Abstract
Cryptococcosis remains a significant cause of morbidity and mortality world-wide; particularly among AIDS patients. Yet, to date, there are no licensed vaccines clinically available to treat or prevent cryptococcosis. In this review, we provide a rationale to support continued investment in Cryptococcus vaccine research, potential challenges that must be overcome along the way, and a literature review of the current progress underway towards developing a vaccine to prevent cryptococcosis.
Keywords: Cryptococcus, Cryptococcal Vaccine, Cryptococcosis, Cryptococcus neoformans, Cryptococcus gattii
A Call to Arms: Why a Vaccine is Warranted
Cryptococcus neoformans and Cryptococcus gattii, the predominant etiological agents of cryptococcosis, can cause severe pneumonia and disease of the central nervous system (CNS) and other tissues (bone, skin, etc.). Disease severity oftentimes depends on the immune status of the individual and whether appropriate antifungal therapy is available and/or rendered in a timely manner. Cryptococcal meningoencephalitis is the most common disseminated fungal disease in AIDS patients and is responsible for 15% of AIDS-related deaths globally [1]. Cryptococcosis accounts for 7–8% of the invasive fungal diseases in solid organ transplant (SOT) recipients with 20% of C. gattii cases occurring in SOT recipients [2]. C. neoformans has a world-wide distribution and predominantly causes disease in immunocompromised individuals, including AIDS patients and those undergoing immunosuppressive therapies [3–9]. Nevertheless, C. neoformans is the leading cause of cryptococcosis in Southeast Asia in HIV negative patients with no apparent underlying disease [10, 11]. C. gattii has been previously reported to cause disease in healthy individuals with no apparent underlying condition [12, 13]. However, C. gattii also causes a significant proportion of cryptococcal disease in HIV positive individuals residing in sub-Saharan Africa which is perhaps symptomatic of the enormous burden of AIDS [14, 15]. In addition, the presence of anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies appears to increase the risk for dissemination of C. gattii to the CNS of otherwise immune competent patients [16, 17]. This later study suggests that more comprehensive immunological evaluation of patients with cryptococcosis due to C. gattii may reveal additional correlates of immune dysfunction resulting in a predisposition to C. gattii meningoencephalitis and cause us to revisit the perception of C. gattii as a primary pathogen. The geographical range of C. gattii has historically been understood to be primarily in the tropical and subtropical climates of Australia, New Zealand, and Southeast Asia [18]. However, the geographical range of C. gattii has clearly expanded to include temperate climates of Vancouver Island, British Columbia, Canada, the Pacific Northwest, Northeast, Southwest, and Southeast regions of the United States and Mediterranean Europe [15, 19–25] suggesting that more individuals will be at risk for developing cryptococcosis.
The morbidity and mortality rates due to cryptococcosis are unacceptably high in resource-limited settings such as sub-Saharan Africa, South Asia and Southeast Asia [1]. Access to three mainstay drugs used to treat cryptococcosis (i.e., amphotericin B, flucytosine and fluconazole) in resource-limited settings is hampered by high drug costs, inadequate drug stocks or supply chains, and difficulty managing potential adverse side effects of conventional formulations of amphotericin B in patients [26]. Regrettably, despite earnest efforts, there is currently no clinically available vaccine to combat cryptococcosis.
The incidence of HIV-associated cryptococcosis has significantly declined in countries with ready access to antivirals owing to immune reconstitution resulting from the widespread use of antiretroviral therapy [27, 28]. However, the use of antiretroviral drugs to treat HIV infection is also associated with the development of Cryptococcus-related immune reconstitution inflammatory syndrome (IRIS) which is also life-threatening [29–31]. Cryptococcus-associated IRIS is also observed in solid organ transplant (SOT) recipients following receipt of antifungal therapy and reduction of immunosuppressive drugs [3, 32–34]. Additionally, patients who are HIV negative or are not organ transplant recipients may be among the most at risk groups for mortality attributed to cryptococcosis in resource-available settings; perhaps because of delayed diagnosis due to lack of clinical suspicion and the myriad of other underlying diseases that are present within this patient population [35]. There are also genetic risk factors that may attribute to increased susceptibility to cryptococcosis in HIV positive and HIV negative patients [36–38].
The overwhelming majority of Cryptococcus exposures do not progress to cryptococcal disease. Thus, it is more prudent to select appropriate at-risk populations as candidates to receive a Cryptococcus vaccine. The pervasive presence of Cryptococcus in the environment indicates that exposure of a high number of persons with defective or suppressed immunity or some underlying genetic risk factor(s) is a concern. Consequently, individuals with compromised immunity and/or some pre-disposing genetic risk factors are obvious targets for an anti-cryptococcal vaccine. Also, certain individuals predicted to have an exceptionally high risk for developing cryptococcosis (i.e., patients scheduled to receive organ transplants, otherwise healthy HIV positive persons, and immune competent persons in endemic areas observed to contain C. gattii) would be ideal candidates for vaccination as a prophylactic measure. A more difficult but necessary challenge will be to prospectively identify patient populations with no apparent risk factors but who are expected to have higher mortality due to delayed diagnosis or additional underlying diseases as candidates for vaccination. Vaccines designed to prevent cryptococcosis will need to be broadly effective against the most virulent members of the C. neoformans and C. gattii species complexes and consider the current and predicted immune status of the patient. While there are a number of factors that will generally need to be considered to devise a viable vaccine candidate [Reviewed in 39] an effective cryptococcal vaccine will additionally need to 1) confer protection in persons with suppressed T cell mediated immunity, 2) remain effective during the subsequent development of immune suppression, 3) prevent reactivation of latent disease without generating an over-exuberant immune response like that observed with IRIS, and 4) contain individual proteins that would not induce deleterious T cell activation and proliferative responses to Cryptococcus that may enhance pathogenesis [40]. Taken together, the constant and ready exposure of high-risk populations, rise in medical procedures predicted to increase the susceptibility of patients to Cryptococcus disease, lack of an active and well-resourced pipeline for the development of novel classes of anti-fungal drugs, and current public health burden of cryptococcosis underlines the need for a “call to arms” to develop a cryptococcal vaccine. This review highlights the need for continued investment into cryptococcal vaccine development, stresses major obstacles that need to be overcome, and discusses promising approaches towards creating a viable Cryptococcus vaccine.
Overcoming Obstacles to Cryptococcal Vaccine Development
The consensus of studies using animal models demonstrate that cell-mediated immunity (CMI) by T helper (Th)1-type CD4+ T cells is necessary for protection against cryptococcosis [41–47]. Results obtained from animal models mirror clinical observations by showing that cryptococcosis is typically most aggressive in individuals with impaired T cell function (i.e., persons with AIDS, lymphoid malignancies, and recipients of immunosuppressive therapies) [48–50]. Th1-type CD4+ T cells choreograph the protective anti-cryptococcal immune response through the generation of Th1-type cytokines including interleukin-2 (IL-2), IL-12, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). These cytokines, in turn, induce increased lymphocyte and phagocyte recruitment to the lungs and activation of anti-cryptococcal delayed-type hypersensitivity (DTH) responses resulting in increased cryptococcal uptake and antimicrobial phagocyte activity [41, 42, 51]. The apparent dependency of intact T cell function on the generation of protection against cryptococcosis questions the premise for developing an anti- cryptococcal vaccine that will elicit protection in persons who present with low CD4+ T cell counts or a vaccine that will remain protective in vaccinated individuals following the onset of immune suppression. However, studies utilizing animal models have provided proof that protection following immunization can be maintained in immunocompromised hosts [52, 53].
Studies by Huffnagle et al. have shown that CD8+ T cells may compensate for the loss of CD4+ T cells to facilitate protection against cryptococcosis [54]. Other studies show mice depleted of CD4⁺ or CD8⁺ T cells prior to and during pulmonary infection with a C. neoformans strain genetically engineered to produce IFN-γ, designated H99γ, were able to resolve the initial infection and were completely protected against a subsequent otherwise lethal challenge with wild-type (WT) C. neoformans [53, 55]. Similarly, immune competent mice immunized with C. neoformans strain H99γ and subsequently rendered CD4+ or CD8+ T cell deficient during challenge with WT C. neoformans were completely protected, as evidenced by 100% survival and sterilizing immunity [53, 55]. Interestingly, 80% of H99γ immunized mice depleted of both CD4+ and CD8+ T cell populations survived a secondary challenge with WT yeast. Also, mice immunized with a C. neoformans strain genetically engineered to lack the enzyme sterylglucosidase 1 (Sgl1; see Glossary), which results in the accumulation of sterylglucosides (Figure 1, Key Figure), and then depleted of CD4+ T cells were protected from a subsequent infection with WT C. neoformans [52]. These studies support the premise that a cryptococcal vaccine can induce protection in CD4+ T cell deficient hosts and/or elicit protective immunity after CD4+ T cell counts are significantly reduced. However, a question remains as to what arm of the host immune response or cell population(s) provide protection in T cell deficient persons.
Figure 1. Cellular Location of Potential Cryptococcal Vaccine Targets and/or Components.
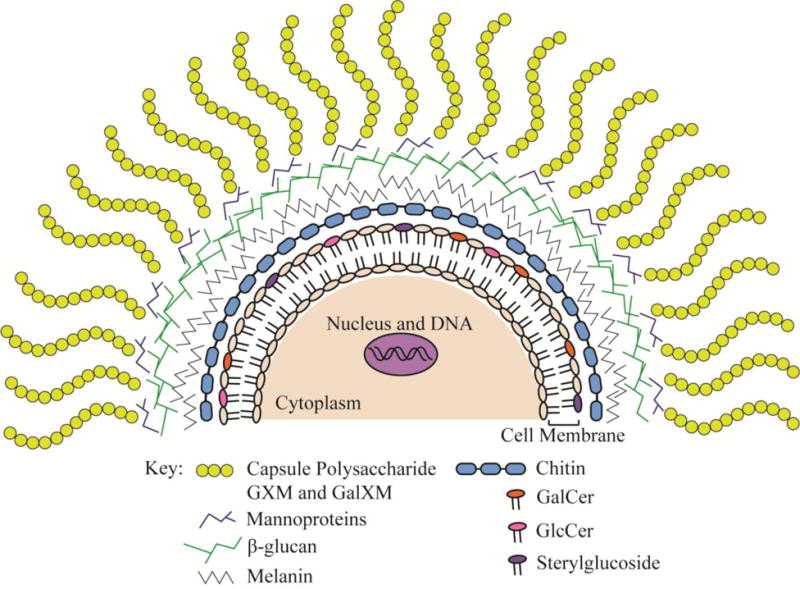
Various strategies to develop an effective anti-cryptococcal vaccine include targeting the capsule [62–70], engineering recombinant proteins of mannoproteins [88–90], targeting or utilization of β-glucans [71, 109], targeting melanin [73], engineering mutant chitosan deficient strains [77], and utilizing cryptococcal glycolipids as vaccine candidates or adjuvants [52, 110, 115]. GXM: glucuronoxylomannan; GalXM: galactoxylomannan; GalCer: galactosylceramide; GlcCer: glucosylceramide.
Studies by Netea et al. showed that an initial exposure of monocytes/macrophages to a microbe or antigen enhances their innate immune responses against reinfection with the same or different pathogen, a concept coined “trained immunity” [56, 57]. Altogether, the studies showed that exposure of monocytes and macrophages to the fungal cell wall component β-glucan induced changes in the epigenetic programming of the monocytes/macrophages leaving them able to respond in a memory-like fashion following re-stimulation with a potential pathogen that is independent of adaptive immunity [58]. These findings suggest that therapies targeting monocytes and/or macrophages could potentially provide protective immunity in the exact populations most at risk for developing cryptococcosis. Immune-based therapies and/or vaccines designed to augment monocyte/macrophage responses against C. neoformans are plausible strategies considering the critical role of STAT1-mediated M1 macrophage activation in mediating protection [59]. Nonetheless, a recent study using a mouse model to investigate the long-term relevance of this phenomenon suggested that the impact of β-glucan training of monocytes/macrophages may be transient in vivo; significantly subsiding within 3 weeks [60]. Thus, more experiments are needed to establish whether innate cells can provide long-term protection against fungal diseases including cryptococcosis.
Antibody-mediated immunity (AMI) has been shown to contribute to vaccine-mediated protection against cryptococcosis in persons with suppressed cell-mediated immunity. The polysaccharide capsule of Cryptococcus which is predominantly comprised of the polysaccharides glucuronoxylomannan (GXM) and galactoxylomannan (GalXM) and to a lesser extent, <1%, mannoproteins (MP) (Figure 1) [Reviewed in 61] is its most prominent virulence determinant and an obvious target for development of antibody-mediated therapies. However, cryptococcal polysaccharides have profound suppressive effects on immune responses [Reviewed in 61] and polysaccharide-only vaccines generally do not induce strong or long lasting immune responses associated with immunological memory. To overcome this, conjugate vaccines consisting of GXM combined to either tetanus toxoid (TT) or Pseudomonas aeruginosa exoprotein A (rEPA) were developed and shown to induce high antibody titers and elicit partial protection against cryptococcosis in mice [62, 63]. Mice actively immunized with GXM covalently linked to tetanus toxoid (GXM-TT) or by passive immunization with GXM-TT elicited antiserum demonstrated 70–80% protection against systemic C. neoformans infection [64]. Studies also showed that mice immunized with a peptide mimetic of C. neoformans GXM, peptide 13 (P13), recognized by human anti-GXM antibodies [65] conjugated to various protein carriers demonstrated prolonged survival and lowered serum GXM levels following an otherwise lethal C. neoformans challenge [66] or after establishment of a chronic infection [67] compared to controls. Passive immunization with serum from P13-conjugate vaccinated mice to naïve mice also conferred partial protection against cryptococcal challenge [67, 68]. Casadevall et. al. developed a murine monoclonal antibody (mAb), 18B7, to C. neoformans polysaccharide that underwent phase I clinical studies in HIV+ patients with cryptococcal antigenemia [69, 70]. Patients receiving mAb 18B7 showed a modest reduction in serum cryptococcal antigen titers before eventually returning to baseline levels and the treatment was well tolerated at certain doses [70]. However, no clinical studies focusing on the therapeutic potential of mAb 18B7 are on-going.
Additional studies included utilizing mAbs generated against C. neoformans cell wall components such as β-glucans [71], glucosylceramide [72], and melanin (Figure 1) [73]. An anti-β-glucan mAb 2G8 targeting β(1, 3)-linked glucans of the brown alga Laminaria digitata inhibited capsule formation and growth of C. neoformans in vitro and reduced fungal burden in mice given a systemic cryptococcal infection [71]. Mice passively immunized with mAbs against glucosylceramide exhibited reduced inflammatory responses and prolonged survival to lethal infection with C. neoformans [72]. mAbs to melanin were able to reduce the growth rate of melanized C. neoformans in vitro and mice passively immunized with mAbs against melanin also demonstrated prolonged survival [73]. Antibodies directed against C. neoformans are able to aid in phagocytosis and modulation of the inflammatory response, and modulate metabolism of the yeast rendering it more susceptible to antifungal drugs [74]. While it is generally agreed that AMI contributes to protective anti-cryptococcal host immune responses, there is no conclusive evidence demonstrating that AMI alone can provide protection against cryptococcosis in the absence of T cell-mediated immunity. However, mAb therapies and/or vaccines intended to elicit protective anti-Cryptococcus antibody responses may delay onset of disease or reduce disease severity in a host environment where T cell-mediated immunity is diminished but not absent.
The murine model of experimental pulmonary cryptococcosis is the predominant animal model used to evaluate the efficacy of various vaccine strategies against cryptococcosis. However, mice of different genetic backgrounds have differential susceptibility to cryptococcosis [75, 76]. Dissimilar levels of sensitivity to experimental cryptococcal infection can complicate interpretation of survival data and other immunological results attained from murine studies. For example, studies by Upadhya et al. showed that CBA/J, 129, and A/J mice vaccinated with a chitosan-deficient (Figure 1) C. neoformans strain, cda1Δ2Δ3Δ, demonstrated similar levels of protection against challenge with virulent C. neoformans and significantly more protection compared to that observed in BALB/c mice followed by C57BL/6 mice [77]. Also, C. neoformans strain 52D is more virulent in C57BL/6 compared to BALB/c mice which can resolve pulmonary infection with this strain [78]. Most commercially available transgenic mice are readily available only on the C57BL/6 background which may pose a problem if the phenotype of the vaccine-mediated immune response is not effectively replicated in this mouse model. Fortunately, most transgenic mouse lines can be re-derived or bred onto a different mouse background; albeit at some sacrifice in time and expense. Also, vaccine candidates can be tested in multiple inbred and/or outbred mouse strains, humanized mice, or a rabbit model of cryptococcal meningitis [79] as alternatives.
Cryptococcal Vaccines
Perhaps an indication of the difficult challenge before us is that, to date, no vaccines are approved for clinical use to prevent any fungal disease. Additionally, we are still working to understand the requirements for inducing protective immunity against cryptococcosis and defining which combination of cryptococcal antigen, adjuvant, delivery system, delivery route, and several other factors are needed to establish an effective and viable Cryptococcus vaccine. Herein, we discuss the strengths and weaknesses of various strategies to develop an attractive vaccine candidate.
Heat-killed or Attenuated Mutant Strains as Cryptococcal Vaccine Candidates
An ideal vaccine will not only be effective in preventing disease but will have an impeccable safety profile in all populations such as the elderly, young infants, and the immunocompromised [39]. Earlier studies are mixed in regard to the efficacy of vaccination with heat-killed Cryptococcus yeast or attenuated C. neoformans strains to elicit protective immune responses against cryptococcosis in mice [80–83]. Nonetheless, recent studies have shown that mice vaccinated with heat-killed or live/attenuated Cryptococcus strains with specific modifications to their cell wall architecture elicit significant levels of protection against cryptococcosis. Zhai and colleagues investigated the use of C. neoformans hyphal mutants to induce protective anti-cryptococcal immune responses in mice. Vaccination of mice with an attenuated C. neoformans strain engineered to overexpress Znf2, a master regulator of hyphal development, resulted in protective Th1- and Th17-type immune responses and a vaccination dose-dependent increase in protection against challenge with WT C. neoformans [84]. Interestingly, mice immunized with heat-killed Znf2-overexpressing strains also demonstrated 100% protection against subsequent challenge with WT C. neoformans. Studies using the chitosan-deficient (Figure 1) C. neoformans strain cda1Δ2Δ3Δ demonstrated that mice immunized with this avirulent mutant produced Th1-type responses and subsequent protection against infection with WT C. neoformans [77, 85]. Additionally, vaccination with a high dose of heat-killed cda1Δ2Δ3Δ elicited robust protection against an otherwise lethal challenge with WT C. neoformans compared to unvaccinated or heat-killed C. neoformans vaccinated mice [77]. Studies also demonstrated increased cross-protection in mice immunized with the C. neoformans cda1Δ2Δ3Δ strain against challenge with C. gattii compared to unvaccinated mice.
Potential strengths for the use of attenuated Cryptococcus strains as vaccines include low production costs and the capacity for these strains to induce stronger, longer-lasting immune responses compared to vaccination with heat-killed organisms [39, 86]. However, there are concerns regarding reversion of an attenuated strain to WT virulence levels or simply that vaccination in immunosuppressed individuals could cause a dysregulated immune response. The probability for reversion of attenuated strains to the fully virulent phenotype can be significantly reduced by genetically introducing several mutations into the attenuated strain. However, the efficacy of attenuated strains to induce protective immune responses typically declines as it is weakened. Since a majority of patients suffering from severe cryptococcosis are immunocompromised, a non-viable mutant strain vaccine would be a safer alternative and the results of recent studies demonstrate that heat-killed Cryptococcus mutants may, unlike initially observed, be utilized to elicit vaccine-mediated protection against cryptococcosis.
Crude Extracts and Recombinant Proteins as Potential Vaccine Candidates
Vaccines based on crude fungal extracts or recombinant proteins have the potential advantage of being relatively low cost and safer than attenuated Cryptococcus strains because there is no danger regarding reversion. A significant body of work is available to demonstrate that vaccination with various crude Cryptococcus extracts or protein fractions and/or individual proteins elicit protective host immune responses against experimental cryptococcal infection in mice. Mice vaccinated with a C. neoformans culture filtrate antigen (CneF) in complete Freund’s adjuvant (CFA) were partially protected against a subsequent cryptococcal infection compared to mice immunized with heat-killed C. neoformans (HK-C.n.) in CFA, HK-C.n. in saline alone, or mice immunized with saline alone [82]. Fractionation of CneF revealed mannoprotein (MP) (Figure 1) as the primary antigenic component responsible for the stimulation of anti-cryptococcal CMI responses in mice [87]. A screen for C. neoformans antigens that stimulate T cell responses resulted in the discovery of MP98, which contains a chitin deacetylase motif with the ability to convert chitin into chitosan [88] and MP88 [89] whose function remains unknown. MP88 and MP98 were shown to have extensive N-and O-linked mannosylation which were demonstrated to be essential for antigen recognition and optimal T cell responses [90, 91]. These data suggest that vaccine designs involving recombinant proteins should consider the contribution of post-translational modifications on the antigen’s potential immunogenicity. MPs have also been shown to induce proinflammatory cytokine and chemokine production from dendritic cells [92, 93]. However, not all MP fractions stimulate pro-inflammatory immunological responses [94] and MP fractions or any additional antigens identified that are immune stimulatory may require the appropriate adjuvant to induce the desired host immunological response [95].
Immunogenic cryptococcal antigens are often pre-selected for analysis based on their serological activity [96–99]. While immune serum has traditionally been used to identify putatively protective cryptococcal antigens, immunoreactivity to serum antibody does not ensure that the identified antigens will also induce protective T cell responses against Cryptococcus. In addition to MPs, Mandel et al. have identified and cloned a gene, designated DHA1, which encodes a protein that induces DTH responses in mice [100]. Biondo et al. used a 25-kDa extracellular polysaccharide deacetylase from C. neoformans in a vaccine strategy showing prolonged survival and reduced fungal burden in mice [101, 102]. Studies in our laboratory utilized a dual immunological and a proteomic approach to identify potential immunogenic cell wall (CW) and cytoplasmic (CP) associated proteins from both C. neoformans and C. gattii that may serve as attractive vaccine candidates [99, 103, 104]. C. neoformans CW and CP proteins were fractionated and screened for both immunoreactivity against serum antibody and the ability to stimulate a protective Th1-type cytokine response [104]. Mice immunized with individual protein fractions that elicited antibody and Th1-type cytokine responses exhibited significantly prolonged survival against experimental pulmonary challenge with C. neoformans. For C. gattii, mice immunized with individual CW and/or CP protein preparations also showed significantly prolonged survival against challenge with a virulent C. gattii strain [103]. Immunodominant proteins identified in these studies included proteins involved in stress responses (particularly heat shock proteins, HSPs), carbohydrate metabolism, signal transduction, amino acid biosynthesis, and protein synthesis [99]. HSPs may be of particular interest considering that hsp70 is highly abundant and immunogenic in vivo during pulmonary cryptococcosis [97, 98]. Hsp90 has been shown to govern fungal resistance to a number of anti-fungal drugs including azoles and echinocandins [105–108]. Several of the identified immunodominant proteins were similar between C. neoformans and C. gattii [103, 104] and could provide attractive candidates for the development of a prophylactic sub-unit vaccine effective for the treatment and/or prevention of both C. gattii and C. neoformans.
A successful vaccine will require the right mixture of antigen(s) combined with an appropriate adjuvant and/or antigen delivery system to induce preferably protective T cell mediated immunity against cryptococcosis. Specht et al. evaluated the use of β-glucan particles (GPs) (Figure 1), an antigen-delivery platform, packaged with alkaline extracts made from C. neoformans and C. gattii capsule mutant strains as a potential vaccination strategy against cryptococcosis [109]. Mice immunized with GPs containing immune-stimulatory and protective protein extracts derived from capsule deficient C. neoformans or C. gattii strains mounted antigen-specific CD4+ T cell recall responses and displayed prolonged protection against a subsequent otherwise lethal challenge with C. neoformans and C. gattii [109]. The delivery platform appeared to function as both an adjuvant and a vaccine delivery system. The promise of this system is that various Cryptococcus antigen and adjuvant combinations can be packaged within GPs and tested for their efficacy to prevent cryptococcosis in various animal model systems.
Recent studies have demonstrated the potential of glycolipids to serve as a component of a vaccine formulated to prevent cryptococcosis [52, 110]. Glucosylceramide (GlcCer) and SG, discussed earlier, are two major glycolipids found in fungi (Figure 1) [111]. Mice administered purified GlcCer, derived from Candida utilis, prior to challenge with C. neoformans showed significantly increased survival compared to mock vaccinated mice [110]. A significant reduction in pulmonary fungal burden and no dissemination to the brain were observed in GlcCer vaccinated mice compared to untreated mice. Studies have demonstrated both the safety profile [112] and flexibility [Reviewed by 113, 114] of glycolipids to tailor immune responses against a particular pathogen. A potential concern associated with the use of glycolipids, particularly the glycolipid galactosylceramide (GalCer) (Figure 1), as adjuvants is the induction of hyporesponsiveness in particular patient populations (i.e. immunosuppressed) and a number of side effects have been observed in mice given GalCer, including hepatotoxicity and abortion [Reviewed by 113, 115]. Nonetheless, studies using GlcCer suggest that GlcCer may be utilized as an adjuvant or principal component of a vaccine formulation to prevent Cryptococcus infections. Altogether, formulations comprised of an immunodominant protein, antigen, or peptide matched with an appropriate adjuvant and/or antigen delivery system would potentially be a safer option compared to a live-attenuated vaccine.
Concluding Remarks
Given the substantial morbidity and mortality associated with cryptococcosis, there is a need for continued, if not increased, investment in research to develop a vaccine to prevent cryptococcosis. A prophylactic anti-cryptococcal vaccine will dramatically alleviate the burden associated with cost and accessibility to mainstay anti-fungal drugs used to treat patients suffering from cryptococcal meningoencephalitis in resource-limited settings. Incidences associated with Cryptococcus-related IRIS in resource-limited and resource-available settings will also be alleviated if such a vaccine is available and broadly distributed. However, a successful Cryptococcus vaccine will need to be safe enough for administration to immune suppressed patient populations and elicit protective immunity in T cell deficient persons. A live or attenuated Cryptococcus vaccine will likely not be approved for use in immunocompromised patient populations. Heat-killed Cryptococcus mutants remain a viable option and their protective efficacy may extend beyond the Cryptococcus serotype used for vaccination. A subunit vaccine containing antigens from multiple Cryptococcus serotypes is also likely to provide broad protection against multiple etiological agents of cryptococcosis and be safely administered to immune suppressed populations. The addition of adjuvants such as GalCer or GlcCer, perhaps packaged within an antigen delivery system such as GPs, is also likely to enhance the immunogenicity of the vaccine and conceivably augment or “train” innate cell responses against Cryptococcus. Nonetheless, the efficacy of any Cryptococcus vaccine candidate to induce protection against cryptococcosis will need to be verified using an immune deficient animal model system to mimic immune suppression in human populations. Although many questions remain to be answered (see Outstanding Questions), studies using small animal models and engineered Cryptococcus mutants provide proof that a vaccine against Cryptococcus is feasible.
Outstanding Questions.
CD4+ T cell mediated immune responses appear to be critical towards eliciting protective immunity against cryptococcosis. Nevertheless, is it wise to prioritize antigens that preferentially activate CD4+ T cell cytokine recall responses for inclusion in Cryptococcus vaccine formulations considering that a significant segment of cryptococcosis patients are CD4+ T cell deficient?
What is the appropriate target population for a Cryptococcus vaccine and when should they be vaccinated?
Is it essential that all Cryptococcus vaccine candidates be found effective at inducing protection against cryptococcosis in a T cell deficient animal model system prior to any further serious consideration?
What is the criteria for determining which approaches and/or mutant strains or antigens are successful and should be moved forward to clinical trials?
Should a Cryptococcus vaccine have demonstrable protection against virulent strains of the C. neoformans and C. gattii species complexes?
What are the next steps to move from the preclinical work using mice into clinical trials? Are there additional animal models that should be used or developed?
Is it possible for a Cryptococcus vaccine to elicit protection in persons who present with low CD4+ T cell counts (i.e., AIDS patients or persons on immune suppressive therapies to prevent organ transplant rejection) or be protective in vaccinated individuals following the onset of immune suppression?
What are the mechanisms responsible for protective vaccine-mediated immune responses against cryptococcosis in hosts with suppressed CD4+ T cell-mediated immune responses?
Will the projected use of a Cryptococcus vaccine in resource-rich countries make it financially feasible to justify vaccination of select populations in resource-limited nations? If not, is there appropriate support to underwrite an effort to implement an anti-cryptococcal vaccination program in resource-limited settings?
Trends.
Expansion of Cryptococcus in unique patient populations and environmental niches highlight the need for a prophylactic vaccine to prevent cryptococcosis caused by virulent C. neoformans and C. gattii species.
Vaccination with heat-killed Cryptococcus mutants containing cell wall modifications appear to induce protective immune responses against cryptococcosis in mice.
Experimental studies show potential for eliciting long lasting protective immunity against cryptococcosis in CD4+ T cell deficient hosts.
Administration of fungal glycolipids such as glucosylceramide demonstrate significant protection against subsequent Cryptococcus infection in mice.
Mice vaccinated with innovative β-glucan antigen-delivery platform containing immune stimulatory Cryptococcus fractions induce protective anti-cryptococcal immune responses against virulent strains of C. neoformans and C. gattii.
Acknowledgments
The authors would like to thank Dr. Chrissy M. Leopold Wager for critical reading of the manuscript. This work is supported by research grants 2RO1 AI071752 and 3RO1 AI071752-08S1 from the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (F.L.W. Jr.) and grant GM060655 from the National Institute of General Medical Sciences of the NIH and grant 5R01AI071752-09 from the NIAID (M.C.C.V).
Glossary
- β-glucans
a critical component of the fungal cell wall composed of β-1,3-linked polymers of glucose with β-1,6 branches. β-glucans have immunostimulatory properties via activation of the alternative pathway of complement and serve as a ligand for the pattern recognition receptor Dectin-1, expressed on phagocytes
- β-glucan particles (GPs)
spherical hollow shells purified from Saccharomyces cerevisiae that are primarily composed of β-1,3 glucan and are devoid of proteins, lipids, and mannans. The inner hollow cavity of GPs can be loaded with various compounds and agents (i.e., protein antigens, cytokines, small interfering RNA, DNA, etc.) for delivery to antigen presenting cells. GPs have intrinsic immunostimulatory properties allowing them to serve as an antigen-presenting cell-targeted delivery system and an adjuvant
- Chitin
a β(1,4)-linked homopolymer of N-acetylglucosamine, a simple polysaccharide and essential component of the cell walls of all fungi. Encompasses both immune stimulatory and immune suppressive properties and enhanced chitin levels reduce susceptibility to echinocandin
- Chitosan
deacetylated chitin; an integral component of the inner portion of the cell wall and required for the virulence of C. neoformans.
- Galactoxylomannan (GalXM)
a relatively minor component of Cryptococcus polysaccharide capsule, compared to glucuronoxylomannan, constituting about 5–8% of the capsular mass. Structurally, GalXM has an α-(1→6)-galactan backbone containing four potential short oligosaccharide branch structures
- Glucosylceramide (GlcCer)
a glycolipid produced by protozoa, mammalian cells, plants, and multiple fungal species such as C. neoformans and Candida utilis. GlcCer can be found in the plasma membrane, cell wall, and extracellular vesicles of C. neoformans
- Glucuronoxylomannan (GXM)
a major component of Cryptococcus polysaccharide capsule accounting for about 90–95% of the Cryptococcus capsule’s mass. Structurally, it consists of a linear α-(1,3)-mannan main chain with β-(1,2)-glucuronic acid residues attached to every first mannose forming the basic core. This component is known to suppress host immune responses via multiple mechanisms
- Heat-Shock Proteins (HSPs)
synthesized by cells in response to an environmental stress and also act as molecular chaperones that are constitutively expressed and facilitate the synthesis and folding of proteins. HSPs are known to be targets of both the cellular and humoral immune responses in the host
- Mannoprotein (MP)
cell wall polysaccharide composed of mannosylated glycoproteins. MPs are a heterogeneous family of proteins that are defined by their ability to adhere to a Concanavalin A (Con A) affinity column. MPs are highly immunogenic and immune stimulatory
- Melanin
a natural pigment typically located in the cell wall of Cryptococcus that attributes to virulence by protecting Cryptococcus from host factors such as altering cytokine responses, and decrease phagocytosis
- Peptide Mimetic
a peptide that mimics a natural ligand or defined epitope and are often used to either stimulate or antagonize host immune responses
- Sterylglucosidase 1
an enzyme that metabolizes cryptococcal sterylglucosides. Genetic elimination of this gene in C. neoformans results in the accumulation of sterylglucosides
- Sterylglucosides
class of glycolipids produced by animals, plants, bacteria and various fungi including C. neoformans and Candida albicans. Sterylgucosides have been shown to have immunomodulatory properties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajasingham R, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. The Lancet Infectious diseases. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoham S, Marr KA. Invasive fungal infections in solid organ transplant recipients. Future microbiology. 2012;7:639–655. doi: 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Husain S, et al. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerging infectious diseases. 2001;7:375–381. doi: 10.3201/eid0703.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon-Chung KJ, et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb Perspect Med. 2014;4:a019760. doi: 10.1101/cshperspect.a019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu TB, et al. Molecular mechanisms of cryptococcal meningitis. Virulence. 2012;3:173–181. doi: 10.4161/viru.18685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powderly WG. Cryptococcal meningitis and AIDS. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- 7.Saag MS, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;30:710–718. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 8.Sorrell TC, et al. Cryptococcal transmigration across a model brain blood-barrier: evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes and infection. 2016;18:57–67. doi: 10.1016/j.micinf.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 9.van der Horst CM, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. The New England journal of medicine. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 10.Chau TT, et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis. 2010;10:199. doi: 10.1186/1471-2334-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, et al. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis. 2008;14:755–762. doi: 10.3201/eid1405.071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lizarazo J, et al. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl Trop Dis. 2014;8:e3272. doi: 10.1371/journal.pntd.0003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marr KA, et al. Cryptococcus gattii infection in healthy hosts: a sentinel for subclinical immunodeficiency? Clin Infect Dis. 2012;54:153–154. doi: 10.1093/cid/cir756. [DOI] [PubMed] [Google Scholar]
- 14.Litvintseva AP, et al. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in Sub-Saharan Africa. The Journal of infectious diseases. 2005;192:888–892. doi: 10.1086/432486. [DOI] [PubMed] [Google Scholar]
- 15.Hagen F, et al. Autochthonous and dormant Cryptococcus gattii infections in Europe. Emerg Infect Dis. 2012;18:1618–1624. doi: 10.3201/eid1810.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo T, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014;5:e00912–00914. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon-Chung K, Saijo T. Is Cryptococcus gattii a Primary Pathogen? Journal of Fungi. 2015;1:154. doi: 10.3390/jof1020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120:123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- 19.Byrnes EJ, 3rd, et al. First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PloS one. 2009;4:e5851. doi: 10.1371/journal.pone.0005851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walraven CJ, et al. Fatal disseminated Cryptococcus gattii infection in New Mexico. PLoS One. 2011;6:e28625. doi: 10.1371/journal.pone.0028625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCulloh RJ, et al. Cryptococcus gattii genotype VGI infection in New England. Pediatr Infect Dis J. 2011;30:1111–1114. doi: 10.1097/INF.0b013e31822d14fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galanis E, Macdougall L. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 2010;16:251–257. doi: 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Datta K, et al. Cryptococcus gattii: Emergence in Western North America: Exploitation of a Novel Ecological Niche. Interdiscip Perspect Infect Dis. 2009;2009:176532. doi: 10.1155/2009/176532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datta K, et al. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerging infectious diseases. 2009;15:1185–1191. doi: 10.3201/eid1508.081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emergence of Cryptococcus gattii– Pacific Northwest, 2004–2010. MMWR Morb Mortal Wkly Rep. 2010;59:865–868. [PubMed] [Google Scholar]
- 26.Loyse A, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. The Lancet Infectious diseases. 2013;13:629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 27.Aberg Judith A, et al. A Pilot Study of the Discontinuation of Antifungal Therapy for Disseminated Cryptococcal Disease in Patients with Acquired Immunodeficiency Syndrome, following Immunologic Response to Antiretroviral Therapy. The Journal of Infectious Diseases. 2002;185:1179–1182. doi: 10.1086/339680. [DOI] [PubMed] [Google Scholar]
- 28.Dromer F, et al. Epidemiology of HIV-associated cryptococcosis in France (1985–2001): comparison of the pre- and post-HAART eras. Aids. 2004;18:555–562. doi: 10.1097/00002030-200402200-00024. [DOI] [PubMed] [Google Scholar]
- 29.Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395–401. doi: 10.1016/S1473-3099(07)70085-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen SC, et al. Antifungal Therapy and Management of Complications of Cryptococcosis due to Cryptococcus gattii. Clin Infect Dis. 2013;57:543–551. doi: 10.1093/cid/cit341. [DOI] [PubMed] [Google Scholar]
- 31.Sungkanuparph S, et al. Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49:931–934. doi: 10.1086/605497. [DOI] [PubMed] [Google Scholar]
- 32.Pappas PG, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, et al. Cryptococcosis in solid organ transplant recipients: current state of the science. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;47:1321–1327. doi: 10.1086/524738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh N, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40:1756–1761. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 35.Bratton EW, et al. Approaches to antifungal therapies and their effectiveness among patients with cryptococcosis. Antimicrobial agents and chemotherapy. 2013;57:2485–2495. doi: 10.1128/AAC.01800-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu XP, et al. Association of Fcgamma receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients. PloS one. 2012;7:e42439. doi: 10.1371/journal.pone.0042439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou XT, et al. Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. The Journal of infectious diseases. 2011;203:1686–1691. doi: 10.1093/infdis/jir152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohatgi S, et al. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. mBio. 2013;4:e00573–00513. doi: 10.1128/mBio.00573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine MM, Sztein MB. Vaccine development strategies for improving immunization: the role of modern immunology. Nat Immunol. 2004;5:460–464. doi: 10.1038/ni0504-460. [DOI] [PubMed] [Google Scholar]
- 40.Mody CH, et al. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect Immun. 1999;67:936–941. doi: 10.1128/iai.67.2.936-941.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olszewski MA, et al. Mechanisms of cryptococcal virulence and persistence. Future microbiology. 2010;5:1269–1288. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak KL, et al. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PloS one. 2009;4:e6854. doi: 10.1371/journal.pone.0006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchanan KL, Doyle HA. Requirement for CD4(+) T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infection and immunity. 2000;68:456–462. doi: 10.1128/iai.68.2.456-462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huffnagle GB, et al. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 45.Huffnagle GB, et al. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infection and immunity. 1991;59:1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huffnagle GB, et al. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. The Journal of experimental medicine. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mody CH, et al. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. Journal of immunology. 1990;144:1472–1477. [PubMed] [Google Scholar]
- 48.Chang CC, et al. HIV and co-infections. Immunol Rev. 2013;254:114–142. doi: 10.1111/imr.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pappas PG. Cryptococcal infections in non-hiv-infected patients. Trans Am Clin Climatol Assoc. 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 50.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507–544. v–vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Hole CR, Wormley FL., Jr Vaccine and immunotherapeutic approaches for the prevention of cryptococcosis: lessons learned from animal models. Frontiers in microbiology. 2012;3:291. doi: 10.3389/fmicb.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rella A, et al. Role of Sterylglucosidase 1 (Sgl1) on the pathogenicity of Cryptococcus neoformans: potential applications for vaccine development. Frontiers in microbiology. 2015;6:836. doi: 10.3389/fmicb.2015.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wozniak KL, et al. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clinical and vaccine immunology: CVI. 2011;18:717–723. doi: 10.1128/CVI.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindell DM, et al. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. Journal of immunology. 2005;174:7920–7928. doi: 10.4049/jimmunol.174.12.7920. [DOI] [PubMed] [Google Scholar]
- 55.Wormley FL, Jr, et al. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infection and immunity. 2007;75:1453–1462. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Netea MG, et al. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 57.Kleinnijenhuis J, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintin J, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leopold Wager CM, et al. STAT1 Signaling within Macrophages Is Required for Antifungal Activity against Cryptococcus neoformans. Infection and immunity. 2015;83:4513–4527. doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Valtanen P, et al. Evaluation of trained immunity by beta-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunology and cell biology. 2017 doi: 10.1038/icb.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaragoza O, et al. The capsule of the fungal pathogen Cryptococcus neoformans. Advances in applied microbiology. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Devi SJ, et al. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infection and immunity. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nussbaum G, et al. Molecular and idiotypic analyses of the antibody response to Cryptococcus neoformans glucuronoxylomannan-protein conjugate vaccine in autoimmune and nonautoimmune mice. Infection and immunity. 1999;67:4469–4476. doi: 10.1128/iai.67.9.4469-4476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devi SJ. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996;14:841–844. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, et al. Peptide epitopes recognized by a human anti-cryptococcal glucuronoxylomannan antibody. Infection and immunity. 1997;65:1158–1164. doi: 10.1128/iai.65.4.1158-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fleuridor R, et al. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. Journal of immunology. 2001;166:1087–1096. doi: 10.4049/jimmunol.166.2.1087. [DOI] [PubMed] [Google Scholar]
- 67.Datta K, et al. Therapeutic efficacy of a conjugate vaccine containing a peptide mimotope of cryptococcal capsular polysaccharide glucuronoxylomannan. Clinical and vaccine immunology: CVI. 2008;15:1176–1187. doi: 10.1128/CVI.00130-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maitta RW, et al. Efficacy of immune sera from human immunoglobulin transgenic mice immunized with a peptide mimotope of Cryptococcus neoformans glucuronoxylomannan. Vaccine. 2004;22:4062–4068. doi: 10.1016/j.vaccine.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 69.Casadevall A, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrobial agents and chemotherapy. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larsen RA, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrobial agents and chemotherapy. 2005;49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rachini A, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infection and immunity. 2007;75:5085–5094. doi: 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodrigues ML, et al. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clinical and vaccine immunology: CVI. 2007;14:1372–1376. doi: 10.1128/CVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosas AL, et al. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infection and immunity. 2001;69:3410–3412. doi: 10.1128/IAI.69.5.3410-3412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012;11:447–456. doi: 10.1016/j.chom.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll SF, et al. Susceptibility to progressive Cryptococcus neoformans pulmonary infection is regulated by loci on mouse chromosomes 1 and 9. Infection and immunity. 2012;80:4167–4176. doi: 10.1128/IAI.00417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shourian M, et al. The Cnes2 locus on mouse chromosome 17 regulates host defense against cryptococcal infection through pleiotropic effects on host immunity. Infection and immunity. 2015;83:4541–4554. doi: 10.1128/IAI.00697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Upadhya R, et al. Induction of Protective Immunity to Cryptococcal Infection in Mice by a Heat-Killed, Chitosan-Deficient Strain of Cryptococcus neoformans. mBio. 2016;7:e00547–16. doi: 10.1128/mBio.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoag KA, et al. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. American journal of respiratory cell and molecular biology. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 79.Perfect JR, et al. Chronic cryptococcal meningitis: a new experimental model in rabbits. The American journal of pathology. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- 80.Fromtling RA, et al. Immunization of mice with an avirulent pseudohyphal form of Cryptococcus neoformans. Mycopathologia. 1979;68:179–181. doi: 10.1007/BF00578527. [DOI] [PubMed] [Google Scholar]
- 81.Fromtling RA, et al. Immunization of mice with stable, acapsular, yeast-like mutants of Cryptococcus neoformans. Sabouraudia. 1983;21:113–119. [PubMed] [Google Scholar]
- 82.Murphy JW, et al. Antigen-induced protective and nonprotective cell-mediated immune components against Cryptococcus neoformans. Infection and immunity. 1998;66:2632–2639. doi: 10.1128/iai.66.6.2632-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wormley FL, Jr, et al. Evaluation of host immune responses to pulmonary cryptococcosis using a temperature-sensitive C. neoformans calcineurin A mutant strain. Microb Pathog. 2005;38:113–123. doi: 10.1016/j.micpath.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Zhai B, et al. Development of Protective Inflammation and Cell-Mediated Immunity against Cryptococcus neoformans after Exposure to Hyphal Mutants. mBio. 2015;6:e01433–15. doi: 10.1128/mBio.01433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baker LG, et al. Cell wall chitosan is necessary for virulence in the opportunistic pathogen Cryptococcus neoformans. Eukaryotic cell. 2011;10:1264–1268. doi: 10.1128/EC.05138-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pirofski LA, Casadevall A. Use of licensed vaccines for active immunization of the immunocompromised host. Clinical microbiology reviews. 1998;11:1–26. doi: 10.1128/cmr.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy JW, et al. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988;56:424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levitz SM, et al. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci U S A. 2001;98:10422–10427. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, et al. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-Cell responses. Infection and immunity. 2002;70:5485–5493. doi: 10.1128/IAI.70.10.5485-5493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Specht CA, et al. Contribution of glycosylation to T cell responses stimulated by recombinant Cryptococcus neoformans mannoprotein. The Journal of infectious diseases. 2007;196:796–800. doi: 10.1086/520536. [DOI] [PubMed] [Google Scholar]
- 91.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS yeast research. 2006;6:513–524. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 92.Pietrella D, et al. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005;73:820–827. doi: 10.1128/IAI.73.2.820-827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dan JM, et al. Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS One. 2008;3:e2046. doi: 10.1371/journal.pone.0002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coenjaerts FE, et al. Potent inhibition of neutrophil migration by cryptococcal mannoprotein-4-induced desensitization. J Immunol. 2001;167:3988–3995. doi: 10.4049/jimmunol.167.7.3988. [DOI] [PubMed] [Google Scholar]
- 95.Mansour MK, et al. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infection and immunity. 2004;72:1746–1754. doi: 10.1128/IAI.72.3.1746-1754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biondo C, et al. Characterization of two novel cryptococcal mannoproteins recognized by immune sera. Infection and immunity. 2005;73:7348–7355. doi: 10.1128/IAI.73.11.7348-7355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kakeya H, et al. Heat shock protein 70 (hsp70) as a major target of the antibody response in patients with pulmonary cryptococcosis. Clin Exp Immunol. 1999;115:485–490. doi: 10.1046/j.1365-2249.1999.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kakeya H, et al. A 77-kilodalton protein of Cryptococcus neoformans, a member of the heat shock protein 70 family, is a major antigen detected in the sera of mice with pulmonary cryptococcosis. Infection and immunity. 1997;65:1653–1658. doi: 10.1128/iai.65.5.1653-1658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young M, et al. A proteomic-based approach for the identification of immunodominant Cryptococcus neoformans proteins. Proteomics. 2009;9:2578–2588. doi: 10.1002/pmic.200800713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mandel MA, et al. The Cryptococcus neoformans gene DHA1 encodes an antigen that elicits a delayed-type hypersensitivity reaction in immune mice. Infect Immun. 2000;68:6196–6201. doi: 10.1128/iai.68.11.6196-6201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Biondo C, et al. Identification and cloning of a cryptococcal deacetylase that produces protective immune responses. Infect Immun. 2002;70:2383–2391. doi: 10.1128/IAI.70.5.2383-2391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biondo C, et al. Induction of T helper type 1 responses by a polysaccharide deacetylase from Cryptococcus neoformans. Infect Immun. 2003;71:5412–5417. doi: 10.1128/IAI.71.9.5412-5417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaturvedi AK, et al. Vaccine-mediated immune responses to experimental pulmonary Cryptococcus gattii infection in mice. PloS one. 2014;9:e104316. doi: 10.1371/journal.pone.0104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaturvedi AK, et al. Identification and characterization of Cryptococcus neoformans protein fractions that induce protective immune responses. Proteomics. 2013;13:3429–3441. doi: 10.1002/pmic.201300213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins N, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011;7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5:e1000471. doi: 10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh SD, et al. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009;5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 109.Specht CA, et al. Protection against Experimental Cryptococcosis following Vaccination with Glucan Particles Containing Cryptococcus Alkaline Extracts. mBio. 2015;6:e01905–01915. doi: 10.1128/mBio.01905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mor V, et al. Glucosylceramide Administration as a Vaccination Strategy in Mouse Models of Cryptococcosis. PloS one. 2016;11:e0153853. doi: 10.1371/journal.pone.0153853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watanabe T, et al. Sterylglucoside catabolism in Cryptococcus neoformans with endoglycoceramidase-related protein 2 (EGCrP2), the first steryl-beta-glucosidase identified in fungi. The Journal of biological chemistry. 2015;290:1005–1019. doi: 10.1074/jbc.M114.616300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giaccone G, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 113.Kim S, et al. Glycolipid ligands of invariant natural killer T cells as vaccine adjuvants. Expert review of vaccines. 2008;7:1519–1532. doi: 10.1586/14760584.7.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parekh VV, et al. The in vivo response of invariant natural killer T cells to glycolipid antigens. International reviews of immunology. 2007;26:31–48. doi: 10.1080/08830180601070179. [DOI] [PubMed] [Google Scholar]
- 115.Yanagisawa K, et al. Hyporesponsiveness to natural killer T-cell ligand alpha-galactosylceramide in cancer-bearing state mediated by CD11b+ Gr-1+ cells producing nitric oxide. Cancer research. 2006;66:11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]


