SUMMARY
Mycobacterial cell wall lipids bind the conserved CD1 family of antigen-presenting molecules and activate T-cells via their T-cell receptors (TCRs). Sulfoglycolipids (SGLs) are uniquely synthesized by Mycobacterium tuberculosis, but tools to study SGL-specific T-cells in humans are lacking. We designed a novel hybrid synthesis of a naturally occurring SGL, generated CD1b tetramers loaded with natural or synthetic SGL analogs, and studied the molecular requirements for TCR binding and T-cell activation. Two T-cell lines derived using natural SGLs are activated by synthetic analogs independently of lipid chain length and hydroxylation but differentially by saturation status. By contrast, two T-cell lines derived using an unsaturated SGL synthetic analog were not activated by the natural antigen. Our data provide a bioequivalence hierarchy of synthetic SGL analogs and SGL-loaded CD1b tetramers. These reagents can now be applied to large-scale translational studies investigating the diagnostic potential of SGL-specific T-cell responses or SGL-based vaccines.
Keywords: Human, T-cells, Mycobacteria, Tuberculosis, Antigen-presentation, CD1, T-cell receptor, Lipid
eTOC BLURB
Sulfoglycolipids (SGLs) are uniquely synthesized by Mycobacterium tuberculosis (M.tb) and recognized by human T-cells. James, Yu et al. describe a new hybrid synthesis for key antigenic determinants of SGLs and the development of SGL-specific tetramers that can now be applied to large-scale translational studies.
INTRODUCTION
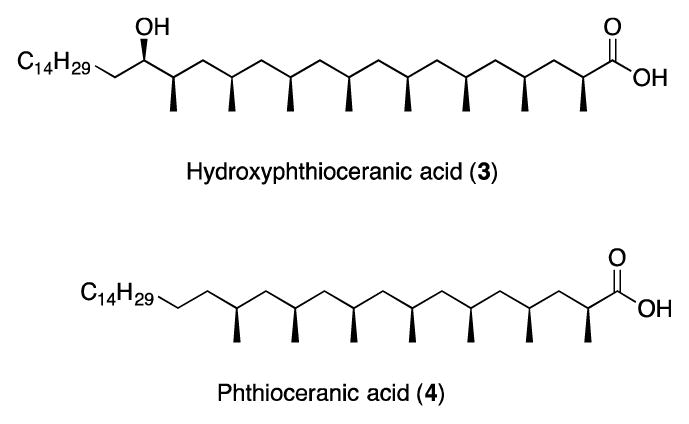
Mycobacterium tuberculosis (M.tb) caused over nine million infections and 1.4 million deaths worldwide in 2015, making it the leading infectious cause of mortality (WHO, 2016). The waxy cell wall of M.tb contains many unique lipids that are recognized by human T-cells when bound to conserved CD1 molecules at the surface of antigen-presenting cells (Beckman et al., 1994). Sulfoglycolipids (SGLs) are a family of cell wall lipids found only in M.tb that consist of a sulfotrehalose core with up to four acyl chains that differ in length and composition (Goren, 1970). Among the acylations there is typically a phthioceranic or hydroxyphthioceranic acid with multiple methyl groups (Goren et al., 1971; Layre, Paepe, et al., 2011). SGLs were first described in virulent M.tb, and SGL synthesis is regulated by PhoP, a transcription factor expressed by M.tb that has a well-established role as a virulence factor (Goren, 1970; Chesne-Seck et al., 2008; Lee et al., 2008). Later studies allowed identification and characterization of a family of sulfoglycolipids (acylated trehalose 2′-sulphate) that were detectable only in M.tb and their amounts correlated with the relative virulence in guinea pigs (Goren, Brokl and Schaefer, 1974). Presence of SGL in virulent bacteria, but not in environmental strains or vaccine strains, is also supported by lipidomic profiling of the cell wall of M.tb and the M. bovis BCG vaccine strain (Layre, Sweet, et al., 2011). The preferential expression of SGLs by pathogenic M.tb strains suggests that SGL-specific T-cells could be harnessed to develop novel lipid-based strategies for specifically diagnosing or treating tuberculosis. For such clinical applications, the availability of contaminant-free, chemically well-defined antigens is crucial (Gilleron et al., 2004).
Diacylated SGL (Ac2SGL) is one of only eight cell wall lipids from M.tb that have been identified as CD1-presented antigens for human T-cells (Beckman et al., 1994; Sieling et al., 1995; Moody et al., 1997; Gilleron et al., 2004; Layre et al., 2009). Proof-of-principle that Ac2SGL is presented by CD1b to T-cells was obtained using two in vitro derived T-cell clones named Z4B27 and Z4A26 derived from a healthy donor with latent M.tb infection (Gilleron et al., 2004). In addition, recall responses to Ac2SGL by primary lymphocytes isolated from patients depends on prior exposure to M.tb but is not affected by a history of vaccination with BCG (Gilleron et al., 2004). This is in contrast to the tuberculin skin test (TST), which is commonly used to diagnose M.tb infection in humans but is limited by false positive responses in BCG-vaccinated populations (Farhat et al., 2006). Further translational work on Ac2SGL-specific T-cells has been hampered for two main reasons. First, Ac2SGLs exist at low abundance in the M.tb cell wall, so purifying a sufficient quantity to perform a large-scale clinical study is economically and logistically prohibitive. Characteristic markers for SGL-specific T-cells other than an antigen-specific T-cell receptor (TCR) are not known, so there are no tools to facilitate their identification in ex vivo blood or tissue samples.
Synthetic analogs of Ac2SGL have been reported previously, and their biological potency compared to the natural compounds has been assessed using Z4B27 and Z4A26 T-cell clones (Guiard et al., 2008, 2009; Gau et al., 2013). These data have revealed that the presence of a hydroxyl group, the number of C-methyl substituents on the acyl chains, the configuration of the chiral centers, and the respective localization of the two different acyl chains on the sugar moiety govern TCR recognition and T-cell activation (Guiard et al., 2009). Comparing C16, C24, and C32 long chain tri-methylated fatty acids revealed increased stimulatory capacity of analogs with the longer alkyl chain lengths (Gau et al., 2013). These findings were supported by a crystal structure which showed that part of the Ac2SGL lipid tail is exposed to the solvent above the binding plane, explaining why the methyl groups on the tail of the lipid are important for antigenicity (Garcia-Alles et al., 2011).
We designed a novel hybrid synthesis of a naturally occurring Ac2SGL by combining an iterative conjugated addition protocol and a lithiation/borylation protocol. This method substantially increases the yield of hydroxyphthioceranic acid, a key antigenic determinant of Ac2SGL. An important tool to study antigen-specific polyclonal T-cells directly ex vivo is by a fluorescent probe, called a tetramer, which binds selectively to antigen-specific T-cells within a mixed population. Here, we generated CD1b tetramers loaded with natural and two synthetic versions of Ac2SGL antigens and used these reagents to study human Ac2SGL-specific T-cells. Our data reveal the fine specificity of human T-cells for Ac2SGL and demonstrate bio-equivalence of one new synthetic analog and natural Ac2SGL, which will permit extensive translational studies of Ac2SGL-specific T-cells.
RESULTS
Natural and Synthetic Ac2SGL Antigens
Ac2SGL purified from M.tb (referred to as natural Ac2SGL) is a family of molecules containing a sulfotrehalose core, which is diacylated with a phthioceranoyl or a hydroxyphthioceranoyl group at the 3 position and a palmitoyl or stearoyl group at the 2 position (Goren et al., 1971; Layre, Paepe, et al., 2011). The hydroxyphthioceranoyl group varies in alkyl chain length (C22-C42) and in the number of methyl groups, ranging from 2 to 11 (Layre, Paepe, et al., 2011). The combined effect of positional, chain length, and methylation variants leads to thousands of known molecular SGL variants cataloged in the MycoMass database and other sources (Layre, Sweet, et al., 2011; Sartain et al., 2011). Four different analogs differing only in the structure of the alkyl chain in position 3 of the sulfotrehalose core were used in this study (Fig 1A). The first molecule has a sulfotrehalose core with a palmitoyl and a tetramethylated unsaturated fatty acid (compound 21c of reference (Gau et al., 2013)), which we will refer to as SL37 Ac2SGL (Fig. 1A). Two additional compounds, SL27 (SGL12 of reference (Guiard et al., 2009)) and SL29 Ac2SGL (SGL8 of reference (Guiard et al., 2009)), have the same sulfotrehalose core with a palmitic acid linked as in SL37 Ac2SGL, but the tetramethylated fatty acid is eight carbons shorter. SL27 Ac2SGL is further distinguished from SL29 due to the unsaturation on the first methyl group in the chain (Fig 1A). The fourth molecule, called AM Ac2SGL exactly mimics the structure of one of the compounds in the natural Ac2SGL mixture, and is reported here.
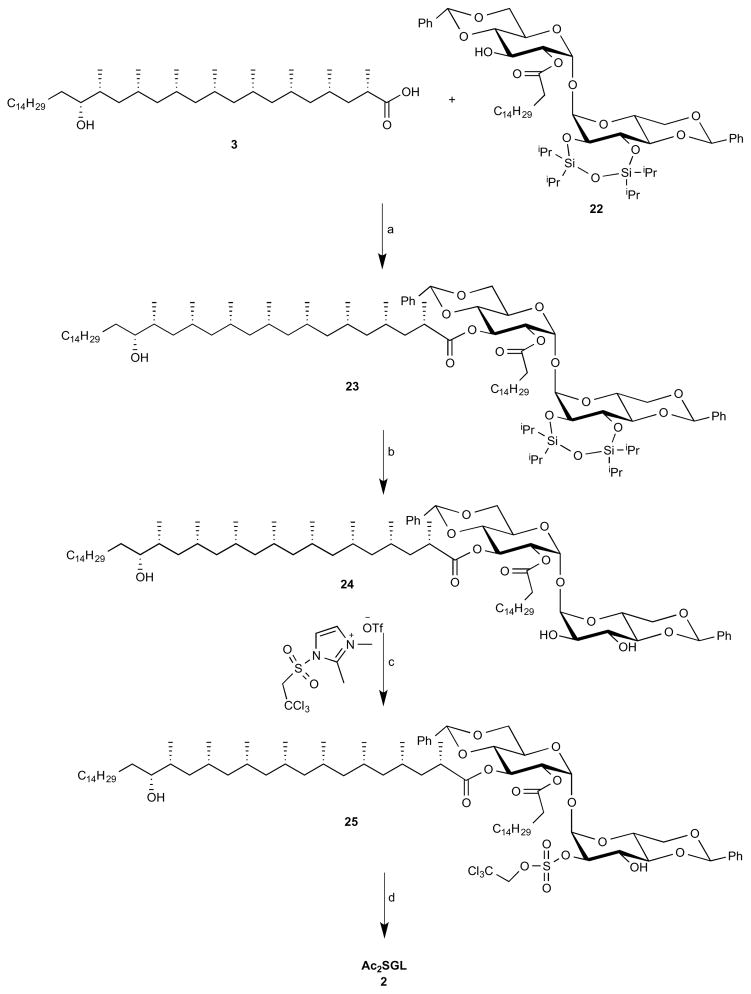
Figure 1. Diacylated sulfoglycolipid (Ac2SGL) antigens.
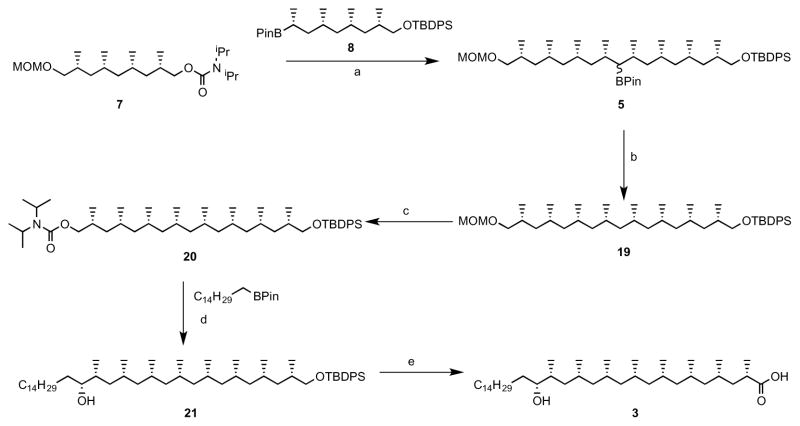
(A) Structures of natural Ac2SGL purified from M.tb and three synthetic forms that have been previously described: SL37 Ac2SGL, SL27 Ac2SGL, SL29 Ac2SGL. Synthesis of a fourth analog (AM Ac2SGL) is reported here. These compounds are used to probe the specificity of T-cell responses to Ac2SGL antigens. (B) Hybrid synthesis method for hydroxyphthioceranic acid consisting of an iterative conjugated addition and lithiation/borylation. TBDPS = tert-butyldiphenylsilyl, MOM = methoxymethyl, Pin = pinacolato. (C) Regioselective addition of hydroxyphthioceranoic acid to the trehalose core followed by 2′-O-sulfation and deprotection to yield AM Ac2SGL. Validation of the compounds by mass and NMR spectrometry is shown in the STAR Methods.
The main challenge in synthesizing a compound that is chemically identical to the natural species is the stereoselective preparation of hydroxyphthioceranic acid. Geerdink et al. previously published a first total synthesis of hydroxyphthioceranic acid containing tetra-acylated SGL by applying copper-catalyzed asymmetric conjugate addition and allylic substitution as the key steps (Geerdink and Minnaard, 2014). Soon thereafter the groups of Schneider and Aggarwal reported syntheses of hydroxyphthioceranic acid based on asymmetric hydrogenation and a lithiation/borylation/protodeborylation strategy, respectively (Pischl et al., 2013; Rasappan and Aggarwal, 2014). Still, these approaches comprise a large number of steps. We designed a novel hybrid synthesis of hydroxyphthioceranic acid by combining the efficient asymmetric Cu-catalyzed iterative conjugated addition protocol with the lithiation/borylation strategy in the later stage of synthesis (Fig. 1B). This strategy was effective and led to a higher yield and more efficient synthesis of hydroxypthioceranic acid. To subsequently assemble a fully synthetic Ac2SGL that recapitulates a natural compound, hydroxyphthioceranic acid was regioselectively esterified to the trehalose core using Yamaguchi conditions (Fig. 1C). The existing route could be improved, as it turned out that protection of the hydroxyl group in hydroxyphthioceranic acid was not required for the esterification to trehalose (Geerdink and Minnaard, 2014). Regioselective 2′-O-sulfation was carried out, followed by removal of the benzylidene acetals and TCE group using a combination of Pd(OH)2-/C and Pd-/C leading to the final compound, termed AM Ac2SGL. The structures of the compounds were confirmed by NMR and mass spectrometry (STAR Methods). Thus, we generated pure reagents in high yield that would allow us to probe the structural features required for antigen recognition by T-cells.
Generation of Ac2SGL -specific tetramers and T-cell lines
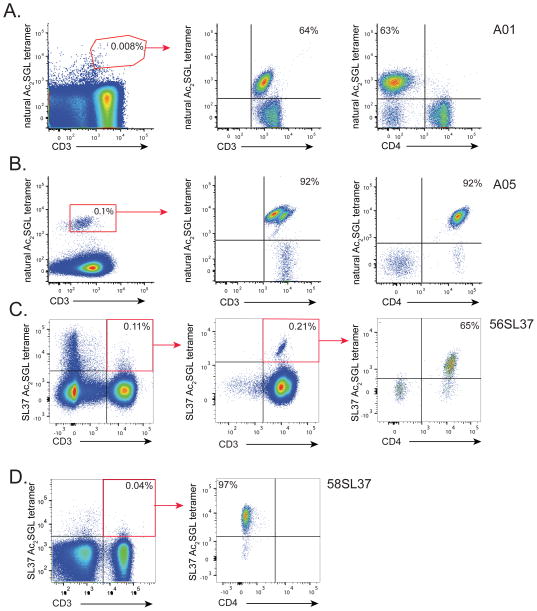
We next used natural and synthetic Ac2SGL preparations to generate CD1b-Ac2SGL tetramers that would enable us to sort and expand T-cells specific for these lipid antigens. CD1b-Ac2SGL tetramers are multimers of CD1 proteins that are fluorescently labeled and loaded with Ac2SGL to take advantage of high avidity to bind polyclonal T-cells even when present in low numbers of total blood cells. This powerful technique can be used to detect antigen specific T-cells in patients, isolate T-cell clones of interest, or investigate T-cell fine specificity for structurally related antigens. Based on a long term goal of detecting SGL-specific T-cells in large cohorts of tuberculosis patients, we first generated CD1b tetramers loaded with natural Ac2SGL by adapting our recently published method for generating human CD1b tetramers (Kasmar et al., 2011). Subsequently, peripheral blood mononuclear cells (PBMC) from a subject with latent tuberculosis were stained with the tetramer along with markers to label T-cells and cell viability (Figure 2A, left). Even though we did not yet confirm that the tetramers were sufficiently loaded, we detected a rare population (0.008%) of T-cells that stained with CD1b-natural Ac2SGL tetramer. These T-cells were sorted and expanded in vitro. We named the resulting T-cell line A01 and noted that 64% of A01 T-cells stained with CD1b-natural Ac2SGL tetramer (Figure 2A, center). These results suggest stable loading of CD1b with natural Ac2SGL. We used an antibody against the CD4 co-receptor to discover that the majority of Ac2SGL-specific T-cells within the A01 T-cell line did not express CD4 (Figure 2A, right). These results are in contrast to mycobacterial glucose monomycolate-specific T-cells, which commonly express CD4 (Kasmar et al., 2011; Van Rhijn et al., 2013). We generated another T-cell line (A05) specific for natural Ac2SGL by first stimulating PBMC with the antigen in the presence of monocyte-derived dendritic cells and subsequently sorting using CD1b-natural Ac2SGL tetramer (Figure 2B, left). We noted that 92% of A05 T-cells stained with CD1b-natural Ac2SGL tetramer (Figure 2B, center) and predominantly express the CD4 co-receptor (Figure 2B, right). Further, we generated SL37 Ac2SGL-loaded CD1b tetramers and isolated SL37 Ac2SGL-specific T-cells from two additional subjects with latent tuberculosis to create two additional T-cell lines, named 56SL37 and 58SL37, one from each subject. We noted that these two T-cell lines differed with respect to the expression of the CD4 co-receptor (Figures 2C and 2D). These results validate CD1b tetramers loaded with natural Ac2SGL or SL37 Ac2SGL and describe four new SGL-specific T-cell lines.
Figure 2. Generation of SGL-specific T-cell clones.
T-cell lines were generated from peripheral blood mononuclear cells (PBMC) by sorting rare T-cells that bound to Ac2SGL-loaded tetramers followed by in vitro expansion. Specificity of the resulting T-cell lines was confirmed by staining with the same tetramer used in the sort and reveal greater than 100-fold enrichment of antigen-specific T-cells. CD4 co-receptor expression was also examined using a specific antibody. (A) A01 T-cell line lacks CD4 expression and was created after two rounds of in vitro expansion after sorting with natural Ac2SGL-loaded tetramers (red polygon). (B) A05 T-cell line expresses CD4 and was generated by first stimulating PBMC with natural Ac2SGL in the presence of monocyte-derived dendritic cells and sorting with natural Ac2SGL tetramer following in vitro expansion (red box) (C) 56SL37 T-cell line expresses CD4 and was created after multiple round of in vitro expansion and re-sorting using SL37 Ac2SGL-loaded tetramers (red box). (D) 58SL37 T-cell line generated in a manner similar to 56SL37 but lacks CD4 expression. Sorting data are representative of a single experiment, but tetramer staining of T-cell lines was confirmed in two or more experiments.
T-cell specificity for natural and synthetic Ac2SGL analogs
We then explored the reactivity of the newly derived T-cell lines to natural and synthetic SGLs. We noted that natural Ac2SGL and AM Ac2SGL stimulated A01 with half-maximal effective concentration (EC50) of 0.04 μg/ml and 0.006 μg/ml respectively in an IFN-γ ELISPOT assay (Figure. 3A). Similarly, AM Ac2SGL is a potent antigen for A05, with an estimated EC50 value of 0.0006 μg/mL (Figure 3A). However, SL37 Ac2SGL was a much less potent antigen for both A01 and A05, with an average EC50 of 0.11 μg/ml. In addition, SL27 Ac2SGL, which has a shorter methylated carbon chain than SL37 Ac2SGL, had an average EC50 of 0.01 μg/ml, while SL29 Ac2SGL, which lacks the unsaturation in methylated carbon chain, has an EC50 of 0.009 μg/ml, similar to that of AM Ac2SGL (Figure 3B). Collectively, the low EC50 estimates using AM Ac2SGL and SL29 Ac2SGL to activate both the A01 and A05 T cell lines demonstrate the importance of using fully saturated analogs similar to that present in natural Ac2SGL to induce strong activation of the T-cells. Further, these data suggest that the extra methylations and hydroxyl group present in AM Ac2SGL and lacking in SL29 Ac2SGL are not required to reproduce the activity of the native mixture (Figure 1A).
Figure 3. Fine specificity of SGL-specific T-cell lines.
(A) IFN-γ production by A01 and A05 in response to titrating amounts of natural Ac2SGL, AM Ac2SGL, and SL37 Ac2SGL as measured by an IFN-γ ELISPOT. (B) IFN-γ production by A01 and A05 in response to titrating amounts of AM Ac2SGL, SL29 Ac2SGL, and SL27 Ac2SGL as measured by an IFN-γ ELISPOT. (C) IFN-γ production by C56SL37 in response to 5 μg/ml SL37 Ac2SGL, AM Ac2SGL, or whole mycobacterial lipid extract. T-cell clone activation was blocked using the anti-CD1b antibody BCD1b.3 (10 μg/ml). (D) A01(left) and A05 (right) was stained with mock loaded CD1b tetramer (shaded histogram) or CD1b loaded with either natural or AM Ac2SGL (open histograms). Data are representative of two or three independent experiments. Error bars represent standard deviation of triplicate wells in an ELISPOT assay.
The T-cell lines C56SL37 and C58SL37, which were derived using SL37 Ac2SGL-loaded tetramers, were activated by SL37 Ac2SGL as expected, and this was blocked by anti-CD1b (Figure 3C and data not shown). However, neither AM Ac2SGL nor M.tb lipid extract containing natural Ac2SGL were stimulatory for C56SL37 and C58SL37. Because the three Ac2SGL variants tested here share an identical sulfated trehalose head group and 2-palmitoylation, the data confirm that T-cells display specificity for the acylation at the 3 position of the Ac2SGL synthetic analogs (Guiard et al., 2009; Gau et al., 2013). Finally, we stained A01 using CD1b tetramers treated with natural Ac2SGL or AM Ac2SGL to validate its ability to recognize each lipid variant. Tetramers treated with natural Ac2SGL or the synthetic analog AM Ac2SGL stained equivalently sized populations of the A01 and A05 T-cell lines (Figure 3D). Together, these data suggest that AM Ac2SGL and SL29 Ac2SGL are bio-equivalent to natural Ac2SGL and provide biological validation of the stereoselective preparation of hydroxyphthioceranic acid described above (Figure 1B).
CD1b surface point mutants abrogate T-cell activation by Ac2SGL
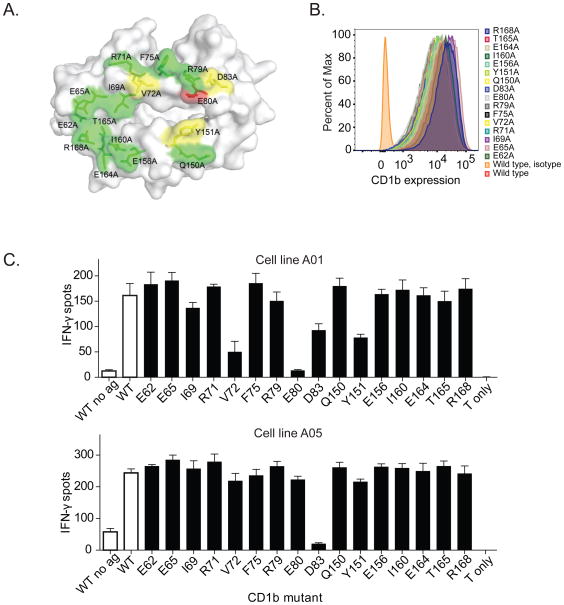
Next, we asked whether mutations in the CD1b binding surface would influence recognition of Ac2SGLs. We generated a library of mutations in the α1 and α2 domains of CD1b to selectively modify the surface available for contacting the TCR (Figure 4A). The mutations are not predicted to change the antigen-binding cleft. C1R cells were transduced with the mutant CD1b constructs and used as antigen presenting cells for T-cells. Importantly, all of these transfectants expressed similar levels of CD1b at the cell surface (Figure 4B). We examined the effect of each of these mutations on activation of A01 and A05 T-cell lines in response to AM Ac2SGL. Amino acid D83 markedly reduced activation of both A01 and A05 T-cell lines (Figure 4C). Additionally, we noted that amino acids Y151, E80, and V72, which are located near the center of the CD1b platform near the site of the expected SGL headgroup protrusion, significantly abrogated T-cell activation of the A01 but had more modest effects on A05 (Figure 4C). These data emphasize the critical nature of centrally located residues in both the α1 and α2 domains of CD1b on T-cell recognition of SGLs (Garcia-Alles et al., 2011).
Figure 4. Effect of CD1b point mutations on SGL antigen recognition.
(A) Location of CD1b point mutations in stably transfected C1R cells. The figure is based on PDB entry 1GZP (Gadola et al., 2002). Substitutions at residues colored green did not show an effect on A05 T-cell activation, while residues colored yellow showed moderate inhibition, and residues colored red showed complete inhibition (B) Expression of CD1b by each of the transfected C1R cells. Isotype indicates staining with an isotype control antibody. (C) IFN-γ production by A05 and A01 T-cell clone in the presence of AM Ac2SGL and transfected C1R cells. WT = wild type. Controls are shown in as white bars and CD1b mutants are shown as black bars. Data are representative of three independent experiments with triplicate wells. Error bars represent SEM of triplicate wells.
Synthetic Ac2SGL analogs are recognized by diverse T-cell receptors
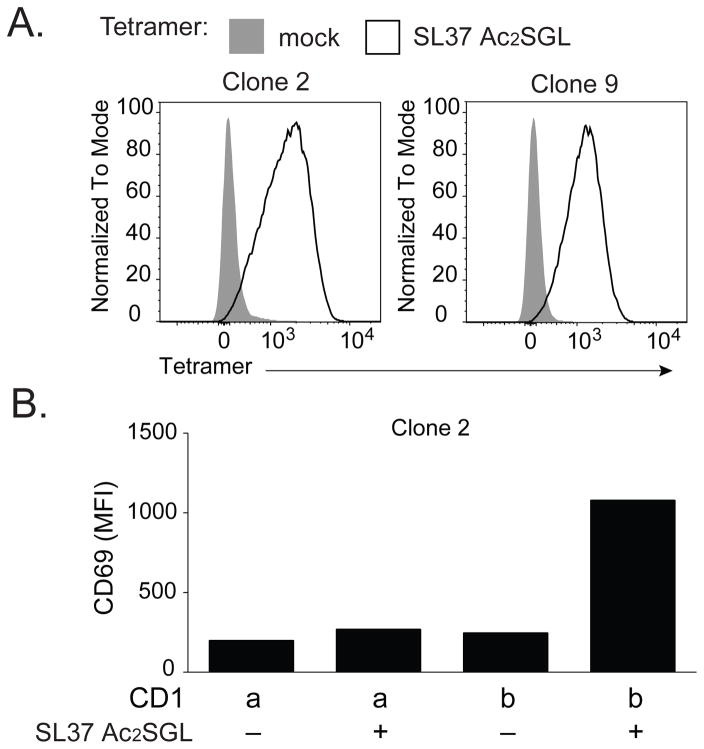
Finally, we used commercial immunosequencing or single-cell multiplex PCR to obtain the sequences for the TCR α and β chain of A01, C56SL37, and C58SL37. We further confirmed the TCR sequence by staining all three lines with the predicted anti-TCR Vβ antibody (data not shown). We found no overlap in dominant TCR sequences among the three lines (Table 1). Unlike recent results with mycobacterial glucose monomycolate-reactive clones, this survey of three Ac2SGL-specific TCRs failed to demonstrate conservation of TCRs that bind antigens with identical head groups but differ in the composition of only one of two lipid tails. To test whether the TCR alone is sufficient to convey antigen specificity, we transduced Jurkat 76 cells that lack CD4 and CD8 co-receptor molecules with the dominant C58SL37 TCR α and β chain. We tested two TCR-transduced clones that stained with SL37 Ac2SGL-loaded tetramers, but not with mock loaded tetramers (Figure 5A). These data confirm the identity of the dominant clone within the C58SLS37 T-cell line. Stimulation with CD1b-transfected, but not CD1a-transfected antigen presenting cells, activated TCR-transduced cells in the presence but not in the absence of SL37 Ac2SGL (Figure 5B). Together, these data reveal that TCR interaction with CD1b and SGL antigen is sufficient to mediate T-cell activation.
Table 1. TCRs expressed by SGL-specific T-cell clones.
T-cell receptor gene usage, CDR3 region amino acid sequence, of Ac2SGL and SL37 specific T-cell cones. Variable and joining gene segment names were assigned using the International ImMunoGeneTics (IMGT) Database.
| Clone | TCR | Variable | CDR3 | Joining |
|---|---|---|---|---|
| A01 | α | TRAV8-6 | CAVKAGYSSASKIIF | TRAJ3 |
| β | TRBV12-4 | CASKGKQGPEQFF | TRBJ2-1 | |
| 56SL37 | α | TRAV14 | CAMRRGFQKLVF | TRAJ8 |
| β | TRBV4-1 | CASSQALLTGSYEQYF | TRBJ2-7 | |
| 58SL37 | α | TRAV17 | CATPNTGGFKTIF | TRAJ9 |
| β | TRBV6-2 | CASSSPFRRASVGELFF | TRBJ2-2 |
Figure 5. TCR transduction confers antigen specificity.
(A) Jurkat cells were transduced with the dominant TCR-α and TCR-β of C58SL37 and clones were isolated by limiting dilution. Two independent clones, clone 2 and clone 9, were stained with SL37 Ac2SGL-loaded tetramers or mock loaded tetramers. (B) C1R cells (20,000) transfected with CD1a or CD1b were loaded with SL37 Ac2SGL, mixed with clone 2 cells (100,000) and incubated overnight before staining with an antibody against CD69 as a marker of T-cell activation. MFI = mean fluorescence intensity. Data are representative of four independent experiments.
DISCUSSION
The discovery that human T-cells recognize bacterial lipid antigens bound to CD1 proteins opened new areas of investigation with potential applications in pathogen-specific immunity (Porcelli, Morita and Brenner, 1992; Beckman et al., 1994). However, key reagents and tools to conduct studies in human populations have been lacking. Here, we describe four main results that will advance translational studies of CD1-restricted T-cells in tuberculosis patients. First, we report a new hybrid synthesis strategy for key antigenic determinants of Ac2SGL that resulted in substantially higher yields of synthetic Ac2SGL than previous schemes. Second, we provide chemical and immunologic data demonstrating the equivalence of the new synthetic Ac2SGL compound to the natural ligand. Third, we report the generation and validation of Ac2SGL-loaded CD1b tetramers, a new tool that will allow us to probe the phenotypes and functions of Ac2SGL-specific T-cells ex vivo. Finally, we provide insights in cross-reactivity patterns among human Ac2SGL-specific T-cell lines that will guide choices concerning the development of synthetic Ac2SGL analogs into vaccine components.
Because SGLs are normally produced in many hundreds of forms, but will be developed as vaccines and recall reagents using chemically simplified versions, understanding whether multiple forms are bioequivalent is essential. Our data show that synthetic analogs of SGLs are not equivalent, and understanding the specific chemical basis of divergence is important. Previous studies have demonstrated that phthioceranic acid and hydroxyphthioceranic acid are key antigenic determinants of Ac2SGL, but synthesis of these complex lipids required multiple steps and were typically not of sufficient yield to enable large scale clinical studies (Gilleron et al., 2004; Guiard et al., 2009; Geerdink, Horst, Lepore, Mori, Puzo, Anna K. H. Hirsch, et al., 2013; Geerdink and Minnaard, 2014; Rasappan and Aggarwal, 2014). The novel hybrid synthesis scheme we present here is much simpler and results in yields that enable validation of a new immunologic tool to probe SGL-specific human T-cell responses ex vivo. The A01 and A05 T-cell lines that we isolated based on recognition of natural Ac2SGL cross-react strongly with AM Ac2SGL, a synthetic molecule that is a component of the natural Ac2SGL mixture that includes eight methylated carbons on the hydroxyphthioceranic acid and corresponds to the major acyl form at m/z 1249.9. It also cross-reacts strongly with SL29 Ac2SGL, which has a fully saturated tetramethylated phthioceranic acid. By contrast, A01 reacts weakly with SL37 Ac2SGL, a molecule that is not identical to any form of naturally occurring Ac2SGL. Further, we show that T-cell lines from two different blood donors that were isolated based on recognition of SL37 Ac2SGL are unable to recognize natural Ac2SGL. Thus, even though the Z4B27 T-cell clone originally reported to recognize natural Ac2SGL was broadly cross reactive and recognized nature-identical and modified versions of Ac2SGL, this is not a general rule and has implications for the use of synthetic Ac2SGL in future designs of vaccines against tuberculosis (Gilleron et al., 2004).
Our findings have immediate relevance for the development of novel whole cell and subunit vaccines for tuberculosis. MTBVAC is an attenuated strain of M.tb that recently completed Phase I clinical trials and has entered efficacy testing (Spertini et al., 2015). MTBVAC contains inactivating mutations in fadD26 and phoP, thus it would not be expected to express SGLs and induce Ac2SGL-specific T-cell responses (Arbues et al., 2013). Induction and expansion of SGL-specific T-cells could be measured using tetramers and used as a surrogate marker of infection with M.tb in efficacy studies of MTBVAC. Another approach would be to consider vaccinating with lipid antigens themselves. CD1-restricted T-cells have anti-bacterial effector functions and lyse M.tb-infected target cells in vitro (Stenger et al., 1997; Rosat et al., 1999; Busch et al., 2016). Humanized mouse models have shown that these cells can provide protection against M.tb challenge in vivo (Zhao et al., 2015). In a recently published guinea pig vaccination model, natural and SL37 Ac2SGL containing liposomes conferred modest protection to M.tb challenge (Larrouy-Maumus et al. 2017). These results may reflect suboptimal cross-reactivity with SGL antigens present in M.tb and could be improved if T-cells were primed using a different synthetic analog, such as AM or SL29 Ac2SGL. Our data show that there is a human T-cell repertoire for natural and synthetic forms of Ac2SGL, so it might be possible to use synthetic analogs other than SL37 to prime T-cells by immunization. The high yields that are possible through the novel synthetic scheme we describe here should enable investigators to optimize vaccination strategies in small animal models by comparing various doses, routes, and formulations. The immunogenicity of these vaccines in humans can then be evaluated using the SGL-loaded CD1b tetramers.
The newly developed Ac2SGL-loaded CD1b tetramers will be useful in probing the functions of Ac2SGL-specific T-cells because it is generally not known which functional classes exist among lipid-specific T-cells in vivo. Tetramer staining can be used in combination with staining for markers of T-cell function, including cytokine production, or to isolate single Ac2SGL-specific T-cells for RNA sequencing. Further, the TCR repertoire of Ac2SGL-specific T-cells can now be studied using tetramers. We describe three newly discovered TCRs here with different patterns of reactivity against natural and synthetic SGLs. High throughput sequencing of T-cells that bind to tetramers loaded with natural or AM Ac2SGL could potentially lead to the identification of a highly relevant new population of invariant T-cells. It is worth noting that one of our T-cell clones contained a TRAV17 to TRAJ9 rearrangement. Though the CDR3 regions are markedly different, we have previously reported TRAV17 to TRAJ9 rearrangements among a group of CD1b and glucose monomycolate-specific T-cells that are called “LDN5-like T-cells” (Van Rhijn et al., 2014). Thus, TRAV17 and TRAJ9 may be part of a general molecular pattern that is associated with CD1b recognition.
We used CD1b point mutants to study the influence of specific residues located at the CD1b surface on T-cell activation and found that mutation of V72, E80, D83, or Y151 reduces T-cell activation. This reduction could be caused by diminished direct interaction between these residues and the TCR. An alternative possibility is that these residues are required to “lock” the SGL antigen in the CD1b binding groove, so that lack of T-cell activation reflects a lack of antigen. Supporting the latter model is the published crystal structure of CD1b bound to SL27 Ac2SGL, which reveals a dramatic conformational change and decreased groove volume compared to unloaded soluble CD1b in which E80 and Y151 are responsible for occluding the F′ entrance of the CD1B binding pocket (Garcia-Alles et al., 2011). Further, the authors show that replacement of E80 and Y151 with alanine completely abrogated the activation of T-cell clone Z4B27 to both natural Ac2SGL as well as SL27 Ac2SGL. These published data are consistent with our results and suggest shared molecular mechanisms underlie recognition of CD1b-presented SGL antigens from natural and synthetic sources. Our data further implicate a role of D83 and V72 in T-cell recognition of natural Ac2SGL that may differ from SL27 Ac2SGL.
Our findings may also have implications for the improved diagnosis of M. tb infection in humans. The current standard for the diagnosis of latent tuberculosis infection is the IFN-γ release assay (Pai et al., 2014). This assay detects memory T-cell responses to ESAT-6 and CFP-10, two secreted proteins that are specific to M. tb. While this test has improved specificity over the traditional tuberculin skin test (TST), neither test accurately predicts which patients will eventually develop active disease. We recently published that T-cell responses to mycobacterial proteins and lipids are poorly correlated in a South African cohort, revealing the complementary nature of immunity to these two classes of antigens (Seshadri et al., 2015). The data presented in the current manuscript suggest measuring SGL-specific T-cell responses using tetramers could improve existing diagnostic algorithms by providing complementary information. Unlike MHC tetramers, SGL-CD1b tetramers can be applied independently of genetic background because CD1 genes are structurally non-polymorphic (Han et al., 1999). SGL-loaded CD1b tetramers could also be used to detect SGL-specific T-cell responses in the blood or lungs of patients with suspected active tuberculosis disease. Recent data have revealed that T-cells specific for ESAT-6 and CFP-10 that expressed an activated phenotype was able to distinguish latent infection from active disease (Adekambi et al., 2015). SGL-CD1b tetramers could similarly be incorporated into multi-parameter flow cytometry assays designed to measure the activation status of lipid-specific T-cells.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Chetan Seshadri (seshadri@u.washington.edu).
Individuals seeking to establish SGL-loaded CD1b tetramers in their own labs will first need to complete a materials transfer agreement (MTA) with the NIH Tetramer Core Facility to receive soluble biotinylated CD1b monomers. Synthetic AM Ac2SGL is available via the TB Vaccine Accelerator funded by the Bill & Melinda Gates Foundation upon request from Dr. Branch Moody.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human Studies
T-cell lines were derived from U.S. and South African adults with latent tuberculosis infection. The gender of these subjects is not reported and the samples were chosen cryopreserved repositories on the basis of convenience. Informed consent was obtained from all subjects.
Approving Bodies
Blood was donated with informed consent by asymptomatic tuberculin positive subjects with no clinical or radiographic evidence of active tuberculosis, as approved by the institutional review boards of the Lemuel Shattuck Hospital and Partners Healthcare. The study was also approved by the IRB of the University of Washington and University of Cape Town.
Primary T-cell Cultures
The cell line A01 was isolated from cryopreserved, CD14-depleted peripheral blood mononuclear cells (PBMC) from a South African adult with latent tuberculosis infection as defined by a positive QuantiFERON-TB Gold blood test. PBMC were thawed and washed in warm RPMI 1640 (Gibco, Waltham, MA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 10 mL/mL Benzonase (Millipore, Billerica, MA) and enumerated using Trypan blue exclusion. T cell media consists of RPMI 1640 (Gibco, Waltham, MA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/mL Penicillin and 100 mg/mL Streptomycin (Gibco, Waltham, MA), 55 mM 2-mercaptoethanol (Gibco, Waltham, MA), 0.3X Essential Amino Acids (Gibco, Waltham, MA), 60mM Non-essential Amino Acids (Gibco, Waltham, MA), 11mM HEPES (Gibco, Waltham, MA), and 800mM L-Glutamine (Gibco, Waltham, MA). PBMC were then plated in a 24-well plate at a density of three million cells per well in T cell media and allowed to rest overnight at 37°C in humidified incubators supplemented with 5% CO2. The following day, PBMC were washed and blocked with 50% human serum (Valley Biomedical, Winchester, VA) in PBS supplemented with 0.2% BSA (Sigma, St. Louis, MO) (FACS buffer) for 10 min at 4°C. The samples were washed twice with PBS and stained with Aqua Live/Dead stain (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Following two additional PBS washes, cells were resuspended in 50 μL FACS buffer and 1 uL of either mock loaded CD1b tetramer or Ac2SGL-loaded CD1b tetramer and incubated at room temperature for 40 minutes in the dark. Finally, cells were stained with anti-CD3-Phycoerythrin-Texas Red (ECD) (Beckman Coulter, Brea, CA), washed twice in T cell media and filtered through a cell strainer tube (Falcon, Tewksbury, MA) prior to sorting. Tetramer-positive T cells were sorted using a FACS Aria II (BD, San Jose, CA) cell sorter equipped with 407nm, 488nm, and 641nm lasers.
Sorted T cells were washed and resuspended in T cell media supplemented with 10% human serum and divided among eight wells of a 96-well plate to create a T cell line. Irradiated PBMC (150,000 cells per well) were added as feeder cells along with phytohaemagglutinin (Remel, San Diego, CA) at a final concentration of 1.6 mg/ml. After two days in culture at 37°C, 5% CO2, 10 mL natural IL-2 (Hemagen, Columbia, MD) was added to each well. Half the media was replaced every two days with T cell media supplemented with 10% human serum and natural IL-2. When the cell clusters were large and round (approximately after eight days of growth), they were pooled into a 24-well plate. After 10 days in culture, the cell line was screened by tetramer staining or functional response to Ac2SGL. We then further expanded the T cell line using a modified version of a previously published rapid expansion protocol (Riddell et al., 1992). Briefly, 200,000 T cells were mixed with 5 million irradiated EBV-transformed B cells and 25 million irradiated PBMC as feeder cells in 25 ml T cell media. Anti-CD3 (clone OKT3) was added a final concentration of 30 ng/mL, and the mixture was incubated overnight at 37°C, 5% CO2. The following day, recombinant IL-2 (rIL-2) (UWMC Clinical Pharmacy) was added to a final concentration of 50 U/mL. On day 4, the cells were washed twice in T cell media to remove OKT3, and fresh media supplemented with rIL-2 at 50 U/mL was added. Half the media was replaced every three days or split into new T25 tissue culture flasks (Costar, St. Louis, MO) as determined by cell confluency. After 13 days in culture, the line was screened by tetramer staining and then cryopreserved on day 14. Sorting data are representative of a single experiment, but tetramer staining of T-cell lines was confirmed in two or more experiments.
The cell lines C58SL37 and C56SL37 were derived from PBMC from a latent tuberculosis patient and a random blood bank donor, respectively. Cells were resuspended in 50 μl FACS buffer and 1 uL of either mock loaded CD1b tetramer or Ac2SGL-loaded CD1b tetramer and incubated at room temperature for 20 minutes in the dark. Finally, cells were stained with anti-CD3-Fitc (clone SK7, BD, San Jose, CA), washed twice in T cell media and filtered through a cell strainer tube (Falcon, Tewksbury, MA) prior to sorting. Tetramer-positive T cells were sorted using a FACS Aria II (BD, San Jose, CA) cell sorter equipped with 407nm, 488nm, and 641nm lasers. Sorted cells were expanded with the anti-CD3-based rapid expansion method seeding 1000 T cells with 40,000 irradiated EBV-transformed B cells and 200,000 irradiated PBMC per well of a round bottom 96-well plate.
METHOD DETAILS
Isolation of sulfoglycolipids
Natural Ac2SGL was purified from M. tuberculosis as previously described (Gilleron et al., 2004). Briefly, bacterial cultures (strain H37Rv) were suspended in chloroform/methanol (1:1) and filtered four times. The extract was concentrated and further partitioned by addition of water and chloroform. The chloroform was evaporated and redissolved in a minimal volume of chloroform. Acetone was added and incubated overnight at 4°C to form a precipitate. The suspension was centrifuged at 3,000xg for 15 minutes at 4°C. The acetone-soluble phase was fractionated on a silicic column and irrigated by various chloroform/methanol solutions (10, 20, and 30% methanol). Natural Ac2SGL is found in the 20% methanol fraction. This fraction was further purified by reverse phase chromatography, and validated by thin layer chromatography on aluminum-backed silica gel plates (Alugram Sil G; Macherey-Nagel, Germany). Chemical synthesis of SL37 Ac2SGL is the subject of a previous manuscript (Gau et al., 2013).
Synthesis of AM Ac2SGL
Chemical synthesis of AM Ac2SGL consisted of a separate synthesis of hydroxyphthioceranic acid and a subsequent assembly of AM Ac2SGL. Hydroxyphthioceranic acid was synthesized by the combination of efficient asymmetric Cu-catalyzed iterative conjugated addition protocol with lithiation/borylation strategy in the later stage of synthesis (Des Mazery et al., 2005; López et al., 2006; ter Horst, Feringa and Minnaard, 2007a; Casas-Arce et al., 2008; Madduri and Minnaard, 2010; Geerdink, Horst, Lepore, Mori, Puzo, Anna K H Hirsch, et al., 2013; Geerdink and Minnaard, 2014; Rasappan and Aggarwal, 2014).
Fig. 2.
Structures of Hydroxyphthioceranic acid (1) and Phthioceranic acid (2)
1. Retrosynthetic Analysis of Hydroxyphtioceranic Acid
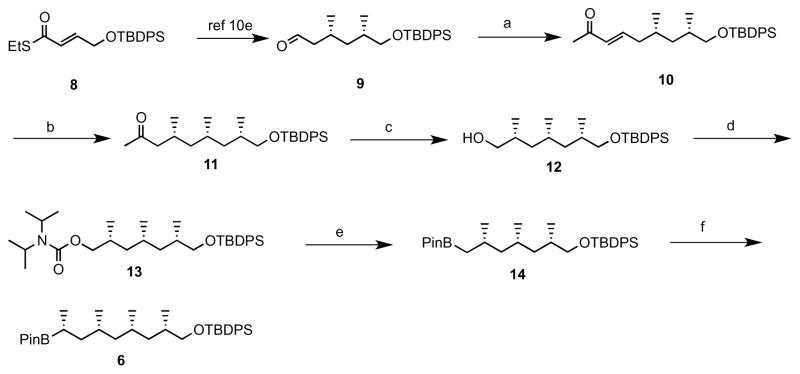
The retrosynthetic analysis outlined in Scheme 1 shows that disconnection of 3 would lead to carbamate 5 and alkyl boronate. Carbamate 5 could be constructed from building blocks 6 and 7 by lithiation/borylation. Boronate 6 would be derived from 8 via Cu-catalysed iterative conjugate additions (Des Mazery et al., 2005; López et al., 2006; ter Horst, Feringa and Minnaard, 2007a, 2007b; Madduri and Minnaard, 2010) followed by lithiation/borylation, and carbamate 7 could be obtained by Cu-catalysed iterative conjugate additions followed by carbamoylation of alcohol.
Scheme 1.
Retrosyntheis of hydroxyphthioceranic acid. TBDPS: tert-butyldiphenylsilyl, PinB: pinacolboronate
1.1 Synthesis of boronate 6
The synthesis of 6 was commenced from known compound 9 (Madduri and Minnaard, 2010) which was readily obtained in 5 steps from compound 8 by Cu-catalysed iterative asymmetric conjugate addition followed by thioester reduction. Horner–Wadsworth–Emmons reaction of 9 with with (OEt)2POCH2COMe gave unsaturated ketone 10 in 85% yield. Third methyl group was installed by Cu-catalyzed asymmetric conjugate addition protocol on 10 to afford 11 in 91% yield with excellent selectivity, which on Baeyer-Villiger oxidation with sodium percarbonate followed by hydrolysis afforded the alcohol in 82% yield (Wu, Harutyunyan and Minnaard, 2014). Boronate 14 was achieved from alcohol in 2 steps with an overall yield of 46% by carbomolation of alcohol and lithiation/borylation of carbamate with pinacolborane, and homologated boronate 6 was afforded by stereoselective deprotonation of ethyl carbamate with sec. BuLi/(−)-spartein followed by treatment with boronate 13 in 62% yield.
1.2 Synthesis of carbamate 7
Synthesis of ketone 15 was prepared from aldehyde 9 using Cu-catalyzed asymmetric conjugate addition protocol. Alcohol 16 was acheived from compound 15 by Baeyer-Villiger oxidation with sodium percarbonate followed by hydrolysis in 77% yield, which on protection of the hydroxyl group as MOM ether 17, followed by deprotection of TBDPS ether with TBAF and carbomoylation of formed alcohol 18 efficiently produced carbomate 7 in 76% over three steps as shown in Scheme 3.
Scheme 3.
a) 1. (CF3CO)2O, sodium percarbonate, DCM, rt, 2. K2CO3, MeOH, rt, 77%; b) MOM-Cl, DIPEA, DCM, rt, 88%; c) TBAF•3H2O, THF, rt, 97%; d) (iPr)2NCOCl, NEt3, toluene, 150 °C, 89%.
1.3 Synthesis of hydroxyphthioceranic acid 3
The end game for the synthesis of hydroxyphthioceranic acid depicted in Scheme 4. The coupling of two bulding blocks achieved by deprotonation of carbamate 7 with sec. BuLi/TMEDA followed by treatment with boronate 8 upon protodeborylation afforded the polydeoxypropionate 19 in 36% yield. Deprotection of MOM ether followed by carbomoylation of formed alcohol produced carbamate 20 in 70% yield over two steps. Long hydrocarbon chain was introduced by stereoselctive deprotonation of carbomate 20 with sec. BuLi/(+)-spartein followed by treatment with boronate, further oxidation of formed boronate with H2O2 afforded 21 in 40% yield. Hydroxyphthioceranic acid was achieved from 21 by deprotection of silyl ether with TBAF followed by oxidation. Crude hydroxyphthioceranic acid was used for the assembly of Ac2SGL without purification.
Scheme 4.
a) Sec. BuLi, TMEDA, ether, −78 °C to 60 °C, b) 1) MeLi, ether, −78 °C, 2) Mn(OAc)3, TBC, DCE, 60 °C, c) 1) ZnBr2, nPrSH, DCM, rt, d) Sec. BuLi, (+)-Spartein, ether, −60 °C to 60 °C, e) 1) TBAF, THF, rt, 2) TEMPO, NaClO2, NaOCl, Phosphate buffer, CH3CN
2. Synthesis of Ac2SGL 2
The end game for the synthesis of Ac2SGL is depicted in Scheme 5 (Guiard et al., 2008, 2009; Leigh and Bertozzi, 2008; Sarpe and Kulkarni, 2014). Hydroxyphthioceranic acid 3 was regioselectively coupled to the trehalose core 22 by using Yamaguchi conditions, affording 23 in 68% yield. The TIPS ether was removed by using a buffered TBAF solution to provide 24 in 79% yield. Using a reported procedure, regioselective 2′-O-sulfation on 24 was carried out to afford 25 in 69% yield. Finally, we attempted to deprotect benzylidene acetals and TCE groups in one-step using hydrogenolysis conditions. The usual hydrogenolysis with H2/Pd(OH)2-C was given the product in low yield. Several hydrogenolysis strategies were attempted, but the combination of Pd(OH)2-C and Pd-C was afforded the Ac2SGL 2 in 71% yield.
Scheme 5.
a) 2,4,6-trichlorobenzoylchloride, Et3N, DMAP, toluene, 68%; b) TBAF (1M in THF, PH=6.5), THF, 40 C, 79%; c) 1,2-Dimethylimidazole, THF, rt, 69%; d) NH4HCO2, Pd(OH)2/C, Pd/C, MeOH/CH2Cl2, 71%.
All reactions were performed under nitrogen atmosphere using dry glassware and dry solvents. MTBE, Et2O, THF, toluene and DCM were taken from an MBraun solvent purification system (SPS-800). TMEDA was freshly distilled from CaH2 and stored under argon atmosphere. (+)-Sparteine was purchased from BOC-Science and stored in a glovebox. (−)-Sparteine was liberated from the commercially available sulfate salt (Nikolic and Beak, 1997). (R, S)-Josiphos.EtOH was purchased from Solvias and stored under argon atmosphere. TLC analysis: Merck silica gel 60/Kieselguhr F254, 0.25 mm. Compounds were visualized using either anisaldehyde stain or potassium permanganate stain. Flash chromatography: SiliCycle silica gel type SiliaFlash P60 (230–400 mesh).
Chemical characterization of AM Ac2SGL and its synthetic intermediates
1H- and 13C-NMR spectra were recorded on a Varian 400-MR (400, 100.6 MHz, respectively). High resolution mass spectra were recorded on a Thermo Scientific LTQ Orbitrap XL. Enantiomeric excess was determined by HPLC (Chiracel OB, 250*4.6 mm, 10 μm) using a PDA detector (Shimadzu).
1. (6S,8S, E)-9-((tert-butyldiphenylsilyl)oxy)-6,8-dimethylnon-3-en-2-one (10)
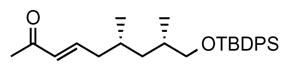
To a solution of (EtO)2POCH2COMe (3.53 g, 18.2 mmol, 1.3 eq) dissolved in THF (93 mL) under nitrogen and cooled to 0 °C, n-butyllithium (10.5 mL, 16.8 mmol, 1.2 eq, 1.6 M in hexane) was added slowly at 0 °C. The reaction mixture was stirred for 10 min at rt. Then aldehyde 9 (5.35 g, 14.0 mmol, 1.0 eq), dissolved in 3 mL THF was slowly added and the reaction mixture stirred at rt for 10 h. The reaction mixture was washed with distilled water and extracted with diethyl ether. The combined organic phases were dried over MgSO4 and concentrated under reduced pressure to yield crude 10. Purification by flash chromatography (8% ether in pentane) afforded α,β-unsaturated ketone 10 as a colorless oil (5.04 g, 85% yield). [α]D22 = −8.5° (c = 1.3, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.69 – 7.67 (m, 4H), 7.45 – 7.37 (m, 6H), 6.76 (dt, J = 15.3, 7.4 Hz, 1H), 6.07 (d, J = 15.9 Hz, 1H), 3.52 (dd, J = 10.0, 5.2 Hz, 1H), 3.46 (dd, J = 9.6, 6.4 Hz 1H), 2.26 – 2.19 (m, 1H), 2.23 (s, 3H), 2.03 – 1.95 (m, 1H), 1.80 – 1.65 (m, 2H), 1.48 – 1.41 (m, 1H), 1.06 (s, 9H), 1.03 – 0.89 (m, 1H), 0.96 (d, J = 6.7 Hz, 3H), 0.88 (d, J = 6.6 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 198.52, 147.22, 135.61, 135.60, 133.94, 132.56, 129.56, 127.61, 68.66, 40.75, 39.80, 33.15, 30.04, 26.90, 26.83, 20.21, 19.32, 17.63. HRMS, calcd for C27H38O2SiNa (M+Na+) 445.253, found 445.253.
2. (4R,6S,8S)-9-((tert-butyldiphenylsilyl)oxy)-4,6,8-trimethylnonan-2-one (11)
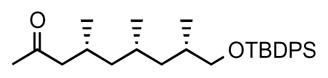
(R, SFe)-Josiphos•CuBr complex (88.0 mg, 0.112 mmol, 1 mol%) was dissolved in t-BuOMe (60 mL) under nitrogen. The mixture was cooled to −80 °C and methylmagnesium bromide (4.46 mL 13.4 mmol, 3 M solution in diethyl ether) was added dropwise over 10 min. After stirring for 10 min, a solution of enone 10 (4.73 g, 11.2 mmol) in t-BuOMe (20 mL) was added via syringe pump over 1.5 h. The reaction mixture was stirred at −80 °C for 18 h, then quenched by the addition of MeOH and allowed to warm to room temperature. Saturated aqueous NH4Cl was added, and after phase separation and extraction of the aqueous phase with diethyl ether, the combined organic phases were dried over MgSO4, concentrated under reduced pressure and purified by column chromatography (5% ether in pentane) to afford 11 as a colourless oil (4.48 g, 91% yield). [α]D22 = −7.4° (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.68-7.65 (m, 4H), 7.44-7.35 (m, 6H), 3.51 (dd, J = 10.0, 5.1 Hz, 1H), 3.42 (dd, J = 9.8, 6.5 Hz, 1H), 2.37 (app q, J = 8.4 Hz, 1H), 2.10 (app t, J = 8.4 Hz, 1H), 2.09 (s, 3H), 1.76-1.68 (m, 1H), 1.51-1.42 (m, 1H), 1.40 – 1.33 (m, 1H), 1.21 – 1.12 (m, 2H), 1.07 (s, 9H), 0.98– 0.88 (m, 2H), 0.94 (d, J = 6.4 Hz, 3H), 0.85 (d, J = 6.4 Hz, 3H), 0.82 (d, J = 6.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 208.66, 135.50, 135.48, 133.90, 133.87, 129.40, 129.39, 127.47, 127.46, 68.62, 50.70, 44.94, 41.12, 33.02, 30.26, 27.58, 26.81, 26.65, 20.64, 20.52, 19.20, 18.00. HRMS, calcd for C28H42O2SiNa (M+Na+) 461.285, found 461.284.
3. (2R,4S,6S)-7-((tert-butyldiphenylsilyl)oxy)-2,4,6-trimethylheptan-1-ol (12)
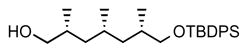
To a mixture of compound 11 (3.27 g, 7.5 mmol) and sodium percarbonate (10 eq, 11.8 g) in DCM (75 mL), trifluoroacetic anhydride (5 eq, 5.25 mL) was added. The reaction was stirred at room temperature for 2 days and filtered through a Celite pad. The filtrate was neutralized with sat. NaHCO3(aq) and the aqueous layer was back-extracted with Et2O. The organic phases were dried over MgSO4, concentrated and purified by column chromatography (10% ether in pentane) to afford the acetate as colorless oil (2.92 g, 86% yield). The product acetate (1.66 g, 3.8 mmol) was dissolved in methanol (5 mL) and potassium carbonate (1.3 eq, 0.67 g) was added. The reaction was stirred at room temperature for 3 h, after which it was diluted with water and extracted with Et2O. The combined organic phases were dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (50% ether in pentane) to give compound 12 as colorless oil (1.49 g, 95%). [α]D22 = −3.4° (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.83 – 7.57 (m, 4H), 7.49 – 7.30 (m, 6H), 3.52 (dd, J = 5.0, 2.6 Hz, 1H), 3.49 (dd, J = 5.0, 3.2 Hz, 1H), 3.41 (dd, J = 9.8, 6.5 Hz, 1H), 3.33 (dd, J = 10.4, 6.8 Hz, 1H), 1.79 – 1.65 (m, 2H), 1.58 – 1.47 (m, 1H), 1.37 (m, 1H), 1.31 – 1.20 (m, 1H), 1.05 (s, 9H), 0.93 (d, J = 6.7 Hz, 3H), 0.91 – 0.82 (m, 2H), 0.89 (d, J = 6.7 Hz, 3H), 0.84 (d, J = 6.5 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 135.63, 135.60, 134.07, 134.04, 129.46, 127.53, 68.72, 68.16, 41.24, 41.21, 33.19, 33.06, 27.75, 26.88, 20.97, 19.30, 18.04, 17.62. HRMS, calcd for C26H40O2SiNa (M+Na+) 435.269, found 435.268.
4. (2R,4S,6S)-7-((tert-butyldiphenylsilyl)oxy)-2,4,6-trimethylheptyl diisopropylcarbamate (13)

Compound 12 (1.20 g, 2.88 mmol) was dissolved in anhydrous toluene (3.5 mL) and diisopropyl carbamoyl chloride (943 mg, 5.76 mmol, 2.0 eq) and dry triethylamine (1.0 mL, 7.2 mmol, 2.5 eq) were added. The reaction mixture was heated at 150 °C in a microwave reactor for 2 h. The mixture was cooled to room temperature, washed with HCl (2 M aq.), dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (10% ether in pentane) to give compound 13 as colorless oil (1.29 g, 83%). [α]D 22 = −6.7° (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.72–7.68 (m, 4H), 7.45–7.37 (m, 6H), 4.03 (dd, J = 10.5, 4.9 Hz, 1H), 3.87 (dd, J = 10.6, 6.7 Hz, 1H), 3.55 (dd, J = 9.8, 5.2 Hz, 1H), 3.45 (dd, J = 9.8, 6.5 Hz, 1H), 2.00 – 1.87 (m, 1H), 1.82 – 1.71 (m, 1H), 1.65 – 1.52 (m, 1H), 1.46 – 1.30 (m, 2H), 1.26 – 1.21 (m, 2H), 1.24 (s, 6H), 1.23 (s, 6H), 1.10 (s, 9H), 0.98 (d, J = 7.2 Hz, 3H), 0.96 (d, J = 7.2 Hz, 3H), 0.94 – 0.90 (m, 2H), 0.98 (d, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 155.90, 135.64, 135.61, 134.04, 134.01, 129.51, 127.58, 69.41, 68.83, 45.06, 41.46, 41.31, 33.21, 30.35, 27.66, 26.93, 20.98, 19.32, 18.51, 17.95. HRMS, calcd for C33H53NO3SiNa (M+Na+) 562.367, found 562.367.
5. tert-butyldiphenyl(((2S,4S,6S)-2,4,6-trimethyl-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)heptyl)oxy)silane (14)
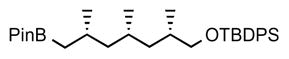
sBuLi (1.7 mL, solution in cyclohexane, 1.4 M, 1.2 eq) was added dropwise to a solution of carbamate 13 (1.09 g, 2.0 mmol, 1.0 eq) and TMEDA (0.39 mL, 2.6 mmol, 1.3 eq.) in anhydrous Et2O (9 mL) at −78 °C. The reaction mixture was stirred for 5 h at −78 °C and then a solution of pinacol borane (0.58 mL, 4.0 mmol, 2.0 eq) in anhydrous Et2O (1 mL) was added slowly and the mixture was stirred for 1 h at −78 °C. The cooling bath was removed, the reaction mixture was warmed to 40 °C and kept at this temperature for 12 h. The reaction mixture was subsequently cooled to 0 °C, diluted with KH2PO4 (1 M aq., 40 mL) and stirred for an additional 10 min. The phases were separated, and the aqueous phase was extracted with ether. The combined organic layers were dried over anhydrous MgSO4, filtered, concentrated and purified by column chromatography (2% ether in pentane) to give boronate 14 (0.57 g, 55%) as colorless oil. [α]D 22 = −4.6° (c = 1.4, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.91 – 7.56 (m, 4H), 7.52 – 7.28 (m, 6H), 3.52 (dd, J = 9.8, 5.1 Hz, 1H), 3.39 (dd, J = 9.8, 6.8 Hz, 1H), 1.76 (m, 2H), 1.55 – 1.45 (m, 1H), 1.36–1.30 (m, 1H), 1.25 (s, 12H), 1.23 – 1.12 (m, 2H), 1.06 (s, 9H), 0.93 (d, J = 6.8 Hz, 3H), 0.91 – 0.85 (m, 2H), 0.89 (d, J = 6.8 Hz, 3H), 0.81 (d, J = 6.8 Hz, 3H), 0.55 (dd, J = 15.3, 8.6 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 135.62, 135.60, 134.13, 134.11, 129.41, 129.40, 127.51, 82.74, 68.98, 47.49, 41.47, 34.11, 33.11, 27.71, 26.88, 26.79, 24.91, 24.74, 23.27, 22.32, 20.80, 19.29, 17.86, 14.05. HRMS, calcd for C32H51BO3SiNa (M+Na+) 545.359, found 545.359.
6. tert-butyldiphenyl(((2S,4S,6S,8R)-2,4,6-trimethyl-8-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nonyl)oxy)silane (6)
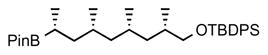
sBuLi (solution in cyclohexane, 1.4 M, 0.93 mL, 1.3 eq) was added dropwise to a solution of ethyl carbamate (0.24 g, 1.4 mmol, 1.4 eq) and (−)-sparteine (0.33 g, 1.4 mmol, 1.4 eq) in anhydrous Et2O (5 mL) at −78 °C. The reaction mixture was stirred for 5 h at −78 °C and then a solution of boronate 14 (0.52 g, 1.0 mmol, 1.0 eq) in anhydrous Et2O (2 mL) was added. The reaction mixture was stirred for 1 h at −78 °C. The cooling bath was removed and the reaction mixture was warmed to 50 °C and kept at this temperature for 12 h. The reaction mixture was cooled to 0 °C and diluted with KH2PO4 (1 M aq., 2 mL) and stirred for an additional10 min. The phases were separated, and the aqueous phase was extracted with ether. The combined organic layers were dried over anhydrous MgSO4, filtered, concentrated and purified by column chromatography (1% followed by 2% ether in pentane) to give pinacol boronate 6 (0.344 g, 62%) as colorless, viscous oil. [α]D 22 = −5.7° (c = 1.3, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.72–7.58 (m, 4H), 7.48–7.39 (m, 6H), 3.54 (dd, J = 10.0, 5.1 Hz, 1H), 3.42 (dd, J = 9.5, 6.7 Hz, 1H), 1.80 – 1.72 (m, 1H), 1.65 – 1.48 (m, 2H), 1.41 – 1.30 (m, 1H), 1.28 – 1.21 (m, 2H), 1.24 (s, 12H), 1.15 – 1.10 (m, 2H), 1.08 (s, 9H), 0.98 (d, J = 7.2 Hz, 3H), 0.95 (d, J = 7.2 Hz, 3H), 0.88 – 0.82 (m, 8H); 13C NMR (101 MHz, CDCl3) δ 135.63, 135.62, 134.13, 134.11, 129.45, 129.43, 127.54, 82.68, 68.87, 45.75, 41.42, 40.75, 33.15, 29.56, 27.45, 26.91, 24.78, 24.74, 20.77, 19.32, 18.05, 16.48. HRMS, calcd for C34H55BO3SiNa (M+Na+) 573.391, found 573.390.
7. (2R,4R,6S,8S)-9-((tert-butyldiphenylsilyl)oxy)-2,4,6,8-tetramethylnonan-1-ol (16)
![]()
To a mixture of compound 15 (4.85 g, 10 mmol) and sodium percarbonate (16 eq, 25 g) in DCM (170 mL), trifluoroacetic anhydride (4 eq, 5.5 mL) was added. The reaction was stirred at room temperature for 16 h, and then a fresh portion of sodium percarbonate (8 eq, 12.5 g) and trifluoroacetic anhydride (2 eq, 2.7 ml) was added. The reaction was stirred for a subsequent 2 days and then filtered through a Celite pad. The filtrate was neutralized with sat. solution of NaHCO3(aq) and the aqueous layer was back-extracted with Et2O (3x). The organic phases were dried over MgSO4, filtered and concentrated. The residue was dissolved in methanol (10 mL) and potassium carbonate (1.1 eq, 1.5 g) was added. The reaction was stirred at room temperature for 3 h, after which it was diluted with water and extracted with Et2O. The combined organic phases were dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (25% ether in pentane) to give compound 16 as colorless oil (3.5 g, 77%). [α]D 22 = −2.5° (c = 0.8, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.79 – 7.58 (m, 4H), 7.49 – 7.31 (m, 6H), 3.55 – 3.49 (m, 2H), 3.42 (dd, J = 9.8, 6.4 Hz, 1H), 3.35 (dd, J = 10.5, 6.9 Hz, 1H), 1.72 (m, 2H), 1.61 – 1.45 (m, 3H), 1.38 (m, 1H), 1.32 – 1.13 (m, 3H), 1.05 (s, 9H), 0.94 (d, J = 6.8 Hz, 3H), 0.92 (d, J = 6.8 Hz, 3H), 0.90 – 0.86 (m, 2H), 0.84 (d, J = 6.4 Hz, 3H), 0.81 (d, J = 6.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 135.62, 135.60, 134.10, 129.45, 129.44, 127.53, 127.52, 68.66, 68.10, 45.55, 41.11, 40.94, 33.18, 33.07, 27.65, 27.56, 26.88, 21.12, 21.00, 19.31, 18.21, 17.71. HRMS, calcd for C29H46O2SiNa (M+Na+) 477.316, found 477.315.
8. (6R,8R,10S,12S)-6,8,10,12,16,16-hexamethyl-15,15-diphenyl-2,4,14-trioxa-15-silaheptadecane (17)
![]()
To a solution of compound 16 (1.34 g, 2.95 mmol) in anhydrous DCM (15 mL) was added N,N-diisopropylethylamine (2.0 eq, 1 mL) and chloromethyl methyl ether (1.5 eq, 0.36 mL), and the reaction mixture was stirred at room temperature overnight. The mixture was diluted with Et2O, washed with HCl (10% aq., 2x) and brine, dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (2% Et2O in pentane) to give compound 17 as colorless oil (1.3 g, 88%). [α]D 22 = −7.5° (c = 1.3, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.84 – 7.55 (m, 4H), 7.50 – 7.29 (m, 6H), 4.60, 4.61 (AB q, J = 6.4 Hz, 2H), 3.51 (dd, J = 9.8, 5.0 Hz, 1H), 3.44 – 3.38 (m, 2H), 3.35 (s, 3H), 3.24 (dd, J = 9.3, 7.1 Hz, 1H), 1.88 – 1.66 (m, 2H), 1.54 (m, 2H), 1.34 (m, 2H), 1.24 – 1.12 (m, 2H), 1.05 (s, 9H), 0.94 (d, J = 6.8 Hz, 6H), 0.92 – 0.85 (m, 2H), 0.84 (d, J = 6.4 Hz, 3H), 0.80 (d, J = 6.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 135.62, 135.60, 134.09, 129.45, 129.43, 127.53, 127.52, 96.57, 73.04, 68.68, 55.05, 45.56, 41.35, 41.12, 33.16, 30.82, 27.59, 27.50, 26.88, 21.04, 20.95, 19.30, 18.39, 18.18. HRMS, calcd for C31H50O3SiNa (M+Na+) 521.342, found 521.342.
9. (2S,4S,6R,8R)-9-(methoxymethoxy)-2,4,6,8-tetramethylnonan-1-ol (18)
![]()
To a solution of 17 (3.28 g, 6.57 mmol) in anhydrous THF (40 mL) was added TBAF·3H2O (1.5 eq, 3.1 g). The reaction mixture was stirred at room temperature overnight and then diluted with NaHCO3 (sat. aq.). The aqueous phase was extracted with diethyl ether (3x). The combined organic phases were dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (15% to 40% Et2O in pentane) to give compound 18 as colorless oil (1.66 g, 97%). [α]D 22 = −11.1° (c = 1.1, CHCl3). 1H NMR (400 MHz, CDCl3) δ 4.60, 4.61 (AB q, J = 6.4 Hz, 2H), 3.54 (dd, J = 10.5, 4.9 Hz, 1H), 3.44 – 3.34 (m, 2H), 3.36 (s, 3H), 3.26 (dd, J = 9.3, 7.0 Hz, 1H), 1.77 (m, 2H), 1.64 – 1.53 (m, 2H), 1.33 (m, 2H), 1.20 (m, 2H), 0.94 (d, J = 6.4 Hz, 3H), 0.93 (d, J = 6.8 Hz, 3H), 0.92 – 0.89 (m, 2H), 0.87 (d, J = 6.4 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 96.58, 73.01, 68.08, 55.07, 45.40, 41.35, 40.98, 33.08, 30.86, 27.57, 21.03, 20.99, 18.38, 17.68. HRMS, calcd for C15H32O3Na (M+Na+) 283.225, found 283.224.
10. (2S,4S,6R,8R)-9-(methoxymethoxy)-2,4,6,8-tetramethylnonyl diisopropylcarbamate (19)
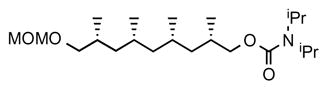
Compound 18 (590 mg, 2.27 mmol) was dissolved in anhydrous toluene (20 mL) and diisopropyl carbamoyl chloride (2 eq, 750 mg) and dry triethylamine (2.5 eq, 0.80 mL) were added. The reaction mixture was heated at 150 °C in a microwave reactor for 2 h. The mixture was cooled to room temperature, washed with HCl (2 M aq.), dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (10% Et2O in pentane) to give compound 19 as colorless oil (781 mg, 89%). [α]D 22 = +2.0° (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 4.60, 4.61 (AB q, J = 6.4 Hz, 2H), 4.00 (dd, J = 10.5, 4.7 Hz, 1H), 3.82 (dd, J = 10.5, 6.8 Hz, 1H), 3.39 (dd, J = 9.3, 5.0 Hz, 1H), 3.34 (s, 3H), 3.23 (dd, J = 9.3, 7.0 Hz, 1H), 1.95 – 1.75 (m, 2H), 1.67 – 1.49 (m, 2H), 1.33 (m, 2H), 1.21 (s, 6H), 1.19 (s, 6H), 1.21 – 1.12 (m, 2H), 0.96 (d, J = 6.8 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H), 0.94 – 0.87 (m, 2H), 0.86 (d, J = 6.8 Hz, 3H), 0.84 (d, J = 6.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 155.95, 96.55, 73.00, 69.28, 55.03, 45.52, 41.36, 41.27, 30.79, 30.25, 27.43, 27.40, 20.91, 20.76, 18.60, 18.31. HRMS, calcd for C22H45NO4Na (M+Na+) 410.324, found 410.324.
11. (6R,8R,10S,12S,14R,16R,18S,20S)-6,8,10,12,14,16,18,20,24,24-decamethyl-23,23-diphenyl-13-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,4,22-trioxa-23-silapentacosane (5) )
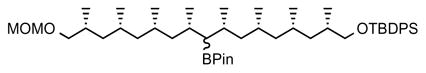
sBuLi (solution in cyclohexane, 1.4 M, 0.93 mL, 1.3 eq.) was added dropwise to a solution of carbamate 7 (0.54 g, 1.4 mmol, 1.4 eq) and TMEDA (0.21 mL, 1.4 mmol, 1.4 eq) in anhydrous Et2O (5 mL) at −78 °C. The reaction mixture was stirred for 5 h at −78 °C and then a solution of boronate 6 (0.55 g, 1.0 mmol, 1.0 eq) in anhydrous Et2O (2 mL) was added slowly and the mixture was stirred for 1 h at −78 °C. The cooling bath was removed and the reaction mixture was warmed to 40 °C and kept at this temperature for 12 h. The reaction mixture was cooled to 0 °C and diluted with KH2PO4 (1 M aq., 2 mL) and stirred for an additional10 min. The phases were separated, and the aqueous phase was extracted with ether. The combined organic layers were dried over anhydrous MgSO4, filtered, concentrated and purified by column chromatography (15% ether in pentane) to give boronate 5 as a mixture of isomers (0.53 g, 67%), as a colorless liquid. [α]D 22 = −3.0 °(c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.68–7.66 (m, 4H), 7.45 – 7.33 (m, 6H), 4.62, 4.61 (AB q, J = 6.4 Hz, 2H), 3.53 (dd, J = 9.8, 4.9 Hz, 1H), 3.47 – 3.38 (m, 2H), 3.36 (s, 3H), 3.25 (dd, J = 9.3, 7.1 Hz, 1H), 1.94 – 1.70 (m, 4H), 1.67– 1.50 (m, 4H), 1.45 – 1.27 (m, 4H), 1.28 – 1.14 (m, 2H), 1.23 (s, 12H), 1.05 (s, 9H), 0.95 (d, J = 6.4 Hz, 3H), 0.94 (d, J = 6.4 Hz, 3H), 0.92 – 0.72 (m, 25H); 13C NMR (101 MHz, CDCl3) δ 135.62, 135.60, 134.13, 134.11, 129.42, 129.41, 127.52, 127.51, 96.57, 82.65, 73.02, 68.68, 55.04, 45.49, 45.45, 44.68, 41.24, 40.88, 33.25, 30.89, 28.74, 28.72, 27.64, 27.57, 27.32, 26.89, 25.05, 21.45, 21.35, 21.13, 21.06, 19.61, 19.59, 19.31, 18.49, 18.25; HRMS, calcd for C49H89BO5SiN (M+NH4 +) 810.656, found 810.661.
12. (6R,8R,10R,12R,14S,16S,18S,20S)-6,8,10,12,14,16,18,20,24,24-decamethyl-23,23-diphenyl-2,4,22-trioxa-23-silapentacosane (19)
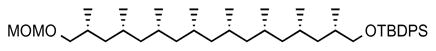
MeLi (solution in hexane, 1.6 M, 0.16 mL, 2.0 eq) was added dropwise to a solution of boronate 5 (100 mg, 0.126 mmol, 1.0 eq) in anhydrous ether (5 mL) at −78 °C. The reaction mixture was stirred for 1 h at −78 °C before being warmed to room temperature. Solvents were subsequently removed under reduced pressure in situ. Then the septum was removed and under a positive pressure of argon, Mn(OAc)3·2H2O (33.8 mg, 0.126 mmol, 1.0 eq) and 4-tertbutyl catechol (104.7 mg, 0.63 mmol, 5.0 eq.) were added to the reaction vessel. The septum was placed back and anhydrous DCE (5 mL) was added to the reaction mixture. The resulting brown solution was heated at 80 °C for 12 h. The reaction mixture was subsequently cooled to room temperature, filtered through a plug of Celite, washed with Et2O (3 × 40 mL) and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography (SiO2, 5% ether in pentane) to give the silyl ether 19 (45.5 mg, 54%) as colorless oil. [α]D 22 = −5.8° (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.80 – 7.53 (m, 4H), 7.51 – 7.27 (m, 6H), 4.63 (s, 2H), 3.52 (dd, J = 9.8, 5.0 Hz, 1H), 3.42 (m, 2H), 3.36 (s, 3H), 3.26 (dd, J = 9.3, 7.1 Hz, 1H), 1.91 – 1.78 (m, 2H), 1.64–1.48 (m, 6H), 1.42–1.32 (m, 2H), 1.26–1.15 (m, 6H), 1.06 (s, 9H), 0.95 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 6.8 Hz, 3H), 0.89 – 0.78 (m, 24H); 13C NMR (101 MHz, CDCl3) δ 135.64, 135.62, 134.12, 134.08, 129.45, 127.54, 96.59, 73.04, 68.70, 55.06, 45.51, 45.36, 45.32, 45.30, 41.36, 41.11, 33.22, 30.88, 27.73, 27.65, 27.56, 26.91, 21.49, 21.47, 21.39, 21.32, 21.17, 21.14, 19.32, 18.43, 18.23; HRMS of 19 could not be obtained due to suppressed ionization.
13. (2R,4R,6R,8R,10S,12S,14S,16S)-17-((tert-butyldiphenylsilyl)oxy)-2,4,6,8,10,12,14,16-octamethylheptadecyl diisopropylcarbamate (20)
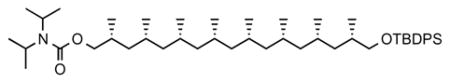
To a stirred solution of 19 (173.6 mg, 0.26 mmol) and n-PrSH (47 μL, 0.51 mmol) in CH2Cl2 (20 mL) was added ZnBr2 (58.0 mg, 0.26 mmol) at 0 °C. After stirring for 10 min at room temperature, the reaction mixture was diluted with CH2Cl2 (100 mL). The reaction was quenched with satd. NaHCO3(aq) at 0 °C, and the mixture was filtered through Celite. The organic phase was separated and the aqueous phase was further extracted with CH2Cl2. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated. The crude product was dissolved in anhyd. toluene (3 mL) and diisopropyl carbamoyl chloride (1.1 eq, 46 mg) and dry triethylamine (2.5 eq, 7 μL) were added. The reaction mixture was heated at 150 °C in a microwave reactor for 2 h. The mixture was cooled to room temperature, washed with HCl (2 M aq.), dried over MgSO4, filtered and concentrated. The product was purified by flash chromatography (10% Et2O in pentane) to give compound 20 as colorless oil (135 mg, 70%). [α]D 22 = −6.6° (c = 0.5, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.87 – 7.52 (m, 4H), 7.39 (m, 6H), 4.16–3.74 (br. s, 2H), 4.03 (dd, J = 10.6, 4.7 Hz, 1H), 3.86 (dd, J = 10.6, 6.8 Hz, 1H), 3.53 (dd, J = 9.6, 5.0 Hz, 1H), 3.42 (dd, J = 9.8, 6.3 Hz, 1H), 2.01 – 1.84 (m, 2H), 1.77–1.72 (m, 2H), 1.65–1.51 (m, 6H), 1.47 – 1.35 (m, 2H), 1.33–1.27 (m, 4H), 1.23 (s, 6H), 1.21 (s, 6H), 1.07 (s, 9H), 0.99 (d, J = 6.6 Hz, 3H), 0.95 (d, J = 6.6 Hz, 3H), 0.91 – 0.78 (m, 24H); 13C NMR (101 MHz, CDCl3) δ 155.96, 135.63, 135.61, 134.10, 134.07, 129.45, 129.44, 127.54, 127.53, 69.30, 68.69, 45.50, 45.45, 45.31, 45.26, 41.23, 41.10, 33.22, 30.32, 27.73, 27.60, 27.57, 27.54, 27.49, 26.91, 21.47, 21.42, 21.39, 21.25, 21.18, 21.00, 19.31, 18.67, 18.24, 15.28. HRMS, calcd for C48H84NO3Si (M+H+) 750.622, found 750.621 (Sohn et al., 2005).
14. (16R,17R,19R,21R,23R,25S,27S,29S,31S)-32-((tert-butyldiphenylsilyl)oxy)-17,19,21,23,25,27,29,31-octamethyldotriacontan-16-ol (21)
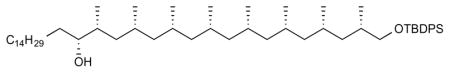
sBuLi (solution in cyclohexane, 1.4 M, 0.12 mL, 1.3 eq) was added dropwise to a solution of carbamate 20 (0.115 g, 0.153 mmol, 1.0 eq) and (+)-spartein (50.2 mg, 0.214 mmol, 1.4 eq) in anhydrous TBME (3 mL) at −60 °C. The reaction mixture was stirred for 5 h at −60 °C and then a solution of boronate (0.104 g, 0.31 mmol, 2.0 eq) in anhydrous TBME (2 mL) was added slowly and the mixture was stirred for 1 h at −60 °C. The cooling bath was removed and the reaction mixture was warmed to 70 °C and kept at this temperature for 16 h. The reaction mixture was cooled to 0 °C and quenched with 2 mL of H2O2 (1 mL of H2O2 in 4 mL of 1 M NaOH) and stirred for 30 min at rt. The reaction mixture was washed with ether and the combined organic layers were dried over anhydrous MgSO4, filtered, concentrated and purified by column chromatography (6% ether in pentane) to give alcohol 21 (51.1 mg, 40%) as colorless liquid. [α]D 22 = +8.7° (c = 0.2, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.91 – 7.56 (m, 4H), 7.40 (m, 6H), 3.62 – 3.49 (m, 2H), 3.43 (ddd, J = 9.7, 6.4, 2.5 Hz, 1H), 1.75 (dt, J = 11.9, 4.1 Hz, 2H), 1.66 – 1.50 (m, 8H), 1.43 (m, 6H), 1.30–1.21 (m, 29H), 1.07 (s, 9H), 0.95 (d, J = 6.8 Hz, 3H), 0.96 – 0.73 (m, 30H). 13C NMR (101 MHz, CDCl3) δ 135.64, 135.61, 134.12, 134.09, 129.44, 127.53, 74.31, 68.70, 45.50, 45.30, 45.27, 41.14, 41.10, 35.09, 34.91, 33.22, 31.93, 30.33, 29.78, 29.70, 29.66, 29.64, 29.37, 27.72, 27.68, 27.65, 27.62, 27.58, 27.55, 26.90, 26.38, 22.70, 21.50, 21.48, 21.45, 21.38, 21.26, 21.16, 19.31, 18.23, 14.13, 14.02. HRMS, calcd for C56H100NaO2Si (M+Na+) 855.739, found 855.738.
Deprotection of 21 followed by oxidation to hydroxyphthioceranic acid 3 was carried out according to our previous work (Geerdink, Horst, Lepore, Mori, Puzo, Anna K H Hirsch, et al., 2013).
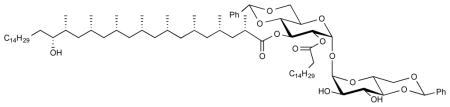
15. (2R,4aR,6R,7R,8S,8aR)-7-(palmitoyloxy)-2-phenyl-6-(((5aR,6R,7aR,10R,11aR,11bS)-2,2,4,4-tetraisopropyl-10-phenylhexahydro-[1,3]dioxino[4′,5;:5,6]pyrano[3,4-f][1,3,5,2,4]trioxadisilepin-6-yl)oxy)hexahydropyrano[3,2-d][1,3]dioxin-8-yl (2S,4S,6S,8S,10R,12R,14R,16R,17R)-17-hydroxy-2,4,6,8,10,12,14,16-octamethyldotriacontanoate (23)

To hydroxyphthioceranic acid 3 (97.5 mg, 0.16 mmol) in benzene (5.0 mL) was added Et3N (2.1 eq, 48 μL, 0.34 mmol) and 2,4,6-trichlorobenzoyl chloride (1.05 eq, 26 μL, 0.17 mmol). After 1 h, protected trehalose (4.0 eq, 639.6 mg, 0.64 mmol) in 5.0 mL of benzene and DMAP (1.1 eq, 21.5 mg, 0.18 mmol) were added. The reaction was stirred for 48 h and quenched with an aq. saturated solution of NaHCO3. The layers were separated and the aqueous layer was extracted with EtOAc. The combined organic layers were dried over MgSO4 and all volatiles were evaporated. The crude product was purified using flash chromatography (gradient elution, 15, 17, 19% ether in pentane) to give pure 23 as a colorless oil (174.1 mg, 68%). [α]D 22 = +27.4° (c = 1.0, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.44 (m, 2H), 7.41 – 7.36 (m, 2H), 7.31 (m, 6H), 5.65 (t, J = 9.8 Hz, 1H), 5.52 (s, 1H), 5.45 (s, 1H), 5.36 (d, J = 3.9 Hz, 1H), 5.11 (d, J = 4.0 Hz, 1H), 5.01 (dd, J = 10.0, 3.6 Hz, 1H), 4.29 (m, 1H), 4.24 – 4.16 (m, 2H), 4.12 (dd, J = 10.0, 4.7 Hz, 1H), 3.89 (dd, J = 8.5, 4.1 Hz, 1H), 3.85 – 3.74 (m, 1H), 3.73 – 3.60 (m, 3H), 3.50 (t, J = 9.1 Hz, 1H), 3.15 – 3.04 (m, 1H), 2.61 – 2.49 (m, 2H), 2.32 (m, 3H), 1.90 – 1.70 (m, 2H), 1.60–1.50 (m, 9H), 1.42 (s, 12H), 1.33 – 0.96 (m, 79H), 0.90 – 0.68 (m, 30H); 13C NMR (101 MHz, CDCl3) δ 175.19, 173.13, 137.60, 137.06, 128.79, 128.55, 127.96, 127.92, 126.11, 125.89, 101.41, 101.08, 94.42, 91.90, 81.09, 79.42, 75.24, 74.27, 73.40, 70.88, 68.72, 68.38, 65.83, 62.81, 62.51, 45.55, 45.31, 45.24, 44.92, 41.11, 39.80, 37.56, 35.07, 34.89, 33.90, 31.92, 30.31, 29.77, 29.71, 29.69, 29.67, 29.65, 29.63, 29.55, 29.47, 29.37, 29.36, 29.14, 29.10, 27.82, 27.56, 27.53, 27.51, 27.34, 27.03, 26.37, 24.61, 22.69, 21.39, 21.37, 21.24, 21.10, 20.68, 20.57, 18.54, 17.44, 17.39, 17.31, 17.20, 17.14, 17.11, 16.99, 15.26, 14.11, 14.01, 12.89, 12.64, 12.28, 11.70. HRMS, calcd for C94H164O15Si2Na (M+Na+) 1613.154, found 1613.155.
16.(2R,4aR,6R,7R,8S,8aR)-6-(((2R,4aR,6R,7R,8R,8aS)-7,8-dihydroxy-2-phenylhexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-7-(palmitoyloxy)-2-phenylhexahydropyrano[3,2-d][1,3]dioxin-8-yl (2S,4S,6S,8S,10R,12R,14R,16R,17R)-17-hydroxy-2,4,6,8,10,12,14,16-octamethyldotriacontanoate (24)
To compound 23 (167 mg, 0.105 mmol) in THF (4.2 mL) was added TBAF (40 eq, 4.2 mL, 4.2 mmol, 1 M solution in THF, acidified to pH = 6.5 with TFA). The mixture was kept at 40 °C for 24 h and afterwards diluted with EtOAc and the organic layer was washed with 10 mL of water and then dried over MgSO4. After all volatiles were evaporated, the product was purified using flash column chromatography (50% EtOAc in pentane) to afford pure 24 (112.1 mg, 79%) as a colorless oil. [α]D 22 = +36.9° (c = 1.3, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.49 – 7.44 (m, 2H), 7.43 – 7.39 (m, 2H), 7.38 – 7.30 (m, 6H), 5.64 (t, J = 9.9 Hz, 1H), 5.51 (s, 1H), 5.50 (s, 1H), 5.40 (d, J = 4.0 Hz, 1H), 5.18 (d, J = 3.8 Hz, 1H), 5.06 (dd, J = 10.0, 4.0 Hz, 1H), 4.32 (dd, J = 10.2, 5.0 Hz, 1H), 4.24 – 3.98 (m, 3H), 3.85–3.79 (m, 1H), 3.78 – 3.64 (m, 4H), 3.51 (t, J = 9.3 Hz, 1H), 3.47 (br. s, 1H), 2.61 (m, 1H), 2.45 (br. s, 2H), 2.41 – 2.24 (m, 2H), 1.83–1.76 (m, 1H), 1.66 – 1.52 (m, 9H), 1.45 – 1.13 (m, 58H), 1.05 –0.73 (m, 38H); 13C NMR (101 MHz, CDCl3) δ 175.52, 172.93, 136.94, 136.76, 129.21, 128.87, 128.22, 128.11, 128.09, 126.26, 125.91, 101.92, 101.19, 94.75, 92.36, 80.84, 79.34, 74.35, 72.20, 71.18, 70.63, 68.63, 68.34, 63.27, 63.04, 45.52, 45.29, 45.24, 45.11, 41.11, 39.86, 37.60, 35.01, 34.85, 33.88, 31.91, 29.76, 29.68, 29.66, 29.64, 29.56, 29.50, 29.35, 29.20, 27.95, 27.61, 27.56, 27.52, 27.45, 27.07, 26.34, 24.63, 22.68, 21.45, 21.41, 21.22, 21.15, 20.70, 20.51, 18.70, 14.11, 13.96. HRMS, calcd for C82H142O14N (M+NH4 +) 1365.043, found 1365.042.
17. (2R,4aR,6R,7R,8S,8aR)-6-(((2R,4aR,6R,7R,8S,8aS)-8-hydroxy-2-phenyl-7-(((2,2,2-trichloroethoxy)sulfonyl)oxy)hexahydropyrano[3,2-d][1,3]dioxin-6-yl)oxy)-7-(palmitoyloxy)-2-phenylhexahydropyrano[3,2-d][1,3]dioxin-8-yl (2S,4S,6S,8S,10R,12R,14R,16R,17R)-17-hydroxy-2,4,6,8,10,12,14,16-octamethyldotriacontanoate (25)
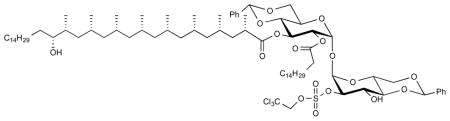
To compound 24 (112 mg, 83 μmol) in DCM (5.5 mL) was added 2,2,2-trichloroethyl sulfuryl 2-methylimidazolium triflate (2 eq, 76 mg, 0.17 mmol, prepared according to literature)(Ingram et al., 2009). The mixture was cooled to 0 °C and 1,2-dimethylimidazole (2.5 eq, 20.0 mg, 0.21 mmol) was added as solution in DCM (2.5 mL) over 4 h. The reaction was allowed to slowly reach rt after which it was stirred for 48 h. The mixture was diluted with DCM and the organic layer was washed with brine (10 mL). The organic layer was dried over MgSO4 and all volatiles were evaporated. The crude mixture was purified using flash chromatography (15% EtOAc in pentane) to afford pure 25 as colorless oil (89.4 mg, 69%). [α]D 22 = +39.8° (c = 1.1, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.50 – 7.41 (m, 4H), 7.40 – 7.35 (m, 3H), 7.31 (dd, J = 5.1, 1.9 Hz, 3H), 5.62 (t, J = 9.9 Hz, 1H), 5.52 (s, 1H), 5.49 (s, 1H), 5.46 (d, J = 3.8 Hz, 1H), 5.36 (d, J = 4.0 Hz, 1H), 5.08 (dd, J = 10.1, 4.0 Hz, 1H), 5.00, 4.83 (AB system, J = 10.7 Hz, 2H), 4.61 (dd, J = 9.6, 3.8 Hz, 1H), 4.43 (dd, J = 10.2, 5.0 Hz, 1H), 4.36 (t, J = 9.5 Hz, 1H), 4.21–4.14 (m, 2H), 4.02 – 3.89 (m, 1H), 3.72 (t, J = 10.4 Hz, 2H), 3.69 (t, J = 10.4 Hz, 1H), 3.56 (t, J = 9.5 Hz, 1H), 3.51 – 3.43 (m, 1H), 3.26 (br. s, 1H), 2.71 – 2.47 (m, 1H), 2.35 (t, J = 7.8 Hz, 2H), 1.79 (m, 1H), 1.66 – 1.49 (m, 9H), 1.47 – 1.07 (m, 68H), 0.84 (m, 30H); 13C NMR (101 MHz, CDCl3) δ 175.40, 172.75, 136.91, 136.43, 129.48, 128.77, 128.34, 127.99, 126.22, 126.09, 102.24, 101.26, 94.17, 93.73, 92.49, 81.08, 80.89, 80.02, 79.00, 77.19, 74.34, 70.56, 68.46, 68.36, 68.15, 63.40, 62.79, 45.52, 45.28, 45.26, 45.17, 41.11, 39.94, 37.60, 34.98, 34.81, 33.83, 31.91, 29.76, 29.69, 29.66, 29.65, 29.63, 29.55, 29.49, 29.35, 29.21, 29.19, 27.97, 27.63, 27.56, 27.49, 27.12, 26.33, 24.62, 22.68, 21.50, 21.42, 21.21, 21.16, 20.73, 20.50, 18.67, 14.11, 13.96. HRMS, calcd for C84H138Cl3O17S (M−H+) 1555.873, found 1555.873.
18. Sodium (2R,3R,4S,5S,6R)-4,5-dihydroxy-2-(((2R,3R,4S,5R,6R)-5-hydroxy-4-(((2S,4S,6S,8S,10R,12R,14R,16R,17R)-17-hydroxy-2,4,6,8,10,12,14,16-octamethyldotriacontanoyl)oxy)-6-(hydroxymethyl)-3-(palmitoyloxy)tetrahydro-2H-pyran-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3-yl sulfate (AM Ac2SGL)
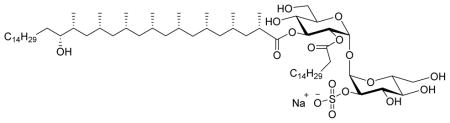
To compound 25 (46.7 mg, 0.03 mmol) was added DCM (2 mL) and MeOH (4 mL). Ammonium formate (20 eq, 37.8 mg, 0.6 mmol) was added and the mixture was stirred until all ammonium formate dissolved. Pd(OH)2 (0.5 eq, 10 mg, 20% weight on carbon) and Pd/C (0.5 eq, 16 mg, 10% weight on carbon) was added and the mixture was placed under 1 bar of H2 atmosphere (balloon) using three vacuum/N2 cycles followed by four vacuum/H2 cycles. The reaction was monitored and after 36 h showed complete disappearance of the starting material. The mixture was filtered over Celite, concentrated, the crude was triturated with ether and CHCl3 and the supernatant separated carefully with a pipette to get the ammonium salt as a white solid. The ammonium salt was flushed over a DOWEX Na+ ion-exchange column (DCM/acetone/MeOH) to give the pure sodium salt as a white solid (27.2 mg, 71%). 1H NMR (400 MHz, CD3OD/CDCl3) δ 5.47 (d, J = 3.6 Hz, 1H), 5.42 (t, J = 9.8 Hz, 1H), 5.29 (d, J = 3.6 Hz, 1H), 4.88 (dd, J = 10.2, 3.5 Hz, 1H), 4.19 (dd, J = 9.0, 3.0 Hz, 2H), 3.95 – 3.84 (m, 2H), 3.70 (m, 4H), 3.59 (t, J = 9.6 Hz, 1H), 3.50 – 3.37 (m, 2H), 2.60 (m, 2H), 2.32 (m, 4H), 1.76 (d, J = 12.3 Hz, 3H), 1.59 (m, 9H), 1.29 (d, J = 6.6 Hz, 58H), 1.04 – 0.75 (m, 32H); 13C NMR (101 MHz, CD3OD/CDCl3) δ 180.57, 176.80, 96.80, 95.87, 80.66, 77.87, 76.64, 76.10, 75.85, 75.22, 74.67, 74.02, 72.77, 64.82, 64.57, 49.51, 49.33, 49.26, 48.94, 45.41, 44.23, 41.14, 39.10, 38.29, 37.42, 35.63, 35.61, 33.48, 33.37, 33.36, 33.33, 33.30, 33.28, 33.25, 33.20, 33.04, 33.01, 32.90, 31.85, 31.63, 31.38, 31.05, 29.88, 28.25, 26.30, 26.28, 24.76, 24.69, 24.51, 24.42, 24.16, 23.87, 21.51, 17.53, 17.10, 17.08. HRMS, calcd for C68H129O17SNa2 (M+Na+) 1295.874, found 1295.875.
Generation of SGL-loaded CD1b tetramers
Soluble biotinylated CD1b monomers were provided by the National Institutes of Health Tetramer Core Facility (Emory University, Atlanta, GA). The loading protocol for CD1b monomers was based on previously published glucose monomycolate loading protocols (Kasmar et al., 2011). Natural Ac2SGL was dried down in a glass tube in a stream of nitrogen and sonicated into a 50 mM sodium citrate buffer at pH 4, containing 0.25% with 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) (Sigma, St. Louis, MO) for two minutes at 37°C. The sonicate was transferred to a microfuge tube, and 20 μg of CD1b monomer was added and incubated in a 37°C water bath for 2 hours with vortexing every 30 minutes. At the end of the incubation, the solution was neutralized to pH 7.4 with 6 μl of 1M Tris pH 9. For SL37 Ac2SGL and AM Ac2SGL, the sonication was performed in 50 mM sodium citrate buffer at pH 7.4, containing 10 mM taurocholate (Sigma, St. Louis, MO) for 30 minutes at 37°C. After addition of CD1b, the mixture was incubated in a 37°C water bath for 2 hours. Finally, 10 μl of Streptavidin conjugated to allophycocyanin or phycoerythrin (Life Technologies, Carlsbad, CA) was added in ten aliquots of 1 μl every 10 minutes. The final product was filtered through a SpinX column (Sigma, St. Louis, MO) to remove aggregates and stored at 4°C until use.
TCR transduction
TCR constructs containing TCRα and TCRβ separated by a 2A linker were synthesized (Biocat, Germany) and inserted into pMIG2 vectors as previously described (Holst et al., 2006). Human embryonic kidney (293T) cells were transfected with TCR expression vector, pMIG expression vector containing CD3δγεζ sequence, packaging vectors pEQ-Pam3(−E) and pVSV-G using FuGENE 6 transfection reagent (Promega)(Szymczak et al., 2004). Retrovirus containing supernatant was collected twice daily and used to transduce the TCR-deficient T cell line Jurkat 76 (male) in the presence of Polybrene (Sigma-Aldrich, St. Louis, MO) for a total of 4 days. Transduction data are representative of four independent experiments.
IFN-γ ELISPOT
We used an IFN-γ ELISPOT to determine the antigen specificity of our T cell lines. EMD Multiscreen-IP filter plates (Millipore, Billerica, MA) were coated with 1D1K antibody (Mabtech, Sweden) diluted 1:400 in PBS and incubated overnight at 4°C. The following day, one thousand T cells were plated 1:50 with K562 cells stably transfected with CD1b or with a mock vector. Lipids antigens were stored in 2:1 chloroform:methanol and appropriate amount was dried using gaseous nitrogen. The lipids were sonicated into media to obtain a 4 μg/mL suspension and plated with the cells at a final concentration of 1 μg/mL. These cultures were incubated at 37°C for approximately 16 hours. The following day, the cells were washed twice with sterile water to lyse the cells, and the plates were incubated with the detection antibody 7-B6-1-biotin (Mabtech, Sweden) diluted 1:1000 in PBS + 0.5% FCS and incubated for two hours at room temperature. The cells were then washed five times with PBS and incubated in ExtrAvidin-Alkaline Phosphatase (Sigma, St. Louis, MO) diluted 1:1000 in PBS and incubated for one hour at room temperature. The wells were then washed five times and incubated with BCIP/NBT substrate (Sigma, St. Louis, MO) for five minutes to develop the membrane. The wells and IFN-γ spots were counted using an ImmunoSpot S6 Core Analyzer (Cellular Technology Limited, Cleveland, OH). ELISPOT assay results are reported as the mean of triplicate wells. Representative data was shown from at least two independent replicates.
T cell receptor sequencing
High-throughput sequencing of TCRs of cell line A01 was performed using the ImmunoSEQ assay (Adaptive Biotechnologies, Seattle, WA) with TCR-β and/or TCR-α/δ assays for each sample using a multiplex PCR approach following by Illumina high-throughput sequencing (Robins et al., 2010; Carlson et al., 2013). The TCRs of C56SL37 and C58SL37 were sequenced by a single cell approach (Wang et al., 2012).
Production of wild-type and mutant CD1b-expressing C1R cells
Gene segments encoding full-length wild-type CD1b or single-site mutant CD1b (E62A, E65A, I69A, R71A, V72A, F75A, R79A, E80A, D83A, Q150A, Y151A, E156A, I160A, E164A, T165A and R168A) were purchased from Thermo Fisher Scientific (Waltham, MA), cloned into the pMIG retroviral vector and transfected into HEK293T (female) cells to produce CD1b containing retrovirus. C1R (female) cells were transduced with retroviral supernatants as previously described (Birkinshaw et al., 2015). Each of the CD1b transduced cell lines were stained with an anti-CD1b monoclonal antibody (SN13; Biolegend, San Diego, CA) and sorted by flow cytometry with a FACSAria III Cell Sorter (BD Biosciences, San Jose, CA) to ensure each line expressed similar levels of CD1b. Data are representative of three independent experiments with triplicate wells. Error bars represent SEM of triplicate wells.
QUANTIFICATION AND STATISTICAL ANALYSIS
Error bars reported represent the standard error of the mean (SEM) or standard deviation (SD), as reported in the Figure legends. SEM and SD were calculated using GraphPad Prism (v.6.0). Estimated EC50 values were calculated based on a nonlinear regression model of the means of triplicate wells in GraphPad Prism (v.6.0). Our data do not violate assumptions of the non-linear regression model. Information regarding experimental replicates can be found in the Methods subheading of STAR Methods.
Scheme 2.
a) (EtO)2POCH2COMe, n-BuLi, THF, rt, 85%; b) MeMgBr, R, S-Josiphos, CuBr.SMe2, t-BuOMe, −75°C, 91%; c) 1. (CF3CO)2O, sodium percarbonate, DCM, rt, 2. K2CO3, MeOH, rt, 82%; d) (iPr)2NCOCl, NEt3, toluene, 150 °C, 83%; e) Sec. BuLi, TMEDA, PinBH, ether, −78°C, 55%; f) EtOCb, Sec. BuLi, (−)-Spartein, ether, −78°C, 62%.
SIGNIFICANCE.
Sulfoglycolipids (SGLs) are a class of bacterial cell wall lipid that is uniquely synthesized by Mycobacterium tuberculosis (M.tb) and recognized by human T-cells. Determining whether synthetic SGLs are bioequivalent to the natural mixture is essential to developing them into clinical products. We describe a new hybrid synthesis for key antigenic determinants of SGL that results in higher yields than previous schemes. We also report a bioequivalence hierarchy of synthetic SGLs and the development of SGL-specific T-cell probes called tetramers. These reagents can now be applied to large-scale translational studies investigating SGLs as vaccine agents or the diagnostic potential of SGL-specific T-cells.
HIGHLIGHTS.
We describe hybrid synthesis of a mycobacterial sulfoglycolipid T-cell antigen.
This sulfoglycolipid (SGL) analog is bioequivalent to the natural compound.
This antigen was used to generate SGL-loaded CD1b tetramers.
Tetramers can be used to identify SGL-specific T-cells in peripheral blood.
Acknowledgments
The work was supported by the University of Washington Department of Medicine and Royalty Research Fund (C.S.), U.S. National Institutes of Health (R01 AI-125189 to C.S.), and Doris Duke Charitable Foundation Clinical Scientist Development Award (C.S.). Bill and Melinda Gates Foundation Vaccine Accelerator Award to D.B.M. The National Health and Medical Research Council of Australia (NHMRC 1113293) and the Australian Research Council (ARC; CE140100011) to D.I.G. DIG is also supported by NHMRC Senior Principal Research Fellowship (1117766). C.A.J was supported by the Molecular Medicine Training Grant (T32 GM095421 07). We thank Prof. C. Schneider, University of Leipzig, for a donation of phthioceranic acid.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, I.V.R. and C.S.; Investigation, C.A.J., K.K.Q.Y., F.J.R, and I.V.R.; Resources, M.G., J.P., A.J.M., V.R.Y., Z.Z.M., E.D, M.K., V.K.A, R.O.E., T.J.S., M.N.T.S., D.I.G., D.G.P.; Writing – Original Draft, I.V.R. and C.S. Writing – Review and Editing, I.V.R., A.J.M, C.A.J., C.S., D.B.M., D.G, M.G., and J.P.; Funding and Project management, D.B.M., C.S; Supervision, I.V.R. and C.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, Day CL, Ray SM, Rengarajan J. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. Journal of Clinical Investigation. 2015;125(5):1827–1838. doi: 10.1172/JCI77990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, Fernandez C, Parra A, Cardona PJ, Vilaplana C, Ausina V, Williams A, Clark S, Malaga W, Guilhot C, Gicquel B, Martin C. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–4873. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Beckman EM, Porcelli Sa, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Birkinshaw RW, Pellicci DG, Cheng TY, Keller AN, Sandoval-Romero M, Gras S, de Jong A, Uldrich AP, Moody DB, Godfrey DI, Rossjohn J. alphabeta T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat Immunol. 2015 Feb 03;16(3):258–266. doi: 10.1038/ni.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch M, Herzmann C, Kallert S, Zimmermann A, Hofer C, Mayer D, Zenk SF, Muche R, Lange C, Bloom BR, Modlin RL, Stenger S. Lipoarabinomannan-responsive polycytotoxic T cells are associated with protection in human tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2016;194(3):345–355. doi: 10.1164/rccm.201509-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung M-W, Parsons JM, Steen MS, LaMadrid-Herrmannsfeldt Ma, Williamson DW, Livingston RJ, Wu D, Wood BL, Rieder MJ, Robins H. Using synthetic templates to design an unbiased multiplex PCR assay. Nature communications. 2013;4:2680. doi: 10.1038/ncomms3680. [DOI] [PubMed] [Google Scholar]
- Casas-Arce E, ter Horst B, Feringa BL, Minnaard AJ. Asymmetric total synthesis of PDIM A: a virulence factor of Mycobacterium tuberculosis. Chemistry. 2008;14(14):4157–4159. doi: 10.1002/chem.200800243. [DOI] [PubMed] [Google Scholar]
- Chesne-Seck ML, Barilone N, Boudou F, Asensio JG, Kolattukudy PE, Martín C, Cole ST, Gicquel B, Gopaul DN, Jackson M. A point mutation in the two1166 component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. Journal of Bacteriology. 2008;190(4):1329–1334. doi: 10.1128/JB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: What is the absolute effect of BCG and non-tuberculous mycobacteria? International Journal of Tuberculosis and Lung Disease. 2006:1192–1204. doi: 10.1136/OEM.2006.028068. [DOI] [PubMed] [Google Scholar]
- Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nature immunology. 2002;3(8):721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- Garcia-Alles LF, Collmann A, Versluis C, Lindner B, Guiard J, Maveyraud L, Huc E, Im JS, Sansano S, Brando T, Julien S, Prandi J, Gilleron M, Porcelli SA, de la Salle H, Heck AJ, Mori L, Puzo G, Mourey L, De Libero G. Structural reorganization of the antigen-binding groove of human CD1b for presentation of mycobacterial sulfoglycolipids. Proc Natl Acad Sci U S A. 2011 Oct 19;108(43):17755–17760. doi: 10.1073/pnas.1110118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau B, Lemétais A, Lepore M, Garcia-Alles LF, Bourdreux Y, Mori L, Gilleron M, De Libero G, Puzo G, Beau JM, Prandi J. Simplified deoxypropionate acyl chains for mycobacterium tuberculosis sulfoglycolipid analogues: Chain length is essential for high antigenicity. ChemBioChem. 2013;14(18):2413–2417. doi: 10.1002/cbic.201300482. [DOI] [PubMed] [Google Scholar]
- Geerdink D, Horst Bter Lepore M, Mori L, Puzo G, Hirsch AKH, Gilleron M, de Libero G, Minnaard AJ. Total synthesis, stereochemical elucidation and biological evaluation of Ac 2 SGL; a 1,3-methyl branched sulfoglycolipid from Mycobacterium tuberculosis. Chem Sci. 2013;4(2):709–716. doi: 10.1039/C2SC21620E. [DOI] [Google Scholar]
- Geerdink D, Horst B, ter Lepore M, Mori L, Puzo G, Hirsch AKH, Gilleron M, de Libero G, Minnaard AJ. Chem Sci. 2. Vol. 4. The Royal Society of Chemistry; 2013. Total synthesis{,} stereochemical elucidation and biological evaluation of Ac2SGL; a 1{,}3-methyl branched sulfoglycolipid from Mycobacterium tuberculosis; pp. 709–716. [DOI] [Google Scholar]
- Geerdink D, Minnaard AJ. Total synthesis of sulfolipid-1. Chem Commun (Camb) 2014 Jan 21;50(18):2286–2288. doi: 10.1039/c3cc48087a. [DOI] [PubMed] [Google Scholar]
- Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated Sulfoglycolipids Are Novel Mycobacterial Antigens Stimulating CD1-restricted T Cells during Infection with Mycobacterium tuberculosis. The Journal of Experimental Medicine J Exp Med. 2004;36491100(5):649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren MB. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv II. Structural studies. Biochimica et Biophysica Acta (BBA)/Lipids and Lipid Metabolism. 1970;210(1):127–138. doi: 10.1016/0005-2760(70)90068-8. [DOI] [PubMed] [Google Scholar]
- Goren MB, Brokl O, Das BC, Lederer E. Sulfolipid I of Mycobacterium tuberculosis, strain H37RV. Nature of the acyl substituents. Biochemistry. 1971;10(1):72–81. doi: 10.1021/bi00777a012. [DOI] [PubMed] [Google Scholar]
- Goren MB, Brokl O, Schaefer WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: Correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infection and Immunity. 1974;9(1):142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard J, Collmann A, Garcia-Alles LF, Mourey L, Brando T, Mori L, Gilleron M, Prandi J, De Libero G, Puzo G. Fatty acyl structures of mycobacterium tuberculosis sulfoglycolipid govern T cell response. Journal of immunology (Baltimore, Md: 1950) 2009;182(11):7030–7037. doi: 10.4049/jimmunol.0804044. [DOI] [PubMed] [Google Scholar]
- Guiard J, Collmann A, Gilleron M, Mori L, De Libero G, Prandi J, Puzo G. Synthesis of diacylated trehalose sulfates: Candidates for a tuberculosis vaccine. Angewandte Chemie - International Edition. 2008;47(50):9734–9738. doi: 10.1002/anie.200803835. [DOI] [PubMed] [Google Scholar]
- Han M, Hannick LI, DiBrino M, Robinson Ma. Polymorphism of human CD1 genes. Tissue antigens. 1999;54(2):122–127. doi: 10.1034/j.1399-0039.1999.540202.x. [DOI] [PubMed] [Google Scholar]
- Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DAA. Generation of T-cell receptor retrogenic mice. Nature Protocols. 2006;1(1):406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- ter Horst B, Feringa BL, Minnaard AJ. Catalytic asymmetric synthesis of mycocerosic acid. Chemical communications (Cambridge, England) 2007a;(5):489–91. doi: 10.1039/b612593j. [DOI] [PubMed] [Google Scholar]
- ter Horst B, Feringa BL, Minnaard AJ. Catalytic asymmetric synthesis of phthioceranic acid, a heptamethyl-branched acid from Mycobacterium tuberculosis. Organic letters. 2007b;9(16):3013–5. doi: 10.1021/ol071078o. [DOI] [PubMed] [Google Scholar]
- Ingram LJ, Desoky A, Ali AM, Taylor SD. O- and N-sulfations of carbohydrates using sulfuryl imidazolium salts. The Journal of organic chemistry. 2009;74(17):6479–85. doi: 10.1021/jo9014112. [DOI] [PubMed] [Google Scholar]
- Kasmar AG, van Rhijn I, Cheng T-Y, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB. CD1b tetramers bind T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. Journal of Experimental Medicine. 2011;208(9):1741–1747. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrouy-Maumus G, Layre E, Clark S, Prandi J, Rayner E, Lepore M, de Libero G, Williams A, Puzo G, Gilleron M. Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine. 2017;35:1395–1402. doi: 10.1016/j.vaccine.2017.01.079. [DOI] [PubMed] [Google Scholar]
- Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M. Chemistry and Biology. 1. Vol. 16. Elsevier Ltd; 2009. Mycolic Acids Constitute a Scaffold for Mycobacterial Lipid Antigens Stimulating CD1-Restricted T Cells; pp. 82–92. [DOI] [PubMed] [Google Scholar]
- Layre E, Paepe DC-D, Larrouy-Maumus G, Vaubourgeix J, Mundayoor S, Lindner B, Puzo G, Gilleron M. Deciphering sulfoglycolipids of Mycobacterium tuberculosis. Journal of lipid research. 2011;52(6):1098–110. doi: 10.1194/jlr.M013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layre E, Sweet L, Hong S, Madigan CA, Desjardins D, Young DC, Cheng TY, Annand JW, Kim K, Shamputa IC, McConnell MJ, Debono CA, Behar SM, Minnaard AJ, Murray M, Barry CE, Matsunaga I, Moody DB. Chemistry and Biology. 12. Vol. 18. Elsevier Ltd; 2011. A comparative lipidomics platform for chemotaxonomic analysis of mycobacterium tuberculosis; pp. 1537–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Krause R, Schreiber J, Mollenkopf HJ, Kowall J, Stein R, Jeon BY, Kwak JY, Song MK, Patron JP, Jorg S, Roh K, Cho SN, Kaufmann SHE. Mutation in the Transcriptional Regulator PhoP Contributes to Avirulence of Mycobacterium tuberculosis H37Ra Strain. Cell Host and Microbe. 2008;3(2):97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Leigh CD, Bertozzi CR. Synthetic studies toward Mycobacterium tuberculosis sulfolipid-I. The Journal of organic chemistry. 2008;73(3):1008–17. doi: 10.1021/jo702032c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López F, van Zijl AW, Minnaard AJ, Feringa BL. Highly enantioselective Cu-catalysed allylic substitutions with Grignard reagents. Chemical communications (Cambridge, England) 2006;(4):409–11. doi: 10.1039/b513887f. [DOI] [PubMed] [Google Scholar]
- Madduri AVR, Minnaard AJ. Formal synthesis of the anti-angiogenic polyketide (−)-borrelidin under asymmetric catalytic control. Chemistry (Weinheim an der Bergstrasse, Germany) 2010;16(38):11726–31. doi: 10.1002/chem.201001284. [DOI] [PubMed] [Google Scholar]
- Des Mazery R, Pullez M, López F, Harutyunyan SR, Minnaard AJ, Feringa BL. An iterative catalytic route to enantiopure deoxypropionate subunits: asymmetric conjugate addition of grignard reagents to alpha, beta-unsaturated thioesters. Journal of the American Chemical Society. 2005;127(28):9966–7. doi: 10.1021/ja053020f. [DOI] [PubMed] [Google Scholar]
- Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, Besra GS, Porcelli SA. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278(5336):283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- Nikolic NA, Beak P. (R)-(+)-2-(DIPHENYLHYDROXYMETHYL)PYRROLIDINE [2-Pyrrolidinemethanol, α,α-diphenyl-, (R)-] Organic Syntheses. 1997;74(23) [Google Scholar]
- Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clinical Microbiology Reviews. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischl MC, Weise CF, Müller MA, Pfaltz A, Schneider C. A convergent and stereoselective synthesis of the glycolipid components phthioceranic acid and hydroxyphthioceranic acid. Angewandte Chemie - International Edition. 2013;52(34):8968–8972. doi: 10.1002/anie.201303776. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Morita C, Brenner M. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360(6404):593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Rasappan R, Aggarwal VK. Synthesis of hydroxyphthioceranic acid using a traceless lithiation–borylation–protodeboronation strategy. Nature Chemistry. 2014;6(9):810–814. doi: 10.1038/nchem.2010. [DOI] [PubMed] [Google Scholar]
- Van Rhijn I, Gherardin Na, Kasmar A, de Jager W, Pellicci DG, Kostenko L, Tan LL, Bhati M, Gras S, Godfrey DI, Rossjohn J, Moody DB. TCR bias and affinity define two compartments of the CD1b-glycolipid-specific T Cell repertoire. Journal of immunology (Baltimore, Md: 1950) 2014;192(9):4054–60. doi: 10.4049/jimmunol.1400158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nature immunology. 2013;14(7):706–13. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science (New York, NY) 1992;257(5067):238–41. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor \beta -chain diversity in \alpha\beta T cells. Blood. 2010;114(19):4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling Pa, Frederique D, Modlin RL, Porcelli Sa, Furlong ST, Brenner MB. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. Journal of immunology (Baltimore, Md: 1950) 1999;162(1):366–371. [PubMed] [Google Scholar]
- Sarpe VA, Kulkarni SS. Expeditious synthesis of Mycobacterium tuberculosis sulfolipids SL-1 and Ac2SGL analogues. Organic letters. 2014;16(21):5732–5. doi: 10.1021/ol5027987. [DOI] [PubMed] [Google Scholar]
- Sartain MJ, Dick DL, Rithner CD, Crick DC, Belisle JT. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel ‘Mtb LipidDB’. Journal of lipid research. 2011;52(5):861–72. doi: 10.1194/jlr.M010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri C, Lin L, Scriba TJ, Peterson G, Freidrich D, Frahm N, DeRosa SC, Moody DB, Prandi J, Gilleron M, Mahomed H, Jiang W, Finak G, Hanekom WA, Gottardo R, McElrath MJ, Hawn TR. T Cell Responses against Mycobacterial Lipids and Proteins Are Poorly Correlated in South African Adolescents. Journal of immunology (Baltimore, Md: 1950) 2015;195(10):4595–4603. doi: 10.4049/jimmunol.1501285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269(5221):227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Sohn J-H, Waizumi N, Zhong HM, Rawal VH. Total synthesis of mycalamide A. Journal of the American Chemical Society. 2005;127(20):7290–1. doi: 10.1021/ja050728l. [DOI] [PubMed] [Google Scholar]
- Spertini F, Audran R, Chakour R, Karoui O, Steiner-Monard V, Thierry AC, Mayor CE, Rettby N, Jaton K, Vallotton L, Lazor-Blanchet C, Doce J, Puentes E, Marinova D, Aguilo N, Martin C. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: A randomised, double-blind, controlled phase I trial. The Lancet Respiratory Medicine. 2015;3(12):953–962. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette a, Brenner MB, Porcelli Sa, Bloom BR, Modlin RL. Differential effects of cytolytic T cell subsets on intracellular infection. Science (New York, NY) 1997;276(5319):1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DAA. Correction of multi-gene deficiency in vivo using a single ‘self1337 cleaving’ 2A peptide-based retroviral vector. Nature biotechnology. 2004;22(5):589–94. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- Wang GC, Dash P, Mccullers JA, Doherty PC, Thomas PG. T Cell Receptor ab Diversity Inversely Correlates with Pathogen-Specific Antibody Levels in Human Cytomegalovirus Infection. no date doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. TUBERCULOSIS: Global Tuberculosis Report 2016 2016 [Google Scholar]
- Wu Z, Harutyunyan SR, Minnaard AJ. Total synthesis of (R,R,R)-γ-tocopherol through Cu-catalyzed asymmetric 1,2-addition. Chemistry (Weinheim an der Bergstrasse, Germany) 2014;20(44):14250–5. doi: 10.1002/chem.201404458. [DOI] [PubMed] [Google Scholar]
- Zhao J, Siddiqui S, Shang S, Bian Y, Bagchi S, He Y, Wang CR. Mycolic acid-specific T cells protect against Mycobacterium tuberculosis infection in a humanized transgenic mouse model. eLife. 2015 Dec;4 doi: 10.7554/eLife.08525. [DOI] [PMC free article] [PubMed] [Google Scholar]