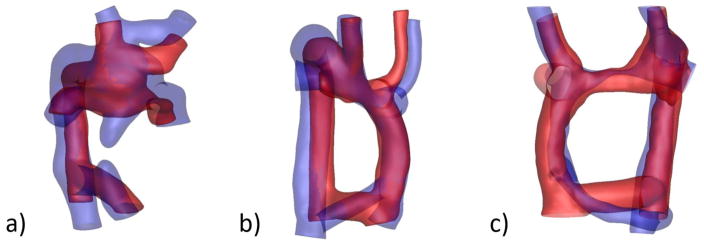
Previous studies, though utilizing small cohorts, have qualitatively shown a high degree of similarity between the anatomies of virtually proposed and surgically implemented options [49]. This is not surprising since the process of creating potential surgical options takes into account anatomical constraints and landmarks and employs commercially available graft sizes. In addition, the proposed options are often created in collaboration with clinicians, resulting in options that are inherently based on their feedback. Fig. 8 shows the proposed and postoperative anatomies overlaid for three example cases.
Fig. 8.
Comparison of proposed (red) and post-operative (blue) anatomies for three representative cases. (a) 8 year follow up, (b) ½ year follow up, (c) 3 year follow up
On the other hand, post-op flow boundary condition prediction is probably the weakest methodological link in the surgical planning paradigm. As it currently stands, many surgical planning cases use pre-operative vessel flow waveforms as boundary conditions for the proposed surgical options in order to simulate blood flow through the “new” connection and calculate relevant clinical metrics (HFD, energy loss, wall shear stress etc.). From a purely physiological standpoint, this simplified prediction method is clearly limited. For single ventricle patients moving from a Glenn to Fontan anatomy (Stage 2–3), there is no reason to think that the complete re-routing of the IVC from the right atrium to a total cavopulmonary anastomosis will result in similar flow waveforms for the vena cava and pulmonary arteries. Furthermore, no evidence exists that even a Fontan revision will produce similar flow waveforms as the preoperative state (pre- to post-operative inlet flow comparison shown in Fig. 7).
The use of sophisticated LPNs has undoubtedly advanced the field and allowed for more complete analysis of proposed surgical options (oxygen saturation and delivery, pressure changes etc.) [45]. However, as with all physiological modeling, limitations remain. Again, due to a lack of data, LPN parameter estimations/ranges are sometimes based on fundamentally different patient populations. In addition, holding certain parameter values/ratios constant between the pre- and post-operative states may not be sufficient to accurately represent a patient. Currently, all modeling is left to use various methods of scaling to account for patient growth, activity level and adaptation over time.
The fundamental idea of patient specific modeling requires a very complex, multifaceted system-level physiology to be accurately represented using basic engineering principles. Researchers have become well-equipped to do this for a patient’s current state, but the physiologic prediction aspect of surgical planning is an ongoing area of interest with much room for improvement. Patient specific modeling is inherently an individualized endeavor; it remains to be seen how accurate predictions can be given the limited amount of clinical data.
Automation
Ideally, the surgical planning process would involve minimal user input in order to encourage clinical use and feasibility. The two steps that require substantial user input include medical image segmentation and CFD/LPN simulation setup. Automation of these two steps could significantly reduce the personnel hours needed for surgical planning.
Current efforts to automate image segmentation have employed machine learning and “active contour” methods to semi-automatically track regions of interest through a set of images [32]. These methods have worked well for brain and coronary artery segmentation, but remains lacking for Fontan segmentation. This shortcoming is primarily due to differences in image quality and patient variability. Automatic segmentation is highly dependent on image quality, and MRI data (most Fontan studies) has a much lower resolution than CT images (used for previous brain and coronary artery segmentations). Moreover, it is common for Fontan patients to have stents, coils, and other medical devices which can create imaging artifacts and affect image quality. From a patient variability standpoint, a TCPC can have between 4–8 vessels, with vessel pathways and connections that vary greatly between patients. Fully automatic segmentation of these highly-varied anatomies has proved to be a substantial task.
Other than the set of flow waveforms and physiologic parameters that are unique to each patient, most simulation parameters including blood properties, solver and method selections, time step sizes, and convergence criteria are consistent across the majority of patients. In general, most aspects of mesh generation and simulation setup should be automated in the future. With proper automation, the potential for parametric studies including shape optimization, robustness analysis and uncertainty quantification could be performed for each surgical option [36, 50–51]. These studies would provide a more thorough analysis and design of each surgical option as well as confidence intervals for the predicted metrics. Furthermore, once the simulations are complete, processing results is a fairly straightforward process and can be easily automated using user defined functions or other post-processing codes.
With future efforts to automate image segmentation, mesh generation and simulation setup/processing, we envision surgical planning requiring only 3–4 hours of user input, with an additional 2 hours per surgical option/condition. Not only does automation save user input time, but also reduces the need for expertise in each of these areas. This would encourage use in the clinic by clinicians without the requirement of an extensive knowledge of fluid mechanics or CFD. A relatively short (one month) surgical planning timeline could be possible with these advances (Fig. 9). Additionally, the use of virtual/augmented reality is quickly approaching the clinical space and may provide additional options/functionality for use in surgical planning.
Fig. 9.
Potential Fontan surgical planning clinical timeline Events color-coded by blue (clinical) and gold (academic)
Future vision - Clinical implementation
Utilization of Fontan surgical planning, including computational modeling and CFD analysis to predict optimal post-operative hemodynamic states, is currently limited to a few select centers with robust BME – pediatric cardiology collaborative research teams. As the methodological advancements mentioned above progress, the field should see a reduction in time for processing of individual cases as well as improved predictive accuracy for the results. These refinements, however, are unlikely to allow routine application at local sites without access to experienced basic scientists for performance of the analyses, so the need for “centers of excellence”, to which complex cases can be routed, seems likely to remain essential.
This fact then leads to an important next question: how should such work be funded? Surgical planning is still in the research stages and is not covered by insurance. There are relatively few funded trials dealing with computational modeling and pre-surgical planning in congenital heart disease, but the Modeling of Congenital Heart Alliance (MOCHA), with participating institutions from both Europe and the United States, serves as an excellent model for others to build on [39, 45, 52]. Our lab and others are working to expand our services to other clinical and research centers, but only with adequate funding can these collaborations continue to thrive.
Summary
Ultimately, the goal of Fontan surgical planning is to provide additional insights into the clinical decision making process. In its current state, surgical planning offers an accurate hemodynamic assessment of the preoperative condition, provides anatomical constraints for potential surgical options, and postoperative predictions if boundary conditions are similar enough between the preoperative and postoperative states. Moving forward, validation with postoperative data is a necessary step in order to assess the accuracy of surgical planning and determine which methodological improvements are needed. Future efforts to automate the surgical planning process will the individual expertise needed and encourage use in the clinic by clinicians. As postoperative physiologic predictions improve, Fontan surgical planning will become an even more effective tool to accurately model patient specific hemodynamics.
Acknowledgments
Sources of Funding: This work was funded by NHLBI Grants HL67622 and HL098252 as well as an American Heart Association Predoctoral fellowship 17PRE33630117.
Abbreviations
- AZ
azygous
- CFD
computational fluid dynamics
- CHD
congenital heart defect
- CMR
cardiac magnetic resonance
- ECC
extracardiac conduit
- HFD
hepatic flow distribution
- IVC
inferior vena cava
- LPA
left pulmonary artery
- LPN
lumped parameter network
- LSVC
left superior vena cava
- PAVM
pulmonary arteriovenous malformation
- PCMRI
phase contrast magnetic resonance imaging
- RPA
right pulmonary artery
- SSFP
steady-state free precession
- SVC
superior vena cava
- TCPC
total cavopulmonary connections
- VENC
velocity encoding
Footnotes
Disclosures: Phillip Trusty - none; Timothy Slesnick – none; Alan Wei - none; Jarek Rossignac – none; Kirk Kanter – none; Mark Fogel – Research grant, modest; Ajit Yoganathan – none.
No animal studies were carried out by the authors for this article.” in the manuscript immediately before the References section.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102(14):1081–1086. doi: 10.1136/heartjnl-2015-307467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rychik J, Goldberg D, Rand E, Semeao E, Russo P, Dori Y, et al. End-organ consequences of the Fontan operation: liver fibrosis, protein-losing enteropathy and plastic bronchitis. Cardiol Young. 2013 May;23:831–840. doi: 10.1017/S1047951113001650. [DOI] [PubMed] [Google Scholar]
- 4.Shah MJ, Rychik J, Fogel MA, Murphy JD, Jacobs ML. Pulmonary AV Malformations After Superior Cavopulmonary Connection : Resolution After Inclusion of Hepatic Veins in the Pulmonary Circulation. 1997;4975(96):0–3. doi: 10.1016/s0003-4975(96)00961-7. [DOI] [PubMed] [Google Scholar]
- 5.Rychik J. The Relentless Effects of the Fontan Paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19(1):37–43. doi: 10.1053/j.pcsu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Rychik J, Veldtman G, Rand E, Russo P, Rome J, Krok K, et al. The precarious state of the liver after a fontan operation: Summary of a multidisciplinary symposium. Pediatr Cardiology. 2012;33(7):1001–1012. doi: 10.1007/s00246-012-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shinohara T, Yokoyama T. Pulmonary Arteriovenous Malformation in Patients with Total Cavopulmonary Shunt: What Role Does Lack of Hepatic Venous Blood Flow to the Lungs Play? Pediatr Cardiology. 2001;22(4):343–346. doi: 10.1007/s002460010243. [DOI] [PubMed] [Google Scholar]
- 8.De Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96(5):682. [PubMed] [Google Scholar]
- 9.De Leval MR, Dubini G, Migliavacca F, Jalali H, Camporini G, Redington A, et al. Use of computational fluid dynamics in the design of surgical procedures: Application to the study of competitive flows in cavopulmonary connections. J Thorac Cardiovasc Surg. 1996;111(3):502–513. doi: 10.1016/s0022-5223(96)70302-1. [DOI] [PubMed] [Google Scholar]
- 10.Khunatorn Y, Mahalingam S, DeGroff CG, Shandas R. Influence of Connection Geometry and SVC-IVC Flow Rate Ratio on Flow Structures within the Total Cavopulmonary Connection: A Numerical Study. J Biomech Eng. 2002;124(4):364. doi: 10.1115/1.1487880. [DOI] [PubMed] [Google Scholar]
- 11.Pekkan K, Whited B, Kanter K, Sharma S, de Zelicourt D, Sundareswaran K, et al. Patient-specific surgical planning and hemodynamic computational fluid dynamics optimization through free-form haptic anatomy editing tool (SURGEM) Med Biol Eng Comput. 2008;46(11):1139–1152. doi: 10.1007/s11517-008-0377-0. [DOI] [PubMed] [Google Scholar]
- 12.Tang E, Restrepo M, Haggerty CM, Mirabella L, Bethel J, Whitehead K, et al. Geometric characterization of patient-specific total cavopulmonary connections and its relationship to hemodynamics. JACC Cardiovasc Imaging. 2014;7(3):215–224. doi: 10.1016/j.jcmg.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soerensen DD, Pekkan K, Sundareswaran KS, Yoganathan AP. New power loss optimized Fontan connection evaluated by calculation of power loss using high resolution PC-MRI and CFD. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2004;2:1144–1147. doi: 10.1109/IEMBS.2004.1403367. [DOI] [PubMed] [Google Scholar]
- 14.Marsden AL, Bernstein AJ, Reddy VM, shadden S, Spilker R, Chan F, et al. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J Thorac Cardiovasc Surg. 2009;137(2):394–403. doi: 10.1016/j.jtcvs.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Tree M, Trusty P, Munz B, Slesnick T, Yoganathan A, Deshpande S, et al. In Vitro Examination of the HeartWare Circulite VAD in the Fontan Circulation. ASAIO. 2016;35(4):S46. doi: 10.1097/MAT.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein S, Bello R, Pizarro C, Fynn-Thompson F, Kirklin J, Guleserian K, et al. The use of the Berlin heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg. 2014;147(2):697–705. doi: 10.1016/j.jtcvs.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande SR, Maher KO, Morales DL. Mechanical Circulatory Support in Children: Challenges and Opportunities. Prog Pediatr Cardiol. 2016;43:31–41. [Google Scholar]
- 18.Pundi KN, Johnson JN, Dearani J, Pundi K, Li Z, Hinck C, et al. 40-Year Follow-Up after the Fontan Operation Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol. 2015;66(15):1700–1710. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 19.Trusty PM, Restrepo M, Kanter KR, Yoganathan AP, Fogel M, Slesnick TC. A pulsatile hemodynamic evaluation of the commercially available bifurcated Y-graft Fontan modification and comparison with the lateral tunnel and extracardiac conduits. J Thorac Cardiovasc Surg. 2016 doi: 10.1016/j.jtcvs.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Sundareswaran KS, de Zélicourt D, Sharma S, Kanter K, Spray T, Rossignac J, et al. Correction of Pulmonary Arteriovenous Malformation Using Image-Based Surgical Planning. JACC Cardiovasc Imaging. 2009;2(8):1024–1030. doi: 10.1016/j.jcmg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Zelicourt D, Marsden A, Fogel M, Yoganathan AP. Imaging and patient-specific simulations for the Fontan surgery: Current methodologies and clinical applications. Prog Pediatr Cardiol. 2010;30(1–2):31–44. doi: 10.1016/j.ppedcard.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogel MA, Khiabani RH, Yoganathan A. Imaging for preintervention planning pre- and post-fontan procedures. Circ Cardiovasc Imaging. 2013;6(6):1092–1101. doi: 10.1161/CIRCIMAGING.113.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slesnick TC, Yoganathan AP. Computational modeling of Fontan physiology: At the crossroads of pediatric cardiology and biomedical engineering. Int J Cardiovasc Imaging. 2014;30(6):1073–1084. doi: 10.1007/s10554-014-0442-8. [DOI] [PubMed] [Google Scholar]
- 24.Wei Z, Trusty PM, Tree M, Haggerty C, Tang E, Fogel M, et al. Can time-averaged flow boundary conditions be used to meet the clinical timeline for Fontan surgical planning? J Biomech. 2016;50:172–179. doi: 10.1016/j.jbiomech.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marino BS, Fogel M, Mercer-Rosa L, Wei A, Trusty PM, Tree M, et al. Poor Fontan Geometry, Hemodynamics, and Computational Fluid Dynamics Are Associated With Worse Quality of Life. Circulation. 2017;(Supp1)(136):A18082. [Google Scholar]
- 26.Fogel MA, Trusty PM, Wei A, Harris M, Whitehead K, Yoganathan A. Cardiac Magnetic Resonance and Computational Fluid Dynamic Parameters Are Important Predictors of Adverse Events in the Fontan Almost 10 Years After Imaging. Circulation. 2017;(Supp1)(136):A18160. [Google Scholar]
- 27.Khiabani RH, Whitehead KK, Han D, Restrepo M, Tang E, Bethel J, et al. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart. 2015;101(2):139–143. doi: 10.1136/heartjnl-2014-306337. [DOI] [PubMed] [Google Scholar]
- 28.Sundareswaran KS, de Zélicourt D, Sharma S, Kanter K, Spray T, Rossignac J, et al. Correction of Pulmonary Arteriovenous Malformation Using Image-Based Surgical Planning. JACC Cardiovasc Imaging. 2009;2(8):1024–1030. doi: 10.1016/j.jcmg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W, Chan FP, Reddy VM, Marsden AL, Feinstein J. Flow simulations and validation for the first cohort of patients undergoing the Y-graft Fontan procedure. J Thorac Cardiovasc Surg. 2015;149(1):247–255. doi: 10.1016/j.jtcvs.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 30.De Moraes TF, Amorim PH. InVesalius-An open-source imaging application. Comput Vis Med Image Process. 2011:405. [Google Scholar]
- 31.Fedorov A, Beichel R, Kalphaty-Cramer J, Finet J, Fillion-Robin J, Pujol S, et al. 3D slicers as an image computing platform for thw quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yushkevich PA, Piven J, Hazlett HC, Smith R, Ho S. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment - freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10(1):1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidhult SL, Carlsson M, Steding-Ehrenborg K, Arheden H, Heiberg E. A new method for vessel segmentation based on a priori input from medical expertise in cine phase-contrast Magnetic Resonance Imaging. J Cardiovasc Magn Reson. 2014;16(Suppl 1):P355. doi: 10.1186/1532-429X-16-S1-P355. [DOI] [Google Scholar]
- 35.Luffel M, Sati M, Rossignac J, Yoganathan A, Haggety C, Restrepo M, et al. SURGEM: A solid modeling tool for planning and optimizing pediatric heart surgeries. Computer-aided Design. 2015 doi: 10.1016/j.cad.2015.06.018. [DOI] [Google Scholar]
- 36.Restrepo M, Luffel M, Sebring J, Kanter K, del Nido P, Veneziana A, et al. Surgical Planning of the Total Cavopulmonary Connection: Robustness Analysis. Ann Biomed Eng. 2014;43(6):1321–1334. doi: 10.1007/s10439-014-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zélicourt D, Ge L, Wang C, Sotiropoulos F, Gilmanov A, Yoganathan A. Flow simulations in arbitrarily complex cardiovascular anatomies - An unstructured Cartesian grid approach. Comput Fluids. 2009;38(9):1749–1762. [Google Scholar]
- 38.Haggerty CM, Restrepo M, Tang E, Zelicourt D, Sundareswaran K, Mirabella L, et al. Fontan hemodynamics from 100 patient-specific cardiac magnetic resonance studies: A computational fluid dynamics analysis. J Thorac Cardiovasc Surg. 2013;148(4):1–10. doi: 10.1016/j.jtcvs.2013.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kung E, Baretta A, Baker C, Arbia G, Biglino G, Corsini C, et al. Predictive modeling of the virtual Hemi-Fontan operation for second stage single ventricle palliation: Two patient-specific cases. J Biomech. 2013;46(2):423–429. doi: 10.1016/j.jbiomech.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Vignon-Clementel IE, Troianowski G, Reddy VM, Feinstein J, Marsden AL. Hepatic blood flow distribution and performance in conventional and novel Y-graft Fontan geometries: A case series computational fluid dynamics study. J Thorac Cardiovasc Surg. 2012;143(5):1086–1097. doi: 10.1016/j.jtcvs.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 41.Corsini C, Baker C, Kung E, Schievano S, Arbia G, Baretta A, et al. An integrated approach to patient-specific predictive modeling for single ventricle heart palliation. Comput Methods Biomech Biomed Engin. 2014;17(14):1572–1589. doi: 10.1080/10255842.2012.758254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corsini C, Baker C, Baretta A, Biglino G, Hlavacek A, Hsia TY, et al. Integration of Clinical Data Collected at Different Times for Virtual Surgery in Single Ventricle Patients : A Case Study. ABME. 2015;43(6):1310–1320. doi: 10.1007/s10439-014-1113-6. [DOI] [PubMed] [Google Scholar]
- 43.Kung E, Pennati G, Migliavacca F, Hsia T, Figliola R, Marsen A, et al. A simulation protocol for exercise physiology in fontan patients using a closed loop lumped-parameter model. J Biomech Eng. 2014;136(8):1–13. doi: 10.1115/1.4027271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiavazzi DE, Baretta A, Pennati G, Hsia TY, Marsden AL. Patient-specific parameter estimation in single-ventricle lumped circulation models under uncertainty. Int jounral numerical methods in biomed eng. 2017;33(3):1–34. doi: 10.1002/cnm.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esmaily-Moghadam M, Hsia TY, Marsden AL. The assisted bidirectional Glenn: A novel surgical approach for first-stage single-ventricle heart palliation. J Thorac Cardiovasc Surg. 2015;149(3):699–705. doi: 10.1016/j.jtcvs.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gewillig M. The Fontan Circulation. Heart. 2005;91(6):839–846. doi: 10.1136/hrt.2004.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsden AL, Vignon-Clementel IE, Chan FP, Feinstein J, Taylor C. Effects of exercise and respiration on hemodynamic efficiency in CFD simulations of the total cavopulmonary connection. Ann Biomed Eng. 2007;35(2):250–263. doi: 10.1007/s10439-006-9224-3. [DOI] [PubMed] [Google Scholar]
- 48.Orlando W, Shandas R, DeGroff C. Efficiency differences in computational simulations of the total cavo-pulmonary circulation with and without compliant vessel walls. Comput Methods Programs Biomed. 2006;81(3):220–227. doi: 10.1016/j.cmpb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Haggerty CM, de Zélicourt DA, Restrepo M, Rossignac J, Spray T, Kanter K, et al. Comparing Pre- and Post-operative Fontan Hemodynamic Simulations: Implications for the Reliability of Surgical Planning. Ann Biomed Eng. 2012;40(12):2639–2651. doi: 10.1007/s10439-012-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang W, Feinstein J, Shadden SC, Vignon-Clementel IE, Marsden AL. Optimization of a Y-graft design for improved hepatic flow distribution in the fontan circulation. J Biomech Eng. 2013;135(1):011002. doi: 10.1115/1.4023089. [DOI] [PubMed] [Google Scholar]
- 51.Schiavazzi DE, Arbia G, Baker C, Hlavacek AM, Hsia TY, Marsden AL, Vignon-Clementel IE. Uncertainty quantification in virtual surgery hemodynamics predictions for single ventricle palliation. Int j numer method biomed eng. 2016;32(3) doi: 10.1002/cnm.2737. [DOI] [PubMed] [Google Scholar]
- 52.Schiavazzi DE, Kung EO, Marsden AL, Baker C, Pennati G, Hsia TY, et al. Hemodynamic effects of left pulmonary artery stenosis after superior cavopulmonary connection: A patient-specific multiscale modeling study. J Thorac Cardiovasc Surg. 2015;149(3):689–696. doi: 10.1016/j.jtcvs.2014.12.040. [DOI] [PubMed] [Google Scholar]