Abstract
According to Parieto-Frontal Integration Theory (P-FIT, Jung & Haier, 2007), individual differences in a circumscribed set of brain regions account for variations in general intelligence (g). The components of g, fluid (Gf) and crystalized (Gc) reasoning, exhibit distinct trajectories of age-related change. Because the brain also ages differentially, we hypothesized that age-related cognitive and neural changes would be coupled. In a sample of healthy middle-aged and older adults, we examined changes in Gf (operationalized by Cattell Culture Fair Test) and Gc (indexed by two vocabulary tests) as well as in structural properties of 19 brain regions. We fitted linear mixed models to the data collected on 73 healthy adults who participated in baseline assessment, with 43 returning for at least one follow-up, and 16 of them contributing four repeated assessments over seven years. We observed age differences as well as longitudinal decline in Gf, contrasted to a lack of age differences and stability in Gc. Cortical thickness and cortical volume exhibited significant age differences and longitudinal decline, which was accelerated in P-FIT regions. Gf (but not Gc) was associated with cortical thickness, but no such relationship was found for cortical volume. Uniformity of cognitive change (lack of reliable individual differences) precluded examination of the coupling between cognitive and brain changes. Cortical shrinkage was greater in high-Gc individuals, whereas in participants with higher Gf cortical volume slower volume shrinkage was observed.
Keywords: aging, brain, intelligence, brain volume, cortical thickness, MRI, frontal, parietal, temporal, cognitive aging
Introduction
Over a century ago, Charles Spearman hypothesized that general intelligence, or g factor, can explain the observed commonality among diverse mental abilities (Spearman, 1904, 1927). Decades later, Spearman’s student Raymond Cattell postulated that intelligence was not a unitary entity and introduced the concepts of fluid (Gf) and crystallized intelligence (Gc) as independent components of Spearman’s g (Cattell, 1943). In contemporary literature, fluid intelligence (Gf) refers to the capacity for logical reasoning and problem-solving that is presumably independent of acquired knowledge(Cattell, 1971) and is typically evaluated by nonverbal tests such as the Cattell Culture Fair IQ test (CFIT, (Cattell and Cattell, 1973), Raven’s Progressive Matrices (Raven, 1981), or the performance subscale of the Wechsler Adult Intelligence Scale (WAIS, (Wechsler, 1958). Crystallized intelligence (Gc), in contrast to Gf, stands for the ability to use acquired and culture-relevant information, and is assessed by tests of vocabulary and general knowledge. Although Gf and Gc are viewed as representation of distinct intelligence components, they are not statistically independent (Carroll, 1993) and correlate with each other, usually greater than r = 0.3 (Flanagan and McGrew, 1998; Li et al., 2004).
In search for its biological foundations, fluid intelligence has been linked to various brain properties and indicators of brain integrity. For example, patients with lesions in the prefrontal (Roca et al., 2010) and parietal cortex (Woolgar et al., 2010) compared to intact controls evidence lower performance on fluid intelligence tests. Increased functional activation in frontal, parietal and anterior cingulate cortices was observed during fluid reasoning tasks (Geake and Hansen, 2005; Masunaga et al., 2008; Prabhakaran et al., 1997). In the temporal lobe, many regions such as posterior superior temporal gyrus (Luo et al., 2003), inferior and middle temporal gyri (Goel and Dolan, 2004; Knauff et al., 2002), as well as the fusiform gyrus (Goel and Dolan, 2004; Luo et al., 2003) also exhibit activation during performance on various fluid intelligence tasks. A recent meta-analysis confirmed the association of gray matter volume estimates in frontal, temporal, and posterior cingulate cortices as well as activation peaks in multiple frontal, parietal, and temporal regions with performance on (predominantly fluid) intelligence tests (Basten et al., 2015).
The described pattern of associations linking multiple cortical regions to Gf performance served as an impetus for developing the Parieto-Frontal Integration Theory (P-FIT) of fluid intelligence (Jung and Haier, 2007). The P-FIT postulates a complex yet circumscribed network of cortical regions as the brain substrate of cognitive operations that constitute fluid reasoning. The proposed network includes the dorsolateral prefrontal cortex, the inferior and superior parietal lobule, the anterior cingulate gyrus, and selected areas within the temporal and occipital lobes. In P-FIT, the temporal and occipital regions are viewed as part of the Gf supporting circuitry due to their contribution to the early processing of sensory information; the parietal cortex is included as the module, in which the products of sensory processing are handled after initial processing in the primary cortices and in interaction with prefrontal regions, with the latter deemed crucial for generating an optimal solution to a given problem; and anterior cingulate constrains the selected response and inhibits other competing process.
Notably, Gf and Gc exhibit distinct trajectories of development and aging (Desjardins and Warnke, 2012; McArdle et al., 2002). Fluid reasoning competence improves rapidly during childhood and adolescence, peaks in early adulthood, and gradually declines throughout the later part of the life span thus acquiring a status of a quintessential age-sensitive ability (Horn and Blankson, 2005). Crystalized intelligence rises during early development and schooling, but does not weaken during normal aging, and may even continue to improve after Gf peaks and starts to wane (McArdle et al., 2000). It follows from P-FIT that decrements in Gf, but not Gc, should be associated with a breach of integrity in any component of the outlined network.
Normal brain aging presents an opportunity for testing this proposition. The cumulative research on healthy aging unequivocally demonstrates that it profoundly alters structural characteristics of the brain, even in the absence of age-related disease (see Fjell et al., 2014; Kennedy and Raz, 2015; Raz and Rodrigue, 2006 for reviews). Moreover, the extant studies converge on the pattern of brain aging that corresponds to the network of structures specified in P-FIT theory of neural bases of intelligence. Thus, it is plausible that cognitive aging characterized by decrements in fluid intelligence would be linked to age-related changes in specific brain structures comprising the P-FIT network.
To date, testing this hypothesis has been hampered by the lack of longitudinal evidence that is necessary for assessment of the brain and cognitive aging and their relationship (Hofer and Sliwinski, 2001; Lindenberger et al., 2011; Maxwell and Cole, 2007). Although a recent longitudinal study has reported coupling of changes in brain structure and Gf (Persson et al., 2016), it was limited to two measurement occasions. The current study aimed at evaluating the relationship between longitudinal changes in Gf and in gross structural properties of the cerebral cortex in a sample of middle-aged and older adults who have been assessed up to four times over a relatively long period. Our objectives were as follows.
First, we modeled change in Gf and Gc over time, and evaluate individual differences therein. Second, in the same fashion, we modeled change and individual differences in change of cortical thickness and volume in the cortical regions specified by P-FIT theory, while contrasting distinct regions that are considered the brain substrates of Gf with the sensory and motor regions regarded as controls. Third, we assessed the relationship of Gf with regional volume and cortical thickness. Finally, depending on the presence of statistically reliable individual differences in change, we planned to examine the coupling of brain and cognitive changes and lead-lag relationships between changes in these two domains.
Based on the surveyed literature, we hypothesized that Gf, but not Gc, would be negatively related to age at baseline and would decline over time. Likewise, we expected smaller cortical volume and thickness in older participants at baseline and significant reduction of both over the follow-up period. In addition, we hypothesized that shrinkage of the tertiary association cortices - prefrontal and parietal - would accelerate over time. Furthermore, better Gf performance was hypothesized to be associated with larger and thicker prefrontal and parietal association cortices and change in Gf was expected to be coupled with cortical changes in the regions identified in P-FIT as critically important for fluid intelligence. In evaluating associations between structural parameters of the cortex and fluid intelligence, we took advantage of a multi-occasion longitudinal design with a theoretical maximum of 5548 data points1 and planned to examine whether steeper decline in Gf would be associated with faster thinning of association cortices and whether changes in the brain structure led or trailed changes in Gf. The latter would depend on finding individual differences in change the variables of interest.
Methods
Participants
Participants were healthy volunteers from the metropolitan Detroit area. They attained at least high school education, were native English speakers and exhibited strong right-hand preference (75% and above on the Edinburgh Handedness Questionnaire; (Oldfield, 1971). Persons who reported a history of cardiovascular disease, neurological or psychiatric conditions, diabetes, head injury with a loss of consciousness for more than 5 min, thyroid problems, history of drug and alcohol abuse were excluded from participation in the study. Persons who were taking anti-seizure medication, anxiolytics, or antidepressants were excluded as well. Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) were used to screen probable cases of dementia and depression, and only participants who scored at least 26 on MMSE and below 16 on CES-D were enrolled in the study. All participants provided informed consent for participation in this study, which was approved by university human investigations committee.
The pool of eligible participants (see Table 1 for descriptive statistics) consisted of 73 persons, age 49 years and older, of whom 43 returned for at least one follow-up. Three additional participants who were assessed ad baseline but did not return were excluded because they did not meet the health criteria: two had cancer and the third one had a colloidal cyst in the brain. The participants who returned for follow-ups did not differ from the rest of the pool in age, education, sex, ethnicity, and hypertension diagnosis (all p > 0.45). However, the 43 individuals returning for follow-up measures had higher MMSE than 30 who did not return: M ± SD: 28.9 ± 1.1 vs. 28.0 ± 1.1, t (71) = 3.188, p = 0.002.
Table 1.
Descriptive statistics of longitudinal measures.
| Interval from baseline (month)
|
N | Age (year)
|
Sex composition (% men) | N (%) with Hypertension Diagnosis | |||||
|---|---|---|---|---|---|---|---|---|---|
| mean | SD | range | mean | SD | range | ||||
| Baseline | - | – | – | 73 | 63.96 | 8.89 | 49.50 - 83.33 | 27:46 (37%) | 24 (33%) |
| 1st follow-up | 16.08 | 1.77 | 13 - 23 | 37 | 65.71 | 9.57 | 50.75 - 84.67 | 14:23 (38%) | 13 (35%) |
| 2nd follow-up | 31.21 | 2.97 | 27 - 39 | 28 | 66.91 | 9.84 | 52.17 - 85.67 | 12:16 (43%) | 11 (39%) |
| 3rd follow-up | 90.33 | 5.99 | 81 - 102 | 24 | 71.34 | 9.56 | 57.17 - 91.17 | 12:12 (50%) | 9 (38%) |
Cognitive measures
Fluid intelligence
The Cattell Culture Fair Intelligence Test (CFIT, Form 3B, (Cattell and Cattell, 1973) was administrated to measure fluid intelligence. The test consists of four subtests, each containing 10 to 14 nonverbal reasoning problems of a gradually increasing difficulty. The subtests cover various aspects of abstract reasoning such as detecting similarity in designs, completing matrices, and solving nonverbal syllogisms, with participants having to derive the rules required for solving the problems. Participants could finish the entire tests, but the items that had been completed at standard times allotted to each subtest were recorded and used for computation of the total timed score that was used in the following analyses.
Crystalize intelligence
Gc was assessed by two multiple-choice vocabulary tests (V-2 and V-3) from the Educational Test Services Kit of Factor-Referenced Tests (Ekstrom et al., 1976). V-2 consisted of 18 items and V-3 contained 24 items, all of which were 5-choice synonym tests. Participants could finish the entire tests, but the items that had been completed at standard times of 4 minutes for V-2 and 6 minutes for V-3 were noted. Subjects were instructed not to guess unless they could eliminate one or more answer choices as wrong. The indices of performance were the number of correct items minus the number of incorrect items multiplied by .25 (penalty for guessing), separately for V-2 and V-3. Total performance on the timed V-2 and V-3 tests are used in the following analyses.
MRI protocol
Images for all four waves were acquired on the same 1.5 Tesla Siemens Magnetom Sonata MRI system (Siemens Medical Systems, Erlangen, Germany). The same hardware, including the head coil was used across sessions, although unavoidable routine manufacturer software updates occurred. The cortical surface was reconstructed from a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence acquired in the coronal plane with the following parameters: repetition time (TR) = 800 ms, echo time (TE) = 3.93 ms, inversion time (TI) = 420 ms, field of view (FOV) = 192×192 mm, acquisition matrix = 256×256 mm, flip angle = 20°, and voxel size = 0.75×0.75×1.5 mm3, 144 slices.
Image processing
To extract reliable cortical thickness and volume estimates, images were semi-automatically processed using FreeSurfer longitudinal stream (Reuter et al., 2012). A within-subject template was created for each individual participant (Reuter and Fischl, 2011; Reuter et al., 2010), and subsequent processing was performed using the common information from the template, thus increasing the reliability and statistical power (Reuter et al., 2012). The white and gray matter surfaces reconstructed from FreeSurfer were inspected by an operator with extensive training in MRI neuroanatomy (PY) and manually edited, if necessary. All cases required manual editing in orbito-frontal or/and temporal regions, including removal of the dura and orbital tissue that were misclassified as gray matter. Two cases needed manual removal of skull from the dorsal prefrontal cortex. Cortical thickness was computed as the average distance between pial surface and gray-white matter boundary within each region of interest (ROI).
Selection of ROIs for analysis
In FreeSurfer, the cortex in each hemisphere is divided into 34 neuroanatomically labeled regions (Desikan et al., 2006; Fischl et al., 2004), for which volume and cortical thickness were calculated. However, to test the specific hypotheses derived from P-FIT model, we selected 19 relevant regions divided into two groups: target (16) and control (3). Targets were the regions that were expected to be associated with fluid intelligence performance according to the P-FIT model: Caudal and rostral middle frontal cortex, inferior frontal cortex (pars opercularis, pars orbitalis, pars triangularis), superior frontal gyrus, superior and inferior parietal lobules, supramarginal gyrus, precuneus, caudal and rostral anterior cingulate gyri, temporal lobe (superior, middle, and inferior gyri), fusiform gyrus. Control regions were pericalcarine cortex, precentral gyri, and postcentral gyri. The results from two hemispheres were combined. i.e. volumes of left and right ROIs were added up, whereas thickness estimates of left and right cortices were averaged.
Statistical analyses
Statistical analyses were carried out in three steps. First, in two separate models, cross-sectional age differences and longitudinal changes in Gf and Gc were evaluated using two-level linear mixed effects models with measurement occasions nested within individuals. Second, cross-sectional age differences and longitudinal change in cortical thickness and volume were gauged using two crossed random effects models including all relevant brain regions across all participants and all measurement occasions. As described above, to test for differential effects as posited by P-FIT theory, a contrast between target (1) and control (0) regions was included in the model as a dummy variable. Third, cognitive and cortical measures were linked by adding the two cognitive measures and their interaction with time to the second model. Throughout the analyses, missing values were handled by full information maximum likelihood estimation (FIML). In addition to p-values based on Satterthwaite’s approximation as implemented in the lmerTest package (Kuznetsova et al., 2016), 95% bootstrap confidence intervals were constructed from up to 500 bootstrap samples per model.
Modeling longitudinal change in Gf and Gc
Growth curve modeling was used to estimate the trajectories of change in Gf and Gc. The analyses were conducted using the lme4 package (Bates et al., 2016) in R (Team, 2014). All parameters were separately estimated for Gf and Gc.
Modeling re-test effects
In longitudinal studies, participants’ performance can improve because of repeated exposure to tests. Repetition-related gains occurred in many cognitive tasks, including fluid intelligence (Rabbitt et al., 2004), processing speed (Ferrer et al., 2005), and memory (Ferrer et al., 2005; Salthouse et al., 2004). These gains are stable and persist for several years after the last exposure (Salthouse et al., 2004). Thus, the rate of age-related decline could be underestimated, if repeated-exposure effects in the longitudinal data are not taken into account (Ferrer et al., 2005; Rabbitt et al., 2001). In the current study, we tried to separate the repeated-testing gain and age-related longitudinal change in cognitive abilities by capitalizing on the differences in the delays between the testing occasions, and modelling time and repetition effects separately. While older adults with above-high school educations are usually familiar with multiple-choice tests of vocabulary and general knowledge, most often this is not the case for tests of fluid intelligence such as the CFIT that was used in the present study. Thus, repeated assessments may bring performance gains simply by familiarization with the testing procedure and material and increasing the comfort level of the participants. The most significant improvement is usually obtained after the first repeated exposure, and the magnitude of performance gains tends to diminish sharply thereafter (Rabbitt et al., 2004). We controlled for such re-test effects by introducing into our models a step function that captured the expected increase in CFIT performance following the first exposure to the test at the individual level.
Cross-sectional age differences were controlled for by including age at the first measurement occasion (baseline) as a control variable. To ease interpretation, age at baseline was grand-mean centered at 64 years. Time intervals were computed at an individual level as the distance from the first measurement occasion. The complete model is schematically presented in Figure 1.
Figure 1.
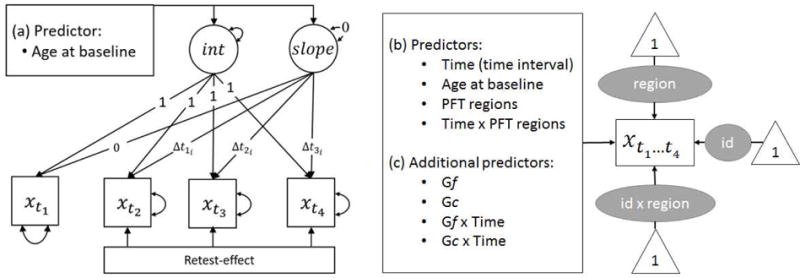
Three statistical models of change in cortical thickness, volume, and cognitive measures (Gf and Gc). (a) Latent growth curve model of Gf and Gc (denoted as x) across four measurement occasions., with age at baseline as cross-sectional predictor. (b) Crossed-random effects model for the analysis of cortical thickness and volume (denoted as ) with three random effects for brain region (region), person (id), and their interaction (id × region). Time, age at baseline, PFT regions membership, and the Time × PFT regions interaction were included as predictors. (c) To link cognitive and cortical measures, model (b) was augmented by the additional variables: Gf, Gc, Gf × Time, Gc × Time. The variable Time denotes the time intervals Δti in age from the first measurement occasion, for everyone.
Modeling longitudinal change in cortical thickness and volume
In two separate models, we evaluated change in cortical thickness and volume using a crossed random effects model that included all relevant brain regions across all participants and all occasions (totaling 2945 non-missing data points per analysis). As before, the lme4 package (Bates et al., 2016) was used to carry out the analyses and cross-sectional age differences were controlled for by including grand-mean-centered age at baseline assessment as a control variable. The dummy variable PFT regions was introduced to capture the grouping of control versus target regions, while differences in change across the regions were captured by the interaction between PFT regions and Time. To mitigate potential scaling artifacts, units of cortical thickness were multiplied by 100, whereas units of volume were divided by 100. The model is depicted in Figure 1b.
Linking cognitive and cortical measures
Cortical and cognitive measures were linked by including Gf and Gc simultaneously as additional predictors in the crossed random effects models for cortical thickness and volume. After controlling for potential main effects, we were interested in the degree to which aging related changes in cortical measures of thickness and volume may differ as a function of Gf, Gc respectively. To this end, we included the corresponding interactions to the model. The model is depicted in Figure 1c.
Results
Change in Gf and Gc
The results of two-level linear mixed models of longitudinal change in Gf and Gc are presented in Tables 2a and b and depicted in Figure 2 which includes all observations. As hypothesized, cross-sectional comparisons revealed lower Gf in older adults (bage at baseline = −0.20, = −3.58, p < 0.01) and no age differences in Gc (bage at baseline = 0.02, t = 0.18, p = 0.86). Also, as expected, we observed a modest decline in Gf (bTime = −0.21, t = −2.01, p < 0.05) but no change in Gc (bTime = −0.09, t = −0.66, p = 0.51). As shown in Tables 2a and 2b, a significant re-test effect was observed for Gf (bReexposure = 1.49, t = 2.65, p < 0.01) but not for Gc (bReexposure = 0.46, t = 0.65, p = 0.51). Age-related acceleration of decline was noted neither for Gf (bTime × baseline age = −0.01, t = −1.65, p = 0.10) nor for Gc (bTime × baseline age = 0.00, t = −0.34, p = 0.73). The standard deviation of the random effect for Gf, was estimated at 3.80 (bootstrapped 95% CI [2.94, 4.52]) with a residual standard deviation of 2.35 (bootstrapped 95% CI [1.98, 2.64]). For Gc, a random effect standard deviation was 6.73 (bootstrapped 95% CI [5.41, 7.89], with a residual standard deviation of 2.88 (bootstrapped 95% CI [2.43, 3.23]. No reliable individual differences in change over time were observed in either score.
Table 2.
Results of a two-level linear mixed model for longitudinal change in (a) Gf and (b) Gc.
| (a) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gf | Estimate | SE | df | t | Pr(> |t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 23.36 | 0.52 | 89.75 | 44.67 | <0.001 | 22.28 | 24.42 |
| Time | −0.21 | 0.11 | 92.14 | −2.01 | 0.048 | −0.43 | 0.00 |
| Re-test | 1.49 | 0.56 | 97.83 | 2.65 | 0.009 | 0.43 | 2.69 |
| Age at baseline | −0.20 | 0.06 | 76.40 | −3.58 | 0.001 | −0.32 | −0.07 |
| Time × baseline age | −0.01 | 0.01 | 93.81 | −1.65 | 0.103 | −0.03 | 0.00 |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gc | Estimate | SE | df | t | Pr(> |t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 26.98 | 0.86 | 82.57 | 31.48 | <0.001 | 25.21 | 28.70 |
| Time | −0.09 | 0.13 | 91.96 | −0.66 | 0.510 | −0.34 | 0.22 |
| Re-test | 0.46 | 0.70 | 95.04 | 0.65 | 0.514 | −1.00 | 1.91 |
| Age at baseline | 0.02 | 0.10 | 75.69 | 0.18 | 0.861 | −0.17 | 0.20 |
| Time × Age at baseline | 0.00 | 0.01 | 92.91 | −0.34 | 0.734 | −0.03 | 0.02 |
Figure 2.
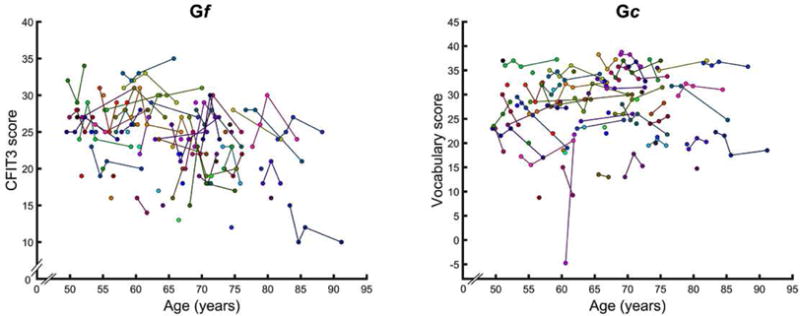
Trajectories of change in Gf and Gc indices over time, with addition of participants who dropped out after baseline assessment and had only one observation.
Removal of one observation with a negative Vocabulary score at baseline (see Fig. 2) did not alter any of the findings.
Change in cortical thickness and volume
Results of two crossed random effects analyses including all relevant brain regions across all participants and all occasions are presented in Tables 3a and 3b, with trajectories of change depicted in Figures 3a and 3b. Compared to their younger counterparts, at baseline, older participants had thinner cortices (bage at baseline = −0.57, t = −5.21, p < 0.001) and smaller cortical volumes (bage at baseline = −0.29, t = −5.44, p < 0.001) across the examined regions. Furthermore, both indices of gray matter structure showed significantly longitudinal shrinkage (thickness: bTime = −0.36, t = −3.10, p < 0.01; volume: bTime = −0.48, t = −9.74, p < 0.001), and declines in cortical thickness and volume of P-FIT target regions (but not the primary sensory and motor ROIs) accelerated over time (thickness: bTime×PFT regions = −0.31, t = −2.44, p < 0.05; volume: bTime×PFT regions = −0.12, t = −2.27, p < 0.05). Substantial individual differences were observed in cortical thickness and volume: random effect standard deviation for thickness (7.66 (bootstrapped 95% CI [6.30, 9.06]); random effect standard deviation for volume: 3.60 (bootstrapped 95% CI [2.85, 4.29]). These individual differences were observed across regions (random effect standard deviation thickness: 20.88 (bootstrapped 95% CI [13.47, 26.52]); random effect standard deviation volume: 45.74 (bootstrapped 95% CI [29.46, 58.32])). Individuals-by-region interaction was also noted: random effect standard deviation for thickness: 11.83 (bootstrapped 95% CI [11.32, 12.31]); random effect standard deviation for volume: 7.65 (bootstrapped 95% CI [7.35, 7.94]). However, we found no reliable individual differences in shrinkage over time, and therefore there was no justification for the inclusion of random slopes.
Table 3.
Results of two crossed-random linear mixed models for analyzing longitudinal change in (a) cortical thickness and (b) volume.
| (a) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cortical thickness | Estimate | SE | df | t | Pr(>|t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 200.59 | 12.12 | 19.22 | 16.55 | <0.001 | 178.04 | 228.18 |
| Time | −0.36 | 0.12 | 1684.91 | −3.10 | 0.002 | −0.60 | −0.12 |
| Age at baseline | −0.57 | 0.11 | 71.94 | −5.21 | <0.001 | −0.75 | −0.36 |
| P-FIT regions | 48.46 | 13.17 | 19.01 | 3.68 | 0.002 | 20.25 | 76.24 |
| Time × P-FIT regions | −0.31 | 0.13 | 1708.95 | −2.44 | 0.015 | −0.56 | −0.05 |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cortical volume | Estimate | SE | df | t | Pr(>|t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 73.03 | 26.42 | 18.87 | 2.76 | 0.014 | 24.39 | 133.54 |
| Time | −0.48 | 0.05 | 1620.85 | −9.74 | <0.001 | −0.58 | −0.37 |
| Age at baseline | −0.29 | 0.05 | 71.90 | −5.44 | <0.001 | −0.38 | −0.18 |
| P-FIT regions | 9.60 | 28.78 | 18.86 | 0.33 | 0.742 | −52.71 | 70.15 |
| Time × P-FIT regions | −0.12 | 0.05 | 1630.59 | −2.27 | 0.024 | −0.23 | −0.01 |
Note: Significance level is p < 0.025 after adjusting for multiple comparisons.
Figure 3.
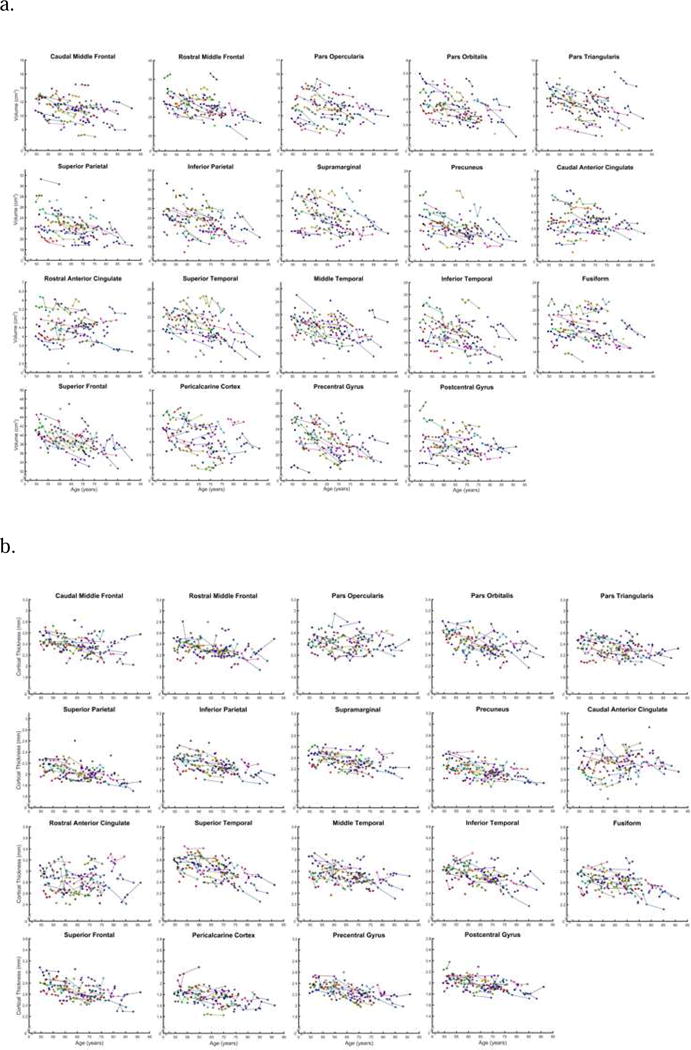
Trajectories of change in regional volume (a) and cortical thickness (b) in all ROIs examined. Observations pertaining to individuals who dropped out after one assessment are also included.
Linking cognitive and cortical measures
To link cognitive and cortical measures, we added Gf and Gc along with their interactions with time to the analyses described above. Results are in Tables 4a and 4b. Higher Gf was associated with greater cortical thickness (bGf = 0.27, t = 4.28, p < 0.001), but not cortical volume (bGf = −0.03, t = −1.14, p = 0.26), and no relationship between Gc and brain structural indicators was observed (see Tables 4ab). Of note, people with higher Gc exhibited steeper declines in cortical thickness (bGc×Time = −0.04, t = −4.44, p < 0.001) and volume (bGc×Time = −0.02, t = −6.51, p < 0.001) over time. In contrast, the interactions between Gf and time showed a weaker, nonsignificant trend in the same direction for cortical thickness (bGc×Time = −0.02, t = −1.90, p = 0.058), but a robust positive association between that index and cortical volume change (bGc×Time = 0.02, t = 5.20, p < 0.001), indicating that participants with higher Gf experienced slower brain shrinkage.
Table 4.
Results for two crossed-random linear mixed models linking cognitive measures to (a) cortical thickness and (b) volume.
| (a) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cortical thickness | Estimate | SE | df | t | Pr(>|t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 200.81 | 12.12 | 19.23 | 16.57 | <0.001 | 178.20 | 228.43 |
| Age at baseline | −0.51 | 0.11 | 72.89 | −4.52 | <0.001 | −0.70 | −0.29 |
| Gf | 0.27 | 0.06 | 1609.44 | 4.28 | <0.001 | 0.15 | 0.39 |
| Gc | −0.02 | 0.05 | 1519.07 | −0.33 | 0.745 | −0.11 | 0.08 |
| Time | −0.33 | 0.11 | 1680.88 | −2.84 | 0.005 | −0.56 | −0.09 |
| P-FIT regions | 48.46 | 13.17 | 19.00 | 3.68 | 0.002 | 20.26 | 76.24 |
| Gf × Time | −0.02 | 0.01 | 1582.32 | −1.90 | 0.058 | −0.04 | 0.00 |
| Gc × Time | −0.04 | 0.01 | 1565.86 | −4.44 | <0.001 | −0.05 | −0.02 |
| Time × P-FIT regions | −0.31 | 0.12 | 1702.54 | −2.47 | 0.014 | −0.56 | −0.05 |
| (b) | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cortical volume | Estimate | SE | df | t | Pr(>|t|) | Confidence Interval
|
|
| 2.50% | 97.50% | ||||||
| (Intercept) | 73.02 | 26.42 | 19.16 | 2.76 | 0.012 | 24.38 | 133.52 |
| Age at baseline | −0.28 | 0.05 | 73.39 | −5.25 | <0.001 | −0.38 | −0.17 |
| Gf | −0.03 | 0.03 | 1627.32 | −1.14 | 0.255 | −0.08 | 0.02 |
| Gc | 0.01 | 0.02 | 1596.56 | 0.68 | 0.498 | −0.03 | 0.05 |
| Time | −0.49 | 0.05 | 1620.44 | −10.08 | <0.001 | −0.59 | −0.38 |
| P-FIT regions | 9.60 | 28.78 | 19.15 | 0.33 | 0.742 | −52.71 | 70.15 |
| Gf × Time | 0.02 | 0.00 | 1585.81 | 5.20 | <0.001 | 0.01 | 0.03 |
| Gc × Time | −0.02 | 0.00 | 1572.30 | −6.51 | <0.001 | −0.03 | −0.02 |
| Time × P-FIT regions | −0.12 | 0.05 | 1628.47 | −2.31 | 0.021 | −0.23 | −0.02 |
Note: Significance level is p < 0.025 after adjusting for multiple comparisons.
Subsidiary analyses
To facilitate interpretation of the findings, we examined the effects of attrition on both cognitive measures. This analysis revealed lower Gf in the participants who had only a baseline measure (M ± SD: 21.9 ± 5.0) compared to participants who had at least two assessments: 24.4 ± 4.4; t (71) = 0.029. In contrast, no such difference was observed in Gc: 12.4 ± 5.5 for dropouts versus 13.1 ± 6.6 versus for those who came for at least one additional evaluation; t(71) = 0.359. Because of imbalanced design with respect to sex, we re-evaluated all models with sex as nuisance covariate, but found neither significant sex differences nor changes in other findings. In addition, because persons with hypertension constituted a sizeable minority of the sample, we tested hypertension diagnosis as an additional covariate. That addition was inconsequential for the findings. Therefore, to conserve degrees of freedom, we retained the models without these covariates.
Discussion
In this seven-year four-wave study of middle-aged and older adults, we observed a decline in fluid intelligence (Gf) over time. Advanced age was associated with poorer Gf performance at the inception of the study. Repeated testing, however, resulted in Gf gains. As expected, Gc evidenced neither longitudinal decline, nor cross-sectional age differences. Significant shrinkage and cortical thinning were observed over the course of this study in the collection of regions that was designated by P-FIT theory as putative brain substrate of intelligence. The differential change across cortical regions was consistent with previous studies demonstrating that association (prefrontal and parietal) cortices were more vulnerable to aging than the primary sensory and motor regions (Fjell et al., 2009; Pfefferbaum et al., 2013; Raz et al., 2010; Raz et al., 2005; Resnick et al., 2003).
We hypothesized positive associations between Gf and cortical size at baseline, and coupling between the change rates of cortices and Gf. One of the two cortical measures – thickness – was indeed positively associated with Gf, whereas no significant association with cortical volume was noted. Neither cortical thickness nor volume were related to Gc. Unfortunately, the relationship between brain shrinkage and cognitive decline could not be modeled, because of the lack of individual differences in Gf change, which is not an uncommon finding (e.g., Raz et al., 2008). We did, nonetheless observe two important links between aging of the brain and cognition. Higher crystallized intelligence, which remained stable over time, was associated with greater volume reduction, and thinning of the cerebral cortex. In contrast, higher fluid intelligence predicted slower cortical volume shrinkage, while exhibiting a non-significant link to faster cortical thinning.
The connection between high cognitive performance at baseline and slower shrinkage of regional brain volume is in line with previously reported findings (e.g., Persson et al., 2016; Raz et al., 2008). Fluid and crystallized intelligence tests are differentially sensitive to aging, and it is possible that persons who preserved higher levels of Gf in this sample that was composed of middle-aged and older adults, were particularly resilient in the face of forces that drive brain shrinkage. Persons with higher cognitive capital did not necessarily have bigger brains as evident from the lack of correlation between Gf and brain volume at baseline. They could have been better in brain maintenance and less prone to behaviors and life styles that accelerate brain aging (Nyberg et al., 2012).
By comparison, the association between higher Gc and greater brain decline may reflect the differences in sensitivity of cognitive measures to brain changes and their stability over time. According to cognitive reserve hypothesis (Stern, 2002), persons with higher cognitive attainment operationalized as higher education, advanced reading comprehension skills and higher IQ can endure, in comparison to their less able counterparts, greater degree of brain deterioration without showing cognitive decline. Cognitive reserve is a formative construct (Jones et al., 2011) that is defined by multiple indicators earlier or “pre-morbid” cognitive performance level. These indicators (e.g., education) are expected to remain stable over time but nonetheless correlate with important age-sensitive abilities, such as executive functions (Siedlecki et al., 2009). In the context of our study, Gc may represent cognitive reserve that was acquired long before the commencement of measurements, whereas Gf, which is modestly correlated with Gc, denotes a time-sensitive part of reserve that has already deteriorated enough to be better synchronized with brain decline.
Similarly, brain measures may vary in their potential of reflecting brain reserve (Satz, 1993). The propensity of larger brains, populated with greater number of neurons and inter-neuronal connections may be less vulnerable to the ravages of aging than smaller ones with lesser number of neurons, thinner neuropil, and less dense white matter connections. In that context, MRI-derived brain volume, which most likely reflects the volume of neuropil (Kassem et al., 2013) may be a more sensitive index of decline than cortical thickness. A coupling of two age-sensitive indices of brain and cognition may produce the pattern of a positive association observed for Gf and volume. Sensitive index of cognition coupled with a less sensitive brain index would produce a pattern of association, such as the weak association between Gf and cortical thickness observed here.
Another plausible reason for the observed associations between Gc and Gf with brain shrinkage may be selection bias. In this sample, we have not observed a clear positive attrition bias noted in some longitudinal studies (e.g., Lindenberger, Singer, & Baltes, 2002). The dropouts did not differ from those who remained in the study on almost all relevant indicators: age, sex, hypertension, and Gc. A modest positive attrition bias was noted, however, on Gf. Because persons who dropped out after one assessment had lower Gf than those who returned for at least one subsequent evaluation, the association between fluid reasoning and brain shrinkage could have been underestimated.
We found partial confirmation to the “bigger is better” hypothesis of relationship between cortical size and fluid intelligence measures, which are heavily dependent on executive functions (Pietschnig et al., 201; Yuan and Raz, 2014). Fluid intelligence was positively associated with cortical thickness, but the association between Gf and cortical volume was not significant. The effects of Gf on rates of cortical change, as discussed above, also differed with respect to cortical thickness and volume. Thus, it is possible that because cortical thickness, and volume are determined by different neurobiological and neurodevelopmental factors, they are differentially associated with cognitive functions and age-related changes therein. According to the radial unit hypothesis, cortical surface area is determined by the number of radial columnar units, whereas cortical thickness is determined by the number of cells within the units (Rakic, 2009). Thus, our results suggest that the cell numbers within radial columnar units in P-FIT region, and possibly denser connections among those units may be associated with Gf. These are, of course, highly speculative interpretations that need to be examined in future research, with translational harmonization of MRI approaches to animal models being a critically needed development in this regard.
Limitations of the current study
Small sample size and homogeneity stemming from stringent health screening criteria could account for failure to observed individual differences in Gf change. Small number of participants who underwent multiple MRI assessment precluded evaluation of lead-lag relationships between the brain and cognition. Lack of functional brain measures did not permit examination of potential mediating role of age-related functional changes in relationship between cortical shrinkage and cognitive performance. Limited scope of this study also precluded examination of possible additional contributors to the observed brain maintenance. All these limitations need to be addressed in future studies.
It is important to re-iterate that the current study was limited to investigation of age-related changes in Gf and shrinkage of brain regions proposed by P-FIT theory of intelligence. Multiple alternate models of the relationship between intelligence and brain regions posit different brain areas and aggregations thereof as the cerebral substrates of higher cognitive functions. For example, multiple-demand regions hypothesis (Duncan and Miller, 2013) and a model emphasizing brain's learning and control architecture (Chein and Schneider, 2012; Cole et al., 2011) hypothesize that complex cognitive operations are served by a smaller collection of more aggregated brain regions than postulated by P-FIT. Many studies forgo hypothesizing regional specificity altogether and using the total brain volume as a correlate of intelligence, with modest but positively associations observed (Pietschnig et al., 2015). The optimal level of region aggregation and differentiation in search for brain basis of intelligence remains unclear. Thus, although a direct empirical comparison of various theoretical accounts of neural substrates of cognition is a worthy goal for future research, it falls outside the scope of this study that inquired whether one widely discussed and empirically investigated model, P-FIT, could be harnessed for explaining age-related cognitive changes.
Conclusion
In summary, we modeled age differences and age-related change in fluid and crystallized intelligence, as well as shrinkage in cortical regions that varied in their putative relevance to intelligence. Although we observed significant decline in fluid intelligence and significant shrinkage of the relevant brain regions, we could not examine the degree of coupling between the two processes. We found evidence for the effects of cognitive reserve and brain maintenance on brain aging. These findings indicate that brain regions viewed in P-FIT theory as pivotal for intelligence may play a role in cognitive aging and that this hypothesis should be further explored in larger and less selected samples.
Supplementary Material
Acknowledgments
This study was supported in part by grants R03-AG024630 and R37/R01 AG011230 to NR. We thank Cheryl Dahle for help in data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
19 brain areas × 73 participants × 4 time points
References
- Basten U, Hilger K, Fiebach CJ. Where smart brains are different: a quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 2015;51:10–27. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P, Bolker MB. Package ‘lme4’. R Package Version 1.1-10 2016 [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. Cambridge University Press; 1993. [Google Scholar]
- Cattell RB. The measurement of adult intelligence. Psychological Bulletin. 1943;40:153–193. [Google Scholar]
- Cattell RB. Abilities: their structure, growth, and action 1971 [Google Scholar]
- Cattell RB, Cattell A. Measuring intelligence with the culture fair tests. Institute for Personality and Ability Testing; Champagne, IL: 1973. [Google Scholar]
- Chein JM, Schneider W. The brain’s learning and control architecture. Current Directions in Psychological Science. 2012;21:78–84. [Google Scholar]
- Cole MW, Etzel JA, Zacks JM, Schneider W, Braver TS. Rapid transfer of abstract rules to novel contexts in human lateral prefrontal cortex. Frontiers in human neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Desjardins R, Warnke AJ. Ageing and skills: a review and analysis of skill gain and skill loss over the lifespan and over time. OECD Education Working Papers 2012 [Google Scholar]
- Duncan J, Miller EK. Adaptive neural coding in frontal and parietal cortex. 2nd. Oxford University Press; 2013. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Ferrer E, Salthouse TA, McArdle JJ, Stewart WF, Schwartz BS. Multivariate modeling of age and retest in longitudinal studies of cognitive abilities. Psychol Aging. 2005;20:412–422. doi: 10.1037/0882-7974.20.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative, A.s.D.N. What is normal in normal aging? Effects of aging, amyloid and Alzheimer's disease on the cerebral cortex and the hippocampus. Progress in neurobiology. 2014;117:20–40. doi: 10.1016/j.pneurobio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan DP, McGrew KS. Interpreting Intelligence Tests from Contemporary Gf-Gc Theory: Joint Confirmatory Factor Analysis of the WJ-R and KAIT in a Non-White Sample. Journal of School Psychology. 1998;36:151–182. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. Neuroimage. 2005;26:555–564. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition. 2004;93:B109–121. doi: 10.1016/j.cognition.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding ageing. Gerontology. 2001;47:341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Horn JL, Blankson N. Foundations for better understanding of cognitive abilities. In: Flanagan D, Harrison P, editors. Contemporary intellectual assessment: Theories, tests, and issues. Guilford Press; New York: 2005. pp. 41–67. [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17(4):593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. discussion 154-187. [DOI] [PubMed] [Google Scholar]
- Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2012;47(2):645–61. doi: 10.1007/s12035-012-8365-7. 2013 Apr. Epub 2012 Nov 9. [DOI] [PubMed] [Google Scholar]
- Kennedy K, Raz N. Brain Mapping: An Encyclopedic Reference. Academic Press; Elsevier Cambridge: 2015. Normal aging of the brain; pp. 603–617. [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: a functional MRI study. Brain Res Cogn Brain Res. 2002;13:203–212. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff P, Christensen R. lmerTest: Tests for Random and Fixed Effects for Linear Mixed Effect Models (lmer Objects of lme4 Package): R Package Version 2.0–30. 2016 Available online at: https.cran.rproject.org/web/packages/lmerTest/index.html.
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Singer T, Baltes PB. Longitudinal selectivity in aging populations: separating mortality-associated versus experimental components in the Berlin Aging Study (BASE) J Gerontol B Psychol Sci Soc Sci. 2002;57(6):474–P82. doi: 10.1093/geronb/57.6.p474. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Von Oertzen T, Ghisletta P, Hertzog C. Cross-sectional age variance extraction: what's change got to do with it? Psychology and aging. 2011;26:34. doi: 10.1037/a0020525. [DOI] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: an fMRI study. Brain Res Cogn Brain Res. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Masunaga H, Kawashima R, Horn JL, Sassa Y, Sekiguchi A. Neural substrates of the topology test to measure fluid reasoning: An fMRI study. Intelligence. 2008;36:607–615. [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12:23. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Dev Psychol. 2002;38:115–142. [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Meredith W, Bradway KP. Modeling the dynamic hypotheses of Gf–Gc theory using longitudinal life-span data. Learning and Individual Differences. 2000;12:53–79. [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. Memory aging and brain maintenance. Trends Cogn Sci. 2012;16:292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N. Regional brain shrinkage and change in cognitive performance over two years: The bidirectional influences of the brain and cognitive reserve factors. Neuroimage. 2016;126:15–26. doi: 10.1016/j.neuroimage.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietschnig J, Penke L, Wicherts JM, Zeiler M, Voracek M. Meta-analysis of associations between human brain volume and intelligence differences: How strong are they and what do they mean? Neurosci Biobehav Rev. 2015;57:411–32. doi: 10.1016/j.neubiorev.2015.09.017. 2015, Oct. Epub 2015 Oct 9. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cerebral cortex. 2016;26:4101–4121. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran V, Smith JA, Desmond JE, Glover GH, Gabrieli JD. Neural substrates of fluid reasoning: an fMRI study of neocortical activation during performance of the Raven's Progressive Matrices Test. Cogn Psychol. 1997;33:43–63. doi: 10.1006/cogp.1997.0659. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, McInnes L. Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2004;59:P84–97. doi: 10.1093/geronb/59.2.p84. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39:532–543. doi: 10.1016/s0028-3932(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature Reviews Neuroscience. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F, Duncan J. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Schroeder DH, Ferrer E. Estimating retest effects in longitudinal assessments of cognitive functioning in adults between 18 and 60 years of age. Dev Psychol. 2004;40:813–822. doi: 10.1037/0012-1649.40.5.813. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A Formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB. Construct Validity of Cognitive Reserve in a Multi-Ethnic Cohort: The Northern Manhattan Study. J Int Neuropsychol Soc. 2009;15(4):558–569. doi: 10.1017/S1355617709090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. "General Intelligence," Objectively Determined and Measured. The American Journal of Psychology. 1904;15:201–292. [Google Scholar]
- Spearman C. The abilities of man. Macmillan; New York: 1927. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Team, R.C. R: A language and environment for statistical computing. Vol. 2013 R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4. Williams & Witkins; Baltimore, MD: 1958. [Google Scholar]
- Woolgar A, Parr A, Cusack R, Thompson R, Nimmo-Smith I, Torralva T, Roca M, Antoun N, Manes F, Duncan J. Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proc Natl Acad Sci U S A. 2010;107:14899–14902. doi: 10.1073/pnas.1007928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42C:180–192. doi: 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


