Abstract
PURPOSE
Ultrashort echo time (UTE) sequences with a minimal nominal TE of 8 µs have shown promise for direct imaging of myelin protons (T2, < 1 ms). However, there is still debate about the efficiency of 2D slice-selective UTE sequences in exciting myelin protons because the half excitation pulses used in these sequences have a relatively long duration (e.g., 0.3-0.6 ms). Here we compared UTE and inversion-recovery UTE (IR-UTE) sequences used with either hard or half excitation pulses (durations 32 µs or 472 µs, respectively) for imaging myelin in native and deuterated ovine brain at 3T.
METHODS
Freshly-frozen ovine brains were dissected into ~2 mm thick pure white matter and ~3-8 mm thick cerebral hemisphere specimens, which were imaged before and/or after different immersion time in D2O.
RESULTS
Bi-component T2* analysis of UTE signals obtained with hard excitation pulses detected an ultrashort T2 component (STC) fraction (fS) of 0-10% in native specimens, and up to ~86% in heavily deuterated specimens. fS values were significantly affected by the TIs used in IR-UTE sequences with either hard or half excitation pulses in native specimens, but not in heavily deuterated specimens. The STC T2*s were in the range of 150-400 µs in all UTE and IR-UTE measurements obtained with either hard or half excitation pulses.
CONCLUSION
Our results further support myelin protons as the major source of the ultrashort T2* signals seen on IR-UTE images, and demonstrate the potential of IR-UTE sequences with half excitation pulses for directly imaging myelin using clinical scanners.
Keywords: T2*, bi-component, myelin, white matter, ultrashort echo time, inversion recovery
INTRODUCTION
Conventional imaging of brain tissue commonly detects three distinct pools: a long T2 pool associated with free water in cerebrospinal fluid (T2, ~2 s), an intermediate T2 pool involving extra- and intra-cellular water (T2, ~100 ms), and a short T2 pool composed of water trapped in myelin bilayers and other associated macromolecules (myelin water) (T2, 1–10 ms) (1,2). Studying free water signals is helpful for detecting increased permeability of the blood-brain barrier in stroke and other pathological conditions (3–5). Charactering extra- and intra-cellular water signals have been useful for probing not only cellular structural characteristics but also functional activation (6–9). Investigating myelin water indirectly provides insights about the integrity of myelin and myelin loss (10–12). These water protons can be characterized using currently available clinical magnetic resonance imaging (MRI) techniques with TEs of 1 ms and longer, and are all referred to as long T2 components (LTCs) in this study.
Unlike most water protons, the semi-solid highly-constrained lipid protons trapped in myelin sheaths, named as myelin protons hereafter (13), have ultrashort T2 (< 1 ms) and are not directly detectable with most conventional magnetic resonance imaging (MRI) sequences (14). Direct imaging of myelin protons may improve the specificity of MRI for the evaluation of neurological diseases that are characterized by demyelination and remyelination, such as multiple sclerosis (15,16), and may also be of value in monitoring therapeutic response. In recently developed two-dimensional ultrashort echo time (2D UTE) sequences, nominal TEs as short as 8 μs can be achieved through half-pulse excitation, variable rate selective excitation (VERSE), radial ramp sampling and fast transmit/receive switching (17,18), making it possible to directly image myelin protons. Furthermore, inversion-recovery UTE (IR-UTE) sequences can provide robust suppression of signals from LTCs using an adiabatic IR preparation pulse and therefore have the potential to directly and selectively image myelin with high contrast (19).
Although UTE imaging of myelin protons has been investigated by several groups (14,18,20–23), there is still debate about whether UTE sequences, especially when used with half excitation pulses (where the pulse duration is on the order of hundreds of microseconds), can directly detect myelin proton signals that have a reported T2* of ~ 300 µs (22) or shorter (14,21). This study aimed to compare UTE and IR-UTE sequences used with either short hard or longer half excitation pulses for probing myelin proton signals at 3T, and meanwhile to probe the specificity and sensitivity of these sequences to myelin proton signals by comparing the ultrashort T2 component (STC) fraction and T2* (i.e., fS and T2S*) seen in fresh and deuterated ovine brain WM. Deuterons in deuterium oxide (D2O) have a magnetic resonance frequency ~6.5 times lower than that of protons and are not detectable with proton MRI (24). By immersing tissue specimens in highly purified D2O, it is possible to replace the majority, if not all, of the long T2 water protons with deuterons (14). The specimens were expected to have different proportions of 1H MRI visible LTCs after they were immersed in D2O for different durations, while myelin protons would survive the D2O exchange (14) and still be detectable with UTE and IR-UTE sequences. If myelin protons are the major source of the ultrashort T2 signals and the sequences are sensitive to proportional changes of these signals, T2S* might not change but fS would change significantly after exchange.
METHODS
Pulse sequences and contrast mechanisms
Figure 1 shows diagrams of the 2D IR-UTE pulse sequences and the associated contrast mechanisms. Either a short hard pulse (rectangular shape, duration 32 µs, bandwidth = 8.2 kHz) or a half pulse (half sinc shape, VERSE corrected, duration 472 µs, bandwidth = 2.7 kHz) was used for signal excitation (Figs. 1A–1B). Each sequence contained an adiabatic IR preparation pulse (Silver–Hoult pulse, duration 8.64 ms, bandwidth = 1.4 kHz) and a minimal nominal TE of 10 µs. The basis for contrast seen on the IR-UTE images for selective myelin proton imaging in white matter (WM) is illustrated in Figures 1C and 1D. The inversion time (TI) is chosen to null the signals from LTCs (TInull) in WM (WML). At the time the UTE acquisition starts (TE = 10 µs), LTCs in grey matter (GML) have non-zero negative magnetization (because GML has a longer T1 than WML), while STCs in WM and grey matter (i.e., WMS and GMS) have non-zero positive magnetizations (Fig. 1C). Therefore, on the IR-UTE images obtained at WML TInull and TE = 10 µs, the WM signal comes from STCs while the grey matter signal contains mixed contributions from both LTCs and STCs. When the selected TI is incrementally offset from WML TInull, WM signals in the image will be increasingly contaminated by LTCs, leading to inaccurate measurement of STC signals (presumably mainly from myelin protons) (Fig. 1D). WML TInull can be estimated from IR-UTE images obtained with varying TIs at a later yet still short TE (e.g., TE = 2.2 ms). At this TE, the magnetization of STCs is zero or near zero due to fast signal decay, while that of LTCs is largely unchanged, allowing selective measurement of the signals from LTCs for the estimation of their T1 and TInull.
Figure 1.
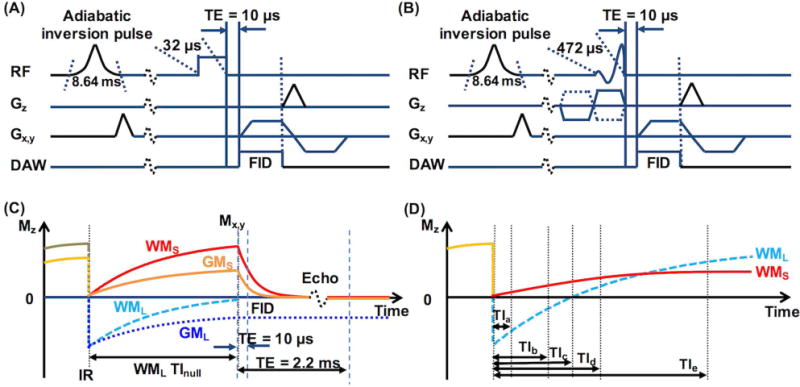
Diagrams of the 2D IR-UTE pulse sequences with a TE of 10 µs used with (A) a short hard excitation pulse (rectangular shape, duration 32 µs, bandwidth = 8.2 kHz) followed by 2D radial ramp sampling, and (B) a half excitation pulse (duration 472 µs, bandwidth = 2.7 kHz) followed by 2D radial ramp sampling. (C) Illustration of the contrast mechanism for imaging ultrashort T2 components in white matter (WMS) using IR-UTE with the inversion time (TI) set for nulling of signals from the long T2 components (WML), with the TI termed as WML TInull. In magnitude IR-UTE images, the signal in white matter quickly decays to zero with increasing TE, while the signal in grey matter is still high at TE = 2.2 ms because it is dominated by long T2 components (GML). (D) MZ of WML and WMS plotted against different TIs at the time UTE acquisition starts. At TIc, signals from WML are nulled. At TIa, which is much shorter than TIc, signal in the image is dominated by WML. At TIb, which is only slightly shorter than TIc, signal from WMS is cancelled out by that from WML. At TId and TIe, signals from WMS and WML coexist in the image but with different fractions. Note that the time parameters in the diagrams were not proportionally illustrated.
Hard excitation pulses have higher power and shorter duration than half excitation pulses, and thus should be more efficient and reliable in exciting extremely short T2 protons (25). However, a half pulse has to be used for 2D slice-selective imaging. In this study, the slice-selective gradients were turned off in the half pulse excitation experiments based on the following three considerations. First, the specimens were carefully prepared into pure WM specimens (~2 mm thick) or thin coronal hemisphere slabs (~3-8 mm thick), thus a region of interest (ROI) could be defined in WM without contamination from neighboring grey matter when the specimen was excited without slice selection. Second, errors induced by eddy currents in regular half pulse excitation experiments could be eliminated by turning off the slice-selective gradients, thus providing more accurate estimations of T2S* and fS, as well as allowing a more direct comparison between the hard pulse and half pulse excitations. Third, regular 2D UTE and IR-UTE imaging using half excitation pulses would require two excitations per scan to achieve slice selection. The total scan time could therefore be halved by turning off the slice-selective gradients.
Specimen preparation and experimental design
Five freshly-frozen ovine brains were thawed and dissected into six ~2 mm thick ~ 2 × 5 mm2 pure WM short blocks, three ~2 mm thick ~ 4 × 10 mm2 pure WM long segments from three consecutive slabs of the same brain, one ~8-mm thick cerebral hemisphere slab, and three ~3-mm thick cerebral hemisphere slabs. Two WM short blocks, all three WM long segments and all three ~3-mm thick cerebral hemisphere slabs were subject to D2O exchange. Each WM short block was immersed in 5 mL D2O (99.9%, Sigma-Aldrich, St. Louis, MO) for 8 hrs with D2O refreshed four times (8-hr 5-pass D2O exchange). Each WM long segment was immersed in 5 mL D2O for one hour, two hours (two 1-hr consecutive periods with D2O refreshed once), or 27 hours (1-hr, 1-hr, 2-hr & 23-hr consecutive periods with D2O refreshed three times, i.e. 27-hr 4-pass D2O exchange), respectively. Each cerebral hemisphere slab was immersed in 10 mL D2O and subject to 27-hr 4-pass D2O exchange.
All the pure WM short blocks were imaged using a 5-mm diameter transmit/receive solenoid coil to achieve high signal to noise ratio (SNR). All the pure WM long segments and cerebral hemisphere slabs were imaged with a 7.6-cm receive-only surface coil that can be used clinically. First, three native WM short blocks were imaged with UTE sequences and IR-UTE sequences with varying TIs using hard excitation pulses. Briefly, one deuterated WM short block was imaged with the UTE sequence using hard excitation pulse, and one native and one deuterated WM short blocks were subject to free induction decay (FID) acquisition to evaluate water signal loss after D2O exchange. Next, the ~8 mm thick cerebral hemisphere slab was imaged in the native condition with UTE and IR-UTE sequences using both hard and half excitation pulses. Lastly, the three WM long segments were imaged after progressive D2O exchange, and the three ~3-mm thick cerebral hemisphere slabs were all imaged before and after 27-hr D2O exchange with UTE and IR-UTE sequences using half excitation pulses.
Data acquisition
Both single-slice 2D UTE and IR-UTE sequences were implemented on a 3T Signa TwinSpeed scanner (GE Healthcare Technologies, Milwaukee, WI) which had a maximum gradient strength of 40 mT/m and a maximum slew rate of 150 mT/m/ms. The UTE sequence was performed with TR = 1000 ms and a series of TEs ranging from 10 µs to up to 30 ms. At each imaging time point, the IR-UTE sequence was first used with TR/TE = 1000/2.2 ms, and a series of TIs (50, 100, 300, 500, and 800 ms) to measure the WML T1 and determine WML TInull. The same IR-UTE sequence was then used with TR = 1000 ms, TI = WML TInull and/or TIs of 5-130 ms longer than WML TInull, and a series of TEs ranging from 10 µs to 30 ms to measure fS and T2S* in WM. The acquisition matrix was 96 × 96 and the scan time was 97s per acquisition. T2-weighted multi-slice FSE images were acquired with TR/TE = 3000/40 ms, slice thickness = 1 mm, and no slice gap to guide the ROI definition in WM on the UTE and IR-UTE images. FID signals were acquired on an 11.7T small bore preclinical scanner (Bruker BioSpec 117/11 USR/R, Rheinstetten, Germany). Briefly, the specimen was placed inside a 5 mm NMR proton free tube and examined using a custom made proton free coil with a block excitation pulse (pulse width = 20 µs), TR = 20 s, 4096 points and sweep bandwidth = 50 kHz.
Data analysis
The WML T1 and TInull were calculated offline in each manually-defined ROI using a single component three parameter fitting model in Matlab (The Mathworks Inc. Natick, MA, USA). T2S* and fS were calculated using a previously published bi-component fitting model in Matlab (26):
| [1] |
where is the UTE or IR-UTE MR signal, AS and AL are the signal amplitudes of the STCs and LTCs, T2S* is the STC T2*, and T2L* is the LTC T2*. was defined as AS/(AS+AL). Initially, all datasets were subjected to bi-component fitting. However, in some cases with exceptionally high fractions of LTCs or STCs, the two components were not clearly separated (i.e. both T2* values < 1 ms or both > 20 ms) using Eq. 1. Thus, to improve algorithmic stability, these cases were reanalyzed using a single component fitting model:
| [2] |
where is the signal intensity at the minimum TE. The key fitting parameters are provided in Supporting Information. All images used for analysis and presentation are magnitude images. Each FID was subject to Fourier transform as well as automatic phase and baseline corrections in iNMR (http://www.inmr.net), and displayed as a spectrum.
RESULTS
Figure 2 and Figure 3 show results from one native WM short block imaged using hard pulse excitation. Figure 2A shows obvious signal intensity variation in the magnitude images acquired with different TIs at TE = 2.2 ms, reflecting varying relative contributions from STCs and LTCs. The signal intensity was lowest at TI = 300 ms, suggesting significant suppression of the signals from LTCs (Fig. 2A). The T1 map (Fig. 2B) calculated from the images in Fig. 2A showed a near uniform structure inside the specimen with marginal regions showing slightly longer T1s (the map scale was 400-460 ms), validating that the specimen was pure WM (T1 of grey matter would be in the range of ~800 - 1000 ms when measured using the same IR-UTE sequences (27)), though its margins might have been contaminated or injured during handling. Figure 2C shows magnitude images acquired with different TEs and no IR preparation. Figures 2D–2F show magnitude images acquired with different TEs and varying TIs. At TI = 270 ms (i.e., WML TInull), the signal intensities dropped significantly with increasing TE (Fig. 2D). At TIs of 275 ms and 295 ms (Figs. 2E–2F), the signal decay against TE was slower but still faster than that on the images acquired without IR preparation (Fig. 2C). Figure 3 shows bi-component analyses of the magnitude images acquired without and with IR preparation using different TIs (ROI was defined in the center of the specimen as illustrated in Fig. 2B). The was low (7.9%) in the measurement without IR preparation (Fig. 3A), and slightly higher (32.9%) at TI = 295 ms (Fig. 3D) and much higher (93.0%) at TI = 270 ms in the measurements with IR preparation (Fig. 3B), whereas the T2S* was all in the range of 150 – 400 µs (Figs. 3A–3D).
Figure 2.
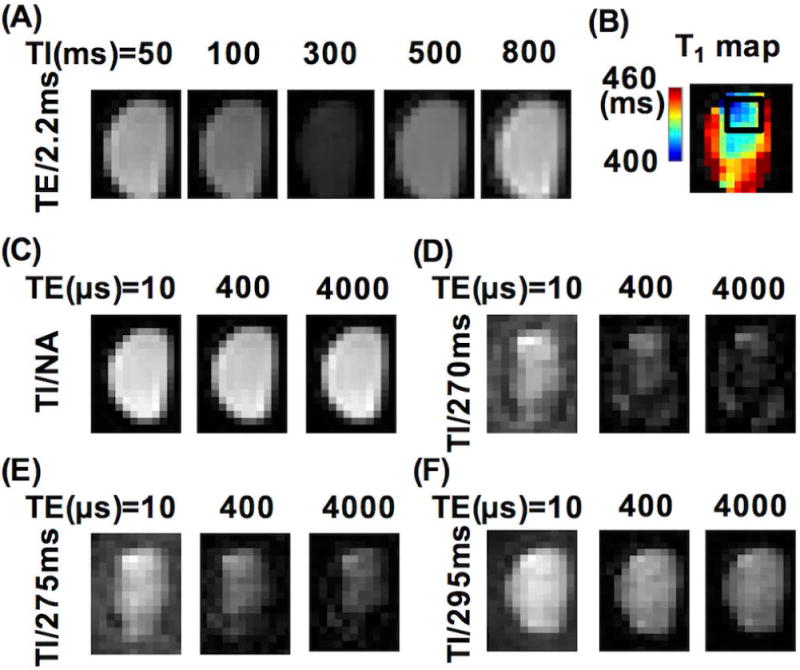
Representative magnitude images (acquired with hard excitation pulses) of one native WM short block. (A) Images obtained with varying inversion times (TIs) at TE = 2.2 ms. (B) T1 map calculated from the images in (A) showing a relatively homogeneous center and a rim with a slightly longer T1. The black box shows the region of interest (ROI) used for quantitative T2* analyses. (C) Images obtained with different TEs and without inversion recovery preparation (labeled as ‘TI/NA (not applicable)’). (D-F) Images obtained with different TEs and varying TIs. There was an obvious signal intensity decrease with the increase of TE in the images at TIs = 270 ms and 275 ms, but not at TI = 295 ms.
Figure 3.
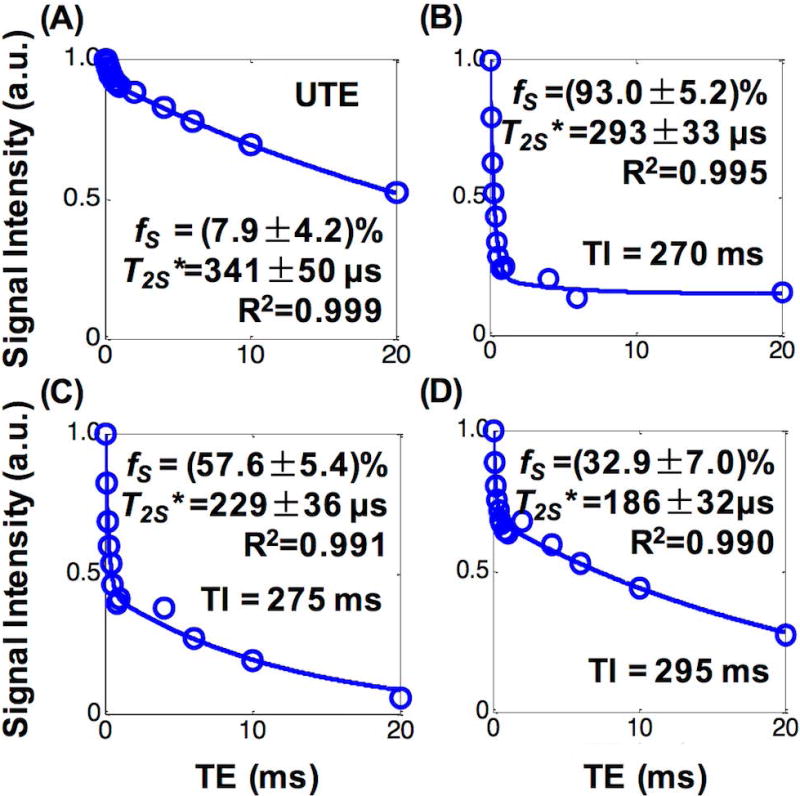
Representative bi-component T2* fitting curves of one native WM short block. (A) Measurement with hard excitation pulses and no IR preparation. (B-D) Measurements with hard excitation pulses and varying TIs. All measurements provided different ultrashort T2 component fractions (fS) but similar T2* (T2S*) values.
The Supporting Information Figure S1 shows enlarged spectra from one native (Supporting Fig. S1A) and one deuterated (Supporting Fig. S1B) WM short blocks. In the native specimen (Supporting Fig. S1A), the area under the water peak was much larger than that of the lipid peak and contributed ~88.5% of the total FID signal. In the deuterated specimen (Supporting Fig. S1B), the water peak was extremely narrow and contributed only ~24.6% to the total FID signal. Figure 4 shows the bi-component fitting results of the magnitude images obtained with hard excitation pulse and no IR preparation from another deuterated WM short block that was subject to an identical deuteration procedure, revealing a fS of ~86%. This fS was much higher than that in a native specimen (~7.9%, Fig. 3A). This difference in fS between the native and the deuterated specimens detected with imaging was consistent with the water fraction reduction after D2O exchange revealed by the FID measurements. The magnitude images (inserts in Fig. 4) from the deuterated specimen showed much faster signal decay with increasing TE than those of the native specimen (Fig. 2C), supporting unexchangeable STCs (presumably mainly myelin protons) as the major source of the UTE signals.
Figure 4.
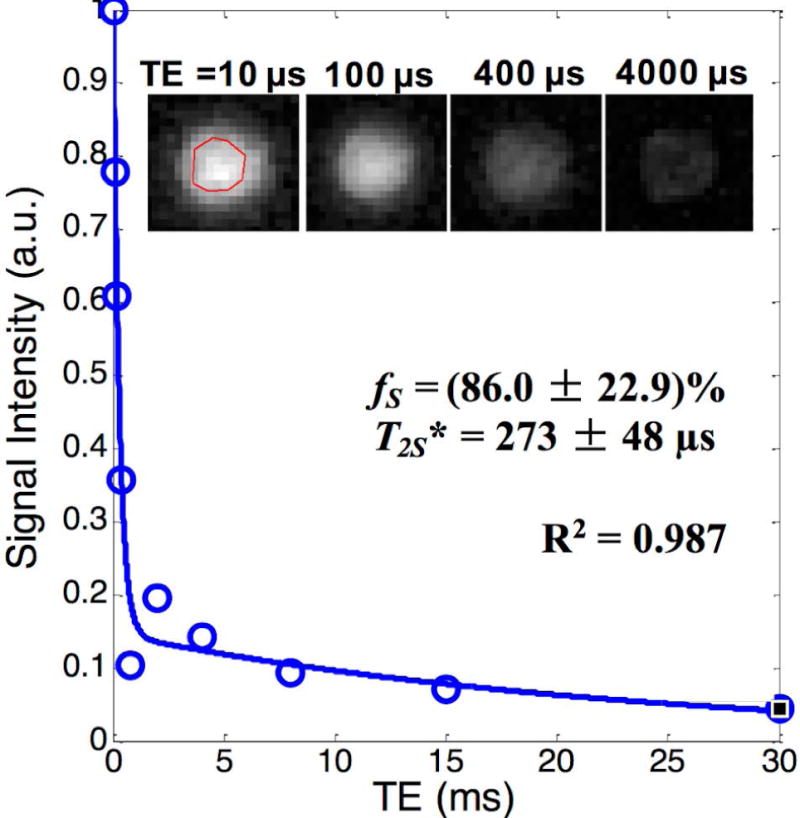
Bi-component T2* analysis of images acquired with hard excitation pulse and no IR preparation from a WM short block after a 27-hr 4-pass D2O exchange, showing a much higher ultrashort T2 component fraction with similar T2* (i.e., fS and T2S*) compared with a native WM short block shown as in Fig.3A. Inserts are the images of the WM short block after 27-hr D2O exchange which showed fast signal decay with the increase in TE.
The Supporting Information Figure S2 illustrates the procedure of ROI definition in the ~8-mm thick native cerebral hemisphere slabs. Figure 5 summarizes the bi-component T2* fitting results from the ~8-mm thick native cerebral hemisphere specimen (Figs. 5A–5C) shown in Supporting Information Figure S2 imaged using both hard and half excitation pulses, and the average results from three native WM short block specimens imaged using hard excitation pulses (Figs. 5D–5F). fS and T2S* were identified in all specimens when measured with the UTE sequences using hard excitation pulses. However, UTE signals of the hemisphere specimen imaged using half excitation pulses showed single component decay, and the T2* value was similar to T2L* in the bi-component measurements with hard excitation pulses (Figs 5A–5C). In the IR-UTE experiments, fS decreased with TI when measured either with hard or half excitation pulses (Figs. 5A&5D), and was consistently lower when measured with half pulse excitation than with hard pulse excitation at various TIs greater than WML TInull (Fig. 5A). In all measurements, T2S* was in the range of 150 – 250 µs (Figs. 5B&5E), and T2L* changed significantly with the increase in TI when hard pulse excitation was used (Figs. 5C&5F). These results demonstrated the efficiency of the half pulse UTE and IR-UTE sequences in measuring T2S* and detecting fS changes in the presence of different proportions of LTCs.
Figure 5.
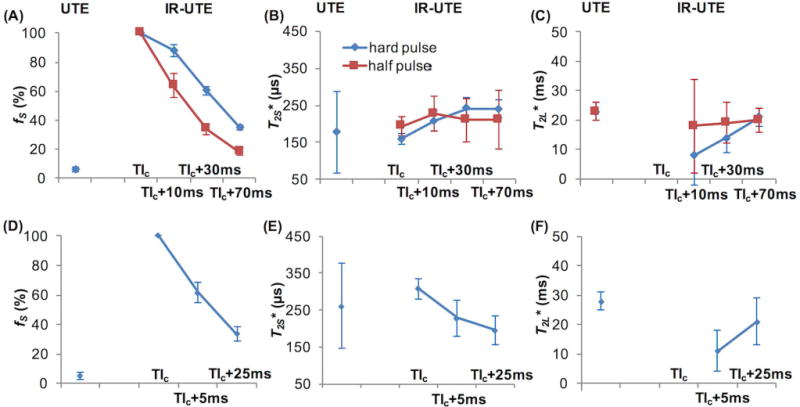
T2* fitting results from the ~8-mm thick hemisphere slab imaged using both hard and half excitation pulse sequences (A–C) and the average results from three pure white matter short blocks imaged using hard excitation pulse sequence (D–F). IR-UTE images were acquired with TI = WML TInull (labeled as TIc, equivalent to that in Fig. 1D, on the x-axes of the figures) and 5-70 ms longer. The ultrashort T2 component fraction (fS) was very low when measured with UTE (<10%), much higher when measured with IR-UTE at WML TInull, and decreased as TI increased from WML TInull. The UTE sequence used with half pulse excitation did not detect the ultrashort T2 component in this native specimen. fS was consistently lower when measured with half pulse excitation than with hard pulse excitation at various TIs greater than WML TInull (A&D). The ultrashort T2 component T2* (T2S*) values were all in the range of 150 – 250 µs (B&E), and the long T2 component T2* (T2L*) changed significantly with the increase in TI when hard pulse excitation was used (C&F). At WML TInull, the T2* signal followed a single component decay (R2 > 0.99) (F). These results demonstrated the efficiency of the half pulse UTE and IR-UTE sequences in measuring T2S* and detecting fS changes in the presence of different proportions of LTC signals.
Figure 6 shows T2S* and fS measured (using half pulse excitation) from three WM long segments that were subjected to 1-hr (No.1), 2-hr (No.2) and 27-hr (No.3) D2O exchanges, respectively. T2S* was similar across all specimens measured with either UTE sequences or IR-UTE sequences with two different TIs (85 ms apart). The UTE sequence detected an increase of fS from ~9.5% to ~47.1% in specimens No.1 to No.3 (Fig. 6A), consistent with progressive D2O exchange. The IR-UTE signals of the specimen after 27-hr D2O exchange (No.3) could be characterized using single component fitting at both the TIs that were used, suggesting fS was less dependent on TI as the D2O exchange progressed.
Figure 6.

T2* fitting curves of UTE and IR-UTE signals (all half excitation pulses) from three pure white matter long segments that were subject to 1-hr (left column, No.1), 2-hr (middle column, No.2) and 27-hr (right column, No.3) exchange with D2O, respectively. (A) The UTE sequence detected an increase of fS from ~9.5% to ~47.1% with D2O exchange time in specimens No.1 to No.3. (B) The IR-UTE images obtained at WML TInull showed single component T2* signal decay, which changed little with D2O exchange time. (C) The IR-UTE images obtained at 85 ms longer than WML TInull (labled as “WML TInull + 85 ms” in figure) showed bi-component T2* signal decays in specimens after 1-hr and 2-hr D2O exchange, and single component T2* signal decay in the specimen after 27-hr D2O exchange. fS, ultrashort T2 component fraction; T2S*, ultrashort T2 component T2*.
Table 1 shows T2S* and fS measured (using half pulse excitation) from three hemisphere slabs before and after 27-hr exchange with D2O. Before exchange with D2O, the UTE signals showed single component decay with a long T2* (~ 24 ms), and fS and T2S* values could not be obtained. Meanwhile, the IR-UTE signals could be characterized through single-component T2* fitting at TI = 295 ms (i.e., WML TInull), or through bi-component T2* fitting at TI = 365 ms with fS values of less than 20% in all specimens. After exchange with D2O, the UTE signals contained a fS of ~40% and the IR-UTE signals showed single-component decay at both TIs used (i.e., TIs = 250 ms and 380 ms). T2S* was in the range of 200-400 µs either before or after exchange in all specimens. These results further support that protons that were unexchangeable with D2O can be measured by UTE and IR-UTE sequences when used with half excitation pulses.
Table 1.
Comparison of UTE and IR-UTE results obtained with half excitation pulses in three cerebral hemisphere slabs before and after these specimens were subjecte to 27-hr 4-pass D2O exchange.
| Native | Deuterated | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| UTE | IR-UTE (TI = 295 ms) |
IR-UTE (TI = 365 ms) |
UTE |
IR-UTE (TI = 250 ms) |
IR-UTE (TI = 380 ms) |
|
| T2S* | ||||||
|
| ||||||
| No. 1 | n.a. | 339 ± 34 µs | 210 ± 69 µs | 239 ± 46 µs | 297 ± 21 µs | 229 ± 39 µs |
| No. 2 | n.a. | 246 ± 28 µs | 202 ± 22 µs | 241 ± 48 µs | 245 ± 10 µs | 262 ± 35 µs |
| No. 3 | n.a. | 215 ± 50 µs | 205 ± 64 µs | 215 ± 50 µs | 247 ± 21 µs | 262 ± 42 µs |
|
| ||||||
| fS | ||||||
|
| ||||||
| No. 1 | n.a. | ≈100%# | 18.0 ± 2.1 % | 42.4 ± 0.4 % | ≈100%# | ≈100%# |
| No. 2 | n.a. | ≈100%# | 17.5 ± 1.7 % | 43.1 ± 2.2 % | ≈100%# | ≈100%# |
| No. 3 | n.a. | ≈100%# | 16.0 ± 1.7 % | 39.1 ± 1.3 % | ≈100%# | ≈100%# |
n.a., signals on the UTE images showed single component decay with long T2* (22 ± 2 ms); #, signals on the IR-UTE images showed single component decay with oultrashort T2*.
DISCUSSION
The present study used a bi-component T2* fitting model as a simplified means to quantify fS and T2S* in WM. In UTE images obtained with hard pulse excitation, fS was found to be ~4-8% in native specimens, and up to 86% in deuterated specimens. In IR-UTE images obtained with either hard or half pulse excitation, fS was close to 100% at WML TInull and decreased significantly with increasing TI in native specimens, but not in deuterated specimens. T2S* was found to be 150 – 400 µs in both native and deuterated specimens imaged using either hard pulse or half pulse excitations, and was consistent with previously reported values in myelin extract and nerve tissue (14,22).
Results obtained with hard or half excitation pulses
This study first tested the UTE and IR-UTE sequences with hard excitation pulses in pure WM short blocks using a 5-mm diameter solenoid coil that produces high SNR. The results showed that the fS was strongly dependent on the choice of TI while T2S* was not. When TI was increased by 5 ms and then by 25 ms from WML TInull, fS decreased from ~100% to ~62% and then to ~34%, respectively. Then the sequences were used to image a native cerebral hemisphere slab using both hard and half excitation pulses. The UTE sequence failed to identify the WMS when used with half excitation pulses, and the IR-UTE sequence detected consistently lower fS values using half excitation pulses than hard excitation pulses over a range of TIs that were offset from WML TInull. These results are consistent with the expectation that the half excitation pulses had lower efficiency than hard excitation pulses in detecting STCs. As compared with the half excitation pules, the hard excitation pulses have shorter durations (32 µs vs. 472 µs), thus broader bandwidth (8.2 kHz vs. 2.7 kHz) and higher efficiency in exciting ultrashort T2 components (20,23,25). However, with TI set at WML TInull, T2S* could be measured through single component fitting of the multi-TE IR-UTE signals obtained using half excitation pulses, with the results similar to those obtained using hard excitation pulses. Furthermore, the IR-UTE sequences used with either hard or half excitation pulses were able to provide stable T2S* values and detect the differences in fS when varying TIs were used. These results support the view that it is possible to use the IR-UTE sequences with half excitation pulses to detect STCs using clinical whole-body MR scanners.
Comparison of T2S* and fS in native and deuterated specimens
This study included three D2O exchange experiments. First, two WM mini blocks were subjected to an identical 8-hr 5-pass D2O exchange procedure and imaged using hard excitation pulses. After exchange, the specimens showed a very narrow HDO peak in the FID spectrum at 11.7T, and an fS as high as ~86% on UTE images at 3T, versus ~7.9% in the native specimen. In the TE range of 10 µs to 4 ms, the UTE images of the deuterated specimen showed marked signal intensity decay with increasing TE, in contrast to the barely observable changes the native specimen. This experiment confirmed significant water signal loss in the deuterated specimen, and proved that UTE sequences used with hard excitation pulses are sensitive to proportional water/myelin proton content changes in biological tissue. Second, three pure WM long segments were subjected to different durations of D2O exchange and imaged using half excitation pulses. When the specimens were imaged using UTE sequences, fS was observed to increase from ~9.5% (1-hr, one-pass exchange) to ~47.1% (27-hr, four-pass exchange). When the specimens were imaged using IR-UTE sequences, fS was shown to be less affected by the choice of TI as the D2O exchange duration increased. This experiment demonstrated that UTE and IR-UTE sequences used with half excitation pulses are also sensitive to proportional water/myelin proton content changes in biological tissue. Lastly, three cerebral hemisphere slabs were imaged using half excitation pulses both before and after a 27-hr 4-pass D2O exchange. Results obtained from these specimens further confirmed an increase in fS after D2O exchange (fS = ~39-42% vs. unmeasurable) when measured using UTE sequences. In all three experiments, when the UTE or IR-UTE sequences were used with either hard or half excitation pulses, the T2S* values were consistently in the range of 150 - 400 µs both before and after exchange with D2O. Furthermore, these T2S* values were comparable with those obtained using the same protocols from myelin powder as well as a myelin paste in D2O (22). These results jointly suggest that the ultrashort T2* signals survived D2O exchange, and provide evidence to support that a) myelin, presumably the non-exchangeable methylene groups (14), is the major source of the ultrashort T2* signals seen in WM on IR-UTE images, and b) half pulse IR-UTE sequences are sensitive to myelin concentration changes and therefore could be used for direct quantification of myelin loss.
Because myelin has a tightly organized, closely packed, highly stable structure with a hydrophobic core (28), complete replacement of water protons in nervous tissue with deuterons may be difficult, and the efficiency may depend on the form and the type of tissue the myelin is in. For example, Horch et al. detected a loss of ~95% of the LTCs in a frog sciatic nerve (peripheral nerve), but only ~68% in a rat optic nerve (cranial nerve) after both nerves were incubated in D2O buffer for 2 hrs (14). This might be associated with the structural and chemical differences of myelin tissue in the two nerves (29). Even after a rat optic nerve was immersed in D2O buffer for 10 days, only a loss of ~82% of LTCs was observed (14). All the specimens used in the present study were much larger in size than the frog sciatic or rat optic nerves (~ 1 mm or smaller in diameter) used in the Horch et al.’s study (14). This might explain why our UTE experiments only detected an fS of ~86% (instead of ~100%) in a WM short block (small specimen) after 8-hr, 5-pass D2O exchange, and ~39%-47% in the larger specimens (one WM long segment and three cerebral hemisphere slabs) after 27-hr, 4-pass D2O exchange. The relatively lower fS in the larger specimens relative to that in the small specimen might be due to difference in the efficiency of D2O exchange, the lower efficiency of half pulse excitation relative to hard pulse excitation, and the lower SNR obtained with the 76-mm surface coil compared to the 5-mm solenoid coil.
Comparison of magnetization transfer (MT) and IR-UTE measurements of myelin tissue
The restricted proton pool (STCs) in white matter include not only protons in myelin lipids but also those in proteins and other semi-solid membrane components. According to literature (13,14) and our own results, the ultrashort T2* signals (T2*, ~ 300 µs) primarily arise from protons in methyl (–CH3) and methylene (–CH2) groups of phospholipids in myelin tissue. If the observed short T2* signals were mainly from proteins, they would not survive D2O exchange because protons in amino (-NH2) and carboxyl (-COOH) groups in the majority of proteins could be exchanged with deuterons in D2O. Protons in proteolipid proteins (PLPs), which are highly hydrophobic, may survive D2O exchange, but the PLPs to lipid ratio in brain myelin is about 1:9 (30), so the contribution from these protons, if any, would be very small. MT is another MR contrast mechanism that has been used to probe the restricted proton pool, by measuring the magnetization exchange between the restricted proton pool and the free proton pool (10,31,32). Carboxyl (-COOH) groups on cholesterol and galactocerebrosides are thought to be the major interaction sites between the restricted pool and the free pool for magnetization exchange (33–35). Therefore, MT contrast and the measured contrast in this study provide different aspects of information of the restricted proton pool. Furthermore, MT ratio (MTR) derived from MT imaging is an indirect measure, while the IR-UTE sequence provides a direct measure of ultrashort T2 components. Therefore, we believe the measured contrast is more specific to myelin lipid protons than MTR.
Limitations
The WML TInull was estimated in this study using the same IR-UTE sequence as that used for T2S* measurement, with the intention of eliminating sequence dependent T1 measurement inaccuracy (36). However, the T1 signals of LTCs were modeled using a single-component fitting model and the T2* signals were fitted using the magnitude images for simplicity. It has been reported that there are two or more T1 relaxation pools in brain tissue that are associated with distinctive T2 relaxation rates (37,38) and these might have different T1s. Therefore, our approach might induce insufficient nulling of LTC signals, as suggested by the observation that the value of fS was not 100% in native WM in IR-UTE experiments at WML TInull (Fig. 2E). Fitting the signal in complex images would provide more accurate estimation of fS. Besides, more advanced inversion recovery UTE sequences are currently under investigation by our team (39) and another group (40) to simultaneously suppress long T2 water signals with a broad range of T1s. Second, our fS values were relatively small compared with the known myelin tissue concentration in the wet mass of WM (41,42). This might be due to insufficient nulling of LTCs. In addition, one previous NMR spectroscopy experiment at 9.4T revealed multiple T2* components in purified bovine myelin extract (suspended in D2O), with 26.4% of the total signal having an effective lifetime of < 25 µs, 51.8% of < 0.1 ms, and 91.6% of < 1 ms (at 20 °C) (21). The super-short T2 components (T2, 0.01–0.1 ms) are probably not accessible by our UTE/IR-UTE imaging experiments at 3T. Third, a simplified bi-component model was used in this study to fit the T2* signals in the magnitude IR-UTE images. The T2L* has limited biophysical meaning, because it is associated with multiple water pools. Multi-component models might be desirable in order to fully capture the tissue relaxation properties. However, such analysis can be very challenging and is prone to errors; it needs further investigation. Fourth, plain D2O was used in this study to replace H2O in the specimens. Ideally D2O buffer should be used to mimic physiological osmotic pressure and pH effects. Nonetheless, the T2S* values before and after D2O exchange were relatively consistent in our study, suggesting that the T2S* was not significantly affected by incubation in plain D2O, possibly because the methylene groups, which are known to form hydrophobic nonpolar hydrocarbon chains (28), were not interchangeable with or inaccessible by D2O (14). Fifth, cross-relaxation between macromolecules and water was not considered in this study. Such cross-relaxation can produce apparent shortening of T1 and T2 (31,43), but its effects on ultrashort T2* values are not fully understand. However, it is unlikely to have significantly affected the ultrashort T2* signals measured in this study. In this study, the WML TInull was directly determined via fitting the IR-UTE images obtained with different TIs. Cross-relaxation induced T1-shortening would only result in a shorter estimated WML TInull. Meanwhile, the ultrashort T2* values were largely consistent in specimens before and after different durations of D2O exchange, further suggesting our measurements were unlikely significantly contaminated by cross-relaxation between the restricted proton pool and the free water pool. This might be further confirmed through comparing the T2* fitting results obtained with UTE experiments with or without magnetization transfer preparation. Lastly, the 2D IR-UTE sequences typically employ slice-selective half excitation pulses for in vivo studies. Eddy currents associated with the slice-selective gradients may lead to out-of-slice signal contamination (20,44), thus affecting T2* analysis. This error can be reduced by measuring the slice-selective gradients, adding pre-compensation gradients, and/or using the measured readout gradients for re-gridding (45). In this study we used non-selective gradients to minimize eddy currents and speed-up data acquisition. The use of non-selective pulses is valid as the focus of this paper is to further demonstrate that myelin protons are directly detectable with 2D IR-UTE sequences on a clinical MR scanner. Further work is needed to increase the robustness of this sequence by using advanced gradient calibration and image reconstruction techniques.
CONCLUSION
The fS values were significantly affected by the TIs used in IR-UTE sequences with either hard or half excitation pulses in native specimens, but not in heavily deuterated specimens. T2S* was in the range of 150-400 µs in all measurements made with either hard or half pulse excitation UTE and IR-UTE sequences. The major source of the ultrashort T2* signals seen in WM on IR-UTE images is likely to be myelin protons, presumably methylene groups. Half pulse IR-UTE sequences have the potential for direct qualitative and quantitative myelin imaging.
Supplementary Material
Supporting Figure S1 Spectra from two WM short blocks examined either in the native condition or after 27-hr immersion in D2O. (A) Spectrum from one WM short block examined in the native condition. The integrated area under the water peak: the total area = 1:1.13, suggesting a ~88.5% contribution from water to the total signal. (B) Spectrum from one WM short block examined after 27-hr immersion in D2O (four changes of D2O with intervals of 1-hr, 1-hr, 2-hr and 23-hr). The integrated area under the water peak: the total area = 1:4.07, suggesting a ~24.6% contribution from water to the total signal.
Supporting Figure S2 Illustration of the procedure for defining the region of interest (ROI) in white matter for myelin proton T2* quantification in cerebral hemisphere slabs. The images were from the ~8-mm thick cerebral hemisphere slab. Yellow boxes show the final ROI. (A-H) Eight consecutive T2-weighted fast spin echo (FSE) images (TR/TE = 3000 ms/40 ms, slice thickness = 1 mm, and no slice gap). (I) The maximum intensity projection (MIP) of (A) to (H). (J) The IR-UTE image (TR/TI/TE= 1000/300/2.2 ms, half pulse excitation, slice-selective gradient turned off) showing similar contrast between white matter (low signal) and grey matter (high signal) as (I). The ROI was first manually drawn on the IR-UTE image (J), copied to the multi-slice T2-weighted FSE images (A-H) and then refined to exclude any GM contamination.
Acknowledgments
The authors acknowledge grant support from National Institute of Neurological Disorders and Stroke, NIH (1R01 NS092650).
References
- 1.Laule C, Vavasour IM, Kolind SH, Li DK, Traboulsee TL, Moore GR, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4(3):460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp do PM, Zakszewski E, Field AS. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1(6):423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, Chowdhury D, Cvoro V, Dennis MS. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008;39(4):1327–1332. doi: 10.1161/STROKEAHA.107.500124. [DOI] [PubMed] [Google Scholar]
- 5.Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer’s disease: a case-control MRI study. Psychiatry Res. 2009;171(3):232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 7.Cao P, Hyder F, Zhou IY, Zhang JW, Xie VB, Tsang A, Wu EX. Simultaneous spin-echo and gradient-echo BOLD measurements by dynamic MRS. NMR Biomed. 2017;30(9) doi: 10.1002/nbm.3745. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D. Diffusion, confusion and functional MRI. Neuroimage. 2012;62(2):1131–1136. doi: 10.1016/j.neuroimage.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Mollink J, Kleinnijenhuis M, Cappellen van Walsum AV, Sotiropoulos SN, Cottaar M, Mirfin C, Heinrich MP, Jenkinson M, Pallebage-Gamarallage M, Ansorge O, Jbabdi S, Miller KL. Evaluating fibre orientation dispersion in white matter: Comparison of diffusion MRI, histology and polarized light imaging. Neuroimage. 2017;157:561–574. doi: 10.1016/j.neuroimage.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naumova AV, Akulov AE, Khodanovich MY, Yarnykh VL. High-resolution three-dimensional macromolecular proton fraction mapping for quantitative neuroanatomical imaging of the rodent brain in ultra-high magnetic fields. Neuroimage. 2017;147:985–993. doi: 10.1016/j.neuroimage.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TD, Spincemaille P, Gauthier SA, Wang Y. Rapid whole brain myelin water content mapping without an external water standard at 1.5T. Magn Reson Imaging. 2017;39:82–88. doi: 10.1016/j.mri.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Ortiz E, Levesque IR, Pike GB. Multi-gradient-echo myelin water fraction imaging: Comparison to the multi-echo-spin-echo technique. Magn Reson Med. 2017 doi: 10.1002/mrm.26809. [DOI] [PubMed] [Google Scholar]
- 13.Ramani A, Aliev AE, Barker GJ, Tofts PS. Another approach to protons with constricted mobility in white matter: pilot studies using wideline and high-resolution NMR spectroscopy. Magn Reson Imaging. 2003;21(9):1039–1043. doi: 10.1016/s0730-725x(03)00207-8. [DOI] [PubMed] [Google Scholar]
- 14.Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med. 2011;66(1):24–31. doi: 10.1002/mrm.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews PM, Roncaroli F, Waldman A, Sormani MP, De Stefano N, Giovannoni G, Reynolds R. A practical review of the neuropathology and neuroimaging of multiple sclerosis. Pract Neurol. 2016;16(4):279–287. doi: 10.1136/practneurol-2016-001381. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, Cross AH, Song SK. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138(Pt 5):1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27(6):825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Waldman A, Rees JH, Brock CS, Robson MD, Gatehouse PD, Bydder GM. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology. 2003;45(12):887–892. doi: 10.1007/s00234-003-1076-z. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage. 2014;87:32–41. doi: 10.1016/j.neuroimage.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson PE, Gurney PT, Nayak K, Gold GE, Pauly JM, Nishimura DG. Designing long-T2 suppression pulses for ultrashort echo time imaging. Magn Reson Med. 2006;56(1):94–103. doi: 10.1002/mrm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A. 2012;109(24):9605–9610. doi: 10.1073/pnas.1115107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheth V, Shao H, Chen J, Vandenberg S, Corey-Bloom J, Bydder GM, Du J. Magnetic resonance imaging of myelin using ultrashort Echo time (UTE) pulse sequences: Phantom, specimen, volunteer and multiple sclerosis patient studies. Neuroimage. 2016;136:37–44. doi: 10.1016/j.neuroimage.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson PE, Conolly SM, Pauly JM, Nishimura DG. Using adiabatic inversion pulses for long-T2 suppression in ultrashort echo time (UTE) imaging. Magn Reson Med. 2007;58(5):952–961. doi: 10.1002/mrm.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller S, Seelig J. Invivo Nmr Imaging of Deuterium. Journal of Magnetic Resonance. 1987;72(3):456–466. [Google Scholar]
- 25.Carl M, Bydder M, Du J, Takahashi A, Han E. Optimization of RF excitation to maximize signal and T2 contrast of tissues with rapid transverse relaxation. Magn Reson Med. 2010;64(2):481–490. doi: 10.1002/mrm.22433. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Ma L, Chang EY, Shao H, Chen J, Chung CB, Bydder GM, Du J. Effects of inversion time on inversion recovery prepared ultrashort echo time (IR-UTE) imaging of bound and pore water in cortical bone. NMR Biomed. 2015;28(1):70–78. doi: 10.1002/nbm.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan SJ, Ma Y, Chang EY, Bydder GM, Du J. Inversion recovery ultrashort echo time imaging of ultrashort T2 tissue components in ovine brain at 3 T: a sequential D2 O exchange study. NMR Biomed. 2017 doi: 10.1002/nbm.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien JS. Stability of the Myelin Membrane. Science. 1965;147(3662):1099–1107. doi: 10.1126/science.147.3662.1099. [DOI] [PubMed] [Google Scholar]
- 29.Finean JB, Hawthorne JN, Patterson JD. Structural and chemical differences between optic and sciatic nerve myelins. J Neurochem. 1957;1(3):256–259. doi: 10.1111/j.1471-4159.1957.tb12080.x. [DOI] [PubMed] [Google Scholar]
- 30.Kalwy SA, Smith R. Mechanisms of myelin basic protein and proteolipid protein targeting in oligodendrocytes (review) Mol Membr Biol. 1994;11(2):67–78. doi: 10.3109/09687689409162223. [DOI] [PubMed] [Google Scholar]
- 31.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 32.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14(2):57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 33.Ceckler TL, Wolff SD, Yip V, Simon SA, Balaban RS. Dynamic and Chemical Factors Affecting Water Proton Relaxation by Macromolecules. Journal of Magnetic Resonance. 1992;98(3):637–645. [Google Scholar]
- 34.Koenig SH, Brown RD, Spiller M, Lundbom N. Relaxometry of Brain - Why White Matter Appears Bright in Mri. Magnet Reson Med. 1990;14(3):482–495. doi: 10.1002/mrm.1910140306. [DOI] [PubMed] [Google Scholar]
- 35.Kucharczyk W, Macdonald PM, Stanisz GJ, Henkelman RM. Relaxivity and Magnetization-Transfer of White-Matter Lipids at Mr-Imaging - Importance of Cerebrosides and Ph. Radiology. 1994;192(2):521–529. doi: 10.1148/radiology.192.2.8029426. [DOI] [PubMed] [Google Scholar]
- 36.Stikov N, Boudreau M, Levesque IR, Tardif CL, Barral JK, Pike GB. On the accuracy of T1 mapping: searching for common ground. Magn Reson Med. 2015;73(2):514–522. doi: 10.1002/mrm.25135. [DOI] [PubMed] [Google Scholar]
- 37.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- 38.Does MD, Gore JC. Compartmental study of T(1) and T(2) in rat brain and trigeminal nerve in vivo. Magn Reson Med. 2002;47(2):274–283. doi: 10.1002/mrm.10060. [DOI] [PubMed] [Google Scholar]
- 39.Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn Reson Med. 2017 doi: 10.1002/mrm.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harkins KUS, Nyman J, Does M. Robust pore water suppression in cortical bone with multiple adiabatic inversion recovery; Proceedings of the 25th Annual Meeting of ISMRM; Honolulu, Hawaii, USA. 2017. p. 1588. [Google Scholar]
- 41.Malone MJ, Szoke MC. Neurochemical changes in white matter. Aged human brain and Alzheimer’s disease. Arch Neurol. 1985;42(11):1063–1066. doi: 10.1001/archneur.1985.04060100045019. [DOI] [PubMed] [Google Scholar]
- 42.van der Knaap M, Valk J. Myelin and White Matter. In: Heilmann U, Mennecke-Buhler D, editors. Magnetic Resonance of Myelination and Myelin Disorders. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005. pp. 1–19. [Google Scholar]
- 43.Gochberg DF, Gore JC. Quantitative imaging of magnetization transfer using an inversion recovery sequence. Magn Reson Med. 2003;49(3):501–505. doi: 10.1002/mrm.10386. [DOI] [PubMed] [Google Scholar]
- 44.Wansapura JP, Daniel BL, Pauly J, Butts K. Temperature mapping of frozen tissue using eddy current compensated half excitation RF pulses. Magn Reson Med. 2001;46(5):985–992. doi: 10.1002/mrm.1285. [DOI] [PubMed] [Google Scholar]
- 45.Lu A, Daniel BL, Pauly JM, Pauly KB. Improved slice selection for R2* mapping during cryoablation with eddy current compensation. J Magn Reson Imaging. 2008;28(1):190–198. doi: 10.1002/jmri.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1 Spectra from two WM short blocks examined either in the native condition or after 27-hr immersion in D2O. (A) Spectrum from one WM short block examined in the native condition. The integrated area under the water peak: the total area = 1:1.13, suggesting a ~88.5% contribution from water to the total signal. (B) Spectrum from one WM short block examined after 27-hr immersion in D2O (four changes of D2O with intervals of 1-hr, 1-hr, 2-hr and 23-hr). The integrated area under the water peak: the total area = 1:4.07, suggesting a ~24.6% contribution from water to the total signal.
Supporting Figure S2 Illustration of the procedure for defining the region of interest (ROI) in white matter for myelin proton T2* quantification in cerebral hemisphere slabs. The images were from the ~8-mm thick cerebral hemisphere slab. Yellow boxes show the final ROI. (A-H) Eight consecutive T2-weighted fast spin echo (FSE) images (TR/TE = 3000 ms/40 ms, slice thickness = 1 mm, and no slice gap). (I) The maximum intensity projection (MIP) of (A) to (H). (J) The IR-UTE image (TR/TI/TE= 1000/300/2.2 ms, half pulse excitation, slice-selective gradient turned off) showing similar contrast between white matter (low signal) and grey matter (high signal) as (I). The ROI was first manually drawn on the IR-UTE image (J), copied to the multi-slice T2-weighted FSE images (A-H) and then refined to exclude any GM contamination.


