Abstract
Diabetes results from a loss of β-cell function. With the number of people with diabetes reaching epidemic proportions globally, understanding mechanisms that are contributing to this increasing prevalence is critical. One such factor has been circadian disruption, with shift-work, light pollution, jet-lag, increased screen time, all acting as potential contributory factors. Though circadian disruption has been epidemiologically associated with diabetes and other metabolic disorders for many decades, it is only recently that there has been a better understanding of the underlying molecular mechanisms. Experimental circadian disruption, via manipulation of environmental or genetic factors using gene-deletion mouse models, has demonstrated the importance of circadian rhythms in whole body metabolism. Genetic disruption of core clock genes, specifically in the β-cells in mice, have, now demonstrated the importance of the intrinsic β-cell clock in regulating function. Recent work has also shown the interaction of the circadian clock and enhancers in β-cells, indicating a highly integrated regulation of transcription and cellular function by the circadian clock. Disruption of either the whole body or only the β-cell clock leads to significant impairment of mitochondrial function, uncoupling, impaired vesicular transport, oxidative stress in β-cells and finally impaired glucose-stimulated insulin secretion and diabetes. In this review, we explore the role of the circadian clock in mitigating oxidative stress and preserving β-cell function.
Keywords: Circadian clock, Islet, β-cell, Insulin, Oxidative stress, Diabetes, Bmal1, Nrf2
1. Introduction
There is a growing burden of T2D, reaching epidemic proportions. The WHO estimated in 2014 that there are 422 million people with diabetes in the world with the prevalence almost doubling in the last 4 decades [1]. Diabetes is a result of a loss, or decrease, in insulin action. In type 1 diabetes (T1D) there is an autoimmune destruction of pancreatic insulin-producing β-cells, while in type 2 diabetes (T2D) there is often significant insulin resistance with varying degrees of β-cell dysfunction. Thus a decrease in β-cell mass or β-cell function, or both, underlie all forms of diabetes. Obesity is a common risk factor for diabetes and the frequent glucolipotoxicity seen with diabetes and obesity is a well-accepted cause of worsening β-cell dysfunction, there are many other environmental factors that are less well understood, contributing to ongoing β-cell dysfunction. One such factor is disruption of the normal circadian rhythm. The importance of having a normal circadian rhythm and the association with disease, if disrupted, has been known for a long time, especially in at-risk populations, such as in shift-workers [2] with rotational or night shift work. Strong associations between shift-work and risk for metabolic dysfunction, obesity and diabetes have been reported [3–6], with a cumulative excess risk of up to 60% of T2D [6,7]. ARNTL (also referred to as BMAL1), an essential core clock gene, is associated with type 2 diabetes (T2D) [8]. Interestingly, its expression is significantly downregulated in diabetic human islets [9]. In addition, genome-wide association studies have implicated MTNR1b and Cry2, circadian rhythm related genes, in T2D and impaired β-cell function [10–15].
However, it is only recently that with a better understanding of both β-cell dysfunction and the molecular mechanisms of the circadian clock, are there mechanistic connections being made to better understand how circadian disruption leads to diabetes and specifically β-cell dysfunction. With modern day lifestyle and constant work-related disruption of the body circadian rhythms, understanding the molecular pathways mediating circadian regulation of β-cell function is critical and urgently need for addressing this prevalent public health concern. In this review, we will present these interactions with a focus on how the circadian clock affects β-cell function and oxidative stress response.
2. The molecular clock
The circadian rhythm is established by the core components of the molecular clock. The molecular clock comprises of a transcription/ translational feedback loop comprised of the non-redundant transcription factor Bmal1 (Brain and Muscle Arnt like 1, or Arntl) that forms a heterodimer with another transcription factor, Clock (Circadian locomotor output cycles kaput), or its homologue Npas2, to bind to E-box elements in the promoters of target genes (clock-controlled genes). Four of these target genes (Per1, Per2, Cry1 and Cry2) encode proteins that translocate to the nucleus as heterodimers, to inhibit transactivation by Bmal1/Clock on their own promoters, and on those of other clock-controlled genes. The levels of Per1, Per2, Cry1 and Cry2 are also regulated by phosphorylation-mediated degradation. This slow rise in the levels of these proteins, thus sets up a feedback loop that gives rise to oscillations in the expression levels of clock-controlled genes – the circadian rhythm that has a ~ 24 h period. [16–25]. Reverbα and Reverbβ are transcriptional repressors that have E-box elements in their promoters and are clock-controlled genes. In addition, they negatively regulate Bmal1 [26–29] to accord Bmal1 expression a circadian rhythm adding another layer of robustness to the core molecular clock.
3. Central and peripheral clocks
Most cell types, especially those that are differentiated, display robust clock oscillations in their gene expression [30]. These circadian oscillations in gene expression have also been demonstrated in pancreatic islets [31–36] and islets maintained, in culture, ex vivo [34]. The endogenous, or free-running, rhythm in the expression of clock-controlled genes can be entrainable by internal stimuli, such as from the circadian pace-setter located in the suprachisamatic nucleus (SCN) of the hypothalamus or by other external cues. The highly interconnected network of neurons, in the SCN, receive direct input from the retina via the retino-hypothalamic tract. Light is the primary driver of circadian oscillations in the SCN while temperature has also been shown to affect it [37]. On light exposure, the molecular events of transcriptional and post-translational events are set in motion, which result in the circadian oscillations of the expression of clock controlled genes in the SCN. These are communicated to the rest of the body (peripheral clocks), including the β-cells, through neurohumoral pathways [38,39]. While there have been mechanistic studies to characterize the nature of this communication between the central SCN clock and the liver peripheral clock [40], these are lacking for β-cells. Nevertheless, it has been recognized that those tissues, such as the liver, pancreas (including β-cells [41]), muscle etc. are also significantly influenced by, not only the cues from the central clock, regarding the time of the day, but also by nutritional cues [40–44], such as the time and nature of these nutrient cues. When the timing of food is uncoupled from the normal light/dark cycle, many of the metabolically active tissues, such as the liver, reset their circadian oscillations to align with the nutrient cues, indicating the dominance of these cues for these tissues [45,46]. Similarly, activity has also been shown to regulate peripheral clocks [47,48]. This is represented in Fig. 1.
Fig. 1. Interaction of β-cell clock with the central clock and environmental cues.
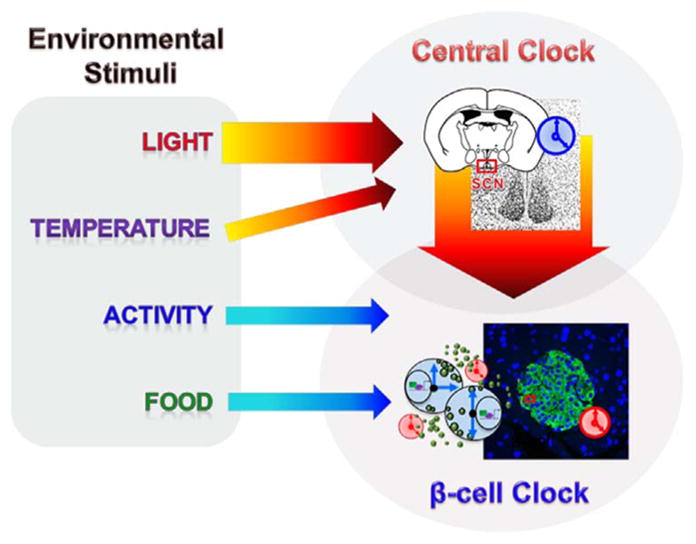
The central clock is entrained by external cues, of which light is the primary entraining signal. Other entraining signals include activity, temperature and food. The central clock regulates the β-cell clock via neurohumoral outputs.
4. Circadian clock regulation of metabolism
The circadian clock regulates whole-body metabolism [49] and this has been demonstrated in human studies, both epidemiological and interventional studies, and in animal models with circadian gene gain-of and loss-of-function studies. Targeted disruptions of clock genes result in striking metabolic disturbances [33–36,45,50–59], highlighting the central role of circadian regulation of cellular metabolism. ~ 10% or more of all transcripts have a circadian rhythm [30,60,61] that is tissue-specific, while a third of all nuclear receptors that play critical roles in metabolic homeostasis [26,62], display circadian rhythm. Furthermore, circadian control of various metabolic pathways appears to be most apparent on rate-limiting steps [60], compelling evidence that it is required for normal homeostasis. Interestingly, metabolic sensors, such as Sirt1, [63–65], AMPK [66] and PGC-1α [67,68] feed back to the core clock. Similar circadian rhythms of transcripts have been recently reported in β-cells regulating insulin secretion [69].
5. Circadian clock and β-cell function
β-cell clock has been studied for over a decade in rodents with a robust oscillation of core clock genes (Fig. 2), with more recent studies demonstrating their existence and function in human islets [70–72]. Indeed, a large number of transcripts in β-cells are rhythmic and are under circadian regulation [34,69,73]. Loss-of-function studies of the components of the core molecular clock in β-cells demonstrated its requirement for normal function (Table 1). We and others have demonstrated that β-cell-specific deletion of Bmal1, embryonic period onwards, which abrogates all rhythmic activity of the β-cell intrinsic clock, leads to profound β-cell dysfunction and diabetes [34–36,69,74]. Loss of circadian function in β-cells led to impaired substrate oxidation, a decrease in glucose-stimulated mitochondrial ATP production, impaired vesicular trafficking all resulting in a significantly blunted glucose-stimulated insulin secretion, the hallmark of β-cell failure seen in diabetes. Similarly, there is a profound effect on β-cell function even if the intrinsic β-cell clock is disrupted only in the adult life [69,75]. Mice with a loss-of-function of the β-cell clock induced only during adult life, have a blunted compensatory β-cell hyperplastic response in response to diet-induced insulin resistance, supporting evidence for the intrinsic circadian clock regulating β-cell proliferation [75]. All this provides convincing evidence that normal circadian oscillations and a functioning cell-autonomous β-cell circadian clock are essential throughout life to maintain normal β-cell function.
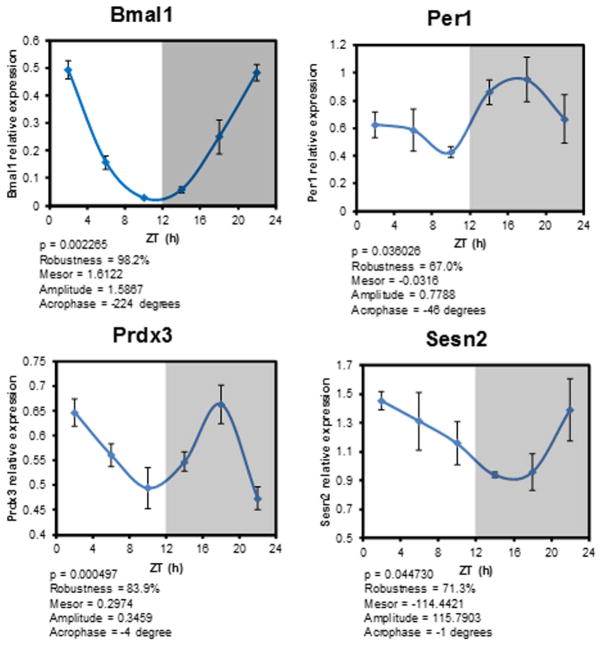
Fig. 2. Core clock and antioxidant gene oscillations in pancreatic islets.
Relative gene expression of Bmal1, Per1 (core clock genes) and of anti-oxidant genes, Senstrin2 (Sesn2) and Peroxiredoxin 3 (Prdx3), are shown after normalization to house keeping genes, Tbp (TATA box binding protein) and topl (topoisomerases I). qRT-PCR from isolated islets that were collected every 4 h is shown. ZT is Zeitgeber time with ZT-0 being when lights are turned on at 7 A.M. Each time point represents islets collected from 4 mice. The gene expression data were fitted to a cosine function (using Acro software V3.5 Dr. Refinetti) and the cocinar parameters are presented below each panel.
Table 1.
Genetic models of core clock gene disruption affecting β-cell function and glucose metabolism.
| Gene disrupted | Metabolic phenotype | Refs. |
|---|---|---|
| Bmal1 (Global) | Impaired gluconeogenesis, adipocyte differentiation, hyperlipidemia, glucose intolerance | [34,50] |
| Bmal1 – since birth (Pancreas using Pdx-1 Cre) | Hyperglycemia, hypoinsulinemia, glucose intolerance, β-cell dysfunction | [34,35] |
| Bmal1 – since birth (β-cell specific using Rip-Cre) | Hyperglycemia, hypoinsulinemia, glucose intolerance, β-cell dysfunction | [36] |
| Bmal1 – only in adult (β-cell specific using Mip-Cre/ERT) | Impaired compensatory hyperplasia in response to diet-induced obesity | [75] |
| Bmal1 – only in adult (β-cell specific using Pdx1-CreER) | Hyperglycemia, hypoinsulinemia, glucose intolerance, β-cell dysfunction | [69] |
| Clock (Global) | Hypertriglyceridemia, hypercholesterolemia, hyperglycemia, hyperleptinemia | [52] |
| Cry1&2 (Global) | Glucose intolerance | [55] |
6. β-cells and oxidative stress
The primary function of β-cells is to sense glucose and secrete proportional amount of insulin [76]. This is achieved by an intricate cellular signaling machinery that, at its core, is composed of an uptake of glucose and subsequent oxidation of glucose to generate ATP. The levels of ATP are sensed by the ATP-dependent potassium channels to regulate membrane depolarization and insulin granule exocytosis. Thus, insulin secretion is tightly linked to plasma glucose levels in the body [77]. This synchronization requires that the glucose uptake into the β-cell be tightly coupled to ATP production, via increased oxygen consumption and mitochondrial oxidative phosphorylation. Since, mitochondrial oxidative metabolism is a large source of intracellular reactive oxygen species (ROS, including superoxides, hydrogen peroxide ec.); β-cells are exposed to potentially damaging amounts of intracellular ROS. In addition, other extra-mitochondrial sources of ROS, including the NADPH oxidase system, have also been shown to be important in β-cell oxidative stress [78]. To compound this, β-cells comparatively have a lesser anti-oxidant capacity, only 15–38% of the ROS scavenging ability [79,80] of most metabolic tissues, such as the liver, putting them at risk for ROS-induced oxidative stress. Indeed this has been hypothesized to be one of the important underlying causes of β-cell failure in many forms of T2D [81].
7. Circadian regulation of β-cell oxidative stress
The regulation of oxidative stress by the circadian clock and Bmal1 has been proposed in the context of the premature aging phenotype seen in mice with global Bmal1 deletion [82–84], and based on conserved E-boxes in the promoters of many antioxidant genes, it was proposed that Bmal1 and the molecular clock control antioxidant genes [82]. Many of the antioxidant enzyme systems that defend against the damage induced by ROS are regulated by the leucine-zipper transcription factor, Nrf2 (Nfe2l2). We and others have shown that Nrf2 expression has a circadian oscillation and is directly under the control of Bmal1 and the circadian clock in β-cells [36] and in other tissues [85,86]. Indeed, in β-cells, there is a circadian oscillation of many critical antioxidant genes that are targets not only of Nrf2, but also Bmal1 (Fig. 2) and are dysregulated with circadian disruption [36,87]. These include genes in the sestrin family (Sesn2), peroxiredoxin family (Prdx3) and critical components of the glutathione system (Gclc and Gclm) [36]. Circadian disruption, thus leads to a dysregulation of mitochondrial function with increased ROS production [36], which when coupled with a decrease in antioxidant gene expression due to an impaired Nrf2 response, results in oxidative stress and β-cell dysfunction. To compound this, increased oxidative stress leads to an upregulation of Ucp2 in the β-cell, initially as a protective mechanism to diminish ROS production in the mitochondria by uncoupling substrate oxidation to phosphorylation of ADP to generate ATP. This uncoupling that result in a loss of oxidative phosphorylation, while beneficial in mitigating mitochondrial ROS production, has detrimental effects in β-cell function. An uncoupled β-cell is unable to couple glucose oxidation with ATP production and loses glucose-stimulated insulin secretion, an essential function. These changes have been demonstrated both in genetic and environmental models of circadian disruption [36]. Thus circadian disruption in the β-cell leads to increased ROS production, uncoupling, decreased antioxidant gene expression, and oxidative stress and culminates in significant impairment in β-cell function and diabetes.
8. Conclusion and future directions
Circadian disruption has become an integral part of modern lifestyle, with increasing number of people in occupations that demand shift-work and travel across time-zones. With mounting evidence demonstrating significant metabolic perturbations with circadian disruption, it has become imperative to decipher the molecular mechanisms underlying circadian regulation of pancreatic β-cells and understand how they interact with other tissues, to regulate whole body metabolism. Recent studies demonstrate that the circadian clock is critical for normal β-cell function and this regulation involves almost all aspects of β-cell biology (pictorially depicted in Fig. 3). However, there remain many questions that need to be answered in future studies. For instance, how does the circadian clock regulate how β-cells adapt and what are the mechanisms underlying acute and chronic adaptation? What is the tipping point when these adaptive mechanisms become pathological? How do β-cells interact with other organs and the central clock? What are the pathways that can be leveraged to prevent, mitigate and reverse circadian disruption induced dysfunction. While the answers to these questions will give us a better understanding about the interactions of the circadian clock and beta cell biology, other studies must be carried out concurrently to translate findings in pre-clinical models to clinical application to prevent and cure circadian disruption induced diabetes.
Fig. 3.
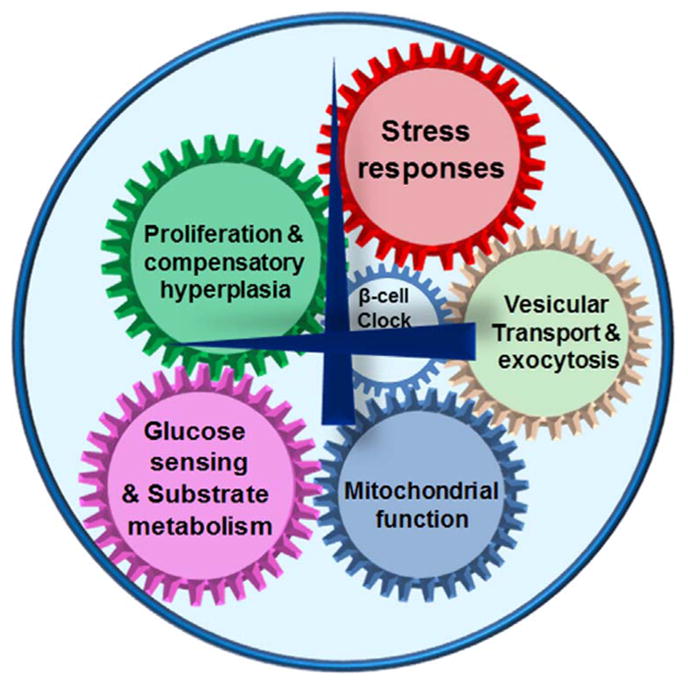
Circadian control of β-cell function. The intrinsic β-cell clock regulated many cellar process critical to normal function, including, glucose sensing and substrate metabolism, mitochondrial function, stress responses, insulin secretion by exocytosis and proliferation. Hence, circadian disruption leads to as failure of stimulus-secretion coupling, poor insulin secretion and diabetes.
Acknowledgments
The work was supported by grants to VY from NIH: R01DK097160; American Diabetes Association: 7-12-BS-210; to KM from NIH: R01DK112794.
Footnotes
Conflicts of interest
There are no potential conflicts of interest.
References
- 1.Roglic G World Health Organization. Global Report on Diabetes. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 2.B.o.L. Statistics. Workers on Flexible and Shift Schedules in 2004 Summary. 2005. [Google Scholar]
- 3.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58(11):747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76(6):424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 5.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75(1):131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 6.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivimaki M, Batty GD, Hublin C. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. PLoS Med. 2011;8(12):e1001138. doi: 10.1371/journal.pmed.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA. 2007;104(36):14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O’Connell PJ, Gonzalez FJ, Kahn CR. Loss of ARNT/ HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van DC, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronn T, Wen J, Yang Z, Lu B, Du Y, Groop L, Hu R, Ling C. A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia. 2009 doi: 10.1007/s00125-009-1297-8. [DOI] [PubMed] [Google Scholar]
- 13.Tuomi T, Nagorny CL, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Ostman B, Soderstrom J, Pesonen AK, Martikainen S, Raikkonen K, Forsen T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067–1077. doi: 10.1016/j.cmet.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Bonnefond A, Karamitri A, Jockers R, Froguel P. The difficult journey from genome-wide association studies to pathophysiology: the melatonin receptor 1B (MT2) paradigm. Cell Metab. 2016;24(3):345–347. doi: 10.1016/j.cmet.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le BO, Lecoeur C, Li NG, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen G, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes G, Tuomi T, Polasek AG, van Dijk KW, van HM, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell MR, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Song C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian clock gene by transgenic BAC rescue. Cell. 1997;89(4):655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89(4):641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, Fruechte EM, Hitomi K, Thresher RJ, Ishikawa T, Miyazaki J, Takahashi JS, Sancar A. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96(21):12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de WJ, Verkerk A, Eker AP, van LD, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 22.Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers JH, van der Horst GT. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286(5449):2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91(7):1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 24.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature. 1997;389(6650):512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 25.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4(2):e1000023. doi: 10.1371/journal.pgen.1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 29.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA. Reverbalpha a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318(5857):1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allaman-Pillet N, Roduit R, Oberson A, Abdelli S, Ruiz J, Beckmann JS, Schorderet DF, Bonny C. Circadian regulation of islet genes involved in insulin production and secretion. Mol Cell Endocrinol. 2004;226(1–2):59–66. doi: 10.1016/j.mce.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Muhlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564(1–2):91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, Ma K, Yechoor VK. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets. 2011;3(6):381–388. doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadacca LA, Lamia KA, Delemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54(1):120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Moulik M, Fang Z, Saha P, Zou F, Xu Y, Nelson DL, Ma K, Moore DD, Yechoor VK. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol Cell Biol. 2013;33(11):2327–2338. doi: 10.1128/MCB.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog ED, Huckfeldt RM. Circadian entrainment to temperature, but not light, in the isolated suprachiasmatic nucleus. J Neurophysiol. 2003;90(2):763–770. doi: 10.1152/jn.00129.2003. [DOI] [PubMed] [Google Scholar]
- 38.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120(7):2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerber A, Esnault C, Aubert G, Treisman R, Pralong F, Schibler U. Blood-borne circadian signal stimulates daily oscillations in actin dynamics and SRF activity. Cell. 2013;152(3):492–503. doi: 10.1016/j.cell.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 41.Damiola F, Le MN, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le MN, Damiola F, Tronche F, Schutz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20(24):7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preitner N, Brown S, Ripperger J, Le-Minh N, Damiola F, Schibler U. Orphan nuclear receptors, molecular clockwork, and the entrainment of peripheral oscillators. Novartis Found Symp. 2003;253:89–99. [PubMed] [Google Scholar]
- 44.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142(6):943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105(39):15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5(2):e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Tahara Y, Shibata S. Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice. Sci Rep. 2016;6:27607. doi: 10.1038/srep27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Shibata S. Phase shifts in circadian peripheral clocks caused by exercise are dependent on the feeding schedule in PER2::LUC mice. Chronobiol Int. 2016;33(7):849–862. doi: 10.3109/07420528.2016.1171775. [DOI] [PubMed] [Google Scholar]
- 49.Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, FitzGerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102(34):12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105(5):683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 54.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12(5):509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda H, Yong Q, Kurose T, Todo T, Mizunoya W, Fushiki T, Seino Y, Yamada Y. Clock gene defect disrupts light-dependency of autonomic nerve activity. Biochem Biophys Res Commun. 2007;364(3):457–463. doi: 10.1016/j.bbrc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 56.Le MG, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, Moschetta A, Schibler U. REVERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7(9):e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REVERB-alpha and REVERB-beta. Nature. 2012;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delezie J, Dumont S, Dardente H, Oudart H, Grechez-Cassiau A, Klosen P, Teboul M, Delaunay F, Pevet P, Challet E. The nuclear receptor REVERBalpha is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26(8):3321–3335. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 60.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 61.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 62.Teboul M, Guillaumond F, Grechez-Cassiau A, Delaunay F. The nuclear hormone receptor family round the clock. Mol Endocrinol. 2008;22(12):2573–2582. doi: 10.1210/me.2007-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450(7172):1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 64.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 66.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 68.Grimaldi B, Sassone-Corsi P. Circadian rhythms: metabolic clockwork. Nature. 2007;447(7143):386–387. doi: 10.1038/447386a. [DOI] [PubMed] [Google Scholar]
- 69.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, Allred AL, Bradfield CA, Dinner AR, Barish GD, Bass J. Pancreatic beta cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stamenkovic JA, Olsson AH, Nagorny CL, Malmgren S, Dekker-Nitert M, Ling C, Mulder H. Regulation of core clock genes in human islets. Metabolism. 2012;61(7):978–985. doi: 10.1016/j.metabol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Pulimeno P, Mannic T, Sage D, Giovannoni L, Salmon P, Lemeille S, Giry-Laterriere M, Unser M, Bosco D, Bauer C, Morf J, Halban P, Philippe J, Dibner C. Autonomous and self-sustained circadian oscillators displayed in human islet cells. Diabetologia. 2013;56(3):497–507. doi: 10.1007/s00125-012-2779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saini C, Petrenko V, Pulimeno P, Giovannoni L, Berney T, Hebrok M, Howald C, Dermitzakis ET, Dibner C. A functional circadian clock is required for proper insulin secretion by human pancreatic islet cells. Diabetes Obes Metab. 2016;18(4):355–365. doi: 10.1111/dom.12616. [DOI] [PubMed] [Google Scholar]
- 73.Rakshit K, Qian J, Ernst J, Matveyenko AV. Circadian variation of the pancreatic islet transcriptome. Physiol Genom. 2016;48(9):677–687. doi: 10.1152/physiolgenomics.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J, Kim M, Li R, Liu V, Fu l, Moore D, Ma K, Yechoor V. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in â-cells. Islets. 2011 doi: 10.4161/isl.3.6.18157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rakshit K, Hsu TW, Matveyenko AV. Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia. 2016;59(4):734–743. doi: 10.1007/s00125-015-3859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wortham M, Sander M. Mechanisms of beta-cell functional adaptation to changes in workload. Diabetes Obes Metab. 2016;18(Suppl 1):S78–S86. doi: 10.1111/dom.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18(2):162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, Carpinelli A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia. 2009;52(12):2489–2498. doi: 10.1007/s00125-009-1536-z. [DOI] [PubMed] [Google Scholar]
- 79.Lenzen S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic beta-cells. BiochimBiophys Acta. 2017;1861(8):1929–1942. doi: 10.1016/j.bbagen.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 80.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20(3):463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 81.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging. 2009;1(12):979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kondratov RV, Antoch MP. The clock proteins, aging, and tumorigenesis. Cold Spring Harb Symp Quant Biol. 2007;72:477–482. doi: 10.1101/sqb.2007.72.050. [DOI] [PubMed] [Google Scholar]
- 84.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20(14):1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, FitzGerald GA. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123(12):5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, Yamamoto M, Chan J, van der Horst GT, Fukada Y, Meng QJ. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J, Liu R, de JD, Kim BS, Ma K, Moulik M, Yechoor V. Circadian control of beta-cell function and stress responses. Diabetes Obes Metab. 2015;17(Suppl 1):S123–S133. doi: 10.1111/dom.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]