Abstract
Adolescence is a period of heightened sensitivity to incentives and relatively weak cognitive control, which may contribute to risky behaviors. Studies of brain activity have generally identified greater activation of the ventral striatum to rewards and less activation of prefrontal regions during control tasks in adolescents compared to adults. Little is known, however, about age-related changes in the functional brain networks underlying incentive processing and cognitive control. This cross-sectional study characterized the effects of incentives on inhibitory control during an oculomotor task using whole-brain functional connectivity analyses. During an fMRI scan, one hundred forty typically developing individuals completed an incentivized antisaccade task consisting of incentive cue, preparation, and response phases. We found that task modulation of control networks increased gradually from childhood to adulthood, whereas a network including ventral striatum and ventromedial prefrontal cortex displayed an adolescent-specific peak in response to the receipt of outcomes, consistent with dual-systems models. Notably, however, greater modulation of salience and motor networks during the preparation phase mediated age-related improvements in antisaccade accuracy, whereas adolescent enhancement of value-related circuitry did not. Relative to neutral cues, both reward and loss cues enhanced task-related connectivity of the salience network when preparing to inhibit a saccade. Altogether, our findings suggest that incentives facilitate inhibitory control by enhancing the salience of one’s responses and that over development, the recruitment of functional networks involved in saliency and motor preparation supports better performance.
Introduction
Although adolescents are often portrayed in popular media as impulsive and lacking forethought, contemporary developmental theories emphasize that cognitive control, including inhibitory control, performance monitoring, and working memory, increases gradually from childhood to adulthood (Casey, 2015; Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). The prevalence of many risky behaviors, however, such as physical fights (Swahn, Bossarte, Palmier, Yao, & Dulmen, 2013), illicit substance use, and non-suicidal self-injury (Muehlenkamp, Claes, Havertape, & Plener, 2012), peaks in adolescence, undermining survival (Molcho, Walsh, Donnelly, Matos, & Pickett, 2015). Adolescents often exhibit heightened behavioral and neural sensitivity to rewards (Richards, Plate, & Ernst, 2013; van Duijvenvoorde, Peters, Braams, & Crone, 2016); are especially influenced by social stimuli, including peers (Telzer, Fuligni, Lieberman, Miernicki, & Galván, 2015); and are more reactive to emotional stimuli such as threat cues (Pfeifer et al., 2011). To resolve the potential dissonance between adolescent-specific peaks in risk-taking and gradual developmental increases in cognitive control, recent accounts have extended classic dual-systems (aka two-mode) theories that posit a separation between a deliberative, self-regulatory system and a more reflexive, emotional system (Epstein, 1994; Kahneman, 2003). More specifically, dual-systems models of adolescent behavior posit that brain changes that accompany puberty rapidly enhance the effective strength of the reflexive system in decision-making relative to a weak deliberative system that develops gradually, leading to risky decisions in affective contexts (Shulman et al., 2016).
Developmental fMRI studies increasingly find evidence for both adolescent immaturities in cognitive control and hyper-reactivity to affective stimuli. For example, activity in the dorsal anterior cingulate cortex (dACC) increases across development during tasks requiring performance monitoring in parallel to behavioral improvements in learning from errors (Velanova, Wheeler, & Luna, 2009). Likewise, developmental improvements in working memory capacity are mediated by gradual increases in executive control regions such as the dorsolateral prefrontal cortex (DLPFC) and decreased activity in the default mode network (Satterthwaite et al., 2013; Thomason et al., 2009). There is also evidence that the refinement of inhibitory control over development depends on top-down signals from executive prefrontal regions (e.g., the anterior insula) to other regions involved in cognitive tasks (Hwang, Velanova, & Luna, 2010; Supekar & Menon, 2012).
A substantial body of human and rodent research supports the notion that adolescents are more reactive to the receipt of rewards, but that they may less sensitive to reward-predictive cues (reviewed in Richards et al., 2013; Spear, 2011). Consistent with the dual-systems perspective, functional neuroimaging studies in humans have typically found adolescent-specific elevated activity in the ventral striatum, a key region in incentive motivation and learning from prediction errors, during reward anticipation (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010) and receipt (Galvan et al., 2006; Silverman, Jedd, & Luciana, 2015; van Duijvenvoorde, Peters, et al., 2016; Van Leijenhorst et al., 2010). These findings are mirrored by animal electrophysiology studies demonstrating adolescent hyper-responsiveness to reward receipt in the striatum and orbitofrontal cortex (Sturman & Moghaddam, 2012).
Although the idea of an imbalance between more impulsive and self-regulatory systems is central to the dual-systems perspective, few developmental studies have adopted a functional network perspective (Casey, 2015). This is a crucial gap in the literature because the reflexive and self-regulatory systems are more likely to reflect the coordination of functional networks than to be captured by differential activations in single regions (Pfeifer & Allen, 2012). Indeed, research over the past decade has shifted toward understanding cognition in terms of the coordination of brain regions in the context large-scale brain networks that exert more complex and robust influence over behavior (Bressler & Menon, 2010). Two recent studies have employed connectivity analyses to describe developmental differences in functional networks underlying cognitive control and incentive processing. First, using an emotional go/no-go task, Somerville and colleagues (2011) found that relative to children and adults, teens had greater difficulty withholding responses to happy faces, an appetitive cue (cf. Hare et al., 2008). The ability to suppress a response was linked with functional connectivity between the dorsal striatum and the right inferior frontal gyrus (a key region in inhibitory control). Second, van Duijvenvoorde and colleagues (2016) used seed correlational analyses of resting-state fMRI data to demonstrate that developmental improvements on a learning task were largely mediated by the enhancement of DLPFC connectivity with the thalamus, whereas age-related increases in self-reported pleasure during a reward task were mediated by enhanced connectivity between the ventral striatum and ventromedial prefrontal cortex.
The present study sought to characterize the development of functional brain networks supporting reward processing and inhibitory control in a large cross-sectional cohort spanning the ages of 10 to 25. Building on previous work in this area (Geier et al., 2010; Hardin & Ernst, 2009; Jazbec et al., 2006; Paulsen, Hallquist, Geier, & Luna, 2015), participants completed an adaptation of the antisaccade task in which they could win or lose points based on the ability to suppress a prepotent motor response, allowing us to examine both the stages of reward processing (Haber & Knutson, 2010) and the interface between incentive motivation and self-regulation. There were three primary aims of the study. First, we sought to characterize age-related changes in the recruitment of control- and incentive-related networks as a function of distinct phases of incentive processing: cue, preparation, and outcome. Second, we were interested in testing whether task-related modulation of these networks mediated age-related improvements in inhibitory control. Third, we sought to describe changes in the within- and between-network connectivity of control and incentive networks from childhood to young adulthood. To test the dual-systems model, for each aim, we were also interested in understanding whether age-related changes were adolescent nonspecific (i.e., linear), emergent (i.e., asymptotic, here operationalized by an inverse function of age), or specific (i.e., quadratic; Casey, 2015). We adopted an information-theoretic approach to adjudicate among potential forms of age-related change in both behavioral and neural data (Burnham & Anderson, 2002).
In a previous report of this task, we found age-related increases in activation of frontoparietal regions and the frontal eye fields (Paulsen et al., 2015). Additional region of interest analyses of these data revealed that reward reactivity of the ventral striatum was differentially associated with sensation seeking in younger versus older individuals (Hawes et al., 2017). Here, we describe results from the same fMRI task in a larger cohort that partially overlaps the previous reports, but with a specific focus on network modulation. Whereas our initial reports aggregated activity across different phases of incentive processing (cue, anticipation, and receipt), the present study separated task-related connectivity by phase, which are differentially implicated in adolescent development (Richards et al., 2013; Spear, 2011). This study focuses on whole-brain functional connectivity, providing a more comprehensive account of networks involved in incentive processing and inhibitory control, whereas we previously addressed activation differences in regions selected a priori.
To date, there have been several regional activation studies of developmental differences in reward processing using paradigms that separate cue, anticipation, and receipt phases (for reviews, see Richards et al., 2013; van Duijvenvoorde, Peters, et al., 2016). Importantly, however, little is known about how incentive processing and inhibitory control in each phase reflect the coordination of functionally connected networks. For example, the ventral striatum is involved in computing reward prediction errors (Glimcher, 2011), whereas the subjective values of alternative outcomes are likely represented in a separate system that includes the ventromedial prefrontal cortex and cingulate cortex (Bartra, McGuire, & Kable, 2013; Cai & Padoa-Schioppa, 2012; Padoa-Schioppa, 2011). Updating subjective value based on prediction errors requires functional coordination of fronto-striatal circuits during different processing phases to guide both valuation and choice (Kable & Glimcher, 2009). Furthermore, the enhancement of inhibitory control by incentives may reflect functional integration of reward and control networks. Although a regional co-activation approach might identify frontal and striatal regions in a reward-by-cognition analysis, it cannot disambiguate whether the activity pattern reflects 1) a transient activation pattern specific to task demands, 2) task-related modulation of a single functionally connected network that includes all regions recruited by the task, or 3) modulation of multiple specialized functional networks that coordinate to handle the task demands. We believe that the enhancement of inhibitory control by incentives likely reflects the coordination of canonical functional networks, not transient task-specific region co-activation, a question that necessitates a whole-brain functional connectivity approach.
To characterize whole-brain task-related connectivity, we employed independent components analysis (ICA), a data-driven technique that decomposes voxelwise fMRI time series into spatially distinct and temporally coherent latent sources (Calhoun, Liu, & Adali, 2009). Although ICA has largely been used to identify resting-state networks (Damoiseaux et al., 2006), it is equally useful for understanding functional connectivity during cognitive tasks (Greicius & Menon, 2004; Kim et al., 2009). We had four hypotheses. First, consistent with prior regional activation literature (Geier et al., 2010), we predicted that task-related modulation of control networks, especially the dorsal attention and salience/cingulo-opercular networks, would increase linearly with age. Second, we predicted that a network including the ventral striatum would be most involved in reward consummation and more strongly recruited among adolescents. Third, we predicted that age-related improvements in inhibitory control would be mediated by greater task-related connectivity in control networks during the preparation phase. Fourth, we hypothesized that over development, there would be greater integration of incentive-related and control networks, potentially reflecting the enhancement of self-regulation. Finally, in exploratory analyses, we tested how the composition of each task-related network changed with development.
Methods
Participants
Participants were 140 typically developing youth and young adults, ranging in age from 10 to 25 (M = 16.52, SD = 3.76). Sixty-six participants (49.3%) were female (see Supplemental Figure 1). Prior to the scan, participants completed an intelligence screening test (Wechsler Abbreviated Scale of Intelligence; Wechsler, 1999) to verify that they had an IQ above 80 (M = 111.04, SD = 11.58). Individuals who reported lifetime diagnoses of neurological disorder, brain injury, pervasive developmental disorder, or psychiatric disorder (in self or first-degree relatives) were ineligible to participate. In addition, participants completed the Youth Self-Report or Adult Self-Report (Achenbach, 2009) and were excluded from the MRI study if they scored in the clinical range (T ≥ 70) on any symptom or substance use scale. All participants had corrected far visual acuity of at least 20/40. Participants and/or their legal guardians provided informed consent or assent prior to participation in this study. Experimental procedures for this study complied with Code of Ethics of the World Medical Association (1964 Declaration of Helsinki) and the Institutional Review Board at the University of Pittsburgh. Participants were compensated $75, plus up to an additional $25 based on task performance. As described above, parts of these data appeared in two previous reports using different analyses and with different scientific goals (122 overlapping participants in Hawes et al., 2017; 82 overlapping participants in Paulsen et al., 2015).
Procedure
Within one week prior to the fMRI scan, participants were tested in our laboratory to confirm that they understood and were able to perform the antisaccade task described below. In the MR scanning environment, eye movements were measured with a long-range optics eye-tracking system (Model 504LRO; Applied Science Laboratories, Bedford, MA) that recorded eye position by pupil-corneal reflection obtained by a mirror mounted on the head coil with a resolution of 0.5° of visual angle. At the beginning of the experimental session and between runs when necessary, a 9-point eye calibration procedure was performed. Using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA), stimuli were projected onto a flat screen positioned behind the magnet and were viewed by participants via a mirror mounted on the head coil.
Participants completed four runs of an incentivized antisaccade task (based on Geier & Luna, 2012) in which each trial consisted of an incentive cue (reward, loss, neutral), response preparation period, and a response period that included auditory performance feedback. The cue phase of each trial lasted 1.5s and indicated whether participants could win five points for correct antisaccade, lose five points for antisaccade failure, or not gain or lose points based on performance (Figure 1). The cue phase was immediately followed by a 1.5s preparatory period consisting of a fixation cross on the screen. Finally, during the 1.5s response phase, a small yellow dot appeared at one of six pseudorandomly selected peripheral location and participants were instructed to look opposite to the dot that appeared on the screen. Correct antisaccades were followed immediately by a cash register sound, whereas participants heard a buzzer sound for incorrect responses. Eye data were scored off-line using ILAB software (Gitelman, 2002) and an in-house scoring suite written in MATLAB (MathWorks, Inc.; details about eye scoring procedures provided in the Supplemental Methods).
Figure 1.
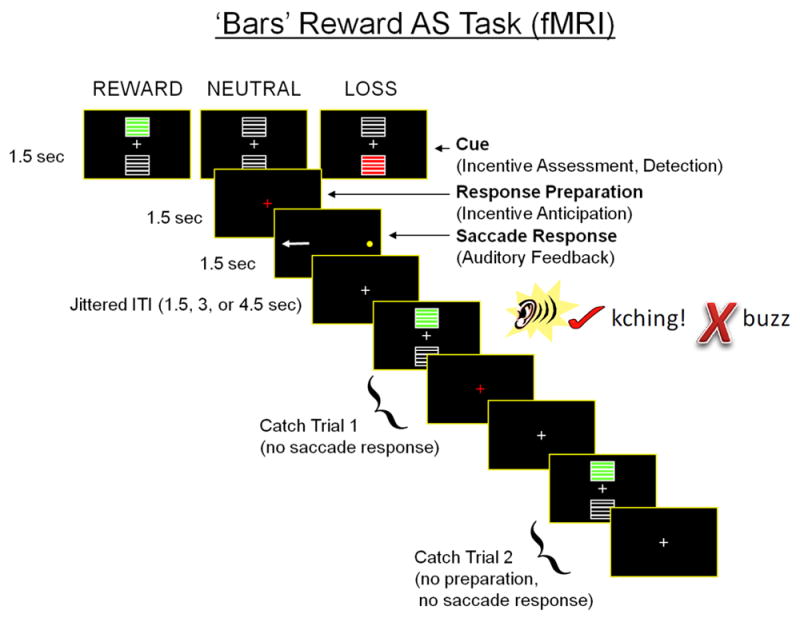
Experimental design of the incentivized antisaccade task. The task consisted of three phases: incentive cue, response preparation, and saccade response, each lasting 1.5 s. Incentive cues were represented rewards (green bars), losses (red bars), or neutral (no bars), and reflected the number of points that could be earned or lost based on antisaccade response accuracy. Participants received immediate auditory feedback during the saccade response phase (“kching” for accurate and “buzz” for inaccurate responses). To improve the separation of the three task phases in data analyses, catch trials that omitted the response phase or the preparation and response phases were included.
Inter-trial intervals varied from 1.5s to 19.5s, following an exponential distribution. Each run included 14 complete trials for reward, loss, and neutral conditions. To better separate hemodynamic responses to each event type, each run also included 18 partial trials: nine with only cue events and nine with cue and preparation events (Ollinger, Shulman, & Corbetta, 2001).
Prior to the scan, participants selected among several potential gift cards (e.g., prepaid Visa, iTunes, or McDonald’s) and were informed that points earned during the task would contribute toward the total value added to the gift card (for details, see Geier & Luna, 2012). Participants were remunerated based on the proportion of points earned out of 280 using the following scale: 0–70 points (US $10), 71–140 (US $15), 141–210 (US $20), 211–280 (US $25.00 or the chosen gift card). Points were tallied at the end of the experiment to determine bonus compensation and participants knew the total points possible, but not the details of the payment schedule. This feature of the design was intended to mitigate concerns about the subjective value of money and to achieve similar levels of motivation across ages.
Of 560 potential runs of fMRI data to be analyzed, 18 were excluded. Eye tracking data for four runs were corrupted and not recoverable. Five fMRI run acquisitions failed due to scanner and experiment problems. We excluded four runs for having poor spatial coverage due to between-run head movement. Finally, we excluded five runs for excessive head movement, as defined by any single movement greater than 5mm root mean squared (RMS) deviation or more than 15% of volumes within a run having greater than 0.3mm relative RMS deviation. The mean framewise displacement (FD) in the dataset was 0.13mm (SD = .04mm). As with other developmental neuroimaging studies, we found a moderate negative correlation between average head movement and age, r(138) = -.29, p < .001. Thus, where possible, motion parameters were included as regressors of no interest.
fMRI Acquisition and Preprocessing
Imaging data were collected using a 3.0 T Siemens Trio scanner at the Magnetic Resonance Research Center, University of Pittsburgh. A single-shot echo-planar imaging sequence sensitive to BOLD contrast was performed. The acquisition parameters were TR = 1.5 s; TE = 25 ms; flip angle = 70°; in-plane resolution of 3.125 mm. Twenty-nine 4-mm thick axial slices with no gap were collected, aligned to the anterior and posterior commissure and covering the entire cortex and part of the cerebellum. A 3D volume magnetization prepared rapid acquisition gradient-echo (MP-RAGE) pulse sequence with 192 slices (1mm slice thickness) was used to acquire structural images in the sagittal plane.
Anatomical scans were registered to the MNI152 template (Fonov, Evans, McKinstry, Almli, & Collins, 2009) using both affine (FSL FLIRT) and nonlinear (FSL FNIRT) transformations. Functional images were preprocessed using tools from NiPy (Millman & Brett, 2007), AFNI (Cox, 1996), and the FMRIB software library (FSL; S. M. Smith et al., 2004). First, large transient spikes in voxel time series were interpolated downward using the AFNI 3dDespike program. Second, slice timing and motion correction were performed simultaneously using a four-dimensional registration algorithm implemented in NiPy (Roche, 2011). Non-brain voxels were removed from functional images by masking voxels with low intensity and by a brain extraction algorithm implemented in FSL BET. The alignment of subjects’ functional images to their anatomical scan was computed using the white matter segmentation of each image and a boundary-based registration algorithm (Greve & Fischl, 2009). Functional scans were then resampled into 3mm isocubic voxels and warped into MNI152 template space using the concatenation of the functional-structural and structural-MNI152 transforms. Images were spatially smoothed using a 5-mm full-width at half maximum kernel (FSL SUSAN). A .008Hz temporal high-pass filter was applied to remove slow-frequency signal changes.
Independent Component Analysis
Because we were explicitly interested in understanding functionally connected brain networks, we used a probabilistic atlas for the MNI152 template (Fonov et al., 2009) to exclude voxels that had a gray matter probability of 0.2 or less, a relatively liberal threshold that largely eliminated voxels in deep cerebral white matter and the ventricles. We also excluded voxels that were not fully sampled across subjects due to coverage differences, which primarily affected inferior cerebellar regions.
Spatial ICA was conducted using the GIFT 3.0a ICA Toolbox for fMRI data (Calhoun et al., 2011). Spatial ICA maximizes the spatial separability of components, while allowing for between-subjects variability in the estimated time courses. To reduce the dimensionality of data and promote ICA convergence, two stages of principal components analysis were performed (Erhardt et al., 2011). First, each task run was reduced to 60 principal components, resulting in a 43189 × 60 matrix per run. Next, principal component matrices for all runs were concatenated and a group PCA reduction to 30 shared components was performed. Finally, we derived 30 independent components from this matrix using the extended infomax ICA algorithm, which has previously been demonstrated to be sensitive to spatiotemporal patterns in fMRI data (Correa, Adali, & Calhoun, 2007). For details about the decision to estimate 30 independent components, see the Supplemental Methods.
Although we estimated 30 ICs, we only interpreted seven as task-relevant based on the following criteria (additional details in Supplemental Methods). First, we excluded one IC with clustering quality Iq < 0.8, indicative of an unreliable component. Second, we excluded eight components whose spatial maps were indicative of artifacts, such as physiological noise or spurious activity at the rim of the brain due to head motion. Third, we excluded two components whose time courses and spectrograms indicated considerable high-frequency power that did not follow the expected 1/f distribution of neural signals. Fourth, we excluded 10 ICs that were not reliably associated with task-related neural activity (see Table S1). Fifth, we excluded two ICs that fractionated at different ICA model orders, suggesting a spatiotemporal component that was vulnerable to the total number of components estimated.
In group ICA, each component consists of a single time course that describes the temporal dynamics of a functional network and a spatial map that quantifies the strength of within-network coupling (more specifically, the temporal similarity of each voxel with the network time course; for details, see Calhoun et al., 2009). In this way, brain regions that are strongly functionally coupled tend to load onto the same component (i.e., they are assigned to the same network). ICA decomposes fMRI data into a set of latent spatiotemporal sources such that BOLD activity in a voxel is represented as a weighted sum of these sources. However, because spatial ICA extracts components with minimal spatial overlap, the majority of BOLD activity in a voxel tends to be captured by a single component. By reversing the dimensionality reduction procedures described above using back-reconstruction, we derived run-specific network time courses and voxel-level spatial maps (Erhardt et al., 2011). Previous simulation studies have demonstrated these to be sensitive to between-subject differences in both spatial and temporal features of functional networks (Allen, Erhardt, Wei, Eichele, & Calhoun, 2012). The resulting spatial maps and network time courses were the basis of analyses of task-related functional coupling, as well as within- and between-network connectivity.
Analyses of Age-related Changes in Functional Networks
Unlike GLM fMRI analyses, where known stimulus timing provides a forward model of expected BOLD activity, spatiotemporal components derived from ICA are based solely on higher-order statistics of the data without assuming a particular temporal model (McKeown et al., 1998). Thus, in the context of task-related fMRI, some components may reflect networks instrumental in the performance of the task, whereas others may reflect unrelated intrinsically connected networks or spatiotemporal sources of noise (e.g., cardiovascular artifacts).
To quantify task-related modulation (TRiM), we regressed run-level network time courses on task design matrices (effects convolved with the canonical double gamma hemodynamic response function; see Supplemental Methods for details). Like conventional voxelwise general linear model (GLM) analyses, the resulting regression coefficients represent the partial effect of the stimulus (e.g., reward cue) on BOLD activity controlling for other effects in the design matrix (Kim et al., 2009). The crucial difference from single voxel analyses, however, is that ICA network time courses represent temporally coherent activity in a set of functionally coupled regions. Thus, the partial association of IC time courses with task effects (i.e., TRiM) reflects both the magnitude of task-related network activity and the temporal correspondence of the network time course with stimulus presentation (similar to regression coefficients in voxelwise GLMs). To characterize TRiM at the group level, we regressed run-level TRiM estimates on age, phase, and incentive in multilevel models (lme4 package in R 3.2.0) with subject as a random effect (i.e., runs were nested within subject). Positive TRiM reflects the tendency for functionally coupled regions comprising a network to increase their activity in response to an experimental stimulus. Conversely, negative TRiM reflects task-induced reductions in network activity.
For TRiM analyses, we controlled familywise error by computing a single general linear hypothesis test for each network using the multivariate distribution of the coefficients of interest (Hothorn, Bretz, & Westfall, 2008). Adjusted p-values are reported such that the Type I familywise error rate is less than .05 per network (i.e., tests of valence, phase, and age were jointly controlled). To reduce the influence of outlier observations on estimates of task-related IC activity, we iteratively re-estimated models excluding the participant with the largest Cook’s distance until the maximum value was less than .029 (Van der Meer, Te Grotenhuis, & Pelzer, 2010). This led to the exclusion of between 1 and 11 participants per model (median = 2).
Analyses of network integration (between-network connectivity)
To examine whether task-related functional networks became more integrated (i.e., functionally connected) over development, we computed correlations among the network time courses. These represent the pairwise functional connectivity of networks across the entire task, similar to what would typically be described in resting-state network analyses (Jafri, Pearlson, Stevens, & Calhoun, 2008). Prior to calculating correlations, subject head motion parameters were regressed out and timecourses were detrended and despiked (Allen et al., 2011). We then tested for changes among the seven task-related networks by regressing pairwise connectivity estimates on age.
Identifying the functional form of age-related change
In order to test for adolescent nonspecific, specific, and emergent patterns of age-related change in behavior and network modulation, we fit linear, quadratic, and inverse functions of age, respectively. Quadratic models included a linear age term as well. To adjudicate among these variants, we selected the model with the lowest corrected Akaike Information Criterion (AICC; Sugiura, 1978). In addition, for tests of age-related changes in within-network connectivity, which involved voxelwise regressions, we retained clusters that were both significant for a given function of age (correcting for multiple comparisons) and had the highest cluster-wise median Akaike weight (Burnham & Anderson, 2002), a measure of relative evidence for one model compared to others in the set of comparisons. This approach was chosen instead of average within-cluster AICC because there are arbitrary scaling differences in the regression log-likelihood function across voxels that render scaling of the AICC incomparable, whereas Akaike weights are scale invariant.
Results
Age-related Improvements in Antisaccade Performance
The average accuracy on the antisaccade task was 91.8% (SD = 8.1%). We fit a binary logistic multilevel model to trial-wise data to characterize developmental changes in accuracy. Consistent with previous developmental literature using the antisaccade task (Luna, Garver, Urban, Lazar, & Sweeney, 2004; Paulsen et al., 2015), an inverse function of age provided the best fit to performance data, with accuracy increasing asymptotically from an average of approximately 83% at age 10 to 97% at age 25, z = 7.24, p < .0001. We also found a significant main effect of trial incentive on accuracy such that performance was significantly higher for rewarded trials than loss and neutral trials, adj. ps < .01. We did not observe a significant Age x Incentive interaction, however, p > .10.
Task-Modulated Functional Networks
We identified seven ICs that were significantly related to at least one aspect of the incentivized antisaccade task (adj. ps < .0001); these were named according to their constituent regions and putative function: Visual, Dorsal Attention (DAN), Motor, Salience (SN), Default Mode (DMN), Valuation, and Auditory (see Figure 2, Table S2). During the cue phase, we found positive task-related modulation (TRiM) in the Visual, Dorsal Attention, and Motor networks, whereas TRiM was negative in the Salience, Default Mode, Valuation, and Auditory networks. Visual network modulation was greater for reward cues than neutral and loss cues (adj. ps < .03 and .001, respectively). Conversely, Auditory network modulation was more negative during the cue phase for reward and loss trials compared to neutral trials (adj. ps < .001).
Figure 2.
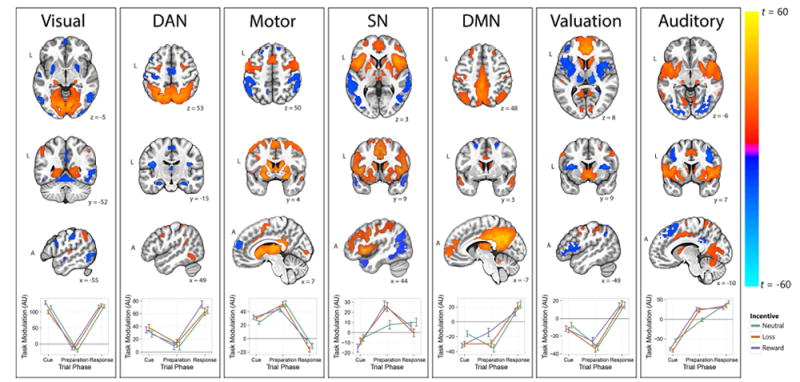
Spatial patterns of task-related functional networks and task-related network activity (details provided in Table S2). Spatial maps were thresholded at |t| > 10 (min. 20 contiguous voxels) for display and represent the strength of temporal correspondence between a region and the time course of the overall network. The bottom panel of each network represents task-related modulation (TRiM) of the network as a function of trial phase and incentive cue. Positive values indicate increases in network activity during the task event (e.g., reward cue), whereas negative TRiM values reflect reductions in network activity. The panels are organized from left to right with respect to task phase, with more cue-related components appearing on the left and more response-related components appearing on the right.
The Visual network included large clusters in bilateral cuneus and lingual gyrus, as well as the lateral geniculate nucleus. The dorsal attention network (DAN) included the frontal eye fields (intersection of precentral sulcus and superior frontal gyrus), intraparietal sulcus, and precuneus. TRiM of the DAN was positive during the cue, preparation, and response phases, but was highest in the response phase (when a voluntary antisaccade is made), moderate during cue, and weakest during preparation (pairwise comparisons significant at adj. p < .001). The Motor network included clusters in bilateral thalamus, dorsal striatum, supplementary motor area, and premotor cortex. The Salience network (SN; also known as the cingulo-opercular network; Dosenbach et al., 2007) was defined by large clusters in the anterior insula/frontal operculum, dorsal cingulate gyrus, middle frontal gyrus, and dorsomedial thalamus. The default mode network (DMN) included the posterior cingulate, precuneus, medial prefrontal cortex, and inferior parietal cortex. The Valuation network included the ventral striatum (VS), medial and ventromedial prefrontal cortex (vmPFC), and lateral orbitofrontal cortex, canonical regions in reward processing (Knutson, Fong, Bennett, Adams, & Hommer, 2003). In addition, the amygdala and thalamus displayed negative coupling with the Valuation network such that activity increases in the vmPFC and VS were accompanied by decreases in amygdala and thalamus. Finally, the Auditory network was composed of bilateral superior temporal gyrus, primary auditory cortex, supplementary motor area, midcingulate cortex, postcentral gyrus, and medial geniculum body.
During the preparation phase, TRiM was positive in the Dorsal Attention, Motor, Salience, and Auditory networks, and negative in the Visual, Default Mode, and Valuation networks. Consistent with a role in motor planning, TRiM of the Motor network increased from the cue phase to the preparation phase (adj. p < .0001). Modulation of the SN during the preparation phase was greater for reward and loss trials compared to neutral trials (adj. ps = .002 and .003, respectively). Negative TRiM of the DMN was weaker for reward trials than neutral trials during the preparation phase (adj. p = .004). Finally, during the preparation phase, we observed greater TRiM of the Auditory network for reward and loss trials than neutral trials (pairwise comparisons significant at adj. p < .0001).
During the response phase, we found positive TRiM in the Visual, Dorsal Attention, Default Mode, Valuation, and Auditory networks, and negative TRiM in the Motor network. Negative TRiM of the Motor network in the response phase was larger for loss trials than reward trials, adj. p = .03.
Developmental Differences in the Modulation of Task-Related Networks
To characterize developmental differences in the recruitment of task-related networks, we examined the association of TRiM estimates and age (results depicted in Figure 3). Fit statistics and tests of model effects are provided in Table 1, and results are described for the best-fitting function of age. During the cue phase, TRiM of the DAN increased linearly with age irrespective of incentive (r = .50; adj. p < .001). Cue-related activity of the SN decreased asymptotically with age, transitioning from no task-related coupling in younger individuals to negative coupling in adults (r = -.18; adj. p = .005). Finally, for the Auditory network, cue-related TRiM became increasingly negative in older individuals (r = -.23; adj. p < .001).
Figure 3.
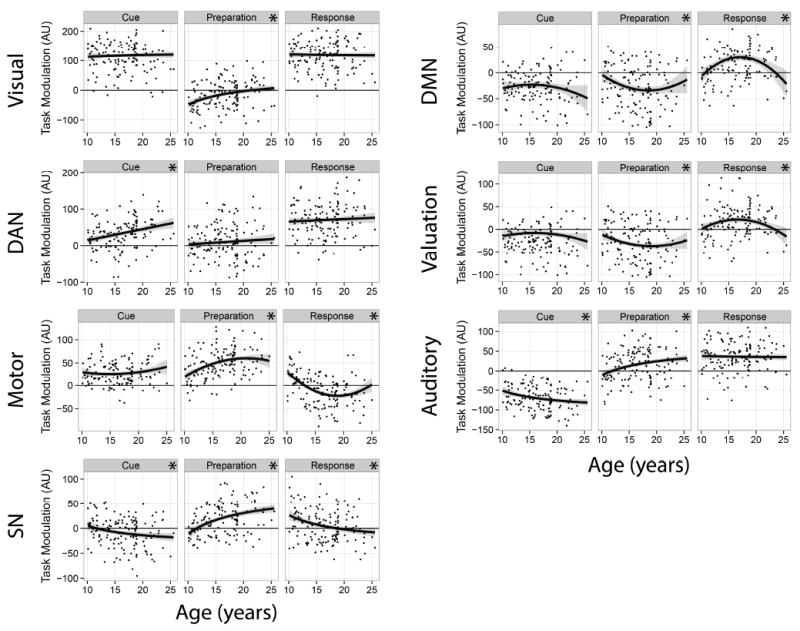
Age-related changes in task-related network modulation as a function of trial phase. Plots represent the strength of the association between network modulation and each phase of the task between ages 10 and 25. The zero point on the vertical axis represents no coupling, on average, of the network with that phase of the task. A regression line representing the best-fitting function of age is overlaid on each panel: linear (DAN), asymptotic (Visual, SN, Auditory), or quadratic (Motor, DMN, Reward). Significant associations are denoted by an asterisk in the subpanel header (see text for a detailed description of these effects).
Table 1.
Effects of age, phase, and valence on task-related network activity.
| Effect | Network | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Visual | DAN | Motor | SN | DMN | Valuation | Auditory | |
|
| |||||||
| Age | 13.52*** (1, 134) | 0.36 (1, 113) | 1.16 (1, 133) | 3.60 (1, 136) | |||
| Age2 | 3.82* (1, 114) | 3.63 (1, 133) | 2.62 (1, 136) | ||||
| 1/Age | 5.59* (1, 132) | 0.24 (1, 136) | 0.44 (1, 137) | ||||
| Phase | 1265.84*** (2, 4512) | 125.17*** (2, 4653) | 261.10*** (2, 3954) | 48.35*** (2, 4653) | 121.05*** (2, 4610) | 127.31*** (2, 4715) | 690.34*** (2, 4688) |
| Valence | 4.19* (2, 4512) | 0.84 (2, 4653) | 0.43 (2, 3954) | 0.25 (2, 4653) | 0.33 (2, 4610) | 0.37 (2, 4715) | 0.74 (2, 4688) |
| Phase × Valence | 6.98*** (4, 4512) | 1.84 (4, 4653) | 4.81*** (4, 3954) | 6.74*** (4, 4653) | 5.90*** (4, 4610) | 0.83 (4, 4715) | 17.56*** (4, 4688) |
| Phase × Age | 3.23* (2, 4653) | 48.28*** (2, 3954) | 4.39** (2, 4610) | 2.57 (2, 4715) | |||
| Phase × Age2 | 22.75*** (2, 3954) | 11.71*** (2, 4610) | 9.36*** (2, 4715) | ||||
| Phase × 1/Age | 12.51*** (2, 4512) | 32.14*** (2, 4653) | 19.07*** (2, 4688) | ||||
| Valence × Age | 0.02 (2, 4653) | 0.01 (2, 3954) | 0.53 (2, 4610) | 0.10 (2, 4715) | |||
| Valence × Age2 | 0.11 (2, 3954) | 0.63 (2, 4610) | 0.03 (2, 4715) | ||||
| Valence × 1/Age | 0.25 (2, 4512) | 0.21 (2, 4653) | 0.02 (2, 4688) | ||||
| Phase × Valence x Age | 0.63 (4, 4653) | 0.78 (4, 3954) | 0.44 (4, 4610) | 0.38 (4, 4715) | |||
| Phase × Valence × Age2 | 1.04 (4, 3954) | 0.60 (4, 4610) | 2.50* (4, 4715) | ||||
| Phase × Valence × 1/Age | 1.21 (4, 4512) | 1.97 (4, 4653) | 2.76* (4, 4688) | ||||
|
| |||||||
| Model AICC Linear | 57660.65 | 59818.43 | 55068.4 | 56864.82 | 58624.31 | 56841.09 | 57284.39 |
| Model AICC Quadratic | 57667.16 | 59831.1 | 55025.45 | 56873.67 | 58602.3 | 56827.82 | 57275.23 |
| Model AICC Inverse | 57656.58 | 59820.49 | 55041.22 | 56855.99 | 58621.57 | 56840.78 | 57273.72 |
Note. Values displayed in cells represent F-statistics of the best-fitting multilevel model for each network. Values in parentheses are the degrees of freedom for the F-tests according to the Kenward-Roger approximation. The relative model fit is represented by the corrected Akaike Information Criterion (AICC) with the best-fitting form of age-related change highlighted for each network. Although we found a significant Phase × Incentive × Age2 effect for the Valuation network (p = .04), pairwise comparisons of Incentive × Age2 effects at each phase did not survive correction for multiple comparison (adj. ps > .10), suggesting that the Phase × Age2 effects were more robust (see Figure 3).
p < .05;
p < .01;
p < .001.
The relative evidence of one model of age-related change over another can be quantified by the difference in AICC, where differences of 0-2 constitute weak evidence, 4-7 are moderate, and 10 or more are large (Burnham & Anderson, 2002). For example, there was only weak evidence that a linear model better described age-related changes of DAN activity compared to the inverse model, but both inverse and linear models were much more likely than the quadratic model.
During the preparation phase, negative modulation of the Visual network weakened asymptotically with development (r = 0.30; adj. p < .001). In the Motor network, preparation-related activity was greatest around age 20 and weaker for younger individuals (age-related multiple R = 0.44; adj. p = .005). Similarly, recruitment of the Salience and Auditory networks increased asymptotically with development, transitioning from little task activity at age 10 to positive activity in adulthood (rs = .33 and .26, respectively; adj. ps < .001). For the Auditory network, age-related increases in TRiM were somewhat greater for Reward and Loss trials (rs = .21 and .27, respectively) than Neutral trials (r = .08; incentive - neutral contrast adj. p = .056). Modulation of the Default Mode and Valuation networks during the preparation phase followed a quadratic pattern, with little task involvement in childhood and adulthood, and negative coupling in late adolescence (multiple Rs = .21; adj. ps < .03).
During the response phase, TRiM of the Motor network was positive in children, negative in late adolescence, and essentially unrelated to the task in adulthood (age-related multiple R = 0.52; adj. p < .0001). Similarly, response-related SN activity decreased with age, transitioning from positive modulation in childhood to essentially no task involvement in adulthood (r = -.24; adj. p < .0001). Response-related activity of the Default Mode and Valuation networks tended to increase between ages 10 and 17, then declined toward zero in adulthood (multiple Rs = .27 and .24, respectively; adj. ps < .01).
Relationship of Task Networks to Antisaccade Performance
Although functional networks were identified on the basis of task-related modulation, this does not necessarily imply a link to performance on the task. For task-modulated networks whose activity was associated with antisaccade accuracy, an important question is whether age-related improvements in performance are mediated by changes in network modulation. We also tested whether age → network → performance effects were moderated by incentive by testing the difference in mediation parameter estimates for Neutral, Reward, and Loss trials, but none of the tests was significant, ps > .20.
Greater TRiM of the Auditory network during the cue phase was associated with poorer performance, β = -.44, p < .001. Such modulation became increasingly negative with development and partially mediated the association between age and performance, β = .16, p = .002, 95% highest posterior density interval (HPDI) = .04 – .42, ΔR2 = .18. Although activity of the DAN during the cue phase increased significantly with age, it was not significantly associated with overall accuracy, p = .14.
Negative modulation of the Visual network during the preparation phase diminished significantly with age, which in turn predicted better performance: age → preparation activity → performance β = .22, p < .0001, 95% HPDI = .10 – .40, ΔR2 = .27. For the Motor network, greater modulation during the preparation phase predicted significantly better performance, β = .46, p < .0001. Moreover, the improvement of performance with age was partially mediated by developmental increases in Motor network modulation, β = .19, p < .0001, 95% HPDI = .07 – .37, ΔR2 = .17. Likewise, greater activity of the SN during the preparation phase was associated with significantly better performance, β = .53, p < .0001, and SN activity partially mediated the age-performance relationship, β = .25, p < .0001, 95% HPDI = .09 – .52, ΔR2 = .23.
During the response phase, greater TRiM of the Motor network was associated with poorer performance, β = -.41, p < .0001. With development, this task modulation diminished, in turn supporting better performance: age → response phase activity → performance β = .18, p < .0001, 95% HPDI = .07 – .36, ΔR2 = .14. Activity of the DMN and Valuation network was not significantly related to performance during any trial phase after correcting for multiple comparisons, nor did DMN or Valuation activity mediate the age-performance relationship.
Age-related Changes in Between-Network Connectivity
We found two significant age-related changes in between-network connectivity that survived the Holm (1979) correction for familywise error rate. Although we tested inverse and quadratic functions of age as above, only linear associations were significant and were consistently preferred by AICC. First, connectivity between the DAN and the Auditory network increased significantly with age, from r = .07 at age 10 to r = .22 at age 25 (Figure 4). Second, coupling of the DAN and SN increased significantly from r = .25 at age 10 to r = .38 at age 25. We also observed a number of correlations among networks that were stable with age, such as a moderate-to-large correlation between the DMN and Valuation networks (Figure 4). Contrary to our hypothesis, we did not observe age-related increases in the integration of control networks with the Valuation network.
Figure 4.
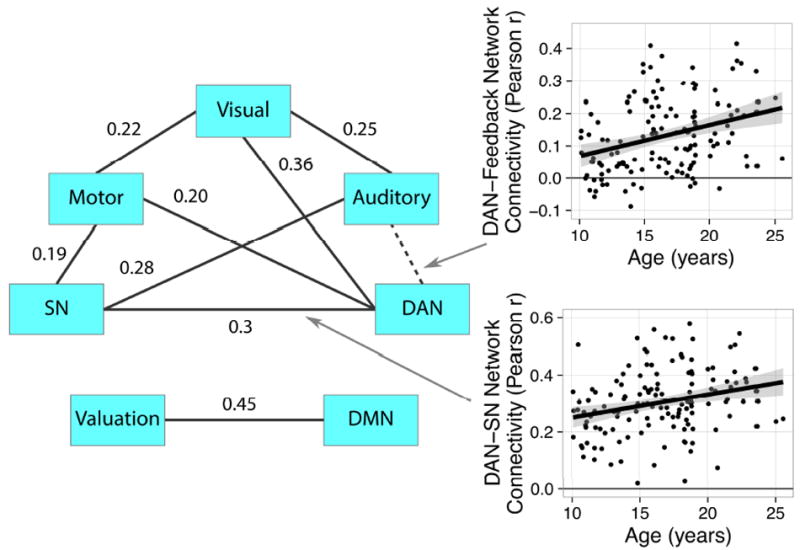
Average correlations among network time courses. Connection labels between two regions reflect the average Pearson correlation between time courses across participants. Only connections that were significant after Holm familywise error correction are displayed. Arrows represent between-network connections that changed with age, with corresponding scatterplots depicting the strength of the association. The dotted arrow between the DAN and Auditory network indicates that the correlation was non-significant the overall sample, but increased with development.
Age-related Changes in Within-Network Connectivity and Composition
In additional exploratory analyses, we characterized age-related changes in network composition and within-network connectivity strength. These analyses inform an understanding of which regions couple or decouple with specific networks over development. Here, we provide a summary of key findings; details for each network are presented in the Supplemental Results and Table S3. In voxelwise analyses of within-network connectivity, the prevailing pattern was that selected regions decoupled from functional networks with age, suggesting network refinement. These changes tended to follow an asymptotic pattern such that within-network connectivity weakened more quickly in younger individuals. This was especially evident for the SN, where within-network connectivity of several regions, including dorsomedial prefrontal cortex, rostrolateral prefrontal cortex, midcingulate, precuneus, and precentral gyrus weakened with age. However, regions considered core to this network such as the anterior insula and dorsal anterior prefrontal cortex were developmentally stable.
Also noteworthy was that sensorimotor regions decoupled from the Visual, DAN, Motor, SN, and Auditory networks with age. For example, in the Motor preparation network, we found that negative coupling of the network with bilateral postcentral gyrus diminished with age, suggesting increasing independence of motor circuitry from somatosensory cortex. We also observed age-related strengthening of key regions within a few networks, such as increasing connectivity strength of bilateral putamen with the Motor network.
Discussion
This is one of the first studies to characterize age-related changes in the composition and coordination of functional brain networks underlying cognitive control and incentive motivation. Using functional connectivity analyses of an incentivized antisaccade task, we found that age-related improvements in inhibitory control were mediated by greater recruitment of networks involved in salience processing and motor preparation. Consistent with our hypotheses, we found that the task-related modulation (TRiM) of control-related networks (Corbetta & Shulman, 2002; Dosenbach et al., 2007), especially the salience and dorsal attention networks, increased gradually with age (Luna et al., 2015). We also identified a network comprising ventral striatum, medial PFC, and ventromedial PFC, which we labeled the Valuation network (Bartra et al., 2013). Recruitment of the Valuation network during the feedback phase was greater in late adolescence than in childhood or adulthood. Contrary to our hypothesis, we did not observe stronger functional coupling of valuation- and control-related networks with development. Altogether, these findings inform and extend the dual-systems model of adolescent neurocognitive development (Shulman et al., 2016) by demonstrating how the relative balance of neurobehavioral systems in adolescents differs at the functional brain network level.
We identified seven task-related networks that largely align with the broader literature on canonical functional brain networks described across a range of resting-state fMRI studies (Cole, Smith, & Beckmann, 2010; Damoiseaux et al., 2006; Dosenbach et al., 2010; Power et al., 2011; S. M. Smith et al., 2009; Yeo et al., 2011). In particular, the composition of Visual, Dorsal Attention, Default Mode, and Salience networks were highly convergent with prior reports on resting-state networks in adults. Although the Auditory network is not commonly discussed in the resting-state literature, its recruitment during the response phase in our study was sensible because auditory feedback was provided to participants to denote accurate or inaccurate responses (see Supplemental Discussion for additional details).
A novel part of this study was to describe how the configuration of functional networks supports information processing at different stages of reward processing, specifically incentive cues, response preparation, and antisaccade execution. During incentive cues, TRiM was positive in the Visual, DAN, and Motor networks (Figure 2), and these networks were functionally coupled (Figure 4). Although our findings do not speak to the direction of connectivity, the joint modulation of Visual and Dorsal Attention networks during the cue phase aligns with evidence that the DAN biases the visual system toward relevant cues.
During the preparation phase, we observed a different configuration of networks: TRiM of the Motor network increased relative to the cue phase, and the Salience and Auditory networks were recruited. Consistent with the role of the salience network (SN) in enhancing attention to behaviorally relevant cues and modulating tonic attention to support accurate responses (Menon & Uddin, 2010; Sadaghiani & D’Esposito, 2014; Seeley et al., 2007), we found that TRiM of the SN was greater during the preparation phase for reward and loss cues than neutral cues. This pattern was mirrored in the Motor network, suggesting that the SN may enhance inhibitory control after incentivized cues via its effect on motor planning circuits. TRiM of the DMN was negative during the cue and preparation phases, consistent with task-related suppression of DMN activity during a cognitively challenging task (Grady et al., 2010).
Finally, the response phase was associated with positive modulation of the Visual, DAN, DMN, Valuation, and Auditory networks. Positive TRiM of the Valuation network during the response phase, but not the cue or preparation phases, is consistent with the role of the ventral striatum and vmPFC in processing the hedonic value of rewarding outcomes (Haber & Knutson, 2010; see Supplemental Discussion for additional details). Interestingly, TRiM was negative in the Valuation network during the cue and preparation phases, indicating functionally coupled increases in the anterior insula and thalamus, but reduced activity in the striatum and vmPFC (Figure 2).
The Mediating Role of Brain Networks in the Development of Inhibitory Control
Corroborating our hypotheses, we found that age-related increases in the modulation of the SN and Motor networks during response preparation mediated the association between age and antisaccade performance. A previous developmental study of incentive effects on sustained attention also observed linear age-related increases in the anterior insula/inferior frontal gyrus, a key node in the SN (Uddin, 2015), irrespective of incentives (A. B. Smith, Halari, Giampetro, Brammer, & Rubia, 2011). Notably, an asymptotic relationship best characterized the association of age with performance and network modulation, suggesting that the developmental refinement of inhibitory control network function matures more slowly as individuals move from adolescence into adulthood. Moreover, whereas on average, the SN was not recruited by children during response preparation, this network was increasingly involved over development (Figure 3). Although we did not find that performance was related to modulation of the DAN, functional connectivity between the DAN and SN increased with age (Figure 4) and the DAN and Auditory networks became coupled. This also aligns with recent resting-state fMRI research indicating that greater functional integration of the SN with other networks supports age-related improvements in inhibitory control (Marek, Hwang, Foran, Hallquist, & Luna, 2015), as well as evidence that changes in SN connectivity are most predictive of brain network maturity (Dosenbach et al., 2010).
Modulation of the DMN and Valuation networks during the response phase (when reward or loss outcomes were delivered) peaked in late adolescence, potentially reflecting an adolescent enhancement of approach-related systems (Ernst, 2014). Although age-related changes in the Valuation network inform our understanding of adolescent-specific enhancement of reward circuitry, modulation of this network was not significantly associated with performance during any phase of the task. Moreover, we did not find evidence that inhibitory control during the antisaccade task depended on the coordination of reward circuitry with control networks (Figure 4). Nor did coupling of the Valuation network with control networks change with age. Altogether, this suggests the possibility that heightened ventral striatal responses to the receipt of rewards in adolescents may reflect hedonic valuation that is largely independent of motivational salience. This interpretation aligns with behavioral and neurobiological research demonstrating a distinction between ‘wanting’ (i.e., incentive motivation) and ‘liking’ (i.e., hedonic value) aspects of rewarding stimuli (Berridge & Robinson, 1998). Consuming hedonic rewards is associated with activity in the ventral striatum and ventral pallidum via opioid neurotransmission (Berridge, Robinson, & Aldridge, 2009). Another possibility is that our incentivized antisaccade task failed to recruit a coordinated response of control- and reward-related networks; the pattern of activity observed in reward paradigms depends substantially on the experimental design (for a review, see Richards et al., 2013).
Conversely, the SN was differentially sensitive to incentives (both reward and loss cues), and greater modulation during response preparation mediated age-related improvements in antisaccade accuracy. These findings support the interpretation that the enhancement of inhibitory control by incentives may be underpinned by greater recruitment of the SN, a crucial network in tonic attention to motivationally relevant stimuli (Sadaghiani & D’Esposito, 2014), rather than paralimbic circuitry. This is consistent with greater enhancement of ‘wanting’ responses to rewards (i.e., incentive motivation, as measured by attention to cues that predict rewards) in adults compared to adolescents (Doremus-Fitzwater & Spear, 2011).
Age-related Refinements of Network Composition
In addition to identifying age-related changes in task-related network activity, we also observed substantial change in the composition of networks between ages 10 and 25. In exploratory analyses, the overall pattern was that canonical regions of each network were developmentally stable in their within-network connectivity strength, whereas potentially ancillary regions tended to decouple with age (Table S3). For example, in younger people, bilateral anterior insula was negatively coupled with the Valuation network, but insular activity became increasing unrelated to this network with age (rs = .57 and .61 for left and right insula, respectively). More generally, our findings suggest that control- and reward-related networks mature in part by sharpening the sensitivity of regional activity to particular cognitive demands (Johnson, 2011). Age-related decreases in within-network connectivity observed in our task-based study also align with similar reductions observed in resting-state research (Betzel et al., 2014; Marek et al., 2015).
Finally, we observed a novel pattern of network integration between the Valuation and Default Mode networks with development. More specifically, we found that a) connectivity of the ventral striatum with the DMN increased with age, b) DMN and Valuation network activity was elevated among adolescents during the response phase, and c) there was moderate connectivity between the Default Mode and Valuation networks that was stable over development. One possibility is that the DMN, which often extends into ventral aspects of the medial prefrontal cortex (e.g., Greicius, Srivastava, Reiss, & Menon, 2004), overlaps with a key network involved in subjective valuation (Bartra et al., 2013). Another possibility is that the self-relevance of rewards may change developmentally, as reflected by functional connectivity between ventral striatum and default mode regions (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). Future research is needed to understand how DMN activity is modulated by the receipt of rewards.
Strengths and Limitations
One strength of our study is that the sample included a relatively large number of participants encompassing late childhood to early adulthood. Relative to some reports that have relied on group comparisons (e.g., adolescents versus adults), the range of ages sampled allowed us to characterize the form of age-related changes, separating adolescent-nonspecific, adolescent-specific, and adolescent-emergent patterns (Casey, 2015). This innovation is important to test specific predictions of the dual-systems theory of adolescent neurocognitive development, such as the gradual maturation of control systems and adolescent peaks in striatal responsiveness to rewards. Our findings build on an emerging body of evidence that has provided initial support for the dual-systems theory using task-based activation analyses (Van Leijenhorst et al., 2010), as well as resting-state functional connectivity (van Duijvenvoorde, Achterberg, et al., 2016). By parsing task-related activity into functionally connected networks using ICA, our study identified canonical networks involved in inhibitory control and incentive processing. Furthermore, our detailed analyses of phase- and valence-specific network modulation provided a useful perspective on how inhibitory control reflects the coordinated activity of several distinct functional modules.
Certain limitations of our study constrain its implications. First, although our findings inform an understanding of average neurodevelopmental changes in typically developing populations, our cross-sectional sample does not provide insight into the pattern of behavioral and neurocognitive changes within an individual over time (cf. Paulsen et al., 2015). For example, in a longitudinal study, Sherman and colleagues (2014) found evidence of increasing functional segregation of the posterior cingulate cortex from control-related regions between ages 10 and 13, whereas in our cross-sectional study, we did not observe age-related changes between control and default mode networks. Furthermore, although our age → network → performance mediation analyses followed from theoretical models of neurocognitive development, our cross-sectional design could not test whether age predicts within-person improvements in performance at a follow-up assessment (i.e., prospective mediation).
Second, although applications of spatial ICA to fMRI data are reasonably sensitive to individual variation in spatial and temporal patterns (Allen et al., 2012), the algorithm nevertheless gives preference to spatial separability defining networks. Recent applications of temporal ICA made possible in part by rapid temporal sampling and long fMRI acquisitions are beginning to inform an understanding of how spatially overlapping networks may contribute to different aspects of cognition (S. M. Smith et al., 2012). Thus, our results may provide only limited information about how the participation of functional regions in multiple task-related networks develops between childhood and adulthood (e.g., Marek et al., 2015). In addition, network labels were chosen based on the modulation profile during the antisaccade task, as well as the composition of each network vis-à-vis previous literature (Yeo et al., 2011). Although our network labels are convergent with extant research, the task-related modulation and network composition observed in our study may not generalize to other tasks with different cognitive demands.
Third, although we found linear increases in DAN modulation from ages 10 to 25 during the cue phase, this was not significantly related to average performance on the antisaccade task. This is inconsistent with previous literature on the role of transient DAN activation to filter out irrelevant stimuli and support top-down control of behavior (e.g., Wen, Yao, Liu, & Ding, 2012). Our failure to corroborate this work may reflect a relative insensitivity of our analytic approach insofar as our measures of network modulation during cues varied at the run level (i.e., the overall association of network time courses with predicted BOLD responses to cue events), not trial level. Thus, to the extent that transient trial-level fluctuations in DAN activity were associated with accuracy, or that the DAN contributed more to latency variations than accuracy, we may have failed to detect a developmental effect.
Fourth, the magnitude of age-related changes in network modulation (Figure 3) varied from small to large according to a common effect size rule of thumb (Cohen, 1988); most effects were moderate in size (see Supplemental Discussion for additional details). Finally, substance use problems were identified based on self-report, not formal drug testing (e.g., urinalysis), which may have failed to eliminate individuals with substance use disorders from our study.
Conclusions
Our study provides new insight into the development of task-related brain networks involved in inhibitory control in the context of incentives. Extending previous work in this area (e.g., Van Leijenhorst et al., 2010), we found that task-related modulation of control networks, especially the dorsal attention and salience networks, increased gradually from childhood to adulthood and that control networks tended to integrate with each other over development. Relative to neutral cues, both reward and loss cues led to greater salience network modulation when preparing to inhibit a saccade. Furthermore, modulation of the salience network during response preparation increased from childhood to adulthood and was linked with better inhibitory control. Conversely, the recruitment of task-irrelevant circuitry (e.g., auditory regions during the visual part of the task) was associated with poorer performance, and this declined into adulthood, potentially reflecting developmental improvements in network switching in response to task demands (Menon & Uddin, 2010).
Consistent with a dual-systems perspective, we found greater recruitment of valuation-related circuits (especially mPFC and ventral striatum; Bartra et al., 2013) during adolescence in response to the receipt of reward and loss outcomes. However, modulation of the Valuation network was not linked with antisaccade accuracy, which may suggest that heightened striatal reactivity to reward receipt does not detract from adolescents’ inhibitory control, whereas striatal reactivity to reward cues may be linked with risky behaviors (Bjork, Smith, Chen, & Hommer, 2011). Importantly, our results speak primarily to how incentives influence performance on inhibitory control tasks and may not generalize to developmental differences in decision-making such as affective versus deliberative contexts (Figner, Mackinlay, Wilkening, & Weber, 2009) or the influence of peers on risky decisions (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011). Altogether, our findings support the interpretation that incentives facilitate greater inhibitory control over development by enhancing functional networks involved in salience processing and motor preparation.
Supplementary Material
Acknowledgments
The authors thank Vince Calhoun for helpful comments and guidance on interpreting measures of task-related connectivity in independent component analysis.
This research was supported by a grant from the National Institute of Mental Health (MH080243) to B. L. Further support was provided by K01 MH097091 to M.N.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2009. [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Calhoun VD, et al. A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. https://doi.org/10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Wei Y, Eichele T, Calhoun VD. Capturing inter-subject variability with group independent component analysis of fMRI data: A simulation study. NeuroImage. 2012;59(4):4141–4159. doi: 10.1016/j.neuroimage.2011.10.010. https://doi.org/10.1016/j.neuroimage.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. https://doi.org/10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. https://doi.org/10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research: Brain Research Review. 1998;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. https://doi.org/10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102(Part 2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. https://doi.org/10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW. Psychosocial problems and recruitment of incentive neurocircuitry: Exploring individual differences in healthy adolescents. Developmental Cognitive Neuroscience. 2011;1(4):570–577. doi: 10.1016/j.dcn.2011.07.005. https://doi.org/10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. https://doi.org/10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multi-model inference: A practical information-theoretic approach. 2. New York: Springer; 2002. [Google Scholar]
- Cai X, Padoa-Schioppa C. Neuronal Encoding of Subjective Value in Dorsal and Ventral Anterior Cingulate Cortex. The Journal of Neuroscience. 2012;32(11):3791–3808. doi: 10.1523/JNEUROSCI.3864-11.2012. https://doi.org/10.1523/JNEUROSCI.3864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45(1 Suppl):S163–172. doi: 10.1016/j.neuroimage.2008.10.057. https://doi.org/10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66(1):295–319. doi: 10.1146/annurev-psych-010814-015156. https://doi.org/10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. https://doi.org/10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Routledge Academic; 1988. [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. https://doi.org/10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. https://doi.org/10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Correa N, Adali T, Calhoun VD. Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magnetic Resonance Imaging. 2007;25(5):684–694. doi: 10.1016/j.mri.2006.10.017. https://doi.org/10.1016/j.mri.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. https://doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. https://doi.org/10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011;125(4):661–667. doi: 10.1037/a0023763. https://doi.org/10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Schlaggar BL, et al. Prediction of individual brain maturity using fMRI. Science (New York, N Y) 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. https://doi.org/10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. Integration of the cognitive and the psychodynamic unconscious. American Psychologist. 1994;49(8):709–724. doi: 10.1037//0003-066x.49.8.709. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. https://doi.org/10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain and Cognition. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. https://doi.org/10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(3):709–730. doi: 10.1037/a0014983. https://doi.org/10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans A, McKinstry R, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Supplement 1):S102. https://doi.org/10.1016/S1053-8119(09)70884-5. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. https://doi.org/10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Luna B. Developmental Effects of Incentives on Response Inhibition. Child Development. 2012;83(4):1262–1274. doi: 10.1111/j.1467-8624.2012.01771.x. https://doi.org/10.1111/j.1467-8624.2012.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. https://doi.org/10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 2002;34(4):605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. Understanding dopamine and reinforcement learning: The dopamine reward prediction error hypothesis. Proceedings of the National Academy of Sciences. 2011;108(Supplement_3):15647–1565. doi: 10.1073/pnas.1014269108. https://doi.org/10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, McIntosh AR, et al. A Multivariate Analysis of Age-Related Differences in Default Mode and Task-Positive Networks across Multiple Cognitive Domains. Cerebral Cortex. 2010;20(6):1432–1447. doi: 10.1093/cercor/bhp207. https://doi.org/10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. https://doi.org/10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. https://doi.org/10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. https://doi.org/10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. Journal of Addiction Medicine. 2009;3(2):47–54. doi: 10.1097/ADM.0b013e31819ca788. https://doi.org/10.1097/ADM.0b013e31819ca788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. https://doi.org/10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes SW, Chahal R, Hallquist MN, Paulsen DJ, Geier CF, Luna B. Modulation of reward-related neural activation on sensation seeking across development. NeuroImage. 2017;147:763–771. doi: 10.1016/j.neuroimage.2016.12.020. https://doi.org/10.1016/j.neuroimage.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6(2):65–70. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. https://doi.org/10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. https://doi.org/10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. https://doi.org/10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174(4):754–62. doi: 10.1007/s00221-006-0520-9. https://doi.org/http://dx.doi.org.ezaccess.libraries.psu.edu/10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Interactive Specialization: A domain-general framework for human functional brain development? Developmental Cognitive Neuroscience. 2011;1(1):7–21. doi: 10.1016/j.dcn.2010.07.003. https://doi.org/10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The Neurobiology of Decision: Consensus and Controversy. Neuron. 2009;63(6):733–745. doi: 10.1016/j.neuron.2009.09.003. https://doi.org/10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: Mapping bounded rationality. American Psychologist. 2003;58(9):697–720. doi: 10.1037/0003-066X.58.9.697. https://doi.org/http://dx.doi.org.ezaccess.libraries.psu.edu/10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Calhoun VD, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Human Brain Mapping. 2009;30(11):3795–3811. doi: 10.1002/hbm.20807. https://doi.org/10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. https://doi.org/10.1016/S1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of Cognitive Processes From Late Childhood to Adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. https://doi.org/10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An Integrative Model of the Maturation of Cognitive Control. Annual Review of Neuroscience. 2015;38(1):151–170. doi: 10.1146/annurev-neuro-071714-034054. https://doi.org/10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLOS Biology. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. https://doi.org/10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. https://doi.org/10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman KJ, Brett M. Analysis of functional magnetic resonance imaging in Python. Computing in Science & Engineering. 2007;9(3):52–55. [Google Scholar]
- Molcho M, Walsh S, Donnelly P, de Matos MG, Pickett W. Trend in injury-related mortality and morbidity among adolescents across 30 countries from 2002 to 2010. The European Journal of Public Health. 2015;25(suppl 2):33–36. doi: 10.1093/eurpub/ckv026. https://doi.org/10.1093/eurpub/ckv026. [DOI] [PubMed] [Google Scholar]
- Muehlenkamp JJ, Claes L, Havertape L, Plener PL. International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child and Adolescent Psychiatry and Mental Health. 2012;6(10):1–9. doi: 10.1186/1753-2000-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating Processes within a Trial in Event-Related Functional MRI: I. The Method. NeuroImage. 2001;13(1):210–217. doi: 10.1006/nimg.2000.0710. https://doi.org/10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Neurobiology of economic choice: a good-based model. Annual Review of Neuroscience. 2011;34:333–359. doi: 10.1146/annurev-neuro-061010-113648. https://doi.org/10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen DJ, Hallquist MN, Geier CF, Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: A longitudinal fMRI study. Developmental Cognitive Neuroscience. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. https://doi.org/10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. https://doi.org/10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. https://doi.org/10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Petersen SE, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. https://doi.org/10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews. 2013;37(5):976–991. doi: 10.1016/j.neubiorev.2013.03.004. https://doi.org/10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. A Four-Dimensional Registration Algorithm With Application to Joint Correction of Motion and Slice Timing in fMRI. IEEE Transactions on Medical Imaging. 2011;30(8):1546–1554. doi: 10.1109/TMI.2011.2131152. https://doi.org/10.1109/TMI.2011.2131152. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, D’Esposito M. Functional Characterization of the Cingulo-Opercular Network in the Maintenance of Tonic Alertness. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu072. bhu072. https://doi.org/10.1093/cercor/bhu072. [DOI] [PMC free article] [PubMed]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, Gur RE, et al. Functional Maturation of the Executive System during Adolescence. The Journal of Neuroscience. 2013;33(41):16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. https://doi.org/10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. https://doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the Default Mode and Central Executive Networks across early adolescence: A longitudinal study. Developmental Cognitive Neuroscience. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. https://doi.org/10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. https://doi.org/10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. https://doi.org/10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011;56(3):1693–1704. doi: 10.1016/j.neuroimage.2011.01.072. https://doi.org/10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. https://doi.org/10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23Suppl 1:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. https://doi.org/10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Ugurbil K, et al. Temporally-independent functional modes of spontaneous brain activity. Proceedings of the National Academy of Sciences. 2012;109(8):3131–3136. doi: 10.1073/pnas.1121329109. https://doi.org/10.1073/pnas.1121329109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. https://doi.org/10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. https://doi.org/10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proceedings of the National Academy of Sciences. 2012;109(5):1719–1724. doi: 10.1073/pnas.1114137109. https://doi.org/10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N. Further analysis of the data by Akaike’s Information Criterion and the finite corrections. Communications in Statistics, Theory, and Methods. 1978;A7:13–26. [Google Scholar]
- Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Computational Biology. 2012;8(2):e1002374. doi: 10.1371/journal.pcbi.1002374. https://doi.org/10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swahn MH, Bossarte RM, Palmier JB, Yao H, Dulmen MHMV. Psychosocial characteristics associated with frequent physical fighting: findings from the 2009 National Youth Risk Behavior Survey. Injury Prevention. 2013;19(2):143–146. doi: 10.1136/injuryprev-2012-040381. https://doi.org/10.1136/injuryprev-2012-040381. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galván A. The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive and Affective Neuroscience. 2015;10(3):389–398. doi: 10.1093/scan/nsu064. https://doi.org/10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JDE. Development of Spatial and Verbal Working Memory Capacity in the Human Brain. Journal of Cognitive Neuroscience. 2009;21(2):316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience. 2015;16(1):55–61. doi: 10.1038/nrn3857. https://doi.org/http://dx.doi.org.ezaccess.libraries.psu.edu/10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Van der, Meer T, Te Grotenhuis, M Pelzer. Influential Cases in Multilevel Modeling: A Methodological Comment. American Sociological Review. 2010;75(1):173–178. [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage. 2016;124(Part A):409–420. doi: 10.1016/j.neuroimage.2015.04.069. https://doi.org/10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Peters S, Braams BR, Crone EA. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience & Biobehavioral Reviews. 2016;70:135–147. doi: 10.1016/j.neubiorev.2016.06.037. https://doi.org/10.1016/j.neubiorev.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks Z, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. https://doi.org/10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. https://doi.org/10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Wen X, Yao L, Liu Y, Ding M. Causal Interactions in Attention Networks Predict Behavioral Performance. The Journal of Neuroscience. 2012;32(4):1284–1292. doi: 10.1523/JNEUROSCI.2817-11.2012. https://doi.org/10.1523/JNEUROSCI.2817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Buckner RL, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. https://doi.org/10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


